ABSTRACT
Poor indoor air quality in healthcare settings has been tied with the increase in hospital-acquired infections. Thus, this systematic review was conducted to assess the levels and compositions of bacteria in indoor hospital air in the Middle East and North Africa (MENA) region. We examined results provided by different search engines published between 2000 and 2021. Our data showed that most studies were conducted in Iran (80.9%) with a bacterial concentration mean of 172.9 CFU/m3. Comparing sensitive and non-sensitive areas of hospitals, no significant difference was detected in the mean bacterial concentration. The most investigated sensitive hospital areas were operating rooms and intensive care units with mean indoor bacterial concentrations of 180.3 CFU/m3 and 204.6 CFU/m3, respectively. Staphylococcaceae, Enterobacteriaceae, Pseudomonadaceae, and Bacillaceae were commonly identified bacterial families. In conclusion, the mean concentrations of the airborne bacteria were within the acceptable limit compared to WHO standards (300 CFU/m3) for the air in areas occupied by immunosuppressed people.
Introduction
The dynamic environment of healthcare settings is crucial and requires attention to ensure healthy indoor air quality (IAQ) to minimize health risks for patients and healthcare workers (Cabo Verde et al. Citation2015; Farraia et al. Citation2019). Several studies have been carried out worldwide on airborne microbes in various districts and indoor settings (Nunes et al. Citation2005; Jayaprakash et al. Citation2017; Horve et al. Citation2020; Abu Rub et al. Citation2021; Nguyen et al. Citation2021; Monteiro et al. Citation2021). In hospitals and healthcare settings, infection control precautions are implemented to prevent the transmission of hospital-acquired infection (HAI) and other communicable diseases (Stockwell et al. Citation2019). These include in-depth cleaning strategies, use of hygiene protocols, and high-technology mechanical ventilation systems that remove potential pathogenic bioaerosols from indoor air; thereby, lowering the spread of infections (Siegel et al. Citation2007; Carling et al. Citation2010; Gao et al. Citation2016; Monteiro and Cabo Verde Citation2017).
Although healthcare settings have focused on maintaining pure IAQ through the employment of infection control precautions the measures are applicable only for a few infections, such as measles and tuberculosis (Bloch et al. Citation1985; Dharmadhikari et al. Citation2012). Nevertheless, evidence for the emergence of other infections in the indoor air of hospitals has been reported, including staphylococci, bacilli, and clostridia spores (Best et al. Citation2010; Hayleeyesus and Manaye Citation2014; Kunwar et al. Citation2019). Methicillin-resistant Staphylococcus aureus (MRSA) and gentamicin-resistant Gram-negative bacteria are also reported in recent studies to cause serious illnesses (Hara et al. Citation2016; Kunwar et al. Citation2019). Airborne pathogens found indoors are often generated from human or non-human sources or triggered by the surrounding outdoor factors (Srikanth et al. Citation2008; Kunwar et al. Citation2019; Stockwell et al. Citation2019).
Bioaerosols are commonly collected using active or passive air sampling techniques with active sampling devices involving a mechanical component (impingers, cyclones, impactors, and filters) (Haig et al. Citation2016). Active air sampling of bioaerosols offers more advantages than passive techniques but requires special equipment and trained staff (Kummer and Thiel Citation2008; Wu et al. Citation2020). Active sampling techniques provide qualitative and quantitative data which accurately detect bioaerosols even with low concentrations and are considered beneficial for research despite the expenses that deter their use (Taha et al. Citation2006; Haig et al. Citation2016). Active air samplers generally draw in a specific air volume over a set time, allowing for the standard measurement of colony-forming unit per cubic meter (CFU/m3) (Sandle Citation2010; Bonifait et al. Citation2015).
The persistence of airborne bacteria in hospitals and healthcare settings is a proliferating infection control problem that remains relatively understudied (Nunes et al. Citation2005; Stauning et al. Citation2020; Abera et al. Citation2021). Data on bacterial concentration is limited, with many studies focusing on outdoor air. Moreover, studies on indoor exposure levels are present, yet few studies focus on the Middle East and North Africa (MENA) region (Ekhaise et al. Citation2008; Chen et al. Citation2021). Hence, a systematic review of the available literature on the MENA region may help identify the data gap on the airborne bacterial concentration in hospitals within the region.
