 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Exploring plant growth-promoting (PGP) bacterial activity of microbial components aggregated by wastewater treatment can reduce dependence on fossil fuel-derived fertilisers. This study describes the isolation and identification of bacteria from microalgae-bacteria flocs (MaB-flocs) generated in high-rate algal oxidation ponds (HRAOP) of an integrated algal pond system (IAPS) remediating municipal wastewater. Amplified 16S rRNA gene sequence analysis determined the molecular identity of the individual strains. Genetic relatedness to known PGP rhizobacteria in the NCBI GenBank database was by metagenomics. Isolated strains were screened for the production of indoles (measured as indole-3-acetic acid; IAA) and an ability to mineralise ,
, and K + . Of the twelve bacterial strains isolated from HRAOP MaB-flocs, four produced indoles, nine mineralised
, seven solubilised P, and one K. Potential of isolated strains for PGP activity according to one-way ANOVA on ranks was: ECCN 7b > ECCN 4b > ECCN 6b > ECCN 3b = ECCN 10b > ECCN 1b = ECCN 5b > ECCN 8b > ECCN 2b > ECCN 12b > ECCN 9b = ECCN 11b. Further study revealed that cell-free filtrate from indole-producing cultures of Aeromonas strain ECCN 4b, Enterobacter strain ECCN 7b, and Arthrobacter strain ECCN 6b promoted mung bean adventitious root formation suggestive of the presence of auxin-like biological activity.
1. Introduction
Intensive non-sustainable land use and agricultural practice to satisfy global commodity and food demand inflict severe damage on arable soil and freshwater resources, increasing pressure on an already strained water-energy-food nexus. A recent review on the contribution of microorganisms in land restoration strongly supports soil microbiota as the functional agents in forming and sustaining soil [Citation1]. Research in our laboratory has indicated that in situ biological production of humic substances represents a dynamic equilibrium involving transforming and processing soil microorganisms that assimilate and bio-convert molecular components within the rhizosphere to increase soil organic matter (SOM) and sustain plant growth [Citation2].
Plant growth-promoting bacteria (PGPB) are potentially bio-stimulants and biofertilisers in agriculture and phytoremediation and have the potential to offset dependence on fossil fuel-derived fertilisers and synthetic chemical pesticides and contribute to increased food security [Citation3–7]. Plant growth-promoting bacteria are rhizospheric, comprise diverse genera, and typically mineralise nutrients. Additionally, these bacteria produce siderophores, phytohormones, 1-amino-cyclopropane-1-carboxylic acid (ACC) deaminase, promote plant growth and development [Citation4,Citation8], enhance stress tolerance [Citation9], and stimulate rhizodeposition [Citation10].
Furthermore, studies in our laboratory on multidrug resistance profiling as a tool for microbial source tracking confirmed that municipal wastewater treatment works (WWTW) receive aggregate effluents from local and regional environments and are point sources of xenobiotics, genetic material but more importantly, bacteria [Citation11]. As mentioned earlier, municipal wastewater remediation was by an integrated algal pond system (IAPS) comprising shallow, paddlewheel-driven, continuously mixed high-rate oxidation ponds [Citation12]. In these high-rate reactors, profuse algal growth is facilitated by nutrient load and solar irradiation, while heterotrophic bacteria consume photosynthetically-generated oxygen to assimilate residual organic matter [Citation13–15].
Aggregation of microalgae-bacteria (MaB) to form stable MaB-flocs occurs in high-rate algal oxidation ponds (HRAOP) due to prolonged periods of elevated pH and high alkalinity, which in turn increases productivity and recovery of biomass [Citation16–20]. Pond productivity is a consequence of microbial mutualism in which photosynthetically-derived O2 supports the mineralisation of inorganic nutrients and conversion of organic matter by heterotrophic bacteria into ammonium, oxyanions such as nitrates and phosphates and CO2, which are then assimilated by microalgae that are aggregated into MaB-flocs [Citation21]. Since high-rate pond-derived bacteria carry out the oxidative breakdown of dissolved and suspended organic matter, it seemed reasonable to suspect that these heterotrophs might be microorganisms involved in transforming and processing organic matter and with characteristics typical of PGP bacteria/rhizobacteria.
It has become obvious that, with limited arable land and reduced water availability, particularly in the peri-urban space, enormous strain/stress is being exerted on local/regional food production systems. Such outcomes invariably lead to decreased biodiversity, species composition and dominance changes, and toxicity effects that further impact food security. Previous research in our laboratory has led to the innovation of IAPS as a wastewater bioremediation process and platform technology for the peri-urban space to support the development of microalgal biorefineries and to mitigate an already strained water-energy-food nexus [Citation12,Citation22–24]. In the present study, we focus entirely on the potential of the bacterial component of MaB-flocs as a source of PGP microbes for incorporation, either singly or in consort, into bio-fertilizers. To date, and as far as we can determine, no known PGP bacteria have been isolated from MaB-flocs. Here, we report on the isolation, biochemical and molecular characterisation of PGP bacterial isolates from MaB-flocs formed in HRAOP of an IAPS treating municipal wastewater.
2. Materials and methods
2.1. Configuration of IAPS and isolation of bacteria from maB-flocs
The IAPS used in this study is located at the Institute for Environmental Biotechnology (EBRU) experimental station at Belmont Valley Wastewater Treatment Works (WWTW), Makhanda, South Africa (33°19′07″ South, 26°33′25″ East) and has a design capacity of 75 m3 d−1 or 500 person equivalents (PE). The system was operated continuously from 1994 to 2021 and comprised an in-pond digester or fermentation pit, an advanced facultative pond, two HRAOPs, and two algal settler ponds configured in series. System configuration, process flow, and water treatment efficiency have been previously reported [Citation12,Citation18,Citation25]. Due to the dereliction of the Belmont Valley WWTW and the ever-diminishing availability of adequately screened and de-gritted wastewater, effective January 2021, it was decided to decommission this demonstration facility.
Samples of mixed liquor were abstracted from a point immediately in front of the paddlewheel near the inlet to the HRAOP, where the water column is well mixed, transferred to the laboratory under aseptic conditions, MaB-floc aggregates were disrupted by gentle mixing and serial dilutions of each sample prepared. Aliquots (100 µL) were applied to 90 mm Petri dishes containing either nutrient agar (NA), Luria–Bertani agar (LBA), or modified American Type Culture Collection agar (ATCC 111A) and incubated at 30°C for 2–5 d. Individual bacterial colonies were picked, and parallel non-overlapping streaks were made using fresh plates to derive pure colonies. Morphological and biochemical characteristics were established following the confirmation of the purity of the isolates [Citation26,Citation27].
2.2. Morphological and biochemical properties of isolated strains
Scanning electron microscopy (SEM) was carried out essentially as described by Olawale et al. [Citation28]. Morphological properties, including shape, colour, diameter, surface, margin, elevation, opacity, and consistency, were determined using previously described methods [Citation29–31]. Biochemical properties to aid identification included Gram stain status and catalase-activity assays [Citation30,Citation32].
