?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In the future, cleaning products must fulfil the principles of green chemistry while maintaining efficacy against bacteria. This study aims to evaluate the detergent properties, ecotoxicity, and anti-biofilm potential of natural saponins compared to synthetic surfactants. We tested sodium dodecyl sulphate, quillaja saponin, escin, and sapogenin for emulsifying capacity, critical micelle concentration, ecotoxicity to yeast, and antibacterial and anti-biofilm potential against bacteria. The results show that the emulsifying capacities of quillaja saponin and sodium dodecyl sulphate are similar, while the critical micelle concentration for quillaja saponin is much lower . Furthermore, the antibacterial and antibiofilm potentials are much higher for quillaja saponin than for synthetic sodium dodecyl sulphate . Moreover, we have shown that natural saponins are less toxic to the S. cerevisiae than synthetic saponin is. All these facts indicate that quillaja is a suitable candidate to replace synthetic products as it meets the requirements of efficacy and safety.
Introduction
Bacterial attachment to the material surfaces is a critical issue in medicine, technology, and household environments where we attempt to maintain the bacterial population at as low a level as possible (Marriott et al. Citation2018). Conditions on the surface (e.g. water, presence of soil, nutrients and beneficial temperatures) promote bacterial adhesion. By cleaning, visible or invisible soil on the surface is removed to prevent bacterial adhesion and, consequently, biofilm growth. Different approaches are used to control soiling and microbial populations, but the chemical approach by applying a detergent on the surface and separating contaminants from the surface is the most predominant (Fink Citation2019). The chemical industry continually places new cleaning products on the market that act as detergents, degreasers, abrasives, acids, and similar. Many chemicals used in cleaning processes are thus deposited in the environment, causing adverse effects on nature and human health (Wieck et al. Citation2018). Growing populations and improving economies in many countries increase the consumption of cleaning products and, therefore, the pressure on the environment (Elorriaga et al. Citation2013). Therefore, the chemical industry has been forced to search for alternative ways to manage surface hygiene and consider the environment. Some cleaning products contain antibiotics to increase their cleaning efficacy, resulting in increased microbial resistance and cross-resistance being shifted from the clinical environment to everyday life (Giuliano and Rybak Citation2015). Therefore, natural cleaning products from plants that are highly effective, act against bacteria, possess a low risk of microbial resistance, have low production costs, and meet many principles of green chemistry have a great advantage. Natural plant extracts contain several active components, including surfactants, acids, flavonoids, phenols, tannins, and other compounds, which remove cells from the surface and act on cell walls and membranes (Zaynab et al. Citation2021). Their mode of action on bacterial cells is non-specific, thus representing a lower risk for resistance. Saponins are natural organic compounds that are categorized according to their chemical structure as steroid, alkaloid, or triterpenoid saponins. Specifically, triterpenoid saponins are the most widely distributed in the plant kingdom, with applications in medicine, pharmacy, and technology (El Aziz et al. Citation2019). Triterpenoid saponins are considered defensive compounds against pathogenic microorganisms, have foaming ability, and are used as emulsifiers in cosmetics and food (Rai et al. Citation2021).
The main plant sources of triterpenoid saponins include soap bark, horse chestnut, and soapberry (Kregiel et al. Citation2017). Several publications have found promising results for natural saponins’ antibacterial properties. For example, Antolak et al. (Citation2018) demonstrated that soap bark tree saponins can enhance the effectiveness of the disinfection processes in the beverage industry. Also, Sewlikar and D’Souza (Citation2017) reported the antimicrobial potential of quillaja saponin against E. coli cells. Saponin escin from horse chestnut was found to have antibacterial activity against E. coli, S. aureus and S. mutants (Idris et al. Citation2020). Kunatsa and Katerere (Citation2021) reported that the extracts from soapy plants (e.g. soapberry and soap bark) can contribute to hand hygiene and help communities to combat pandemic and other communicable infections. Accordingly, this study aimed to analyse the cleaning properties, ecotoxicity profile, and antibiofilm capacity of three triterpenoid saponins: quillaja saponin found in soap bark tree Quillaja saponaria, escin saponin found in horse chestnut Aesculus hippocastanum, and sapogenin found in soapberry Sapindus saponaria and to compare them with synthetic saponin sodium dodecyl sulphate ().