The consequent review may unify the assessment of airborne bacterial concentration in the indoor air of MENA region hospitals and healthcare settings. The main objectives extended to:
Identifying the concentration distribution of airborne bacteria in different hospitals and healthcare settings of the MENA countries.
Comparing the indoor bacterial concentrations between different locations :sensitive (all non-public areas in the hospital, e.g. patient room and treatment room) and non-sensitive within hospitals and healthcare settings.
Assessing the commonly used methodologies for airborne bacteria detection in MENA countries.
Examining the legislated standards and guidelines for IAQ.
Summarizing the dominant family of airborne bacteria presents in indoor hospitals of the MENA region.
Materials and methods
This systematic review was conducted based on PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines for systematic reviews (Moher et al. Citation2009) to assess the concentration levels and species of indoor air bacteria in the healthcare settings of the MENA region.
Research questions were:
What are the bacterial concentration levels and species recovered from indoor hospitals air in the MENA region?
What are the most used techniques for active air sampling?
Do healthcare governments of MENA countries establish any standards for the indoor air of healthcare environments?
Search criteria
A literature search was accomplished through PubMed, SCOPUS, and Web of Science databases for studies published in the English language. Additional articles were included following searching google scholar search engine. The search covered all literature within the databases from 2000 up to 20 June 2021 (21st century only), using the keywords of “Bacteria”, “Indoor,” and “Air.” All keywords were queried with each country’s name of the MENA region. The countries considered as part of the MENA region in this review were: Algeria, Bahrain, Djibouti, Egypt, Iran, Iraq, Israel, Jordan, Kuwait, Lebanon, Libya, Morocco, Oman, Palestine, Qatar, Saudi Arabia, Sudan, Syria, Tunisia, United Arab Emirates, and Yemen. Moreover, the names of the countries, the terms “west bank”, “Gaza”, “UAE”, “United Arab Emirates”, “Emirates”, “KSA”, “Middle East”, “North Africa”, and “MENA” were used to account for alternate names and ensure complete coverage of the region.
The included research databases were searched by filtering out books, reviews, and commentaries. All recovered citations were imported into Zotero, and duplicates were removed using the Zotero built-in “Find Duplicates” feature. Lastly, the titles and abstracts of the remaining citations were screened to eliminate any irrelevant articles.
Inclusion criteria
All references were initially screened and evaluated based on title and abstract. The study selection was according to the following criteria: 1) studies published (in English) between January 2000 and June 2021; 2) studies should be conducted in indoor healthcare settings in the MENA region; 3) studies must include quantitative bacteria data using the standard bioaerosol measurement units (CFU/m3); and 4) samples collected only from the air
Exclusion criteria
The exclusion criteria were specified as follows: 1) Not an original research study that inspects indoor bacteria (i.e. review, chapter in a book, short communications, conference); 2) studies with no quantitative indoor bacteria; 3) collected samples are not air samples such as dust; 4) studies conducted in a location other than health settings; 5) studies not conducted in MENA region; 6) studies investigated specific bacterial species to avoid the bias and 7) studies that didn’t utilize the standards way of expressing microbial concentrations in air as colony-forming units (CFU) per m3.
Study selection
Initial screening of titles and abstracts search was conducted by the authors (LA) and (AJ) for the databases, who identified studies that met all inclusion criteria and none of the exclusion criteria. All full-text studies meeting the initial criteria were then reviewed by the author (LA) for final inclusion in the review. The author (AJ) went through all selected studies for duplicate screening for eligibility and quality assurance. Any disagreements were reviewed independently by another author who had not participated in the screening (HA).
Data extraction
The author (LA) extracted data from included studies using a data collection form. The data collection was with the following variables: country of study; healthcare facility; airborne bacteria level (CFU/m3); identified bacteria; possible source of the bacteria in the air, and standards for indoor airborne bacteria levels (Table S1).