2.3. Molecular characterisation of isolated strains
Genomic DNA was extracted using the Quick-DNATM Fungal/Bacterial Miniprep Kit (Zymo Research), and the 16S rRNA target region was amplified using OneTaq® Quick-Load® 2X Master Mix (NEB) with the universal primers 27F (5′-AGAGTTTGATC MTGGCTCAG-3′) and 1492R(5′-CGGTTACCTTGTTACGACTT-3′) [Citation33,Citation34]. The resultant PCR products were separated by gel electrophoresis and recovered using the ZymocleanTM DNA Gel Recovery Kit (Zymo Research). The extracted fragments were sequenced in the forward and reverse directions (Nimagen, BrilliantDyeTM Terminator Cycle Sequencing Kit) and purified (Zymo Research, ZR-96 DNA Sequencing Clean-up KitTM). Purified fragments were analysed using an ABI 3500XL Analyser (Applied Biosystems, Foster City, CA, USA). Chromatograms were converted to text format using Chromas and entered into the National Centre for Biotechnology Information (NCBI). The Basic Local Alignment Search Tool (BLAST) was used to locate regions of local similarity between sequences and the results were submitted to GenBank using BankIT to obtain accession numbers and the assigned nearest taxonomic designation.
2.4. Phylogenetic analysis
The phylogenetic relatedness of bacterial strains to known PGP rhizobacteria was determined using MEGA version 6. Thirty-four reference nucleotide sequences were sourced from the NCBI GenBank database, and multiple alignments were deduced using the Neighbour-Joining method. The trees in which associated taxa clustered were bootstraps of 1000 replications, and the distances were calculated using the Jukes-Cantor method [Citation35].
2.5. Screening for PGP-like characteristics
2.5.1. Indole-3-acetic acid
To determine the ability of the isolated bacterial strains to produce indole-3-acetic acid (IAA), NB was supplemented with L-trp, and the concentration of indolic compounds was determined using Salkowski’s reagent [Citation36–38]. To determine indole production potential, NB supplemented with L-trp (1 g.L−1) was used as a medium, inoculated with 0.05 mL of each isolated strain seed culture, and incubated for 3 d at 30°C. Thereafter, samples were centrifuged (18,900 × g for 10 min), and the concentration of indoles was quantified by adding two drops of 10 mM H3PO4 to 2 mL supernatant followed by 4 mL Salkowski reagent (prepared by adding 1.0 mL 0.5 M FeCl3 to 50 mL 35% HClO4) and expressed as IAA equivalents. The mixture was allowed to stand at room temperature for approximately 15 min. The quantity of indoles was determined spectrophotometrically at 530 nm, followed by interpolation from a standard curve of authentic IAA (Sigma-Aldrich) and background subtracted to account for any interference from the culture medium as described elsewhere [Citation27].
2.5.2. Ammonium mineralisation
Ammonia production activity was assessed using peptone water (PW) at pH 7.2 with Nessler’s reagent as the indicator [Citation39,Citation40]. Inoculants were added to 50 mL of PW and incubated on a rotary shaker for 3 d at 30°C. Thereafter, 30 mL from each sample was centrifuged (18,900 × g for 10 min), and 5 mL of supernatant from each reacted with 0.5 mL Nessler’s reagent, and the mixture was allowed to stand at room temperature for 5 min for maximum development of brown colour. Nessler’s reagent was prepared fresh by suspending it in 100 mL of distilled water, 10 g of HgCl2, 7 g of KI, and 16 g of NaOH. Quantification of NH3 was by spectrophotometric analysis at 430 nm followed by interpolation from a standard curve for NH4Cl [Citation41] and data expressed as NH4Cl equivalents.
2.5.3. Phosphate and potassium solubilisation
Phosphate solubilisation activity was determined using Pikovskayas (PVK) medium and tri-calcium orthophosphate (Ca3(PO4)2) as the sole source of insoluble phosphate [Citation42]. For screening, 0.05 mL aliquots of seed culture were inoculated into 50 mL PVK broth, and -solubilisation activity was quantified after incubation at 30°C. Cultures were centrifuged (18,900 × g, 10 min) and
in the supernatant determined spectrophotometrically using a Phosphate Test kit (Merck KGaA, Darmstadt, Germany) after interpolation from a standard curve at 690 nm.
For potassium solubilisation activity, Aleksandrow’s medium was used with potassium aluminosilicate (AlKO6Si2) as the insoluble K-containing substrate [Citation43]. Screening involved inoculating 0.05 mL of seed culture to 50 mL of Aleksandrow’s broth and growth in liquid culture for 7 d at 30°C. Aliquots of freshly prepared culture were centrifuged (18,900 × g, 10 min), and the K concentration of the supernatant was determined spectrophotometrically using a K test kit according to the manufacturer’s instructions (Merck KGaA, Darmstadt, Germany).
2.6. Plant material and adventitious rooting bioassay
Mung bean seeds (Vigna radiata (L.) R. Wilczek) were surface-sterilized by immersion in 80% ethanol for 2 min and then washed with distilled water. Seeds were sown in cotton soaked in distilled water in open Petri dishes and incubated in darkness for 3 d at 20°C. Germinated seedlings were transferred to laboratory light, and growth was allowed to proceed under ambient conditions for a minimum of 7 d or until hypocotyls were ∼5 cm long.
Hypocotyls from similar sized 10-d old seedlings were cut to 4.5 cm and placed in test tubes containing 12.5 mL of test solution comprising; bacteria-free supernatant (18,900 × g for 10 min) from cultures of either Aeromonas strain ECCN 4b, Arthrobacter strain ECCN 6b or Enterobacter strain ECCN 7b grown in NB supplemented with L-trp (1 g.L−1), non-inoculated broth as a background control (Con) and NB containing authentic IAA as the positive control (Con+). Explants were incubated in laboratory light under ambient conditions for 10 d. Hypocotyls were assessed daily for adventitious root (AR) formation, and the number of AR was recorded. Where specified, and following a 3 h acclimation period, each hypocotyl’s initial fresh mass (FMt0) was determined. The final fresh mass (FMt10) was determined after 10 d. Thereafter, hypocotyls were oven dried at 50°C to a constant weight, and the final dry mass (DMt10) was determined.
2.7. Data analysis
Data were computed using either the statistical function in Sigma Plot version 11 (Systat Software Inc., San Jose, CA, USA) or Excel (version 16, Microsoft Corporation, Redmond, WA). Where necessary, results were analysed by one-way analysis of variance (ANOVA) and significant differences between measurements for treatments determined (Holm–Sidak method; p < 0.05). Data are presented as the mean of at least three determinations ± standard error (SE). The plant growth-promoting activity potential of novel isolates was assessed using a Kruskal–Wallis one-way ANOVA on ranks in the analysis function in SigmaPlot version 13 (Systat Software Inc., San Jose, CA, USA).
3. Results
3.1. Morphological and physiological characteristics
Following gentle de-aggregation of MaB-flocs from HRAOP, 12 bacterial isolates were purified and considered morphologically distinct based on colony shape, colour, diameter, surface, margin, elevation, density, and consistency (). Colonies were typically 1–4 mm in diameter, circular, smooth, opaque, and homogenous with elevation raised, except for ECCN 11b, which was flat and had a colony diameter <1 mm. Several colonies were pigmented, ranging from white through creamy yellow to orange and orange-brown. Unsurprisingly, all isolates tested positive for catalase which protects cells from oxidative damage and might be expected of bacteria isolated from super-saturated oxygenated wastewater [Citation12,Citation18]. Four isolates, ECCN 4b, ECCN 7b, ECCN 10b, and ECCN 11b, were Gram-negative, while eight, ECCN 1b, ECCN 2b, ECCN 3b, ECCN 5b, ECCN 6b, ECCN 8b, ECCN 9b, and ECCN 12b tested Gram-positive indicative of a peptidoglycan-containing cell wall. More detailed morphological analysis by SEM revealed all bacterial strains to be either coccobacilli (i.e. short rod- or oval-shaped) or rod-shaped ((A–L)).