Materials and methods
Model organisms
For the antibacterial and anti-biofilm assay, the standard strain of Escherichia coli ATCC 35218 was obtained from the Czech Collection of Microorganisms (CCM, Czech Republic) and Pseudomonas aeruginosa ATCC 27853 and Staphylococcus aureus ATCC 25923 from Sigma Aldrich (Virginia, USA). Bacteria from the collection were transferred on nutrient agar and incubated at 37°C for 24 h. After that, a single colony of a strain was transferred from nutrient agar to the nutrient broth (Biolife, Italy) and incubated under the same conditions.
The toxicity of natural and synthetic saponins was assessed with a yeast toxicity assay that serves as a tool for ecotoxicity screening (Yang et al. Citation2021). First, cells of the standard strain Saccharomyces cerevisiae ATCC 24904 (Sigma Aldrich, Virginia, USA) were transferred from the collection to the yeast extract peptone dextrose agar (Sigma Aldrich, Virginia, USA) and incubated at 37°C for 24 h. After that, a single colony of strain was transferred from the yeast extract peptone dextrose agar to the nutrient broth (Biolife, Italy) and incubated under the same conditions.
Chemicals
Natural plant-active substances sapogenin (SA), quillaja saponin (QS), escin (ES) and synthetic sodium dodecyl sulphate (SDS) were purchased (Sigma-Aldrich, USA), and the antibiotic Bacitracin was purchased from Mastdiscs (Mastgroup, Germany).
Critical micelle concentration
The critical micelle concentration (CMC) of the cleaning products was determined by the onset of constancy in surface tension despite the increase in concentration. The surface tension was measured using a Leconde du Nouy tensorometer, with which the tension required to lift the platinum ring off the surface of the sample was measured. All the experiments were provided in three parallels and three repetitions.
Emulsification capacity
The capacity of the surface-active substances to emulsify fats was assessed using the emulsification index. The surface-active substance emulsification index was determined following a standard protocol according to Lawrance et al. (Citation2014). The emulsification index was determined by vigorously mixing 2 mL of each substance at each concentration with 2 ml of paraffin liquid (Sigma-Aldrich, USA) in test tubes, according to Basu et al. (Citation2015). The emulsification capacity was measured as the thickness of the emulsified layer compared to the total height of the solution. The emulsification capacity was defined as the concentration of the substance that was able to emulsify at least 80% of paraffin (EC80).
Antibacterial disk diffusion assay
Testing the antibacterial potential of selected natural and synthetic saponins was provided with a disk diffusion assay (Hudzicki Citation2009). Briefly, for each bacterial strain, .1 mL of a bacterial suspension at concentration .5 McFarland was uniformly spread on nutrient agar (Biolife, Italy). After that, sterile 6 mm blank paper disks Mastdiscs (Mastgroup, Germany) were placed on the surface of each agar plate, and 20 µL of two dilutions of the tested substance was added (.4–25 mg mL−1). In the next step, plates were incubated in aerobic conditions for 24 h at 37°C. After that, the inhibition zone was measured. Antibacterial activity with minimal inhibitory concentration (MIC) was determined to be the lowest concentration of the substance that produced the inhibition zone. All the experiments were performed with five parallels and three repetitions.