Statistical analysis
The data were analyzed with (R) program version 4.1.0 (R Core Team Citation2011) using the mean CFU/m3. Meta-analysis was not performed due to the frequent lack of information on the sample size. The locations were divided into sensitive and non-sensitive s. Sensitive areas house critical, immunocompromised, or immunosuppressed patients where air contamination would pose a significantly higher risk. These include intensive care units (ICUs), operation rooms (ORs), burn patient wards, isolation wards, infectious diseases wards, transplant wards, and hematoncology wards. The non-sensitive areas include all other sections of the hospital. The difference in CFU/m3 between the sensitive and non-sensitive areas was assessed using a student t-test and an analysis of variance (ANOVA) was performed to assess the differences between the sensitive areas (i.e. ICU, OR, and patient wards). A p-value <0.05 was considered significant. All plots were generated using the ggplot2 version 3.3.3 and ggpubr version 0.4.0 packages in R (Wickham Citation2016; Kassambara Citation2020).
Results
Article selection
According to the inclusion and exclusion criteria mentioned in the methodologies section, a total of 630 related articles were found after the initial literature search (). Nevertheless, 490 articles were excluded. Only 140 articles were full-text assessed and reviewed after eligibility screening, and of that, 119 articles were excluded. The mean of CFU/m3 data was extracted from 21 articles eligible to be included in our study. Likewise, we collected any data on the country, duration, year of testing, air sampling location, identified bacteria, source of air contamination, and the legislated standards for IAQ if available for each country. The characteristics of the 21 articles are reported in Table S1. There were 135 valid CFU/m3 values available for analysis. Mean CFU/m3 values were given for that data points (21 studies).
Figure 1. Preferred reporting items for systematic reviews flowchart for selection of studies. N; represents the number of studies.
This systematic review provides an in-depth analysis of hospital indoor bacterial contamination from 2002 to 2021, according to the geographic location of the MENA region country. Few studies were done in this region that met our searching criteria and they were as follows: Iran, Egypt, Jordan, Palestine, and Yemen (Table S1). The most reported studies were conducted in Iran with 80.9% and all other countries representing 19.1%. All studies were conducted in hospitals (42 hospitals), and none were conducted in primary healthcare settings. The sample collection period in the studies ranged from 10days to one year. The typical air sampling methodologies used in the studies included in this review were impaction, impingements, and filtration, with a percentage of 71.43%, 19.05%, 4.86%, respectively. In addition, 4.76%.of the studies did not report the air sample collection method used. Moreover, the primary source of the contamination was hypothesized to be humans.
Total indoor bacterial concentraions
The distributions of indoor airborne bacteria concentrations in hospitals and healthcare settings are shown in . The concentration levels in the figures are generated using average airborne bacterial levels (CFU/m3) (Table S1) in testing areas. Airborne bacterial concentrations in the included studies were classified according to the original country of the conducted studies and categorized based on the sensitivity of the tested areas (Table S1). Sensitive areas were defined as hospital areas with restricted access and/or requiring the wearing of personal protective equipment, such as the operating rooms (Ors) and intensive care units (ICUs), emergency department (ED), and hematoncology wards (Stockwell et al. Citation2019). In contrast, non-sensitive areas were all other publicly accessible areas, such as the computed tomography (CT) scan room, reception, and admission.
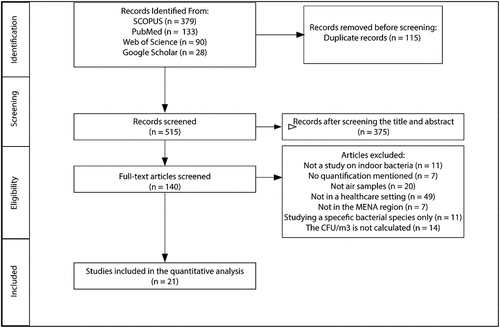
Figure 2. The distribution of bacterial concentrations in the indoor air of healthcare settings in the MENA region by country. N represents the number of distinct sub-locations. Box frames represent the upper quartile and lower quartile. The long line represents the median, and the dots denote outliers.