Figure 1. Scanning electron micrographs showing cell morphology of the twelve bacterial isolates obtained from HRAOP MaB-flocs. ECCN 1b (A); ECCN 2b (B); ECCN 3b (C); ECCN 4b (D); ECCN 5b (E); ECCN 6b (F); ECCN 7b (G); ECCN 8b (H); ECCN 9b (I); ECCN 10b (J); ECCN 11b (K) and ECCN 12b (L).
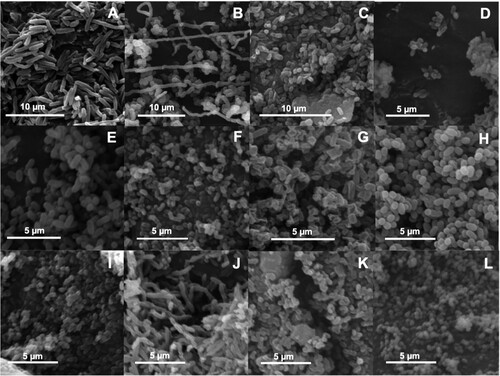
Table 1. Morphological and biochemical characteristics of bacterial colonies of isolates obtained from MaB-flocs formed in the HRAOP of an IAPS supplied municipal sewage.
3.2. Molecular identification of bacterial isolates
Partial 16S rRNA gene sequences from each isolate, ranging from 1389 to 1547 bp, were amplified, and the taxonomic identity was determined following BLAST analysis (). The twelve strains isolated in this study formed nine taxonomic genera comprising seven families, with Bacilliaceae (41.67%) and Microbacteriaceae (16.67%) predominant. Results of sequence similarities showed that five isolates had 100% sequence homology to reference strains (). These were confirmed as Ancylobacter sp. strain ECCN 11b (MW672584), Arthrobacter sp. strain ECCN 6b (MW672569), Bacillus sp. strain ECCN 3b (MW672570), Fictibacillus sp. strain ECCN 2b (MW672569) and Pseudomonas sp. strain ECCN 10b (MW672582).
Table 2. Molecular identity of bacteria isolated from MaB-flocs formed in HRAOP of the Belmont Valley IAPS supplied municipal sewage.
Five other isolates, Aeromonas sp. strain ECCN 4b (MW672571), Bacillus sp. strain ECCN 1b (MW672568), Enterobacter sp. strain ECCN 7b (MW672577), and two Exiguobacterium spp., strains ECCN 5b (MW672573) and ECCN 8b (MW672579) showed 99% sequence homology to reference strains. However, the remaining two Microbacterium spp. strains ECCN 9b (MW672580) and ECCN 12b (MW672585) showed 98% and 91% homology, respectively. Perhaps unsurprisingly, Bacillus was the most represented genus accounting for 41.67%, followed by Microbacterium at 16.67%, while others, including Aeromonas sp., Ancylobacter sp., Arthrobacter sp., Enterobacter sp., and Pseudomonas sp. accounted for 8.33%.
3.3. Phylogenetic relatedness of isolates to PGP rhizobacteria
The twelve bacterial strains isolated from HRAOP mixed liquor MaB-flocs and identified by PCR amplification and analysis of 16S rRNA gene sequences were characterised after phylogenetic analysis of the sequencing relationship with known PGP bacteria reference strains already deposited in the NCBI GenBank database, and the results are shown in .
Figure 2. Dendrogram showing the genetic relationship between the twelve potential PGPR strains (♦) isolated from MaB-flocs generated in HRAOP and reference strains contained in the GenBank database. Assigned strains and GenBank accession numbers are located to the right of each strain. Bootstrap test (1000 replicates) above branches, bootstrap values ≥56% at nodes and bar, substituting 5 nucleotides per 100.
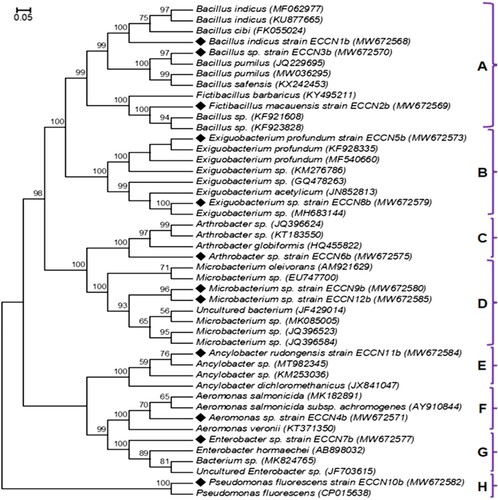
Using the 34 NCBI GenBank-generated reference nucleotide sequences for PGPR, the 12 novel strains grouped into eight distinct but related clusters assigned as; Bacillus (A); Exiguobacterium (B); Arthrobacter (C); Microbacterium (D); Ancylobacter (E); Aeromonas (F); Enterobacter (G) and Pseudomonas (H) as indicated in . However, 15 sequences were grouped individually with respective PGPR relatives and showed 100% bootstrap support at the corresponding master nodes. The clusters all showed high bootstrap values with many PGPR reference strains contained in the GenBank database. Only one cluster, the one containing Pseudomonas sp. strain ECCN 10b (MW672582), was outside of the tree and shared significant similarity (100%) with P. fluorescens (CP015638) as a reference GenBank PGPR sequenced strain.
3.4. Screening for PGP characteristics
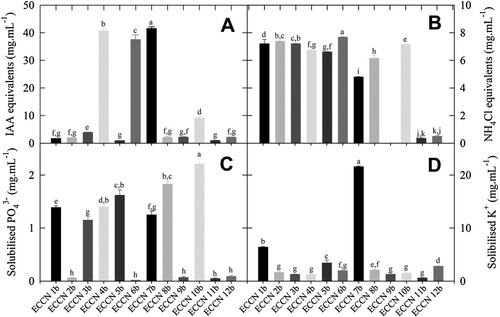
Indole-3-acetic acid production potential was established by monitoring the formation of indolic compounds spectrophotometrically after incubation of each novel isolate in NB supplemented with L-trp, and the results are shown in (A). All strains showed some potential to produce indoles, but the most prominent were; Aeromonas strain ECCN 4b, Arthrobacter strain ECCN 6b, and Enterobacter strain ECCN 7b, in which indole production was ≥ 40 μg.mL−1.
Figure 3. Potential plant growth regulator production and essential nutrient mineralization by bacterial strains isolated from MaB-flocs produced in mixed liquor of the HRAOP of an IAPS supplied municipal sewage. Estimates of indole production (A); ammonium production (B); solubilised phosphate (C); and, solubilised potassium (D) by these bacterial isolates in stationary phase (OD600 = 0.4 at 30°C) were determined spectrophotometrically. Results are the mean ± S.D. Means denoted by a different letter indicate significant differences (p < 0.05).