Bacterial anti-biofilm assay
The anti-biofilm assay was performed following Bohinc et al. (Citation2014) with some modifications as follows. Bacterial cultures of E. coli, P. aeruginosa, and S. aureus were transferred from the nutrient agar into a .9% NaCl solution to achieve a concentration of .5 McFarland (1.5 × 108 CFU mL−1). Next, the tested substances were added to nutrient broth (Biolife, Italy) to give final concentrations of 1MIC, 2MIC, and 3MIC for each substance and bacterial strain based on disk diffusion results. In the 96-well microtiter plates, the bacterial culture was added to the solution to achieve the final concentration of the cell at 106 CFU mL−1 and incubated 24 h at 37°C. In the next step, the mixture was removed and washed 3 times with 200 μL of phosphate buffer saline (PBS). The cells that remained on the surface were stained with 2% crystal violet (Merck, Germany), and the excess dye was removed with PBS. The dye from the cells was remobilized with 200 μL of 96% ethanol. The optical density of the solution was measured at a wavelength of 620 nm by the Infinite 200 PRO microplate reader from Tecan, Austria.
The anti-biofilm (AB) capacity was determined as the percentage of biofilm inhibition, calculated as in EquationEquation (1)(1)
(1) :
where C is the optical density of crystal violet under control treatment, and T is the optical density of crystal violet under treatments with cleaning products.
Statistical analysis was provided using R software version 4.1.1. (Bell Laboratories, New Jersey, U.S.). Normality was checked using the Shapiro–Wilk test (p > .05). One-way analysis of variance (ANOVA) and Tukey’s test was used to determine the significant differences at a significance level of p < .05.
S. cerevisiae ecotoxicity assay
Ecotoxicological testing using S. cerevisiae ATCC 24904 was performed according to Dogra et al. (Citation2021): briefly, yeast culture was transformed from solid media into .9% NaCl solution to achieve a concentration of .5 McFarland. Next, the tested substances were added to 10 mL of nutrient broth (Biolife, Italy) to give final concentrations of .01, .1, 1, 10, and 100 mg mL−1. The yeast culture was added to the solution to achieve the final concentration of the cell at 106 CFU mL−1. After that, the samples were incubated for 1 h at 37°C Next, 20 µL of suspension and 20 µL of methylene blue were mixed on the slide and examined microscopically with Olympus C × 40 and CCD CMOS cameras. The results were expressed as the half-maximal inhibitory concentration (IC50). Non-linear regression analysis was used with dose–response inhibition and statistical significance at p < .05. All the experiments were performed with five parallels and three repetitions.
Conceptual figure () was created with the BioRender.com tool.
Results
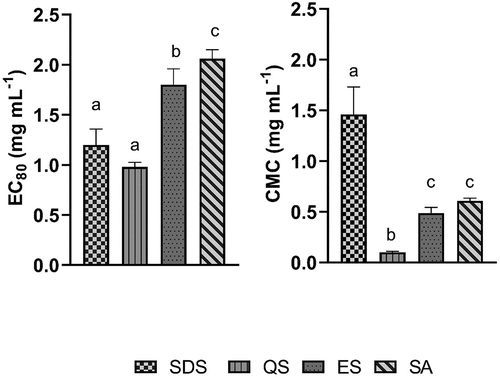
Results show synthetic SDS and natural QS possess low and similar EC80 (SDS 1.2 mg mL−1; QS 1.0 mg mL−1), while for escin and sapogenin, much higher concentrations are required to achieve EC80. In contrast, we demonstrated the lowest CMC for QS (.1 mg mL−1), followed by escin (.5 mg mL−1), sapogenin (.6 mg mL−1) and SDS (1.5 mg mL−1) ().
Figure 2. Emulsification capacity at 80% (Ec80) and critical micelle concentration (CMC) for synthetic and natural surfactants from plants.
Both synthetic and natural surface-active substances have antibacterial properties (). The results show that E. coli is the most resistant to synthetic and natural substances, followed by P. aeruginosa and S. aureus. Among the tested substances, the lowest MIC was observed for natural quillaja saponin and synthetic sodium dodecyl sulphate, while the natural surface-active substances sapogenin and escin showed lower antibacterial activity. Results for inhibition zones demonstrate the largest zones for quillaja saponin and sodium dodecyl sulphate ().
Figure 3. Inhibition zone of synthetic and natural active substances at concentration 12.5 mg mL −1.