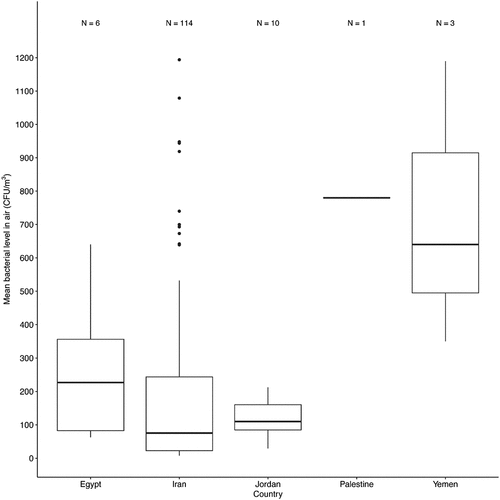
Figure 3. The distribution of bacterial concentrations in the indoor air of healthcare settings in the MENA region is based on the sensitivity of the hospital’s locations. The locations were classified into sensitive and non-sensitive. N represents distinct sub-locations. Box frames represent the upper quartile and lower quartile. The long line represents the median, and the dots denote outliers.
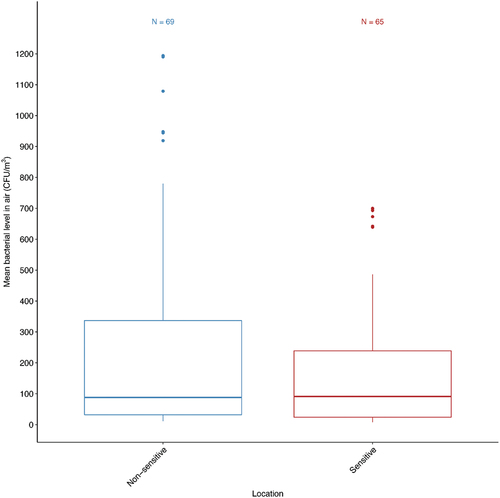
Figure 4. The distribution of bacterial concentrations in the indoor air of healthcare settings in the MENA by sensitive sub-locations. N represents the number of distinct sub-locations. Box frames represent the upper quartile and lower quartile. The long line represents the median, and the dots denote outliers.
Total indoor bacterial concentrations based on countries
Most of the included studies were conducted in Iran with an indoor bacterial concentration median of 75.35CFU/m3 and a mean of 194.37±261.73CFU/m3 and ranged between (7.80–1194 CFU/m3). The total indoor bacterial concentration in Egypt had a median of 226.72CFU/m3 and a mean of 264.97±225.04 CFU/m3 and ranged between (62.41–639.9 CFU/m3), Jordan had a median of 109.8 CFU/m3 and a mean of 117.27±61.19 CFU/m3) and ranged between (29.3–212.3 CFU/m3), Palestine had a median of 780 CFU/m3 and a mean of 780 CFU/m3 , and Yemen had a median of 640 CFU/m3 and a mean of 726.66±426.65 CFU/m3 and ranged between (350–1190 CFU/m3) (). A comparison of the median and mean concentration data between the countries cannot be made since the number of tested areas in all countries (Egypt; n=6, Jordan; n=10, Palestine; n=1 and Yemen; n=3) is low compared to Iran (n=114). Therefore, the data might not necessarily be reflective of real differences.
Total indoor bacterial concentrations in sensitive and non-sensitive areas in healthcare settings
The median and mean total indoor bacterial concentration in sensitive areas were 91.30 CFU/m3 and 175.28±208.55 CFU/m3, respectively, and ranged between (7.80–700 CFU/m3). While in non-sensitive areas, the mean and median were 88 CFU/m3 and 239 CFU/m3, respectively, and ranged between (11.10–1194 CFU/m3). To be more specific, most of the data for sensitive areas fell in the range 23.95– 239CFU/m3, while for non-sensitive zones, it was 32– 337CFU/m3 (). According to the t-test, there was no significant difference between the mean airborne bacterial concentrations in sensitive and non-sensitive areas (P > 0.05).
Total indoor bacterial concentrations within different sensitive areas in healthcare settings
The sub-analyses of sensitive areas involved a range of clinical settings. The most studied sensitive hospital areas were ORs and ICUs with indoor bacterial concentrations (92 median CFU/m3, 180.3 mean±221.34 CFU/m3) and (127 median CFU/m3, 204.63±221.75 mean CFU/m3) respectively (). The highest indoor bacterial concentration was found in the immunosuppressed patient’s ward (median 323 CFU/m3, mean 323230.51 CFU/m3). The lowest was in the isolation ward (median 13.2 CFU/m3, mean 14.66.25 CFU/m3). Also, burns ward recorded a high concentration (200 median CFU/m3, 195.10 mean±101.43 CFU/m3). One-way analysis of variance (ANOVA) was carried out to examine significant differences between the mean concentrations of the sensitive locations, and there was no significant difference between the means (P.05, ANOVA).
