ABSTRACT
Hemispatial neglect is a severe cognitive condition frequently observed after a stroke, associated with unawareness of one side of space, disability and poor long-term outcome. Visuomotor feedback training (VFT) is a neglect rehabilitation technique that involves a simple, inexpensive and feasible training of grasping-to-lift rods at the centre. We compared the immediate and long-term effects of VFT vs. a control training when delivered in a home-based setting. Twenty participants were randomly allocated to an intervention (who received VFT) or a control group (n = 10 each). Training was delivered for two sessions by an experimenter and then patients self-administered it for 10 sessions over two weeks. Outcome measures included the Behavioural Inattention Test (BIT), line bisection, Balloons Test, Landmark task, room description task, subjective straight-ahead pointing task and the Stroke Impact Scale. The measures were obtained before, immediately after the training sessions and after four-months post-training. Significantly greater short and long-term improvements were obtained after VFT when compared to control training in line bisection, BIT and spatial bias in cancellation. VFT also produced improvements on activities of daily living. We conclude that VFT is a feasible, effective, home-based rehabilitation method for neglect patients that warrants further investigation with well-designed randomised controlled trials on a large sample of patients.
Introduction
Hemispatial neglect is a severe neurological disorder, classically defined as a failure to respond to stimuli in the contralesional hemispace (Kerkhoff, Citation2001). Its clinical impact is substantial with a third of patients showing signs of neglect even more than a year after their stroke (Karnath, Rennig, Johannsen, & Rorden, Citation2011; Rengachary, He, Shulman, & Corbetta, Citation2011) and with its diagnosis being a major predictor of stroke disability (Buxbaum et al., Citation2004 Gillen, Tennen, & McKee, Citation2005; Kalra, Perez, Gupta, & Wittink, Citation1997; Katz, Hartman-Maeir, Ring, & Soroker, Citation1999).
In light of these figures, it is perhaps not surprising that, over the last 50 years, an increasing number of cognitive rehabilitation techniques have been developed to improve the recovery of neglect patients (for a recent review see Kerkhoff & Schenk, Citation2012), such as visual scanning training (e.g., Antonucci et al., Citation1995; Kerkhoff, Citation1998; Lawson, Citation1962; Piccardi, Nico, Bureca, Matano, & Guariglia, Citation2006; Pizzamiglio et al., Citation1992), limb activation training (e.g., Brunila, Lincoln, Lindell, Tenovuo, & Hamalainen, Citation2002; Robertson & North, Citation1992, Citation1993, Citation1994; Robertson, Hogg, & McMillan, Citation1998a; Robertson, McMillan, MacLeod, Edgeworth, & Brock, Citation2002; Robertson, North, & Geggie, Citation1992; Samuel et al., Citation2000), sustained attention training (e.g., Robertson, Tegnér, Tham, Lo, & Nimmo-Smith, Citation1995; Robertson, Mattingley, Rorden, & Driver, Citation1998b; Thimm, Fink, Küst, Karbe, & Sturm, Citation2006), prism adaptation (e.g., Frassinetti, Angeli, Meneghello, Avanzi, & Ladavas, Citation2002; Jacquin-Courtois, Rode, Pisella, Boisson, & Rossetti, Citation2008; Rossetti et al., Citation1998; Vaes et al., Citation2016; for a recent review see Newport & Schenk, Citation2012), neck muscle vibration (e.g., Karnath, Citation1994; Karnath, Christ, & Hartje, Citation1993; Schindler & Kerkhoff, Citation2004; Schindler, Kerkhoff, Karnath, Keller, & Goldenberg, Citation2002), caloric (e.g., Cappa, Sterzi, Vallar, & Bisiach, Citation1987; Rubens, Citation1985; Vallar, Sterzi, Bottini, Cappa, & Rusconi, Citation1990) and optokinetic stimulation (e.g., Bisiach, Pizzamiglio, Nico, & Antonucci, Citation1996; Karnath, Citation1996; Kerkhoff, Schindler, Keller, & Marquardt, Citation1999, Citation2006, Citation2012; Pizzamiglio, Frasca, Guariglia, Incoccia, & Antonucci, Citation1990, Citation2004). In addition, a range of new stimulation techniques have been introduced recently spanning from galvanic-vestibular (Kerkhoff et al., Citation2011) and transcranial direct current stimulation (Sparing et al., Citation2009) to transcranial magnetic stimulation (Cazzoli et al., Citation2012; Nyffeler, Cazzoli, Hess, & Müri, Citation2009; Song et al., Citation2009) and functional electric stimulation (Harding & Riddoch, Citation2009; Polanowska, Seniów, Paprot, Leśniak, & Członkowska, Citation2009). Finally, the effects of drug therapy (dopamine agonist rotigone, Gorgoraptis et al., Citation2012) and virtual reality treatments (e.g., Kim, Chun, Yun, Song, & Young, Citation2011; Sedda et al., Citation2013) have also been exploited.
Unfortunately, despite the diversity of the proposed techniques for neglect rehabilitation, a recent Cochrane review concluded that their effectiveness in reducing disability and improving independence remains uncertain (Bowen, Hazelton, Pollock, & Lincoln, Citation2013): there is no evidence for a generalisation of the effects to untrained tasks or functional abilities, most of the effects are not long-lasting and most studies consist of single-case studies or small group studies without an appropriate control group. Moreover, most of the techniques are not easily translated into a clinical setting as they are costly, time-consuming and demand a substantial commitment from both patient and therapist, which is hard to obtain from neglect patients, as they may also suffer from anosognosia (i.e., a lack of insight into their impairments, Appelros, Karlsson, Seiger, & Nydevik, Citation2003). Therefore, neglect rehabilitation remains a challenging problem and currently no rehabilitation approach has been recommended for clinical implementation (Bowen et al., Citation2013). Nevertheless, significant immediate effects in favour of cognitive rehabilitation vs. control treatments on standardised neglect tests justify the need for further well-designed clinical trials (Bowen et al., Citation2013; see also Cappa et al., Citation2005; Champod, Frank, Taylor, & Eskes, Citation2016; Cicerone et al., Citation2005; Priftis, Passarini, Pilosio, Meneghello, & Pitteri, Citation2013).
Visuomotor feedback training (VFT) is a relatively unexplored technique for neglect rehabilitation which involves simple exercises of grasping-to-lift rods at the centre. Importantly, VFT is inexpensive, easy to apply and does not require patient insight into the disorder, so provides all the hallmarks for future wide-scale clinical implementation. The training has its roots in the paradoxical finding that even though patients with left neglect present large rightward biases when asked to point to a rod’s midpoint, such biases are significantly reduced when they grasp that same rod to pick it up (Edwards & Humphreys, Citation1999; Robertson et al., Citation1995; Robertson, Nico, & Hood, Citation1997). It has been postulated that motor responses (such as grasping-to-lift a rod at the centre) allow neglect patients to access visual information not available during perceptual judgements (such as pointing to the perceived centre of a rod) (Robertson et al., Citation1995, Citation1997). In particular, it has been suggested that VFT may improve neglect symptoms via a “dorsal-to-ventral” visual stream recalibration, thus enabling action to ameliorate perception (Harvey, Hood, North, & Robertson, Citation2003; Robertson et al., Citation1995, Citation1997). In line with this, it has been repeatedly shown that despite their severe biases in perceptual tasks neglect patients are able to reach and grasp objects even when positioned in their neglected field (Harvey et al., Citation2001; Harvey & Rossit, Citation2012; Himmelbach & Karnath, Citation2003; McIntosh, Pritchard, Dijkerman, Milner, & Roberts, Citation2001, Citation2004; Rossit et al., Citation2009a, Citation2009b, Citation2009c; Rossit, Fraser, Teasell, Malhotra, & Goodale, Citation2011a, Citation2011b).
To date only a couple of studies have tested the efficacy of VFT in ameliorating neglect (Harvey et al., Citation2003; Robertson et al., Citation1997). In a first within-subject design trial (Robertson et al., Citation1997) 16 neglect patients were asked to repeatedly grasp-to-lift rods at the centre until they were satisfied that they had found the centre (VFT) and, in a control condition, to grasp the centre of a rod without lifting it. Positive effects were found (after VFT only) on line bisection and cancellation tasks up to 20 minutes post-training after only nine trials. In another study (Harvey et al., Citation2003), the immediate and long-term effects of a more intensive version of VFT were further explored in 14 chronic neglect patients. The VFT group (N = 7) was asked to grasp-to-lift rods at the centre whilst patients in the control group (N = 7) grasped and lifted the right side of the rod only. Thus both groups, performed grasp-to-lift actions and received the same amount of training, but only the VFT group received concurrent feedback from their action. Effects were measured before and after an experimenter and home-based sessions and at a one-month follow-up. Patients in the VFT group improved in one third of all neglect standardised tests after the two-day experimenter-led intervention and in 46% of all neglect tests at one-month follow-up. As recently emphasised (Kerkhoff & Schenk, Citation2012) these are promising results which deserve further investigation.