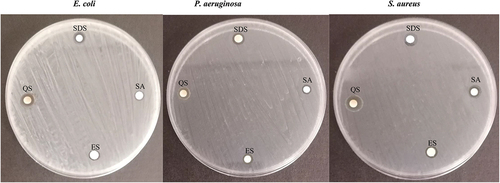
Table 1. Minimal inhibitory concentration of synthetic and natural surface-active substances from plants against E. coli, S. aureus, and P. aeruginosa.
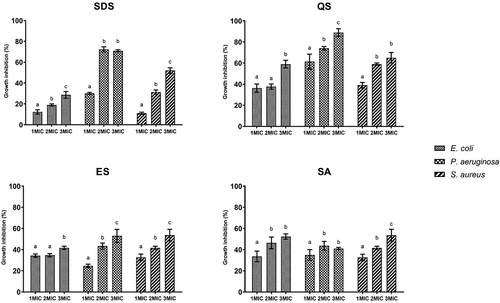
The results of the biofilm inhibition show that increasing the concentration of MIC results in increased anti-biofilm capacity for most of the tested substances. A bacterial-specific strain biofilm inhibition indicates that surface-active substances are less effective against biofilm formation in the case of E. coli and the most against P. aeruginosa. However, the results regarding the substances’ type-specific capacity show that the natural QS is the most effective against all the tested bacteria, followed by synthetic SDS and natural ES and SA ().
Figure 4. Anti-Biofilm potential of synthetic and natural surface-active substances from plants.
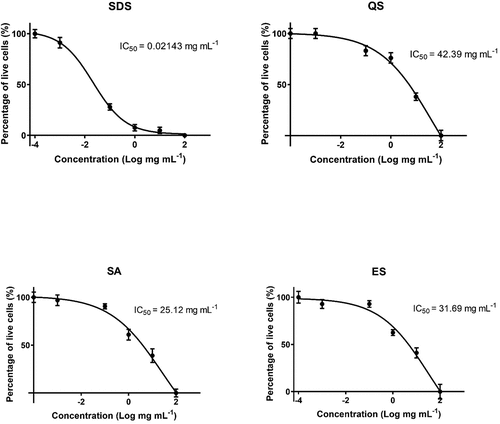
The ecotoxicity assay on model organism S. cerevisiae demonstrates that natural quillaja saponin possesses the lowest acute toxicity (IC50 = 42.39 mg mL−1), followed by escin (IC50 = 31.69 mg mL−1) and sapogenin (IC50 = 25.12 mg mL−1). In contrast, we found the highest acute toxicity for SDS (IC50 = .02 mg mL−1) ().
Discussion
Biofilms represent one of the most significant risks to modern public health due to their ubiquitous nature and resistance. This issue will become even more predominant as the number of bacterial species will increase their resistance in direct response to the enlarged antimicrobial consumption in the current pandemic. The present paper aims to demonstrate alternative approaches to bacterial management, taking into account both safety and efficacy. It has been found that the surface-active properties of QS (e.g. CMC and EC80) are comparable or even better than those of synthetic SDS. The capacity of fat emulsification is a crucial cleaning product characteristic that affects the efficacy. The results of emulsification capacity show some potential as the results of QS (EC80 = 1 mg mL−1) are comparable to SDS (EC80 = 1.2 mg mL−1), reducing >80% of fats, while the results for ES and SA were less promising (EC80 ~2 mg mL−1).
Moreover, Jarzębski et al. (Citation2020) studied the emulsification index of saponins and found that QS can emulsify more than 89% of fats at a concentration of 1 mg mL−1, which is compatible with our findings. Iroha et al. (Citation2015) found a 70% emulsification capacity for SDS at 2 mg mL−1. One reason could be that Iroha and co-workers used kerosene as a fat model instead of paraffin.