Ammonia production ((B)), phosphate ((C)), and potassium ((D)) solubilisation were assayed spectrophotometrically following liquid cultivation of isolates in appropriate media, i.e. either PW, PVK, or Aleksandrow’s medium (AM).
Bacterial NH3 production, measured as NH4Cl equivalents, appeared to be a common characteristic of many bacterial isolates, and most produced significant amounts of NH3, except Microbacterium strain ECCN 9b, Ancylobacter strain ECCN 11b, and Microbacterium strain ECCN 12b ((B)). And for competent strains, including Bacillus strain ECCN 1b, Fictibacillus strain ECCN 2b, Bacillus strain ECCN 3b, Aeromonas strain ECCN 4b, Exiguobacterium strain ECCN 5b, Exiguobacterium strain ECCN 8b, Pseudomonas strain ECCN 10b, and Arthrobacter strain ECCN 6b, the estimated NH3 produced was ≥ 5 μg.mL−1.
Five of the isolates grew successfully on PVK agar, but preliminary screening showed that only Pseudomonas strain ECCN 10b caused the development of clear zones around colonies after 9 d of incubation at 30°C. However, since many isolates do not develop clear zones indicative of phosphate solubilisation in plate assay [Citation44], screening for the release of phosphates from insoluble inorganic phosphate was carried out in a liquid medium. (C) shows that seven of the novel isolates solubilised insoluble inorganic phosphate. Mean values for solubilisation ranged from 1.15 to 2.21 µg.mL−1, with Pseudomonas strain ECCN 10b being the most active. Other competent phosphate-solubilizing bacteria were Exiguobacterium strain ECCN 8b, Exiguobacterium strain ECCN 5b, Aeromonas strain ECCN 4b, Bacillus strain ECCN 1b, Enterobacter strain ECCN 7b, and Bacillus strain ECCN 3b.
Of the 12 isolates, three were able to grow on AM in plate culture, including Bacillus strain ECCN 3b, Pseudomonas strain ECCN 10b, and Enterobacter strain ECCN 7b with the latter, the only bacterium to display a halo or zone around colonies indicative of K+ solubilisation (data not shown). Enterobacter strain ECCN 7b and, to a much lesser extent, Bacillus strain ECCN 1b were the only strains demonstrating a significant ability to solubilise inorganic K+ in a liquid medium ((D)).
To better assess isolate performance in terms of potential PGP activity based on indole and NH3 production and K + - and -solubilising activity, a one-way ANOVA on ranks was carried out using the continuous level data from . Results from all multiple pairwise comparisons with the Tukey test showed that PGP activity potential was based on the production of indolic compounds (i.e. IAA equivalents; P = 0.009) and ammonia (i.e. NH4Cl equivalents; P = 0.011) and less so on solubilisation of K+ and
().
Table 3. Ranking of novel bacterial strains according to PGP activity potential. Strains were isolated from MaB-flocs in mixed liquor of the HRAOP of an IAPS supplied municipal sewage.
3.5. Adventitious root formation
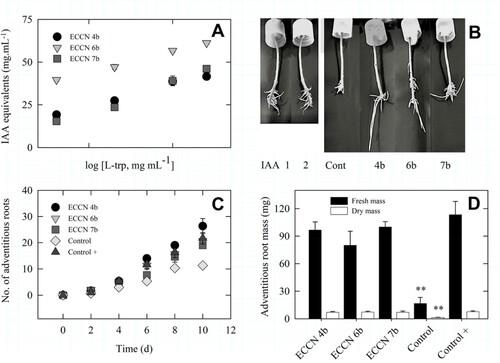
Indole production increased linearly for cultures of the top-ranked strains viz., Enterobacter ECCN 7b, Aeromonas ECCN 4b, and Arthrobacter ECCN 6b in response to increasing concentrations of L-trp ((A)) and the resulting culture filtrates stimulated AR formation by mung bean hypocotyls ((B)). Indeed, more in-depth enumeration revealed that 10−1, 10−2, 10−3, and 10−4 dilutions prepared from each cell-free supernatant serially, 10−3 (i.e. 40–60 µg.mL−1 IAA equivalents) was best for demonstrating AR formation. Results in (C) show that 10−3 dilution of cell-free bacterial culture filtrates from strains ECCN 4b, ECCN 6b, and ECCN 7b all promoted AR and that filtrate from cultures of Aeromonas ECCN 4b was most active. The AR fresh and dry mass resulted in the trend shown in (D). There was no significant difference (P = 0.49; for AR FM; and P = 0.86 for AR DM at P ≤ 0.05) between the positive control (IAA-treated hypocotyls) and those treated with cell-free filtrate from L-trp-supplemented cultures of either Aeromonas ECCN 4b, Enterobacter ECCN 7b, or Arthrobacter ECCN 6b. Interestingly, the dry mass of these AR was almost identical, with no discernible difference in mass detected (P < 0.05).
Figure 4. Adventitious root formation in mung bean hypocotyls by cell-free filtrates from Aeromonas ECCN 4b, Arthrobacter ECCN 6b, and Enterobacter ECCN 7b grown in L-trp-supplemented medium. Indole production, measured as IAA equivalents, as a function of L-trp concentration (A); adventitious roots formed in mung bean hypocotyls treated either with IAA or cell-free bacterial filtrate (B); kinetics of adventitious root formation in mung bean hypocotyls treated either with IAA or cell-free bacterial filtrate (C); and, fresh and dry mass of the adventitious roots formed (D). Results are the mean ± S.D. Means followed by ** indicate significant differences from control + and the cell-free bacterial filtrate treatments (p < 0.05).
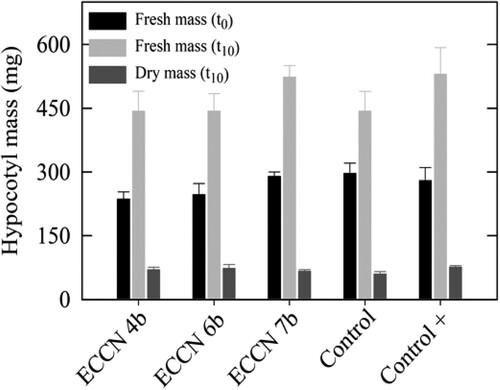
To discount the probability that the results in (D) were due to differences in the physiological status of the mung bean explants, initial fresh mass (FMt0), final fresh mass (FMt10), and final dry mass (DMt10) were determined, and the results are shown in . Statistical analysis and one-way ANOVA revealed no significant difference in either FMt0 (P = 0.086; P ≤ 0.050) or DMt10 (P = 0.053; P ≤ 0.050), confirming uniformity of the mung bean explants used in the bioassay.
Figure 5. Fresh and dry mass of mung bean hypocotyls treated either with IAA or cell-free culture filtrates from L-trp-supplemented cultures of Aeromonas ECCN 4b, Arthrobacter ECCN 6b, and Enterobacter ECCN 7b. Initial fresh mass (FMt0) was determined at start of the experiment (t0) while final fresh mass (FMt10) and dry mass (DMt10) were determined after a 10-d incubation period (t10). Results are the mean ± S.D. (p < 0.05).