Bacterial composition
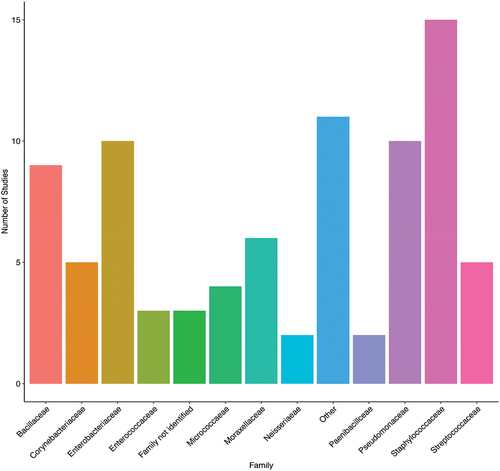
Of the 21 included studies, 18 identified the bacteria after measuring the air quality (Table S1 and ). The most common bacteria found in the air samples belonged to the family Staphylococcaceae (15/18 studies, 83.33%), followed by Enterobacteriaceae (10/18 studies, 55.56%), Pseudomonadaceae (10/15 studies, 55.56%), and Bacillaceae (9/18 studies, 50%). Three of the studies (27.78%) reported the presence of Gram-positive or Gram-negative bacteria without identification.
Figure 5. A. The identified airborne bacterial families amongst the included studies. Each bar represents the number of studies, and the bacterial family was reported. Most studies were from Iran (n=14), while the other countries (Egypt, Jordan, Palestine, and Yemen) had one study each. One study in each of Iran and Jordan had bacterial colonies that were not identified. Other includes bacterial families that were reported in one study only (Alcaligenaceae, Brevibacteriaceae, Caulobacteriaceae, Comamonadaceae, Deinococcaceae, Lactobacillaceae, Microbacteriaceae, Nocardiaceae, Streptomycetaceae, and Xanthomondaceae).
Discussion
Clean and fresh air is a vital requirement for healthy IAQ in all buildings, specifically hospitals and other healthcare settings. This concern has increased recently after the COVID-19 breakout. Hence, our study focused on reviewing the published articles on indoor bacterial concentration in healthcare settings of the MENA region and discussing the indoor air situation.
Based on our analysis, Iran is the country with the highest number of published articles. It showed great interest and dedication to diverse health sector research related to IAQ. Additionally, our study’s results revealed that Iran was the only country among the included studies which adopted guidelines and followed standards for IAQ. These guidelines are mainly from the West and include the Centers for Disease Control and Prevention (CDC), the Healthcare Infection Control Practices Advisory Committee (HICPAC), and the National Institute for Occupational Safety and Health (NIOSH) for the IAQ (Dehghani et al. Citation2018). The other countries within the included articles in the study (Yemen, Palestine, Egypt, and Jordan) were noted to have few publications related to IAQ that met our criteria. This might be attributed to the past few years’ political or economic situations in such countries, which limited their research capability, thereby; resulting in a lower number of research publications.
In terms of measuring the levels of airborne bacterial concentration, several distinct methodologies are used. These can be divided into four groups; the concentration of colony-forming units per cubic meter of air (CFU/m3) (Active); the concentration of colony-forming units (CFU) on settle plates (Passive); measurement of a chemical component of the microbial (cells/m3) of air; and the count under the microscope (Pasquarella et al. Citation2000). For the active methodology, specific active air samplers are used to collect a known volume of air blown onto a collection medium by different techniques (Melymuk et al. Citation2016). Of these active air samplers, the most known and available are impingers, impactors, and filtration samplers (Melymuk et al. Citation2016). Active sampling is the most effective method of quantifying airborne microbes when the concentration is not very high, like in operating rooms and other controlled environments of hospitals (Napoli et al. Citation2012; Fu Shaw et al. Citation2018). According to our inclusion criteria, only active samplers were used in the included studies.