Essential criteria for cleaning products are the capability of micelles to form as they can emulsify the contaminant that would otherwise be insoluble. More specifically, we have demonstrated that QS possesses the lowest CMC (.1 mg mL−1) followed by EC (.5 mg mL−1) and SA (.6 mg mL−1), while SDS is less effective in forming the micelle (1.5 mg mL−1). Furthermore, Jarzębski et al. (Citation2020) found that the CMC varies concerning extraction method between .1 and 1.5 mg mL−1 for Quillaja saponaria extracts, while Ribeiro et al. (Citation2013) found CMC at .2 mg mL−1. However, Alpandi et al. (Citation2021) studied the saponin surface tension and found CMC at .5 mg mL−1, which corresponds to our findings. The findings of the current study are consistent with those of Góral and Wojciechowski (Citation2020), who reported that the CMC for escin is between .3 and .4 mg mL−1. Moreover, Motin et al. (Citation2015) demonstrated CMC for sodium dodecyl sulphate at 2 mg mL−1, which is a bit higher in comparison to our results.
Our study revealed that natural QS has higher antibacterial potential than synthetic SDS. More specifically, we found equal or lower MIC for QS for all tested bacterial strains in comparison to SDS. One reason could be the fact that saponins elucidate the antibacterial mechanism through the cell wall and have membrane-damaging potential (Khan et al. Citation2018). The findings of the current study are consistent with those of Nübling et al. (Citation2017), who tested QS extract against various food-borne pathogens in vitro study and found that the MIC ranged from .5 to 2 mg mL−1. These findings may be explained through observation by Sewlikar and D’Souza (Citation2017), who reported that glucuronic acid and fatty acyl chain act against eukaryotes in QS quillaic acid due to their detergent properties and ability to penetrate the cell membrane by complexing with cholesterol. Ryu et al. (Citation2018) reported that QS causes permeability of the bacterial cell membrane and consequently the cell destruction. Also, Dong et al. (Citation2020) tested saponins on food-borne bacteria and found degradation of the cell wall, as well as disruption of the cytoplasmic membrane and membrane proteins that resulted in leakage of the cell contents.
Furthermore, Wei et al. (Citation2021) analysed SA against several bacterial strains and found MIC for P. aeruginosa at 5 mg mL−1, which supports the results of our study (6.25 mg mL−1). Also, Zaynab et al. (Citation2021) reported that saponins are strongly effective against Gram-positive bacteria and moderately so against Gram-negative bacteria. This finding is similar to that of our research: lower MICs were found for gram-positive S. aureus in comparison to Gram-negative E. coli and P. aeruginosa. The current study found QS can prevent 90% of P. aeruginosa biofilm, 65% of S. aureus biofilm and 60% of E. coli biofilm (). These results are consistent with those of Zhu et al. (Citation2019) that suggest that saponin in the concentration of 9 mg mL−1 can inhibit 50% of S. aureus biofilm biomass. Another study revealed that pre-treatment of biofilm with QS can reduce the consumption of quaternary organic compounds by half (Antolak et al. Citation2018).
According to principles of green chemistry, new cleaning products should not only be more efficient than conventional ones but also less harmful to health and the environment. Our study revealed that all tested natural saponins are substantially less toxic to the ecotoxicity model organism S. cerevisiae than synthetic SDS. The SDS is highly toxic to S. cerevisiae (IC50 = .021 mg mL−1), which is similar to the findings of Hrenovic and Ivankovic (Citation2007), who tested SDS on S. cerevisiae ATCC 64252 and found IC50 at a concentration of .0124 mg mL−1. The results of this study indicate that QS is the least toxic to S. cerevisiae (IC50 = 42 mg mL−1). Also, Fischer et al. (Citation2011) tested quillaja saponin on 16 strains of S. cerevisiae and reported that concentrations below 30 mg mL−1 had no lethal effect, which is comparable to our findings.