In this study, we conducted a controlled trial of VFT in 20-stroke patients with chronic hemispatial neglect. Our main goal was to assess the short and long-term effects on standard neglect tests and critically also on ecological and daily life functioning measures, the Stroke Impact Scale (SIS) in particular. In addition, we sought to investigate the effects of a more feasible protocol of VFT by reducing the number of training sessions (Harvey et al., Citation2003). Following the latest health recommendations (SIGN, Citation2010) that more research is required on rehabilitation methods that target stroke patients living at home, we explored whether VFT is effective when delivered at the patient’s home. Finally, we also examined whether VFT effects were maintained after four-months post-training, a considerably greater follow-up period than previous studies (Harvey et al., Citation2003; Robertson et al., Citation1997).
Methods
Patients
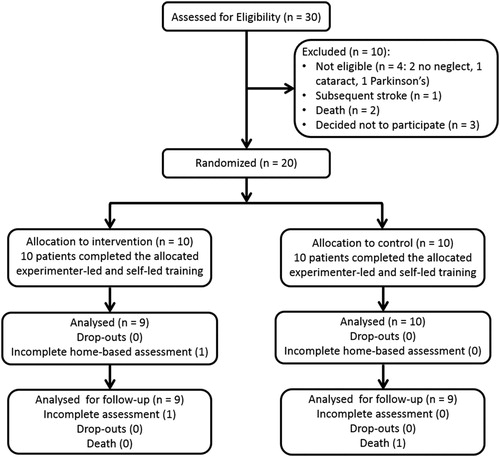
Stroke patients with hemispatial neglect were recruited following incidental referrals by staff at the Southern General Hospital (Glasgow, UK) who were informed that we were aiming to recruit stroke patients with signs of neglect. Inclusion criteria were: stroke lesions as identified by brain imaging report, hemispatial neglect symptoms (in line bisection, BIT or balloons test (as in Rossit et al., Citation2009a, Citation2009b, Citation2009c; Rossit et al., Citation2011a, Citation2011b; see also for clinical/demographic data and neglect cut-offs), ability to follow instructions and medical fitness to participate. Exclusion criteria were: previous or concomitant neurological (e.g., brain tumour, dementia), visual (e.g., cataract) or motor (e.g., arthritis) diseases unrelated to the current stroke. In total 20 neglect patients were allocated to an intervention group and a control group (see for CONSORT flow diagram). The study was approved by the local NHS ethics committee and patients provided informed consent according to the declaration of Helsinki II. Neglect screening was carried out at the hospital while the rest of the trial was performed at the patient’s home. If the time interval between the first neglect screening (at hospital) and the baseline assessment (at the patient’s home) was longer than two months, neglect measures (BIT, line bisection task and Balloons Test) were re-administered to ensure that neglect was still present at baseline.
Design and treatments
To investigate the effects of the VFT we conducted a controlled prospective study using a single-blind design: patients, carers and scorers of our outcome measures remained blind to group assignment throughout the trial, except for the Stroke Impact Scale (SIS). Here, in the majority of cases, the SIS (Proxy version) was rated by the patient’s carer, except for 6 patients (P5, P7, P4, P9, P19 and P20) to which the scale was applied by the experimenter, as there was no carer available. Before the trial experimenters made up a randomised list, and then each consecutive patient enrolled was randomly assigned to either intervention or control groups as determined in the order of this list (complete/unrestricted randomisation) (Blackwell & Hodges, Citation1957; Lachin, Citation1988).
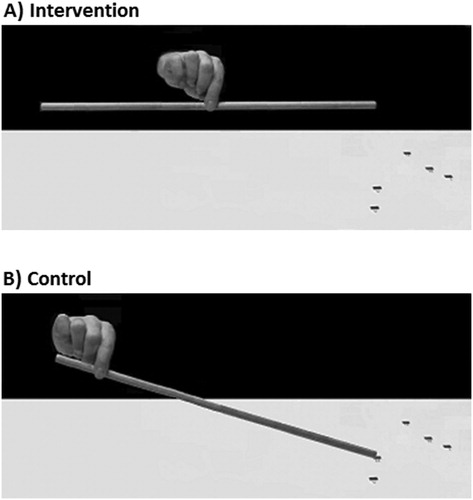
The treatment involved repeated grasping and lifting of rods, using the non-paretic (usually right) limb. Three wooden rods (1.1 cm diameter, 0.63 g in weight) of 50, 75 and 100 cm in length were used. Each rod was presented horizontally on a test mat (160 cm x 30 cm) in front of the patient, with the middle of the mat aligned with the patient’s body midline. In each rod-lifting trial, the rods were placed in different positions (centre, 10 cm to the right or left of the centre) to reduce the possibility that patients reached for rods according to a fixed external reference. At the beginning of each rod-lifting trial, patients were asked to place one of the three rods on a respective position indicated on the mat (). Patients in the intervention group were asked to reach for and grasp the rod with a pincer grip (using the forefinger and the thumb) and try to lift it up in its centre so that it would be balanced ((a)); if they felt that it was not balanced after lifting it, they could repeat the trial until satisfied. Feedback was provided only from the tilting rod and the experimenter did not comment on performance. Patients in the control group were instructed to simply reach for and grasp the rod with a pincer grip (using the forefinger and the thumb) by its non-neglected end (usually the right) and to lift it up from the mat on that side and place it back down again ((b)). In addition, both groups were instructed that whilst lifting a rod they should not move it away (in depth) from its starting position indicated on the mat. The order of the rod-lifting trials (rod size and position) was randomised across sessions and patients. Neither the patients (nor the carers) knew whether the exercises they were performing were part of the control or intervention treatment.
Figure 2. Grasping task performed by the intervention (A) and control (B) groups. The intervention group was asked to grasp the centre of the rod until balanced (A), whilst the control group was asked to grasp the rod at one side (B).
The treatment was delivered in two consecutive phases: experimenter-led and self-led. Following baseline pre-treatment assessment, the experimenter-led session was run for two consecutive times of approximately 30 minutes over two days. Each experimenter-led session consisted of six repetitions totalling 54-rod lifts. After the experimenter-led phase, the patient independently repeated the training for 10 days [sometimes with the help of their carer (e.g,. spouse)], over a period of two weeks (self-led intervention). Each self-led session consisted of 72-rod lifts (8 repetitions). While in hospital patients were encouraged to scan their neglected side but after discharge no other rehabilitation was delivered to patients except this study.
To ensure correct execution and monitor patient adherence to treatment, participants were given a sheet containing the order of all 72 trials (separate sheets, for each of the 10 sessions) and were required to tick each trial they performed. Furthermore, the experimenter also monitored performance via regular (at least two) phone calls to the patients and their carers. An ANOVA revealed no significant differences between the two groups in terms of the time elapsed (in months) between baseline and experimenter-led sessions (mean = 1.6; SE = 0.2), experimenter-led and self-led sessions (mean = 1.8; SE = 0.3) and self-led and follow-up sessions (mean = 4.3; SE = 1.1).
Outcome assessment and measures
Each patient participated in four assessment sessions: baseline pre-treatment; after the two experimenter-led sessions; after the 10 self-led sessions; and follow-up (at four months post-treatment). To reduce learning effects the order of the outcome measures was counterbalanced across sessions and participants.
The primary outcome measures administered at each session included neglect tests [i.e., BIT conventional sub-tests (Wilson, Cockburn, & Halligan, Citation1987), line bisection (Rossit et al., Citation2009a, Citation2009b, Citation2009c; Rossit et al., Citation2011a, Citation2011b) sub-test B of the Balloons Test (Edgeworth, Robertson, & McMillan, Citation1998) and the Landmark Task (Harvey, Milner, & Roberts, Citation1995)]. In addition, during the baseline, self-led and follow-up sessions a room description task (Frassinetti et al., Citation2002), a subjective straight-ahead pointing task (Rode, Rossetti, & Boisson, Citation2001) and the secondary outcome measure, the SIS (UK version 3.0; Duncan, Wallace, Lai, Johnson, et al., Citation1999; Duncan, Wallace, Lai, Studenski, et al., Citation1999; Duncan et al., Citation2002; Duncan, Bode, Lai, & Perera, Citation2003 were also administered. For the landmark task (Harvey et al., Citation1995) patients were presented with 10 horizontal black lines (20 cm x 1 mm) that were already centrally transacted by a vertical mark (6 mm x 1 mm), the landmark. Four lines had landmarks displaced 1 and 2 mm to the left and right of the true centre and the other six were positioned in the true centre. The order of the lines was randomised across participants and sessions. Lines were presented on individual sheets of A4 paper and subjects were asked to point, with their non-paretic limb, to the end of the line closer to the landmark.