4. Discussion
Secondary wastewater treatment involves removing biodegradable organic matter (BOD) and suspended solids (TSS) through oxygenation and either filtration or sedimentation. The process produces a treated effluent with BOD ≤ 25 mg.L−1 and/or TSS ≤ 30 mg.L−1. In nature-based processes such as IAPS, secondary treatment is brought about by the formation and proliferation of MaB-flocs in HRAOP [Citation18,Citation45] facilitated by extracellular polymeric substance production [Citation19,Citation46]. Bio-flocs facilitate water repair and recovery by enhancing the removal of pollutants, N and P, and the adsorption of suspended particulates [Citation14,Citation16,Citation19,Citation25,Citation47,Citation48]. And one calculation revealed that of the available organic carbon in IAPS wastewater, approximately 6 g C.m−2.d−1 is assimilated into MaB-floc biomass [Citation49]. Furthermore, the release of stimulatory products by bacteria that enhance microalgae growth and vice versa has been demonstrated and supports microbial mutualism within the flocs [Citation46,Citation50]. In addition to the effect on microalgae, bacteria (and fungi) produce gibberellins (GA) and auxins (typically IAA) as secondary metabolites. In contrast, others mineralise N, P, and K, and these growth-stimulating properties underpin the group of bacteria referred to as PGP [Citation3,Citation5–8,Citation51–53]. Even so, bacteria can exert both positive and negative impacts attributed to the production of plant hormones. Thus, several plant growth-promoting and phytopathogenic bacteria synthesise various plant hormones, the ethylene precursor 1-ACC [Citation54–60], and more recently, albeit in bio-engineered consortia, strigolactones [Citation61]. And in some cases, the ability to produce a plant hormone increases virulence [Citation62–65]. So, adequate screening must be done to confirm the plant growth-promoting properties of novel microbial isolates.
Bacteria isolated from IAPS-produced MaB-flocs typify those that occur as part of the human microbiome and exist in surface and mineral water and treated and untreated drinking and wastewater [Citation66]. The present study shows these bacteria share a phylogenetic relationship with PGP rhizobacteria. Twelve sequences clustered individually with their respective PGP bacterial relatives, virtually all showing 100% bootstrap support at the corresponding major nodes [Citation27,Citation67]. Furthermore, the potential for PGP activity of these isolates from one-way ANOVA on ranks was: ECCN 7b > ECCN 4b > ECCN 6b > ECCN 3b = ECCN 10b > ECCN 1b = ECCN 5b > ECCN 8b > ECCN 2b > ECCN 12b > ECCN 9b = ECCN 11b with strains Enterobacter ECCN 7b, Aeromonas ECCN 4b, and Arthrobacter ECCN 6b strong producers of indolic compounds, measured as IAA equivalents, and promoters of AR formation in mung bean explants.
Mung bean, a Fabaceae plant, is one of many species of Phaseolus (e.g. P. aureus and P. radiatus) moved to the genus Vigna and, globally, a very important legume crop. Cultivated in tropical and subtropical regions, mung bean is used for human food and as a green manure and livestock feed. Mung bean seeds are a good source of nutrients and are considered a substitute for meat in vegan and vegetarian dishes. Compared to some commonly grown crops, these legumes are among the most water efficient and have one of the smallest carbon footprints of any plant-based source of protein. In fact, they require less water and emit less CO2 to produce than many other row crops. From a research point of view, the mung bean rooting bioassay was developed as a sensitive and relatively specific bioassay for quantifying AR initiation in response to potential plant growth regulator substances with auxin-like activity [Citation68]. The assay is also sensitive to exogenous auxins, and the number of root primordia formed is a function of auxin concentration [Citation69]. Interestingly, while indole- and IAA-induced changes in hypocotyl fresh mass in the present work mirrored the AR response as reported for mung bean by Li et al. [Citation70], the dry mass of both the hypocotyl explants and the AR formed was similar across all treatments. Thus, AR formation in mung bean exposed to exogenous auxin seems to occur independently of carbon, nitrogen and nutrient availability or acquisition at the explant level, and more so on auxin-induced cell de-differentiation, determination of root founder cells, differentiation of these founder root cells into AR, and establishment of sink strength as posited by Druege et al. [Citation71].
Aeromonas and Enterobacter are Gram-negative bacteria, while Arthrobacter is Gram-positive, and all three are found in soil, on plant surfaces, and in fresh and brackish water worldwide. In addition, whereas the Aeromonas and Enterobacter genera comprise some pathogenic strains, the genus Arthrobacter contains mostly strains that are associated with the oxidative breakdown of polymeric compounds and plays an important role in the biodegradation of agrochemicals and pollutants [Citation72,Citation73]. Several Arthrobacter strains appear to possess PGP characteristics; some recent examples include Arthrobacter sp. UKPF54-2 [Citation74], Arthrobacter wenxiniae sp. nov. [Citation75], Arthrobacter sp. GN70 [Citation76], and Arthrobacter nicotinovorans JI39 [Citation77]. Furthermore, Arthrobacter spp. has been isolated from the rhizosphere and shown to promote the growth of legumes [Citation78,Citation79] and rice plants [Citation76]. Similar to Arthrobacter, species of another soil bacterium, Enterobacter, are known to have a wide range of PGP characteristics, including nitrogen fixation, phosphorus mineralisation, production of antibiotics, siderophores, chitinase, ACC-deaminase, and release hydrolytic enzymes that, together with extracellular polysaccharides, enhance soil porosity [Citation80]. Of particular relevance to the present study, as emphasised by these authors, is the ability of Enterobacter spp. to increase root growth, root fresh weight, and promote the release of organic acids into the rhizosphere. Together, this suggests enhanced development of plant root systems by isolates of the genus Enterobacter like ECCN 7b, which, in the present study, along with the isolates Aeromonas ECCN 4b and Arthrobacter ECCN 6b, promoted adventitious root formation in mung bean hypocotyls.
It has been reported that all bacteria produce IAA and that treatment of mung bean cuttings with PGP bacteria enhances adventitious root formation [Citation81]. Thus, for mung bean hypocotyls, it is purported that bacterial production of auxin in parallel with the secretion of 1-aminocyclopropane-1-carboxylate (ACC) deaminase, plant-derived ethylene [Citation82,Citation83], formation of root-produced galactoglucomannan-oligosaccharides [Citation84], H2O2 [Citation70] and salicylic acid [Citation85] act in concert as signalling agents to initiate and drive adventitious root formation. Indeed, the recent observations employing Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway-enrichment show convincingly that phytohormone signal transduction; phenylalanine, pentose, and glucuronate metabolism; photosynthesis; and biosynthesis of phenylpropanoids, sesquiterpenes, triterpenes, and flavonoids are among the pathways most highly regulated in mung bean hypocotyls by the auxin, indole-3-butyric acid [Citation86].
5. Conclusions
As far as the authors are concerned, this study is the first to describe the isolation and molecular characterisation of PGP bacteria from MaB-flocs produced during domestic wastewater treatment. Using MaB-floc biomass generated in HRAOP of an IAPS remediating municipal sewage as the starting material, a suite of PGP bacteria was isolated, the individual strains identified, and PGP properties characterised. Aeromonas strain ECCN 4b, Enterobacter strain ECCN 7b, and Arthrobacter strain ECCN 6b were confirmed as the bacterial isolates with the most prominent PGP activity. Specific knowledge regarding isolation, purification, and morphological, biochemical, and molecular characterisation was derived to support the classification of these bacterial isolates as PGP strains. As a resource for biocatalyst recovery, wastewater-derived bacteria with PGP characteristics could assist in solving some of the contemporary challenges related to the strain on the water-energy-food nexus and food security. For example, generating a cost-effective bio-fertilizer from biomass produced in HRAOP during wastewater treatment may reduce dependence on chemical and fossil fuel-derived fertilisers. And it may make wastewater treatment and beneficiation more attractive to local authorities, particularly if additional income streams are likely. Further studies are therefore underway to investigate in more detail the PGP activity of these isolates and to test each, individually and in consortia, in bioassays, and under field conditions.