Furthermore, products that contain Quillaja saponaria are approved to be safe for human consumption by the United States Food and Drug Administration and the European Union. Also, the Codex Committee on Food Additives and Contaminants allows the use of quillaja saponin at a maximum level of 500 mg kg−1 of body weight (Berlowska et al. Citation2015). EFSA states an acceptable daily intake for humans of 3 mg quillaja saponins per kg of body weight (Younes et al. Citation2019). The results of our study show that the applications of quillaja saponin are far below the concentrations harmful to the environment or humans.
Conclusions
The sustainable development goals of the United Nations aim to transform the world, including responsible consumption and production. Green chemistry is one step toward these goals, but cleaning products must meet levels of both safety and efficacy. Our study demonstrated that natural saponins can represent a suitable candidate for substitutions of synthetic surface-active substances. In particular, QS shows great promise as a highly efficient, antibacterial, and low toxic substance. Not only can QS meet the same criteria of efficacy in comparison to synthetic saponins, but we also demonstrated better micelle formation, as well as antibacterial and biofilm inhibition potential. Above all, QS represents a significantly lower risk for the environment and human health than synthetic substances do and will be an important ingredient of natural cleaning products in the future.
Acknowledgement
RF was supported by the Slovenian Research Agency under the Slovenian-Bosnian bilateral project BI-BA19-20-005: Natural extracts to combat microbial biofilm in the food industry. The authors would like to express their gratitude to Terry T. Jackson for grammar proofing.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Alpandi A, Inasyah F, Sidek A, Husin H, Junin R, Jaafar M. 2021. Critical micelle concentration, interfacial tension and wettability alteration study on the surface of paraffin oil-wet sandstone using saponin. IOP Conf Ser: Mater Sci Eng. 1153(1):012018. doi:10.1088/1757-899X/1153/1/012018.
- Antolak H, Mizerska U, Berłowska J, Otlewska A, Kręgiel D. 2018. Quillaja saponaria saponins with potential to enhance the effectiveness of disinfection processes in the beverage industry. Appl Sci. 8(3):368. doi:10.3390/app8030368.
- Basu A, Basu S, Bandyopadhyay S, Chowdhury R. 2015. Optimization of evaporative extraction of natural emulsifier cum surfactant from Sapindus mukorossi—characterization and cost analysis. Ind Crops Prod. 77:920–931. doi:10.1016/j.indcrop.2015.10.006.
- Berlowska J, Dudkiewicz M, Kregiel D, Czyzowska A, Witonska I. 2015. Cell lysis induced by membrane-damaging detergent saponins from Quillaja saponaria. Enzyme Microb Technol. 75:44–48. doi:10.1016/j.enzmictec.2015.04.007.
- Bohinc K, Dražić G, Fink R, Oder M, Jevšnik M, Nipič D, Godič-Torkar K, Raspor P. 2014. Available surface dictates microbial adhesion capacity. Int J Adhes Adhes. 50:265–272. doi:10.1016/j.ijadhadh.2014.01.027.
- Dogra V, Kaur G, Kumar R, Kumar S. 2021. Toxicity profiling of metallosurfactant based ruthenium and ruthenium oxide nanoparticles towards the eukaryotic model organism Saccharomyces cerevisiae. Chemosphere. 270:128650. doi:10.1016/j.chemosphere.2020.128650.
- Dong S, Yang X, Zhao L, Zhang F, Hou Z, Xue P. 2020. Antibacterial activity and mechanism of action saponins from Chenopodium quinoa Willd. husks against food-borne pathogenic bacteria. Ind Crop Prod. 149:112350. doi:10.1016/j.indcrop.2020.112350.
- El Aziz M, Ashour A, Melad A. 2019. A review on saponins from medicinal plants: chemistry, isolation, and determination. J Nanomed Res. 8(1):282–288.
- Elorriaga Y, Marino DJ, Carriquiriborde P, Ronco AE. 2013. Human pharmaceuticals in wastewaters from urbanized areas of Argentina. Bull Environ Contam Toxicol. 90(4):397–400. doi:10.1007/s00128-012-0919-x.