For the room description task (Frassinetti et al., Citation2002), patients were asked to name 14 novel objects positioned in their living room, along his/her midline (seven on the left and seven on the right. The position of the objects was randomised across sessions and patients. The patients sat in the centre of the room with their back to one of the walls and were blindfolded until the start of the task. A table, placed in the centre of the room in front of the patient contained eight objects, four on the left and four on the right (glue tube, stapler, pencils and booklets). Additionally, along the left and the right side of the room, three objects were positioned on each side (A3 posters, calendar and carton boxes). Patients were asked to name the 14 objects and the experimenter took a note of the number of objects reported on each side.
In the subjective straight-ahead pointing task (Rode et al., Citation2001), patients were blindfolded and sat in front of a horizontal wooden board (87 cm length and 54 cm height, located app. 40 cm from the patient). They were required to point straight-ahead from a resting position while their head was kept aligned with the body’s sagittal axis by the experimenter. Ten pointing trials were performed. After every trial the experimenter registered the end-point of the pointing movement by marking its endpoint on a response sheet of paper that covered the board. The response sheet contained a line that indicated the centre of the board (invisible to the participant as he/she was blindfolded), which was aligned with the patient’s body midline.
The SIS (UK version 3.0; Duncan, Wallace, Lai, Johnson, et al., Citation1999; Duncan, Wallace, Lai, Studenski, et al., Citation1999; Duncan et al., Citation2002, Citation2003) was applied as a secondary outcome measure to assess functional disability. The SIS assesses the following eight domains: strength of the contralesional limbs; contralesional hand function; mobility; emotion; communication; memory and thinking; social participation and activities of daily living/instrumental activities of daily living (ADL/IADL). In addition, the scale contains a question to assess the individual’s global perception of stroke recovery, which ranges from 0 (no recovery) to 100 (full recovery). The scoring of the scale was conducted through a database (in Microsoft Access) provided on-line by the Kansas University Medical Centre (http://www2.kumc.edu/coa/SIS/SIS_pg2.htm).
The outcome assessment was performed by the experimenter (except the SIS) who also delivered the treatment. However, all measures were scored by a treatment-blinded researcher. The following dependent variables were computed for each patient and session: an absolute mean line bisection error (deviation from the centre in mm); a BIT total score; BIT spatial bias scores (the ratio of the difference in the number of targets found on the ipsilesional vs. contralesional side, to the total number of targets found on the line, letter and star cancellation sub-tests of the BIT; Gorgoraptis et al., Citation2012); normalised scores on each domain of the SIS; the lateralised index score in sub-test B of the Balloons Test; the percentage of centred lines reported as being shorter on the neglected side on the Landmark task; the mean absolute displacement from the centre (in degrees) on the straight-ahead pointing task; and the number of items reported on the neglected side on the room description task.
Statistical analysis
The data were analysed separately for all dependent variables with ANOVAs with the factors Time (baseline, experimenter-led, self-led, and follow-up) and Group (intervention, control). Due to the relatively small sample size and the resulting lack of power of the between subject design, we also calculated a gain score for each dependent variable (i.e., each session minus baseline, see also Mizuno et al., Citation2011 for similar analysis). This allowed to us to compare the gain between the two groups, with the data adjusted for each of the participant’s initial status. The α level was set at .05 (2-tailed) and pairwise comparisons were corrected using Bonferroni adjustments. The effect size of partial η2 was also calculated for each variable (a large effect is represented by a partial η2 of at least .138, a moderate effect by .059, and a small effect by .010; Cohen, Citation1988).
Results
Group comparison at baseline
One-way ANOVAs comparing the two groups at baseline in terms of age, time since stroke, presence of hemianopia and extinction, lesion volume and their performance on each outcome measure revealed no significant differences (see for statistics). Lesion analysis (see ) revealed that the two groups suffered lesions in similar brain regions with maximum overlap in the insula and claustrum and supramarginal gyrus white matter (damaged in 63% of intervention patients and in 60% of control patients).
Figure 3. (A) Lesion map for individual patients. B-C) Lesion overlap map summarising the degree of involvement for each voxel in the intervention (B; N = 8) and control (C; N = 5) groups. Lesions were identified by a clinical neurologist (K.M.), who was blind to the design, group assignment and purpose of the study. Lesions were mapped onto 11 axial slices of a T1-weighted template, corresponding to the MNI z coordinates of −24, −16, −8, 0, 8, 16, 24, 32, 40, 50, 60 mm using identical or closest matching transverse slices for each patient using MRIcro software package (Rorden & Brett, Citation2000). Due to technical difficulties at the clinical facility, we were able to obtain and map digital brain scans for 13 patients only (6 MRIs and 7 CTs) as the remaining digital brain scans were either lost or corrupted. Please note however, that all brain scan reports were available and confirmed the presence of a stroke and its location for all our patients. The range of colour scale derives from the absolute number of patient lesions involved in each voxel.
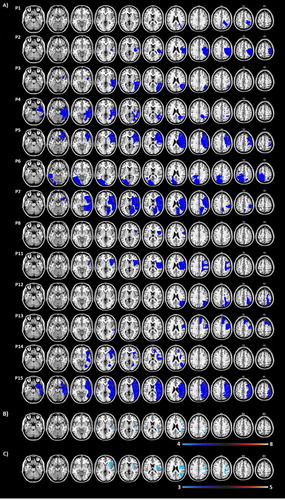
Table 1. Demographical and clinical data of 20 patients with hemispatial neglect in the 2 groups during baseline assessment before treatment.
Table 2. Statistical results for the between-group ANOVA (Intervention vs Control Group) at baseline.
Line bisection
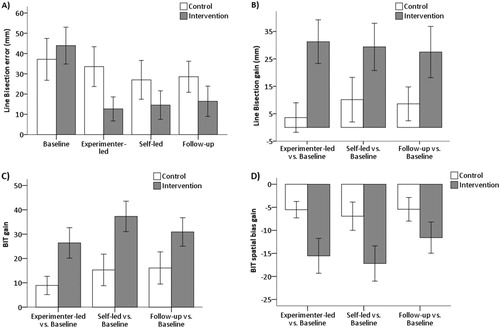
For the bisection error ((A)), the effect of group was not significant (F(1,18) = 0.8, P = 0.377, ), but there was a significant effect of time (F(3,54) = 8.4, P < 0.001,
) and a significant group x time interaction with a large effect size (F(3,54) = 3.4, P = 0.025,
). Post-hoc tests revealed that the intervention group’s bisection errors were significantly reduced at each session when compared to baseline (mean difference experimenter-led vs. baseline: −31.3, p = 0.001; mean difference self-led vs. baseline = −29.4, p = 0.02; mean difference follow-up vs. baseline = −27.5, p = 0.02), whereas for the control group all comparisons failed to reach significance (mean difference experimenter-led vs. baseline: −3.6; mean difference self-led vs. baseline = −10.3; mean difference follow-up vs. baseline = −8.6). In line with these results, the gain scores revealed a significant effect of group, again with a large effect size (F(1,18) = 4.6, p = 0.046,
): the intervention group presented a greater gain (i.e., larger reduction) in their bisection errors when compared to the control group (mean difference = −21.9 mm; (B)). No significant effects of time (F(2,36) = 0.3, p = 0.767,
) or group x time interactions (F(2,36) = 1.1, p = 0.330,
) were found for the bisection gain score.
Figure 4. (A) Mean absolute line bisection error (mm) per session and group. (B) Mean line bisection gain score (mm) per group. (C) Mean BIT gain score per group. (D) Mean spatial bias BIT gain score per group. Note that negative scores for this gain score denote a reduction in the ipsilesional bias (see Methods for more detail). (A-D) Error bars represent standard errors. Gain scores are based on each session vs. baseline.