Author statement
AKC and WLM conceptualised the work; WLM carried out the bacterial isolation and analysed all plant growth promotion parameters; YT and TAK assisted with the collection of metagenomic data and phylogenetic analysis. AKC carried out the statistical analyses and prepared the figures and tables. WLM and AKC prepared a first draft of the manuscript with editorial input from TAK and YT. All authors proofread several iterations and the final submitted paper.
Acknowledgments
This work formed part of a larger project supported by a grant from the Water Research Commission (K1-7164). Wiya L. Masudi is grateful for a bursary from EBRU. Yinka Titilawo and Taobat A. Keshinro are grateful recipients of a prestigious Rhodes University Postdoctoral Research Fellowship and a Rhodes University doctoral scholarship, respectively. The authors thank staff and researchers of EBRU and Biological Sciences for the enabling environment in which this study was conducted.
Data availability
Data will be made available on request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Coban O, De Deyn GB, van der Ploeg M. Soil microbiota as game-changers in restoration of degraded lands. Science. 2022;375(6584):eabe0725. DOI:10.1126/science.abe0725
- Sekhohola-Dlamini LM, Keshinro OM, Masudi WL, et al. Elaboration of a phytoremediation strategy for successful and sustainable rehabilitation of disturbed and degraded land. Minerals. 2022;12(2):111. DOI:10.3390/min12020111
- Lugtenberg B, Kamilova F. Plant-growth-promoting rhizobacteria. Annu Rev Microbiol. 2009;63(1):541–556. DOI:10.1146/annurev.micro.62.081307.162918
- Santoyo G, Moreno-Hagelsieb G, del Carmen Orozco-Mosqued M, et al. Plant growth-promoting bacterial endophytes. Microbiol Res. 2016;183:92–99. DOI:10.1016/j.micres.2015.11.008
- Backer R, Rokem JS, Ilangumaran G, et al. Plant growth-promoting rhizobacteria: context, mechanisms of action, and roadmap to commercialization of biostimulants for sustainable agriculture. Front Plant Sci. 2018;9:1473. DOI:10.3389/fpls.2018.01473
- Basu A, Prasad P, Das SN, et al. Plant growth promoting rhizobacteria (PGPR) as green bioinoculants: recent developments, constraints, and prospects. Sustainability. 2021;13:1140. DOI:10.3390/su13031140
- de los Santos-Villalobosa S, Parra-Cota FI. Current trends in plant growth-promoting microorganisms research for sustainable food security. Curr Res Microbial Sci. 2022;2:100016. DOI:10.1016/j.crmicr.2020.100016
- Glick BR. Bacteria with ACC deaminase can promote plant growth and help to feed the world. Microbiol Res. 2014;169:30–39. DOI:10.1016/j.micres.2013.09.009
- Kang S-M, Shahzad R, Bilal S, et al. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019;19:80. DOI:10.1186/s12866-019-1450-6
- Hallett PD, Marin M, Bending GD, et al. Building soil sustainability from root-soil interface traits. Trends Plant Sci. 2022;27(7):688–698. DOI:10.1016/j.tplants.2022.01.010
- Titilawo Y, Jimoh TA, Cowan AK. Multiple drug-resistant Escherichia coli phylogroups from the Belmont Valley integrated algal pond system. Water Air Soil Pollut. 2021;232(12):485. DOI:10.1007/s11270-021-05440-5
- Laubscher RK, Cowan AK. Elaboration of an algae-to-energy system and recovery of water and nutrients from municipal sewage. Eng Life Sci. 2020;20(7):230–315. DOI:10.1002/elsc.202000007
- Craggs R, Park J, Heubeck S, et al. High rate algal pond systems for low-energy wastewater treatment, nutrient recovery and energy production. N Z J Bot. 2014;52(1):60–73. DOI:10.1080/0028825X.2013.861855
- Mambo PM, Westensee DK, Zuma BM, et al. The Belmont Valley integrated algae pond system in retrospect. Water SA. 2014;40(2):385–391. DOI:10.4314/wsa.v40i2.21
- Ho L, Goethals PLM. Municipal wastewater treatment with pond technology: historical review and future outlook. Ecol Eng. 2020;148:105791. DOI:10.1016/j.ecoleng.2020.105791
- Van Den Hende S, Claessens L, De Muylder E, et al. Microalgal bacterial flocs originating from aquaculture wastewater treatment as diet ingredient for Litopenaeus vannamei (Boone). Aquacult Res. 2014;47(4):1075–1089. DOI:10.1111/are.12564
- Wieczorek N, Kucuker MA, Kuchta K. Microalgae-bacteria flocs (MaB-flocs) as a substrate for fermentative biogas production. Bioresour Technol. 2015;194:130–136. DOI:10.1016/j.biortech.2015.06.104
- Jimoh TA, Cowan AK. Extracellular polymeric substance production in high rate algal oxidation ponds. Water Sci Technol. 2017;76(10):2647–2654. DOI:10.2166/wst.2017.438
- Jimoh TA, Keshinro MO, Cowan AK. Microalgal-bacterial flocs and extracellular polymeric substances: two essential and valuable products of integrated algal pond systems. Water Air Soil Pollut. 2019;230(4):95. DOI:10.1007/s11270-019-4148-3
- Khoo KS, Chia WY, Chew KW, et al. Microalgal-bacterial consortia as future prospect in wastewater bioremediation, environmental management and bioenergy production. Indian J Microbiol. 2021;61(3):262–269. DOI:10.1007/s12088-021-00924-8
- Rawat I, Kumar R, Mutanda T, et al. Dual role of microalgae: Phycoremediation of domestic wastewater and biomass production for sustainable biofuels production. Appl Energy. 2011;88(10):3411–3424. DOI:10.1016/j.apenergy.2010.11.025
- Cowan AK. Bio-refineries: bioprocess technologies for wastewater treatment, energy and product valorization. AIP Conf Proc. 2010;1229:80–86. DOI:10.1063/1.3419705
- Psycha M, Pyrgakis K, Harvey PJ, et al. Design analysis of integrated microalgae biorefineries. Comput Aided Chem Eng. 2014;34:591–596. DOI:10.1016/B978-0-444-63433-7.50083-3
- Jimoh TA, Laubscher RK, Askew DJ, et al. Advanced oxidation as tertiary treatment for recovery of effluent from an integrated algal pond system. Water Environ J. 2021;35:1094–1102. DOI:10.1111/wej.12701
- Mambo PM, Westensee DK, Render DS, et al. Operation of an integrated algae pond system for the treatment of municipal sewage: a South African case study. Water Sci Technol. 2014;69(12):2554–2561. DOI:10.2166/wst.2014.187
- Ruangpan L, Tendencia EA. Laboratory manual of standardized methods for antimicrobial sensitivity tests for bacteria isolated from aquaculture. Iloilo: Southeast Asian Fisheries Development Center, Aquaculture Department; 2004.