On the one hand, the results of our review revealed that impaction sampling of air was the most used among the included studies, followed by impingement and filtration sampling techniques. This is due to its ability to directly collect microorganisms onto solid or semisolid surfaces such as an agar growth medium without the need for post-collection sample processing (Yao and Mainelis Citation2006; Yan et al. Citation2010). Verhoeff et al. conducted a comparison study between commercially different air bio-samplers available for research. The results showed that the Anderson impactor provides the highest yield in CFU/m3, resulting in a successful technique with few limitations (Verhoeff et al. Citation1990).
The IAQ in the sensitive areas of healthcare facilities requires a higher degree of standards than in other areas. The results of our review showed a comparable bacterial concentration infrequent studied sensitive areas (ICU and OR) with medians of 127 and 92 CFU/m3, respectively, which are in the acceptable range of recommended levels by the World Health Organization (WHO) for those people with a suppressed immune system (100 CFU/m3) (Bargard et al. Citation2021, p. 1). On the other hand, the observed highest CFU levels were found in the wards of immunosuppressed patients in two studies only, which was considered atypical since it was reported in those studies only.
According to the included countries, the highest concentrations of airborne bacterial was recorded in Yemen. It exceeded therecommended levels by the WHO, which suggested that the microbial load in work environments should not exceed 300 CFU/m3 and for immunocompromised people should not exceed 100 CFU/m3 as mentioned earlier (Gizaw et al. Citation2016; Dehghani et al. Citation2018). The airborne bacterial concentrations for the sensitive locations (ICUs, burns wards, immunosuppressed patients wards, and ORs) exceeded the recommended levels by WHO.
Unfortunately, no uniform international standard is available on the acceptable range of bacteria in indoor air (Fekadu and Getachewu Citation2015). Every country is followings its own preferred standard, resulting in a wide range of proposed values for acceptable maximum bacterial concentration. For instance, the maximum limit recommended for the total number of indoor bioaerosol particles by the National Institute of Occupational Safety and Health (NIOSH) is 1000 CFU/m3 (Yassin and Almouqatea Citation2010; Dehghani et al. Citation2018). The value recommended by the American Conference of Governmental Industrial Hygienists (ACGIH) is 500 CFU/m3 (Yassin and Almouqatea Citation2010; Dehghani et al. Citation2018). Also, the sanitary standards of the European Commission for non-industrial premises consider less than 50 CFU/m3 as “very low” bacterial loads, 50–100 CFU/m3 as ‘low’, 100–500 CFU/m3 as ‘intermediate’, 500–2000 CFU/m3 as ‘high’ and above 2000 CFU/m3 as ‘very high’ load (Landry et al. Citation2018). Worth notably, the only MENA country that adapted IAQ standards is Iran. As mentioned previously, the other countries of the included studies do not have a clear standard for bacterial loads in indoor air.
Both hospitals’ sensitive and non-sensitive areas were found to have similar total indoor bacterial concentrations with no significant differences. This result was quite surprising, especially when considering the vast differences in the operating conditions and circumstances of these locations in the hospitals. Nevertheless, a possible reason for this comparable result could be the general busyness caused by the patients and healthcare staff in the sensitive areas of the hospitals (Stockwell et al. Citation2019).
Most identified bacteria in the included studies: are Staphylococcaceae, Enterobacteriaceae, Pseudomonadaceae and Bacillaceae, which are typically commensal organisms in humans or environmental (Brown et al. Citation2012). Nevertheless, these organisms are opportunistic pathogens and may threaten hospitalized patients as they are associated with various HAIs. They are not thought of as airborne. However, their concentration in the air could potentially increase the risk through depositing on critical surfaces, as has been previously shown for Staphylococcus aureus (Kozajda et al. Citation2019). Frías-De León, studied the types and abundance of airborne bacteria of two health institutions and found that most frequently isolated genera were Staphylococcus and Bacillus (León MG et al. Citation2016). Similar results were also found in Moussa study which they found that Staphylococcus aureus was the most frequent bacterial contaminant, followed by E. coli and P. aeruginosa (Moussa et al. Citation2020).