Behavioural inattention test (BIT)
For the total BIT score, there was a significant effect of time (F(3,54) = 16.2, p < 0.001, ): all patients presented higher BIT scores at each session when compared to baseline (mean difference experimenter-led vs. baseline = 17.7, p = 0.001; mean difference self-led vs. baseline = 26.3, p < 0.001; mean difference follow-up vs. baseline = 24.0, p < 0.001; ). Moreover, after the self-led session patients also scored significantly higher when compared to the experimenter-led session (mean difference = 8.7, p = 0.016). Nonetheless there was no effect of group, despite a large effect size (F(1,18) = 3.2, p = 0.092,
), and the group x time interaction was not significant despite a moderate effect size (F(3,54) = 2.6, p = 0.059,
). In a similar vein, the BIT spatial bias score (), showed a significant effect of time with a large effect size (F(3,54) = 13.4, p < 0.001,
): there was a reduction in the spatial bias between baseline and all the other phases (mean difference experimenter-led vs. baseline = −10.5, p = 0.001; mean difference self-led vs. baseline = −12.1, p = 0.001; mean difference follow-up vs. baseline = −8.5, p = 0.005). However, and despite moderate effect sizes, the effects of group (F(1,18) = 2.5, p = 0.131,
) and the group x time interaction (F(3,54) = 2.6, p = 0.058,
) were not significant. Importantly though, the ANOVAs on the gain scores revealed significant effects of group (with large effect sizes) both for the total (F(1,18) = 6.7, p = 0.018,
) and spatial bias scores (F(1,18) = 5.2, p = 0.035,
). Compared to the control group, the intervention group had greater gains on the BIT total score (mean difference = 18.1; see (C)) and on the spatial bias score (mean difference = −8.8%; see (D), here reflected as a reduction in error). No significant effects of time or group x time interactions were observed for the total (F(2,36) = 2.3, p = 0.111,
) and spatial bias BIT scores (F(2,36) = 1.7, p = 0.196,
, partial
).
Table 3. Mean for each outcome measure per group and session (standard error in parenthesis). For details on how the scores were computed please refer to the Methods.
Stroke impact scale (SIS)
The ANOVAs performed in each SIS domain revealed significant effects for the ADL/IADL, hand function and global perception of stroke recovery domains (see for descriptive for all SIS domains).
For the ADL/IADL score ((A)), the group x time interaction was significant with a large effect size (F(2,32) = 5.1, p = 0.01, ) although the effects of time or group were not (F(2,32) = 2.8, p = 0.077,
. As can be seen in (A), albeit post-hoc tests not being significant, the control group presented higher scores after the self-led session when compared to baseline (mean difference = 10.7, p = 0.05), whereas the intervention group presented higher scores at follow-up than baseline (mean difference = 10.8, p = 0.06). Similarly, for the ADL/IADL gain scores there were no effects of time or group (F(1,16) = 0.05, p = 0.823,
), but there was significant group x time interaction with a large effect size (F(1,16) = 9.0, p = 0.008,
). Post-hoc tests showed that at follow-up, when compared to the self-led session, the control group significantly deteriorated (mean difference = −10.2, p = 0.036) whilst the intervention group did not deteriorate but their gain increased marginally (mean difference = 8.7, p = 0.067).
Figure 5. (A) Mean ADL/IADL SIS score per session and group. (B) Mean Hand function SIS score per session and group. (A-B) Error bars represent standard errors.
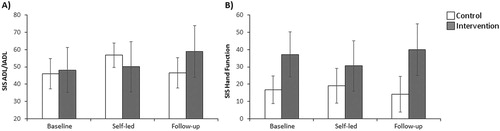
For the hand function score ((B)), there were no significant effects of time or group (F(1,17) = 1.01, p = .328,
), but the group x time interaction was significant and had a large effect size (F(1,17) = 9.7, p = 0.006,
). Post-hoc tests revealed that the intervention group scores were significantly higher at follow-up compared to the self-led session (mean difference = 9.4, p = 0.01), whereas for the control group this difference was not significant (mean difference = 4.8, p = 0.14). However, no significant group differences were observed for the hand function gain score (group: F(1,17) = 1.47, p = 0.242,
; time: F(2,34) = 0.16, p = 0.850,
; group x time: F(2,34) = 1.25, p = 0.300,
).
For the stroke recovery score, there was a significant effect of time (F(2,34) = 6.9, p = 0.003, ): all patients rated their stroke recovery higher at follow-up than at the self-led session (mean difference = 14.7, p = 0.025). No significant effects of group or group x time interaction were found (F(1,17) = 0.09, p = 0.771,
) and no significant effects were observed for the stroke recovery gain score (group: F(1,17) = 0.15, p = 0.703,
; time: F(1,17) = 0.11, p = 0.054,
; group x time: F(1,17) = 1.09, p = 0.311,
).
Balloons test
For the Balloons Test, there was a significant effect of time (F(3,54) = 7.45, p < 0.001, ): the lateralised index score was reduced at each phase when compared to baseline (mean difference exp-led vs. baseline = 12.9, p = 0.02; mean difference self-led vs. baseline = 20.0, p = 0.01; mean difference follow-up vs. baseline = 14.8, p = 0.046). There were no significant effects of group (F(1,18) = 2.01, p = 0.173,
) or time x group interaction (F(3,54) = 1.27, p = 0.293,
). There were also no significant group effects for the Balloons gain score (group: F(1,18) = 0.19, p = 0.664,
; time: F(1,18) = 0.23, p = 0.634,
; group x time: F(1,18) = 3.53, p = 0.076,
).
Landmark, subjective straight-ahead and room description tasks
For the landmark task, only the main effect of time was significant (F(3,54) = 2.8, p = 0.046, ), but post-hoc tests did not reach significance. In addition, no significant main effect of group (F(1,18) = 0.026, p = 0.873,
) or group x time interaction (F(3,54) = 0.653, p = 0.585,
) were observed. In line with this, no significant group effects were observed for the landmark task gain score (group: F(1,18) = 0.85, p = 0.369,
; time: F(2,36) = 2.01, p = 0.148,
; group x time: F(2,36) = 0.54, p = 0.586,
). Moreover, no significant effects were observed for either the raw or gain scores of subject straight-ahead and room description tasks so for brevity these are not reported here but full descriptive statistics can be found in .
Discussion
This study demonstrates, for the first time, that visuomotor feedback training (VFT), a simple training of grasping-to-lift rods, yields a significant improvement of hemispatial neglect, which was observed after just one hour of training and persisted for at least four-months post-training. VFT produced long-term improvements not only in line bisection but, notably, the training effects generalised to untrained tasks, including visual search tasks. Moreover, while the control group deteriorated in ADL/IADL at follow-up the VFT group did not, and instead marginally improved. Notably, the present findings show that visuomotor feedback training produces long-lasting neglect improvements even when applied in a reduced fashion (fewer sessions and trials) and on more severely impaired patients (lower baseline BIT scores than previous studies), thus highlighting the feasibility of this technique to be applied in a real-clinical environment and justifying further larger RCT trials to confirm these results.
Improvements associated with treatment
In particular, we found that visuomotor feedback training produced significant short- and long-term improvements in line bisection performance, a result we were hoping for, as this test can be considered to be most similar to the training procedure.
In terms of the BIT effects, significant improvements were found for all patients but, importantly, these improvements were much greater in the intervention than in the control group. In particular, treatment with visuomotor feedback training was associated with a significantly greater gain in BIT and spatial bias scores (here reflected in a greater reduction in the pathological spatial bias towards the neglected field in cancellation sub-tests). The effects were maintained until the four-months follow-up and found on the most widely used visual search tests to detect neglect in clinical settings (i.e., BIT).
Moreover, visuomotor feedback training also had an effect in the SIS ADL/IADL domain at four-months post-training: the VFT group marginally improved in ADL/IADL whilst the control group deteriorated significantly. Even though this effect will certainly need to be replicated in a larger study, we believe that it is quite encouraging, as the ADL/IADL domain assesses important aspects of the patient’s daily routine (such as eating, dressing, personal hygiene and social activities) and for most patients the SIS was rated by a carer who was blind to group assignment. The fact that the improvements in hand function and ADL/IADL emerged only at four-months post-training, and not immediately after the training sessions, may reflect a late consolidation of learning after training (see also Harvey et al., Citation2003). Alternatively, the fact the SIS was administered by a carer in 14 patients might have hindered the results, as there is evidence that proxies tend to score patients more severely on the SIS than when responses are given by patients themselves (Carod-Artal, Coral, Trizotto, & Moreira, Citation2009). Nevertheless, the present findings suggest that the SIS is sensitive to both the impact of neglect on daily life functioning and to rehabilitation outcomes. In fact, no previous randomised control study has reported such long-lasting improvements in a functional rating scale in neglect rehabilitation studies.