- Titilawo Y, Masudi WL, Olawale JT, et al. Coal-degrading bacteria display characteristics typical of plant growth-promoting rhizobacteria. Processes. 2020;8(9):1111. DOI:10.3390/pr8091111
- Olawale JT, Edeki OG, Cowan AK. Bacterial degradation of coal discard and geologically weathered coal. Int J Coal Sci Technol. 2020;7(2):405–416. DOI:10.1007/s40789-020-00306-3
- Gupta M, Kiran S, Gulati A, et al. Isolation and identification of phosphate solubilising bacteria able to enhance the growth and aloin-A biosynthesis of Aloe barbadensis Miller. Microbiol Res. 2012;167(6):358–363. DOI:10.1016/j.micres.2012.02.004
- Mohite B. Isolation and characterisation of indole acetic acid (IAA) producing bacteria from rhizosphere soil and its effect on plant growth. J Soil Sci Plant Nutr. 2013;13(3):638–649. DOI:10.4067/S0718-95162013005000051
- Zahid M, Abbasi MK, Hameed S, et al. Isolation and identification of indigenous plant growth-promoting rhizobacteria from the Himalayan region of Kashmir and their effect on improving growth and nutrient contents of maize (Zea mays L.). Front Microbiol. 2015;6(207):207. DOI:10.3389/fmicb.2015.00207
- Coico R. Gram staining. Curr Protoc Microbiol. 2006;1:A.3C.1–A.3C.2. DOI:10.1002/9780471729259.mca03cs00
- Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editor. Nucleic acid techniques in bacterial systematics. New York: John Wiley and Sons; 1991. p. 115–175.
- Turner S, Pryer KM, Miao VP, et al. Investigating deep phylogenetic relationships among cyanobacteria and plastids by small subunit rRNA sequence analysis. J Eukaryot Microbiol. 1999;46(4):327–338. DOI:10.1111/j.1550-7408.1999.tb04612.x
- Tamura K, Stecher G, Peterson D, et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. DOI:10.1093/molbev/mst197
- Glickmann E, Dessaux Y. A critical examination of the specificity of the Salkowski reagent for indolic compounds produced by phytopathogenic bacteria. Appl Environ Microbiol. 1995;61:793–796. DOI:10.1128/aem.61.2.793-796.1995
- Gordon SA, Weber RP. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951;26:192–195. DOI:10.1104/pp.26.1.192
- Majeed A, Abbasi MK, Hameed S, et al. Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front Microbiol. 2015;6:198. DOI:10.3389/fmicb.2015.00198
- Zhao Y, Shi R, Bian X, et al. Ammonia detection methods in photocatalytic and electrocatalytic experiments: How to improve the reliability of NH3 production rates? Adv Sci. 2019;2019:1802109. DOI:10.1002/advs.201802109
- Zhou L, Boyd CE. Comparison of Nessler, phenate, salicylate and ion selective electrode procedures for determination of total ammonia nitrogen in aquaculture. Aquaculture. 2016;450:187–193. DOI:10.1016/j.aquaculture.2015.07.022
- Jeong H, Park J, Kim H. Determination of NH4+ in environmental water with interfering substances using the modified Nessler method. J Chem. 2013;2013:e359217, DOI:10.1155/2013/359217
- Sharma SB, Sayyed RZ, Trivedi MH, et al. Phosphate solubilising microbes: sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus. 2013;2(1):587. DOI:10.1186/2193-1801-2-587
- Saha M, Maurya BR, Meena VS, et al. Identification and characterisation of potassium solubilising bacteria (KSB) from Indo-Gangetic plains of India. Biocatal Agric Biotechnol. 2016;7:202–209. DOI:10.1016/j.bcab.2016.06.007
- Nautiyal CS. An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett. 1999;170:265–270. DOI:10.1111/j.1574-6968.1999.tb13383.x
- Lee J, Cho D-H, Ramanan R, et al. Microalgae-associated bacteria play a key role in the flocculation of Chlorella vulgaris. Bioresour Technol. 2013;131:195–201. DOI:10.1016/j.biortech.2012.11.130
- Perera IA, Abinandan S, Subashchandrabose SR, et al. Extracellular polymeric substances drive symbiotic interactions in bacterial-microalgal consortia. Microb Ecol. 2022;83(3):596–607. DOI:10.1007/s00248-021-01772-1
- Van Den Hende S, Vervaeren H, Desmet S, et al. Bioflocculation of microalgae and bacteria combined with flue gas to improve sewage treatment. New Biotechnol. 2011;29(1):23–31. DOI:10.1016/j.nbt.2011.04.009
- Tang C-C, Tian Y, Liang H, et al. Enhanced nitrogen and phosphorus removal from domestic wastewater via algae assisted sequencing batch biofilm reactor. Bioresour Technol. 2018;250:185–190. DOI:10.1016/j.biortech.2017.11.028
- Green FB, Bernstone L, Ludquist TJ, et al. Methane fermentation, submerged gas collection, and the fate of carbon in advanced integrated wastewater pond systems. Water Sci Technol. 1995;31(12):55–65. DOI:10.2166/wst.1995.0458
- Natrah FMI, Bossier P, Sorgeloos P, et al. Significance of microalgal–bacterial interactions for aquaculture. Rev Aquacult. 2013;5:1–14. DOI:10.1111/raq.12024
- Breakfield NW, Collett D, Frodyma ME. Plant growth-promoting microbes – an industry view. Emerg Top Life Sci. 2021;5(2):317–324. DOI:10.1042/ETLS20200313
- Eichmann R, Richards L, Schäfer P. Hormones as go-betweens in plant microbiome assembly. Plant J. 2021;105:518–541. DOI:10.1111/tpj.15135
- Oleńska E, Małek W, Wójcik M, et al. Beneficial features of plant growth-promoting rhizobacteria for improving plant growth and health in challenging conditions: a methodical review. Sci Total Environ. 2020;743:140682. DOI:10.1016/j.scitotenv.2020.140682
- Costacurta A, Vanderleyden J. Synthesis of phytohormones by plant-associated bacteria. Crit Rev Microbiol. 1995;21(1):1–18. DOI:10.3109/10408419509113531
- Frébortová J, Frébort I. Biochemical and structural aspects of cytokinin biosynthesis and degradation in bacteria. Microorganisms. 2021;9(6):1314. DOI:10.3390/microorganisms9061314
- Glick BR, Nascimento FX. Pseudomonas 1-aminocyclopropane-1-carboxylate (ACC) deaminase and its role in beneficial plant-microbe interactions. Microorganisms. 2021;9(12):2467. DOI:10.3390/microorganisms9122467
- Keswani C, Singh SP, Cueto L, et al. Auxins of microbial origin and their use in agriculture. Appl Microbiol Biotechnol. 2020;104:8549–8565. DOI:10.1007/s00253-020-10890-8
- Park S, Kim AL, Hong YK, et al. A highly efficient auxin-producing bacterial strain and its effect on plant growth. J Genet Eng Biotechnol. 2021;19:179), DOI:10.1186/s43141-021-00252-w