- Fink R. 2019. Good hygiene practices and their prevention of biofilms in the food industry. Newcastle upon Tyne: Cambridge Scholars Publishing.
- Fischer MJ, Pensec F, Demangeat G, Farine S, Chong J, Ramírez-Suero M, Mazet F, Bertsch C. 2011. Impact of Quillaja saponaria saponins on grapevine ecosystem organisms. Antonie van Leeuwenhoek. 100(2):197–206. doi:10.1007/s10482-011-9578-x.
- Giuliano CA, Rybak MJ. 2015. Efficacy of triclosan as an antimicrobial hand soap and its potential impact on antimicrobial resistance: A focused review. Pharmacotherapy. 35:328–336. doi:10.1002/phar.1553.
- Góral I, Wojciechowski K. 2020. Surface activity and foaming properties of saponin-rich plants extracts. Adv Colloid Interface Sci. 279:102145. doi:10.1016/j.cis.2020.102145.
- Hrenovic J, Ivankovic T. 2007. Toxicity of anionic and cationic surfactant to Acinetobacter junii in pure culture. Open Life Sci. 2(3):405–414. doi:10.2478/s11535-007-0029-7.
- Hudzicki J. 2009. Kirby-Bauer disk diffusion susceptibility test protocol. Am Soc Microbiol. 23. https://asm.org/getattachment/2594ce26-bd44-47f6-8287-0657aa9185ad/Kirby-Bauer-Disk-Diffusion-Susceptibility-Test-Protocol-pdf.pdf.
- Idris S, Mishra A, Khushtar M. 2020. Phytochemical, ethnomedicinal and pharmacological applications of escin from Aesculus hippocastanum L. towards future medicine. J Basic Clin Physiol Pharmacol. 31(5):20190115. doi:10.1515/jbcpp-2019-0115.
- Iroha O, Njoku O, Ogugua V, Okpashi V. 2015. Characterization of biosurfactant produced from submerged fermentation of fruits bagasse of yellow cashew (Anacardium occidentale) using Pseudomonas aeruginosa. Afr J Environ Sci Technol. 9(5):473–481. doi:10.5897/AJEST2015.1898.
- Jarzębski M, Siejak P, Smułek W, Fathordoobady F, Guo Y, Pawlicz J, Trzeciak T, Kowalczewski PŁ, Kitts DD, Singh A. 2020. Plant extracts containing saponins affects the stability and biological activity of hempseed oil emulsion system. Molecules. 25(11):2696. doi:10.3390/molecules25112696.
- Khan MI, Ahhmed A, Shin JH, Baek JS, Kim MY, Kim JD. 2018. Green tea seed isolated saponins exerts antibacterial effects against various strains of gram positive and gram negative bacteria, a comprehensive study in vitro and in vivo. J Evid Based Complementary Altern Med. Article ID 3486106:12.
- Kregiel D, Berlowska J, Witonska I, Antolak H, Proestos C, Babic M, Babic L, Zhang B. 2017. Saponin-Based, biological-active surfactants from plants. In: Najjar R, editor. Application and characterization of surfactants. Rijeka (Croatia): InTech; pp. 183–205.
- Kunatsa Y, Katerere DR. 2021. Checklist of African Soapy Saponin—rich plants for possible use in communities’ response to global pandemics. Plants. 10(5):842. doi:10.3390/plants10050842.
- Lawrance A, Balakrishnan M, Joseph TC, Palaiya Sukumaran D, Nambali Valsalan V, Gopal D, Ramalingam K. 2014. Functional and molecular characterization of a lipopeptide surfactant from the marine sponge-associated eubacteria Bacillus licheniformis NIOT-AMKV06 of Andaman and Nicobar Islands, India. Mar Pollut Bull. 82(1):76–85. doi:10.1016/j.marpolbul.2014.03.018.
- Marriott NG, Schilling MW, and Gravani RB. 2018. Sanitation and the food industry. In: R. Heldman, Dennis. Principles of food sanitation Sixth Edition. Cham, Switzerland: Springer; pp. 1–17.