A final finding from this study is that the intervention group significantly ameliorated their score on the SIS hand function domain at follow-up (yet only when compared to the self-led session), whilst the control group scores remained unchanged (yet the gain scores did not yield group differences). The SIS hand function domain assesses the patient’s ability to use their impaired hand in functional daily life tasks (such as turning doorknobs, opening a can and picking up small objects). The observation that visuomotor feedback training (involving actions with the non-paretic limb) produced a small hand function amelioration may be because this training improved the patients’ ability to find items on the neglected side (including their paretic hand which gradually increased their hand function). Admittedly, the lack of effects on gain scores for hand function suggests that this finding may be weaker than all the others we have reported and requires further investigations with larger sample of patients.
These findings are promising given that, to our knowledge, this follow-up period is the longest ever tested in a randomised controlled trial of neglect rehabilitation (although longer periods have been reported in studies lacking a control group, e.g., Rabufetti et al., Citation2013; Rusconi & Carelli, Citation2012). These improvements were demonstrated using a randomised, single-blind, attention-controlled design in a group of 20 chronic neglect patients and the intervention and control groups were not significantly different at baseline. Therefore, the positive effects found here were specific to visuomotor feedback training and are not simply due to non-specific factors (e.g., amount of training or attention received) or conventional rehabilitation therapy. In addition, even though the effects were found with a relatively small sample size of 20 patients, it is important to note that this sample size is still amongst the largest ever reported in any neglect rehabilitation trial, including very recent studies by Kerkhoff et al. (Citation2013, Citation2014) showing promising results of “smooth pursuit” training on samples of 50 and 30 patients respectively, and by Mizuno et al. (Citation2011) reporting prism adaptation effects in ADL on a sample of 38 patients.
Outcome measures not affected by treatment
It should be noted that despite the improvement found in the BIT cancellation sub-tests, we did not observe any significant effects of visuomotor feedback training on our other visual search task, the Balloons task, replicating Harvey et al. (Citation2003)’s observation. Possible reasons for this discrepancy might be related to the different sensitivity or task requirement of these tests (for a review of the effects of task demands on neglect and extinction see Bonato, Citation2012).
Moreover, even though significant short- and long-lasting improvements were observed in the line bisection task, no effects were apparent on the landmark test. However, Harvey et al. (Citation2003)’s effects on the landmark task were immediate and were found using the perceptual version of the Landmark task, whereas here we tested the effects on the motor version of the landmark task (i.e., instead of making a perceptual judgement patients were asked to point to the side of line that was shorter). It is thus possible that visuomotor feedback training only affects the perceptual distortion present in neglect patients and not the pre-motor deficits, but future studies will be required to investigate this possibility.
In line with previous findings (e.g., Pisella, Rode, Farnè, Boisson, & Rossetti, Citation2002; Rode et al., Citation2001), most of our patients presented a marked ipsilesional shift at baseline in the subjective straight-ahead pointing task. However, in contrast to studies using prism adaptation (e.g., Frassinetti et al., Citation2002; Pisella et al., Citation2002; Rossetti et al., Citation1998; Sarri et al., Citation2008), visuomotor feedback training did not produce any improvements on this measure. It is possible that prism adaptation produces greater effects on this measure than visuomotor feedback training however, not all patients’ biases improve after wearing prisms and not all patients adapt to the prisms (Rousseaux, Bernati, Saj, & Kozlowski, Citation2006; Sarri et al., Citation2008; Turton, O'Leary, Gabb, Woodward, & Gilchrist, Citation2010). In addition, straight-ahead pointing abnormalities may not be exclusive to neglect patients and may not always correlate with other neglect tests (e.g., Bartolomeo & Chokron, Citation1999; Chokron & Bartolomeo, Citation1997; Farne, Ponti, & Ladavas, Citation1998). Furthermore, we also did not observe any improvements in the room description task due to a clear ceiling effect as the performance of our patient group was already close to normal at baseline. Thus whether visuomotor feedback training also improves tasks performed in extrapersonal space (i.e., outside the reaching space) remains to be determined. Finally there were no differential effects on the overall stroke recovery score but it is possible that this measure may be too broad to reflect subtler changes.
Limitations of the study
Out of necessity, for six patients, the SIS was administered by the experimenter as no carers where available and the experimenter was not blind to treatment allocation so this a constraint of this study (see also above for discussion of carer vs. patient assessment). Moreover, although patients recorded execution of every single trial on a sheet during the home intervention and this was scrutinised after, there was no guarantee that the procedure was in fact adhered to. In future trials it might be better to monitor execution via small cameras/ mobile phones or possibly a wrist device that would monitor rod lifts.
Future considerations
It has been suggested that VFT produces a “dorsal-to-ventral” visual stream re-calibration (Robertson et al., Citation1995, Citation1997; Harvey et al., Citation2003), allowing action processing to improve perception. It could thus be predicted that neglect patients who suffer from damage to dorsal visual stream areas involved in reaching and grasping, such as the superior parietal occipital cortex and intra-parietal sulcus (Culham, Cavina-Pratesi, & Singhal, Citation2006; Rossit, McAdam, McLean, Goodale, & Culham, Citation2013), might not benefit from the treatment. Alternatively, VFT may recruit spared dorsal visual stream regions located in the undamaged hemisphere contralateral to the hand used to perform the training. It is also possible that VFT and prism adaptation (Newport & Schenk, Citation2012; Rode et al., Citation2015) recruit similar sensorimotor processes. Similarly to what happens during prism adaptation, the patients correct their grasping position until successful performance is achieved (i.e., the rod is balanced), a process known as strategic recalibration in the prism literature (Newport & Schenk, Citation2012). Future larger clinical trials involving neuroimaging will be required to establish the exact neural mechanisms behind VFT and for which patients this form of rehabilitation is most suitable for.
To sum up, VFT produces long-lasting improvements in stroke patients with chronic hemispatial neglect. Importantly, we have shown that the effects are present even when administered at the patient’s home, generalise to untrained visual search tasks and that this training impacts on everyday behaviour. Although a larger single-blinded randomised controlled trial will be needed to replicate the present results, this study highlights the promising potential of VFT for future large-scale implementation in the rehabilitation of hemispatial neglect and its feasibility as a home-based intervention. In contrast to most available techniques for neglect rehabilitation, VFT can be easily taught and delivered in either an in- or out-patient setting, it is non-invasive, cost-effective (the cost of the material is negligible), can be conducted by the patients themselves in their homes and does not require patient insight into the disorder.
Acknowledgements
We would like to thank all the stroke patients for their kind cooperation during this trial. We would also like to thank Prof. Valerie Pomeroy and Dr. Fraser W. Smith for most helpful comments on earlier versions of this manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Antonucci, G., Guariglia, C., Judica, A., Magnotti, L., Paolucci, S., Pizzamiglio, L., & Zoccolotti, P. (1995). Effectiveness of neglect rehabilitation in a randomized group study. Journal of Clinical and Experimental Neuropsychology, 17, 383–389. doi: 10.1080/01688639508405131
- Appelros, P., Karlsson, G. M., Seiger, A., & Nydevik, I. (2003). Prognosis for patients with neglect and anosognosia with special reference to cognitive impairment. Journal of Rehabilitation Medicine, 35, 254–258. doi: 10.1080/16501970310012455
- Bartolomeo, P., & Chokron, S. (1999). Egocentric frame of reference: Its role in spatial bias after right hemisphere lesions. Neuropsychologia, 37, 881–894. doi: 10.1016/S0028-3932(98)00150-X
- Bisiach, E., Pizzamiglio, L., Nico, D., & Antonucci, G. (1996). Beyond unilateral neglect. Brain, 119, 851–857. doi: 10.1093/brain/119.3.851
- Blackwell, D., & Hodges Jr., J. L. (1957). Design for the control of selection bias. The Annals of Mathematical Statistics, 28, 449–460. doi: 10.1214/aoms/1177706973
- Bonato, M. (2012). Neglect and extinction depend greatly on task demands: A review. Frontiers in Human Neuroscience, 6, 195. doi: 10.3389/fnhum.2012.00195
- Bowen, A., Hazelton, C., Pollock, A., & Lincoln, N. B. (2013). Cognitive rehabilitation for spatial neglect following stroke. Cochrane Database Systematic Reviews, 7.