Staphylococcaceae is one of the significant pathogenic organisms occupying the health care environment and could cause a serious public health issue espically when it acquires a resistance genes such as methicillin-resistance (mecA genes) which causes hospital and community-acquired infections, resulting in serious consequences (Lakhundi and Zhang Citation2018; Taylor and Unakal Citation2022). Like wise, Enterobacteriaceae and Pseudomonadaceae are a very common pathogenic bacterial families and usually spread in the health care settings between health workers and their patients (Moussa et al. Citation2020). Bacillaceae are highly spread in nature and can be isolated from most environments, some of its species can be considered as a high risk to public health (EFSA Panel on Biological Hazards (BIOHAZ) Citation2016)
Limitations
Based on our data, this systematic review was the first to assess the indoor bacterial concentration and composition of hospitals and healthcare settings’ air in countries of the MENA region. However, we challenged a few limitations. One of the significant obstacles faced was the inability to perform a meta-analysis due to the frequent lack of information on the sample size in most included studies. In addition, the standard way of expressing the bacterial concentration (CFU/m3) in the air was not followed and not reported in most of the published studies. Consequently, this led to the exclusion of the data detected by the passive air sampling method and quantitative polymerase chain reaction, and hence reducing the number of studies that could be eligible according to our inclusion criteria. Another major limitation is the insufficient availability of primary data that is important and required for analyzing this review. To be more specific, some studies failed in detailing the bacterial concentration and composition in the indoor air of hospitals and healthcare settings. Besides, there was an inconsistent reporting of the bacterial concentration among the included studies. However, no modifications were made for sample size and reported values of the bacterial concentration. Consequently, the mean may be CFU/m3 biased.
Conclusions
This review aims to provide a comprehensive summary of the existing knowledge on bacterial concentrations in the indoor air of hospitals and healthcare settings in MENA region countries. As well as analyzing the associated bacterial compositions. The results exceeded the WHO recommended levels of airborne bacteria in the indoor locations and sensitive locations that has immunocompromised patients. The most abundant bacterial families were Staphylococcaceae, Enterobacteriaceae, Pseudomonadaceae, and Bacillaceae, which can put the immunosuppressed patients at high risk. This study also revealed that the most active air sampling technique was impaction and is considered one of the best-recommended air sampling techniques. Moreover, our study unveiled that Iran was the only country that adopted an international IAQ standard among all MENA region countries.
Recommendations
IAQ is a vast and extensive topic in which any single variable could impact the overall performance of air in indoor environments. Most countries of the MENA region showed a distinct interest in that issue in the studied years. Hence, further efforts should be made by countries of the MENA region to investigate the presence of bacterial concentrations in hospitals and especially to understand their potential pathogenicity to prevent HAI transmission.
We recommend the scientific community investigate the issue further, understand the threat, and propose efficient solutions that guarantee the protection of people. Specifically, an international standard should unify the recommended levels of bacterial loads in indoor air environments, especially in hospitals and healthcare settings. In addition, scientists must follow standardized methodologies to express bacterial air contamination (CFU/m3). Therefore, making it easier for researchers worldwide to compare their results with others and in compliance with the international standards.
Our data highlights the importance of a routine bacterial contamination assessment in Primary Health Care Centers, using the standard active sampling techniques. This will help in controlling the risk that is exposed to the patients and healthcare workers. Supplementary Materials: Table S1: Detailed characteristics of the 21 studies included in the review.
Author contributions
Conceptualization, N.E., methodology, L.A., H.A., and A.J.; software, H.A.; resources, N.E.; data curation, N.E.; writing – original draft preparation, L.A., A.J., H.A., and H.A.A.; writing – review and editing, N.E., H.Y.; A.A., H.Q.; project administration, N.E.; funding acquisition, N.E. All authors have read and agreed to the published version of the manuscript.
Supplemental Material
Download MS Word (778.3 KB)Acknowledgments
The authors would like to acknowledge the administrative staff at Biomedical Research Centre for assisting in all the paperwork. .
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data presented in this study are available on request from the corresponding author.
Supplementary material
Supplemental data for this article can be accessed online at https://doi.org/10.1080/09603123.2022.2083087.