- Motin MA, Mia MH, Islam AN. 2015. Thermodynamic properties of sodium dodecyl sulfate aqueous solutions with methanol, ethanol, n-propanol and iso-propanol at different temperatures. J Saudi Chem Soc. 19(2):172–180. doi:10.1016/j.jscs.2012.01.009.
- Nübling S, Hägele F, Wohlt D, Graf B, Schweiggert RM, Carle R, Schmidt H, Weiss A. 2017. Effects of Quillaja saponaria extract and Nα-lauroyl-L-arginine ethyl ester on reducing selected food-borne pathogens in vitro and maintaining quality of fresh-cut endive (Cichorium endivia L.) at pilot plant scale. Food Control. 73:393–400. doi:10.1016/j.foodcont.2016.08.029.
- Rai S, Acharya E, Kafle A, Devkota HP, Bhattarai A. 2021. Plant-Derived saponins: A review of their surfactant properties and applications. Sci. 3(4):44. doi:10.3390/sci3040044.
- Ribeiro BD, Alviano DS, Barreto DW, Coelho MAZ. 2013. Functional properties of saponins from sisal (Agave sisalana) and juá (Ziziphus joazeiro): critical micellar concentration, antioxidant and antimicrobial activities. Colloids Surf A Physicochem Eng. 436:736–743. doi:10.1016/j.colsurfa.2013.08.007.
- Ryu V, McClements DJ, Corradini MG, Yang JS, McLandsborough L. 2018. Natural antimicrobial delivery systems: Formulation, antimicrobial activity, and mechanism of action of quillaja saponin-stabilized carvacrol nanoemulsions. Food Hydrocoll. 82:442–450. doi:10.1016/j.foodhyd.2018.04.017.
- Sewlikar S, D’-Souza DH. 2017. Antimicrobial effects of quillaja saponaria extract against Escherichia coli O157: H7 and the emerging non‐O157 Shiga toxin‐producing E. coli. J Food Sci. 82(5):1171–1177. doi:10.1111/1750-3841.13697.
- Wei M-P, Yu H, Guo Y-H, Cheng Y-I, Xie Y-F, Yao W-R. 2021. Antibacterial activity of Sapindus saponins against microorganisms related to food hygiene and the synergistic action mode of Sapindoside A and B against Micrococcus luteus in vitro. Food Control. 130:108337. doi:10.1016/j.foodcont.2021.108337.
- Wieck S, Olsson O, Kümmerer K. 2018. Not only biocidal products: Washing and cleaning agents and personal care products can act as further sources of biocidal active substances in wastewater. Environ Int. 115:247–256. doi:10.1016/j.envint.2018.03.040.
- Yang S-H, Chen C-H, Chu K-H. 2021. Fecal indicators, pathogens, antibiotic resistance genes, and ecotoxicity in Galveston Bay after Hurricane Harvey. J Hazard Mater. 411:124953. doi:10.1016/j.jhazmat.2020.124953.
- Younes M, Aquilina G, Castle L, Engel KH, Fowler P, Frutos Fernandez MJ, Fürst P, Gürtler R, Gundert‐remy U. 2019. Re‐evaluation of Quillaia extract (E 999) as a food additive and safety of the proposed extension of use. EFSA J. 17(3):e05622.
- Zaynab M, Sharif Y, Abbas S, Afzal MZ, Qasim M, Khalofah A, Ansari MJ, Khan KA, Tao L, Li S. 2021. Saponin toxicity as key player in plant defence against pathogens. Toxicon. 193:21–27. doi:10.1016/j.toxicon.2021.01.009.
- Zhu C, Zhang M, Tang Q, Yang Q, Li J, He X, Ye Y. 2019. Structure and activity of the Camellia oleifera sapogenin derivatives on growth and biofilm inhibition of staphylococcus aureus and Escherichia coli. J Agric Food Chem. 67(51):14143–14151. doi:10.1021/acs.jafc.9b03577.