- Brunila, T., Lincoln, N., Lindell, A., Tenovuo, O., & Hamalainen, H. (2002). Experiences of combined visual training and arm activation in the rehabilitation of unilateral visual neglect: A clinical study. Neuropsychological Rehabilitation, 12, 27–40. doi: 10.1080/09602010143000077
- Buxbaum, L. J., Ferraro, M. K., Veramonti, T., Farné, A., Whyte, J., Làdavas, E., … Coslett, H. B. (2004). Hemispatial neglect: Subtypes, neuroanatomy, and disability. Neurology, 62, 749–756. doi: 10.1212/01.WNL.0000113730.73031.F4
- Cappa, S., Sterzi, R., Vallar, G., & Bisiach, E. (1987). Remission of hemineglect and anosognosia during vestibular stimulation. Neuropsychologia, 25, 775–782. doi: 10.1016/0028-3932(87)90115-1
- Cappa, S. F., Benke, T., Clarke, S., Rossi, B., Stemmer, B., & van Heugten, C. M. (2005). EFNS guidelines on cognitive rehabilitation: Report of an EFNS task force. Journal of Neurology, 12, 665–680.
- Carod-Artal, F. J., Coral, L. F., Trizotto, D. S., & Moreira, C. M. (2009). Self- and proxy-report agreement on the Stroke Impact Scale. Stroke, 40, 3308–3314. doi: 10.1161/STROKEAHA.109.558031
- Cazzoli, D., Mueri, R. M., Schumacher, R., von Arx, S., Chaves, S., Gutbrod, K., … Nyffeler, T. (2012). Theta burst stimulation reduces disability during the activities of daily living in spatial neglect. Brain, 135, 3426–3439. doi: 10.1093/brain/aws182
- Champod, A.S., Frank, R.C., Taylor, K., & Eskes, G.A. (2016). The effects of prism adaptation on daily life activities in patients with visuospatial neglect: A systematic review. Neuropsychological Rehabilitation. doi:10.1080/09602011.2016.1182032
- Chokron, S., & Bartolomeo, P. (1997). Patterns of dissociation between left hemineglect and deviation of the egocentric reference. Neuropsychologia, 35, 1503–1508. doi: 10.1016/S0028-3932(97)00079-1
- Cicerone, K. D., Dahlberg, C., Malec, J. F., Langenbahn, D. M., Felicietti, T., Kneipp, S., … Catanese, J. (2005). Evidence-based cognitive rehabilitation: Updated review of the literature from 1998 through 2002. Archives of Physical Medicine and Rehabilitation, 86, 1681–1692. doi: 10.1016/j.apmr.2005.03.024
- Cohen, J. (1988). Statistical power analysis for the behavior sciences (2nd ed.). Hillsdale, NJ: Erlbaum.
- Culham, J. C., Cavina-Pratesi, C., & Singhal, A. (2006). The role of parietal cortex in visuomotor control: What have we learned from neuroimaging? Neuropsychologia, 44, 2668–2684. doi: 10.1016/j.neuropsychologia.2005.11.003
- Duncan, P. W., Bode, R. K., Lai, S. M., & Perera, S. (2003). Rasch analysis of a new stroke-specific outcome scale: The Stroke Impact Scale. Archives of Physical Medicine and Rehabilitation, 84, 950–963. doi: 10.1016/S0003-9993(03)00035-2
- Duncan, P. W., Lai, S. M., Tyler, D., Perera, S., Reker, D. M., & Studenski, S. (2002). Evaluation of proxy responses to the Stroke Impact Scale. Stroke, 33, 2593–2599. doi: 10.1161/01.STR.0000034395.06874.3E
- Duncan, P. W., Wallace, D., Lai, S. M., Johnson, D., Embretson, S., & Laster, L. G. (1999). The Stroke Impact Scale Version 2.0: Evaluation of reliability, validity, and sensitivity to change. Stroke, 30, 2131–2140. doi: 10.1161/01.STR.30.10.2131
- Duncan, P. W., Wallace, D., Lai, S. M., Studenski, S., Johnson, D., & Embretson, S. (1999). The Stroke Impact Scale (UK English version of SIS). Kansas City: University of Kansas Medical Center.
- Edgeworth, J. A., Robertson, I. H., & McMillan, T. M. (1998). The Balloons Test. Bury St Edmunds: Thames Valley Test Company.
- Edwards, M. G., & Humphreys, G. W. (1999). Pointing and grasping in unilateral visual neglect: Effect of on-line visual feedback in grasping. Neuropsychologia, 37, 959–973. doi: 10.1016/S0028-3932(98)00132-8
- Farne, A., Ponti, F., & Ladavas, E. (1998). In search of biased egocentric reference frames in neglect. Neuropsychologia, 36, 611–623. doi: 10.1016/S0028-3932(97)00164-4
- Frassinetti, F., Angeli, V., Meneghello, F., Avanzi, S., & Ladavas, E. (2002). Long-lasting amelioration of visuospatial neglect by prism adaptation. Brain, 125, 608–623. doi: 10.1093/brain/awf056
- Gillen, R., Tennen, H., & McKee, T. (2005). Unilateral spatial neglect: Relation to rehabilitation outcomes in patients with right hemisphere stroke. Archives of Physical Medicine and Rehabilitation, 86, 763–767. doi: 10.1016/j.apmr.2004.10.029
- Gorgoraptis, N., Mah, Y. H., Machner, B., Singh-Curry, V., Malhotra, P., Hadji-Michael, M., … Husain, M. (2012). The effects of the dopamine agonist rotigotine on hemispatial neglect following stroke. Brain, 135, 2478–2491. doi: 10.1093/brain/aws154
- Halligan, P. W., Manning, L., & Marshall, J. C. (1990). Individual variation in line bisection: A study of four patients with right hemisphere damage and normal controls. Neuropsychologia, 28, 1043–1051. doi: 10.1016/0028-3932(90)90139-F
- Harding, P., & Riddoch, M. J. (2009). Functional electrical stimulation (FES) of the upper limb alleviates unilateral neglect: A case series analysis. Neuropsychological Rehabilitation, 19, 41–63. doi: 10.1080/09602010701852610
- Harvey, M., Hood, B., North, A., & Robertson, I. H. (2003). The effects of visuomotor feedback training on the recovery of hemispatial neglect symptoms: Assessment of a 2-week and follow-up intervention. Neuropsychologia, 41, 886–893. doi: 10.1016/S0028-3932(03)00003-4
- Harvey, M., Jackson, S. R., Newport, R., Krämer, T., Morris, D. L., & Dow, L. (2001). Is grasping impaired in hemispatial neglect? Behavioural Neurology, 13, 17–28. doi: 10.1155/2002/495854
- Harvey, M., Milner, A. D., & Roberts, R. C. (1995). An investigation of hemispatial neglect using the Landmark Task. Brain and Cognition, 27, 59–78. doi: 10.1006/brcg.1995.1004
- Harvey, M., & Rossit, S. (2012). Visuospatial neglect in action. Neuropsychologia, 50, 1018–1028. doi: 10.1016/j.neuropsychologia.2011.09.030
- Himmelbach, M., & Karnath, H. O. (2003). Goal-directed hand movements are not affected by the biased space representation in spatial neglect. Journal of Cognitive Neuroscience, 15, 972–980. doi: 10.1162/089892903770007362
- Jacquin-Courtois, S., Rode, G., Pisella, L., Boisson, D., & Rossetti, Y. (2008). Wheel-chair driving improvement following visuo-manual prism adaptation. Cortex, 44, 90–96. doi: 10.1016/j.cortex.2006.06.003
- Kalra, L., Perez, I., Gupta, S., & Wittink, M. (1997). The influence of visual neglect on stroke rehabilitation. Stroke, 28, 1386–1391. doi: 10.1161/01.STR.28.7.1386
- Karnath, H. O. (1994). Subjective body orientation in neglect and the interactive contribution of neck muscle proprioception and vestibular stimulation. Brain, 117, 1001–1012. doi: 10.1093/brain/117.5.1001
- Karnath, H. O. (1996). Optokinetic stimulation influences the disturbed perception of body orientation in spatial neglect. Journal of Neurology, Neurosurgery & Psychiatry, 60, 217–220. doi: 10.1136/jnnp.60.2.217
- Karnath, H. O., Christ, K., & Hartje, W. (1993). Decrease of contralateral neglect by neck muscle vibration and spatial orientation of trunk midline. Brain, 116, 383–396. doi: 10.1093/brain/116.2.383
- Karnath, H. O., Rennig, J., Johannsen, L., & Rorden, C. (2011). The anatomy underlying acute versus chronic spatial neglect: A longitudinal study. Brain, 134, 903–912. doi: 10.1093/brain/awq355
- Katz, N., Hartman-Maeir, A., Ring, H., & Soroker, N. (1999). Functional disability and rehabilitation outcome in right hemisphere damaged patients with and without unilateral spatial neglect. Archives of Physical Medicine and Rehabilitation, 80, 379–384. doi: 10.1016/S0003-9993(99)90273-3
- Kerkhoff, G. (1998). Cognitive Neurovisual Rehabilitation: A cross-over study in patients with neglect and hemianopia. European Journal of Neuroscience, 10, 375.