- Nascimento FX, Urón P, Glick BR, et al. Genomic analysis of the 1-aminocyclopropane-1-carboxylate deaminase-producing Pseudomonas thivervalensis SC5 reveals its multifaceted roles in soil and in beneficial interactions with plants. Front Microbiol. 2021;12:752288. DOI:10.3389/fmicb.2021.752288
- Shi TQ, Peng H, Zeng SY, et al. Microbial production of plant hormones: opportunities and challenges. Bioengineered. 2017;8(2):124–128. DOI:10.1080/21655979.2016.1212138
- Wu S, Ma X, Zhou A, et al. Establishment of strigolactone-producing bacterium-yeast consortium. Sci Adv. 2021;7:eabh4048. DOI:10.1126/sciadv.abh4048
- Cox CE, Brandl MT, de Moraes MH, et al. Production of the plant hormone auxin by Salmonella and its role in the interactions with plants and animals. Front Microbiol. 2018;8:2668. DOI:10.3389/fmicb.2017.02668
- Dankevych L, Leonova N, Dragovoz I, et al. The synthesis of plant growth stimulators by phytopathogenic bacteria as factor of pathogenicity. Appl Ecol Environ Res. 2018;16(2):1581–1593. DOI:10.15666/aeer/1602_15811593
- Djami-Tchatchou AT, Harrison GA, Harper CP, et al. Dual role of auxin in regulating plant defense and bacterial virulence gene expression during Pseudomonas syringae PtoDC3000 pathogenesis. Mol Plant-Microbe Inter. 2020;33(8):1059–1071. DOI:10.1094/MPMI-02-20-0047-R
- Vrabka J, Niehaus EM, Münsterkötter M, et al. Production and role of hormones during interaction of Fusarium species with maize (Zea mays L.) seedlings. Front Plant Sci. 2019;9(9):1936. DOI:10.3389/fpls.2018.01936
- Vaz-Moreira I, Nunes OC, Manaia CM. Bacterial diversity and antibiotic resistance in water habitats: searching the links with the human microbiome. FEMS Microbiol Rev. 2014;38(4):761–778. DOI:10.1111/1574-6976.12062
- El-Sayed WS, Akhkha A, El-Naggar MY, et al. In vitro antagonistic activity, plant growth-promoting traits and phylogenetic affiliation of rhizobacteria associated with wild plants grown in arid soil. Front Microbiol. 2014;5:651. DOI:10.3389/fmicb.2014.00651
- Blazich FA, Heuser CW. The mung bean rooting bioassay: a re-examination. J Amer Soc Hort Sci. 1979;104(1):117–120. DOI:10.21273/JASHS.104.1.117
- Jackson MB, Harney PM. Rooting cofactors, indole-acetic acid, and adventitious root initiation in mung bean cuttings (Phaseolus aureus). Can J Bot. 1970;48:943–946. DOI:10.1139/b70-132
- Li S-W, Xue L, Xu S, et al. Hydrogen peroxide acts as a signal molecule in the adventitious root formation of mung bean seedlings. Environ Exp Bot. 2009;65:63–71. DOI:10.1016/j.envexpbot.2008.06.004
- Druege U, Hilo A, Pérez-Pérez JM, et al. Molecular and physiological control of adventitious rooting in cuttings: phytohormone action meets resource allocation. Annal Bot. 2019;123:929–949. DOI:10.1093/aob/mcy234
- Westerberg K, Elvang AM, Stackebrandt E, et al. Arthrobacter chlorophenolicus sp. nov., a new species capable of degrading high concentrations of 4-chlorophenol. Int J Syst Evol Microbiol. 2000;50(6):2083–2092. DOI:10.1099/00207713-50-6-2083
- Camargo FAO, Bento FM, Okeke BC, et al. Hexavalent chromium reduction by an actinomycete, Arthrobacter crystallopoietes ES 32. Biol Trace Element Res. 2003;97(2):183–194. DOI:10.1385/BTER:97:2:183
- Shen W, Yu X, Gao N, et al. Genome sequence of Arthrobacter sp. UKPF542, a plant growth-promoting rhizobacterial strain isolated from paddy soil. Microbiol Resour Announc. 2019;8:e01005–19. DOI:10.1128/MRA.01005-19
- Sun YC, Sun P, Xue J, et al. Arthrobacter wenxiniae sp. nov., a novel plant growth-promoting rhizobacteria species harbouring a carotenoids biosynthetic gene cluster. Antonie Van Leeuwenhoek. 2022;115(3):353–364. DOI:10.1007/s10482-021-01701-9 (Erratum in: Antonie Van Leeuwenhoek 2022;115(5):697).
- Chhetri G, Kim I, Kang M, et al. An isolated Arthrobacter sp. enhances rice (Oryza sativa L.) plant growth. Microorganisms. 2022;10:1187. DOI:10.3390/microorganisms10061187
- Jiang Y, Song Y, Jiang C, et al. Identification and characterization of Arthrobacter nicotinovorans JI39, a novel plant growth-promoting rhizobacteria strain from Panax ginseng. Front Plant Sci. 2022;13:873621. DOI:10.3389/fpls.2022.873621
- Aviles-Garcia ME, Flores-Cortez I, Hernández-Soberano C, et al. The plant growth-promoting rhizobacterium Arthrobacter agilis UMCV2 endophytically colonizes Medicago truncatula. Rev Argent Microbiol. 2016;48(4):342–346. DOI:10.1016/j.ram.2016.07.004
- Mekonnen H, Kibret M. The roles of plant growth promoting rhizobacteria in sustainable vegetable production in Ethiopia. Chem Biol Technol Agric. 2021;8:15. DOI:10.1186/s40538-021-00213-y
- Jha CK, Aeron A, Patel BV, et al. Enterobacter: role in plant growth promotion. In: Maheshwari D, editor. Bacteria in agrobiology: plant growth responses. Berlin, Heidelberg: Springer; 2011. p. 159–182. DOI:10.1007/978-3-642-20332-9_8
- Mayak S, Tirosh T, Glick BR. Effect of wild-type and mutant plant growth-promoting rhizobacteria on the rooting of mung bean cuttings. J Plant Growth Regul. 1999;18:49–53. DOI:10.1007/PL00007047
- De Klerk G-J, Hanecakova J. Ethylene and rooting of mung bean cuttings. The role of auxin induced ethylene synthesis and phase-dependent effects. Plant Growth Regul. 2008;56:203–209. DOI:10.1007/s10725-008-9301-8
- Glick BR. Plant growth-promoting bacteria: mechanisms and applications. Scientifica (Cairo). 2012;2012:963401. DOI:10.6064/2012/963401
- Kollárová K, Henselová M, Lišková D. Effect of auxins and plant oligosaccharides on root formation and elongation growth of mung bean hypocotyls. Plant Growth Regul. 2005;46:1–9. DOI:10.1007/s10725-005-5185-z
- Yang W, Zhu C, Ma X, et al. Hydrogen peroxide is a second messenger in the salicylic acid-triggered adventitious rooting process in mung bean seedlings. PLoS One. 2013;8(12):e84580. DOI:10.1371/journal.pone.0084580
- Li S-W, Shi R-F, Leng Y, et al. Transcriptomic analysis reveals the gene expression profile that specifically responds to IBA during adventitious rooting in mung bean seedlings. BMC Genomics. 2016;17:43. DOI:10.1186/s12864-016-2372-4