- Kerkhoff, G. (2001). Spatial hemineglect in humans. Progress in Neurobiology, 63, 1–27. doi: 10.1016/S0301-0082(00)00028-9
- Kerkhoff, G., Bucher, L., Brasse, M., Leonhart, E., Holzgraefe, M., Voelzke, V., … Reinhart, S. (2014). Smooth pursuit “bedside” training reduces disability and unawareness during the activities of daily living in neglect: A randomized controlled trial. Neurorehabilitation and Neural Repair, 28, 554–563. doi: 10.1177/1545968313517757
- Kerkhoff, G., Hildebrandt, H., Reinhar, t. S., Kardinal, M., Dimova, V., & Utz, K. S. (2011). A long-lasting improvement of tactile extinction after galvanic vestibular stimulation: Two Sham-stimulation controlled case studies. Neuropsychologia, 49, 186–195. doi: 10.1016/j.neuropsychologia.2010.11.014
- Kerkhoff, G., Keller, I., Artinger, F., Hildebrandt, H., Marquardt, C., Reinhart, S., & Ziegler, W. (2012). Recovery from auditory and visual neglect after optokinetic stimulation with pursuit eye movements – Transient modulation and enduring treatment effects. Neuropsychologia, 50, 1164–1177. doi: 10.1016/j.neuropsychologia.2011.09.032
- Kerkhoff, G., Keller, I., Ritter, V., & Marquardt, C. (2006). Repetitive optokinetic stimulation induces lasting recovery from visual neglect. Restorative Neurology and Neuroscience, 24, 357–369.
- Kerkhoff, G., Reinhart, S., Ziegler, W., Artinger, F., Marquardt, C., & Keller, I. (2013). Smooth pursuit eye movement training promotes recovery from auditory and visual neglect: A randomized controlled study. Neurorehabilitation and Neural Repair, 27, 789–798. doi: 10.1177/1545968313491012
- Kerkhoff, G., & Schenk, T. (2012). Rehabilitation of neglect: An update. Neuropsychologia, 50, 1072–1079. doi: 10.1016/j.neuropsychologia.2012.01.024
- Kerkhoff, G., Schindler, I., Keller, I., & Marquardt, C. (1999). Visual background motion reduces size distortion in spatial neglect. Neuroreport, 10, 319–323. doi: 10.1097/00001756-199902050-00021
- Kim, Y. M., Chun, M. H., Yun, G. J., Song, Y. J., & Young, H. E. (2011). The effect of virtual reality training on unilateral spatial neglect in stroke patients. Annals of Rehabilitation Medicine, 35, 309–315. doi: 10.5535/arm.2011.35.3.309
- Lachin, J. M. (1988). Properties of simple randomization in clinical trials. Controlled Clinical Trials, 9, 312–326. doi: 10.1016/0197-2456(88)90046-3
- Lawson, I. R. (1962). Visual-spatial neglect in lesions of the right cerebral hemisphere: A study in recovery. Neurology, 12, 23–23. doi: 10.1212/WNL.12.1.23
- McIntosh, R. D., McClements, K. I., Dijkerman, H. C., Birchall, D., & Milner, A. D. (2004). Preserved obstacle avoidance during reaching in patients with left visual neglect. Neuropsychologia, 42, 1107–1117. doi: 10.1016/j.neuropsychologia.2003.11.023
- McIntosh, R. D., Pritchard, C. L., Dijkerman, H. C., Milner, A. D., & Roberts, R. C. (2001). Prehension and perception of size in left visual neglect. Behavioural Neurology, 13, 3–15. doi: 10.1155/2002/252405
- Mizuno, K., Tsuji, T., Takebayashi, T., Fufiwara, T., Hase, K., & Liu, M. (2011). Prism adaptation therapy enhances rehabilitation of stroke patients with unilateral spatial neglect: A randomized, controlled trial. Neurorehabilitation and Neural Repair, 25, 711–720. doi: 10.1177/1545968311407516
- Newport, R., & Schenk, T. (2012). Prisms and neglect: What have we learned? Neuropsychologia, 50, 1080–1091. doi: 10.1016/j.neuropsychologia.2012.01.023
- Nyffeler, T., Cazzoli, D., Hess, C. W., & Müri, R. M. (2009). One session of repeated parietal theta burst stimulation trains induces long-lasting improvement of visual neglect. Stroke, 40, 2791–2796. doi: 10.1161/STROKEAHA.109.552323
- Piccardi, L., Nico, D., Bureca, I., Matano, A., & Guariglia, C. (2006). Efficacy of visuo-spatial training in right-brain damaged patients with spatial hemineglect and attention disorders. Cortex, 42, 973–982. doi: 10.1016/S0010-9452(08)70203-X
- Pisella, L., Rode, G., Farnè, A., Boisson, D., & Rossetti, Y. (2002). Dissociated long lasting improvements of straight-ahead pointing and line bisection tasks in two hemineglect patients. Neuropsychologia, 40, 327–334. doi: 10.1016/S0028-3932(01)00107-5
- Pizzamiglio, L., Antonucci, G., Judica, A., Montenero, P., Razzano, C., & Zoccolotti, P. (1992). Cognitive rehabilitation of the hemineglect disorder in chronic patients with unilateral right brain damage. Journal of Clinical and Experimental Neuropsychology, 14, 901–923. doi: 10.1080/01688639208402543
- Pizzamiglio, L., Fasotti, L., Jehkonen, M., Antonucci, G., Magnotti, L., Boelen, D., & Asa, S. (2004). The use of optokinetic stimulation in rehabilitation of the hemineglect disorder. Cortex, 40, 441–450. doi: 10.1016/S0010-9452(08)70138-2
- Pizzamiglio, L., Frasca, R., Guariglia, C., Incoccia, C., & Antonucci, G. (1990). Effect of optokinetic stimulation in patients with visual neglect. Cortex, 26, 535–541. doi: 10.1016/S0010-9452(13)80303-6
- Polanowska, K., Seniów, J., Paprot, E., Leśniak, M., & Członkowska, A. (2009). Left-hand somatosensory stimulation combined with visual scanning training in rehabilitation for post-stroke hemineglect: A randomised, double-blind study. Neuropsychological Rehabilitation, 19, 364–382. doi: 10.1080/09602010802268856
- Priftis, K., Passarini, L., Pilosio, C., Meneghello, F., & Pitteri, M. (2013). Visual scanning training, limb activation treatment, and prism adaptation for rehabilitating left neglect: Who is the winner? Frontiers in Human Neuroscience, 7, 360.
- Rabufetti, M., Folegatti, A., Spinazzola, L., Ricci, R., Ferrarin, M., Berti, A., & Neppi-Modona, M. (2013). Long-lasting amelioration of walking trajectory in neglect after prismatic adaptation. Frontiers in Human Neuroscience, 7, 382. doi: 10.3389/fnhum.2013.00382
- Rengachary, J., He, B. J., Shulman, G. L., & Corbetta, M. (2011). A behavioral analysis of spatial neglect and its recovery after stroke. Frontiers in Human Neuroscience, 5, 29. doi: 10.3389/fnhum.2011.00029
- Robertson, I. H., Hogg, K., & McMillan, T. M. (1998a). Rehabilitation of unilateral neglect: Improving function by contralesional limb activation. Neuropsychological Rehabilitation, 8, 19–29. doi: 10.1080/713755556
- Robertson, I. H., Mattingley, J. B., Rorden, C., & Driver, J. (1998b). Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature, 395, 169–172. doi: 10.1038/25993
- Robertson, I. H., McMillan, T. M., MacLeod, E., Edgeworth, J., & Brock, D. (2002). Rehabilitation by limb activation training reduces left-sided motor impairment in unilateral neglect patients: A single-blind randomised control trial. Neuropsychological Rehabilitation, 12, 439–454. doi: 10.1080/09602010244000228
- Robertson, I. H., Nico, D., & Hood, B. M. (1997). Believing what you feel: Using proprioceptive feedback to reduce unilateral neglect. Neuropsychology, 11, 53–58. doi: 10.1037/0894-4105.11.1.53
- Robertson, I. H., & North, N. (1992). Spatio-motor cueing in unilateral left neglect: The role of hemispace, hand and motor activation. Neuropsychologia, 30, 553–563. doi: 10.1016/0028-3932(92)90058-T
- Robertson, I. H., & North, N. (1993). Active and passive activation of left limbs: Influence on visual and sensory neglect. Neuropsychologia, 31, 293–300. doi: 10.1016/0028-3932(93)90093-F
- Robertson, I. H., & North, N. (1994). One hand is better than two: Motor extinction of left hand advantage in unilateral neglect. Neuropsychologia, 32, 1–11. doi: 10.1016/0028-3932(94)90064-7
- Robertson, I. H., North, N., & Geggie, C. (1992). Spatiomotor cueing in unilateral left neglect: Three case studies of its therapeutic effects. Journal of Neurology, Neurosurgery & Psychiatry, 55, 799–805. doi: 10.1136/jnnp.55.9.799
- Robertson, I. H., Tegnér, R., Tham, K., Lo, A., & Nimmo-Smith, I. (1995). Sustained attention training for unilateral neglect: Theoretical and rehabilitation implications. Journal of Clinical and Experimental Neuropsychology, 17, 416–430. doi: 10.1080/01688639508405133
- Rode, G., Lacour, S., Jacquin-Courtois, S., Pisella, L., Michel, C., Revol, P., … Rossetti, Y. (2015). Long-term sensorimotor and therapeutical effects of a mild regime of prism adaptation in spatial neglect. A double-blind RCT essay. Annals of Physical and Rehabilitation Medicine, 58, 40–53. doi: 10.1016/j.rehab.2014.10.004
- Rode, G., Rossetti, Y., & Boisson, D. (2001). Prism adaptation improves representational neglect. Neuropsychologia, 39, 1250–1254. doi: 10.1016/S0028-3932(01)00064-1
- Rorden, C., & Brett, M. (2000). Stereotaxic display of brain lesions. Behavioural Neurology, 12, 191–200. doi: 10.1155/2000/421719
- Rousseaux, M., Bernati, T., Saj, A., & Kozlowski, O. (2006). Ineffectiveness of prism adaptation on spatial neglect signs. Stroke, 37, 542–543. doi: 10.1161/01.STR.0000198877.09270.e8
- Rossetti, Y., Rode, G., Pisella, L., Farné, A., Li, L., Boisson, D., & Perenin, M. T. (1998). Prism adaptation to a rightward optical deviation rehabilitates left hemispatial neglect. Nature, 395, 166–169. doi: 10.1038/25988
- Rossit, S., Fraser, J. A., Teasell, R., Malhotra, P. A., & Goodale, M. A. (2011a). Impaired delayed but preserved immediate grasping in a neglect patient with parieto-occipital lesions. Neuropsychologia, 49, 2498–2504. doi: 10.1016/j.neuropsychologia.2011.04.030
- Rossit, S., Malhorta, P., Muir, K., Reeves, I., Duncan, G., Birschel, P., & Harvey, M. (2009a). The neural basis of visuomotor deficits in hemispatial neglect. Neuropsychologia, 47, 2149–2153. doi: 10.1016/j.neuropsychologia.2009.04.015
- Rossit, S., Malhotra, P., Muir, K., Reeves, I., Duncan, G., & Harvey, M. (2011b). The role of right temporal lobe structures in off-line action: Evidence from lesion-behavior mapping in stroke patients. Cerebral Cortex, 21, 2751–2761. doi: 10.1093/cercor/bhr073
- Rossit, S., Malhorta, P., Muir, K., Reeves, I., Duncan, G., Livingstone, K., … Harvey, M. (2009b). No neglect-specific deficits in reaching tasks. Cerebral Cortex, 19, 2616–2624. doi: 10.1093/cercor/bhp016
- Rossit, S., McAdam, T., McLean, D. A., Goodale, M. A., & Culham, J. C. (2013). fMRI reveals a lower visual field preference for hand actions in human superior parieto-occipital cortex (SPOC) and precuneus. Cortex, 49, 2525–2541. doi: 10.1016/j.cortex.2012.12.014
- Rossit, S., Muir, K., Reeves, I., Duncan, G., Birschel, P., & Harvey, M. (2009c). Immediate and delayed reaching in hemispatial neglect. Neuropsychologia, 47, 1563–1572. doi: 10.1016/j.neuropsychologia.2008.08.008
- Rubens, A. B. (1985). Caloric stimulation and unilateral visual neglect. Neurology, 35, 1019–1019. doi: 10.1212/WNL.35.7.1019
- Rusconi, M. L., & Carelli, L. (2012). Long-term efficacy of prism adaptation on spatial neglect: Preliminary results on different spatial components. The Scientific World Journal, 2012, 1–8. doi: 10.1100/2012/618528
- Samuel, C., Louis-Dreyfus, A., Kaschel, R., Makiela, E., Troubat, M., Anselmi, N., … Azouvi, P. (2000). Rehabilitation of very severe unilateral neglect by visuo-spatio-motor cueing: Two single case studies. Neuropsychological Rehabilitation, 10, 385–399. doi: 10.1080/096020100411970
- Sarri, M., Greenwood, R., Kalra, L., Papps, B., Husain, M., & Driver, J. (2008). Prism adaptation aftereffects in stroke patients with spatial neglect: Pathological effects on subjective straight ahead but not visual open-loop pointing. Neuropsychologia, 46, 1069–1080. doi: 10.1016/j.neuropsychologia.2007.11.005
- Schindler, I., & Kerkhoff, G. (2004). Convergent and divergent effects of neck proprioceptive and visual motion stimulation on visual space processing in neglect. Neuropsychologia, 42, 1149–1155. doi: 10.1016/j.neuropsychologia.2004.02.006
- Schindler, I., Kerkhoff, G., Karnath, H. O., Keller, I., & Goldenberg, G. (2002). Neck muscle vibration induces lasting recovery in spatial neglect. Journal of Neurology, Neurosurgery & Psychiatry, 73, 412–419. doi: 10.1136/jnnp.73.4.412
- Scottish Intercollegiate Guidelines Network. (2010). Management of patients with stroke: Rehabilitation, prevention and management of complications, and discharge planning. A national clinical guideline. SIGN publication no. 118. Retrieved from http://www.sign.ac.uk.
- Sedda, A., Borghese, N. A., Ronchetti, M., Mainetti, R., Pasotti, F., Beretta, G., & Bottini, G. (2013). Using virtual reality to rehabilitate neglect. Behavioural Neurology, 26, 183–185. doi: 10.1155/2013/810279
- Song, W., Du, B., Xu, Q., Hu, J., Wang, M., & Luo, Y. (2009). Low-frequency transcranial magnetic stimulation for visual spatial neglect: A pilot study. Journal of Rehabilitation Medicine, 41, 162–165. doi: 10.2340/16501977-0302
- Sparing, R., Thimm, M., Hesse, M. D., Küst, J., Karbe, H., & Fink, G. R. (2009). Bidirectional alterations of interhemispheric parietal balance by non-invasive cortical stimulation. Brain, 132, 3011–3020. doi: 10.1093/brain/awp154
- Thimm, M., Fink, G. R., Küst, J., Karbe, H., & Sturm, W. (2006). Impact of alertness training on spatial neglect: A behavioural and fMRI study. Neuropsychologia, 44, 1230–1246. doi: 10.1016/j.neuropsychologia.2005.09.008
- Turton, A. J., O'Leary, K., Gabb, J., Woodward, R., & Gilchrist, I. D. (2010). A single blinded randomised controlled pilot trial of prism adaptation for improving self-care in stroke patients with neglect. Neuropsychological Rehabilitation, 20, 180–196. doi: 10.1080/09602010903040683
- Vaes, N., Nys, G., Lafosse, C., Dereymaeker, L., Oostra, K., Hemelsoet, D., & Vingerhoets, G. (2016). Rehabilitation of visuospatial neglect by prism adaptation: Effects of a mild treatment regime. A randomised controlled trial. Neuropsychological Rehabilitation, 18, 1–20.
- Vallar, G., Sterzi, R., Bottini, G., Cappa, S., & Rusconi, M. L. (1990). Temporary remission of left hemianesthesia after vestibular stimulation. A sensory neglect phenomenon. Cortex, 26, 123–131. doi: 10.1016/S0010-9452(13)80078-0
- Wilson, B., Cockburn, J., & Halligan, P. W. (1987). Behavioural Inattention Test. Bury St Edmunds: Thames Valley Test Company.