Additional information
Funding
References
- Abbasi F, Jalili M, Samaei MR, Mokhtari AM, Azizi E. 2021. Effect of land use on cultivable bioaerosols in the indoor air of hospital in southeast Iran and its determination of the affected radius around of hospital. Environ Sci Pollu Res. 28(10):12707–12713. doi:10.1007/s11356-020-10357-3.
- Abdollahi A, Mahmoudzadeh S. 2012. Microbial profile of air contamination in hospital wards. Iran J Pathol. 7(3):177–182.
- Abera A, Tilahun M, Tekele SG, Belete MA. 2021. Prevalence, antimicrobial susceptibility patterns, and risk factors associated with enterococci among pediatric patients at Dessie referral hospital, Northeastern Ethiopia. Biomed Res Int. 2021:5549847. doi:10.1155/2021/5549847.
- Abu Rub LIA, Gharbi D, Safi M, Eltai NO, Suhail M, Kotb MM, Yigiterhan O, Alfoldy B. 2021. A preliminary study on microbial air contamination in select schools in Doha, Qatar. Arab J Geosci. 14(24):2707. doi:10.1007/s12517-021-08867-6.
- Al-Shahwani MF. 2005. Bacterial distribution analysis of the atmosphere of two hospitals in lbb, Yemen. East Med Health J. 11(5–6):1115–1119.
- Bargard Z, Najafpoor A, Alidadi H, Pazira M, Ejtehadi M, Ghavami V, Sarkhosh M. 2021. Microbial air monitoring in the pediatric burn ward: experience at the university hospital of Mashhad, Iran. Int J Pediatr-Mashhad. 9(7):14061–14075. doi:10.22038/ijp.2021.45100.3712.
- Best EL, Fawley WN, Parnell P, Wilcox MH. 2010. The potential for airborne dispersal of clostridium difficile from symptomatic patients. Clin Infect Dis. 50(11):1450–1457. doi:10.1086/652648.
- Bloch AB, Orenstein WA, Ewing WM, Spain WH, Mallison GF, Herrmann KL, Hinman AR. 1985. Measles outbreak in a pediatric practice: airborne transmission in an office setting. Pediatrics. 75(4):676–683. doi:10.1542/peds.75.4.676.
- Bonifait L, Charlebois R, Vimont A, Turgeon N, Veillette M, Longtin Y, Jean J, Duchaine C. 2015. Detection and quantification of airborne norovirus during outbreaks in healthcare facilities. Clin Infect Dis. 61(3):299–304. doi:10.1093/cid/civ321.
- Brown SP, Cornforth DM, Mideo N. 2012. Evolution of virulence in opportunistic pathogens: generalism, plasticity, and control. Trends Microbiol. 20(7):336–342. doi:10.1016/j.tim.2012.04.005.
- Cabo Verde S, Almeida SM, Matos J, Guerreiro D, Meneses M, Faria T, Botelho D, Santos M, Viegas C. 2015. Microbiological assessment of indoor air quality at different hospital sites. Res Microbiol. 166(7):557–563. doi:10.1016/j.resmic.2015.03.004.
- Carling PC, Parry MF, Bruno-Murtha LA, Dick B. 2010. Improving environmental hygiene in 27 intensive care units to decrease multidrug-resistant bacterial transmission*. Crit Care Med. 38(4):1054–1059. doi:10.1097/CCM.0b013e3181cdf705.
- Chen H, Du R, Ren W, Zhang S, Du P, Zhang Y. 2021. The microbial activity in PM2.5 in indoor air: as an index of air quality level. Aerosol Air Qual Res. 21(2):200101. doi:10.4209/aaqr.2020.03.0101.
- Dehghani M, Sorooshian A, Nazmara S, Baghani AN, Delikhoon M. 2018. Concentration and type of bioaerosols before and after conventional disinfection and sterilization procedures inside hospital operating rooms. Ecotoxicol Environ Saf. 164:277–282. doi:10.1016/j.ecoenv.2018.08.034
- Dharmadhikari AS, Mphahlele M, Stoltz A, Venter K, Mathebula R, Masotla T, Lubbe W, Pagano M, First M, Jensen PA, et al. 2012. Surgical face masks worn by patients with multidrug-resistant tuberculosis: impact on infectivity of air on a hospital ward. Am J Respir Crit Care Med. 185(10):1104–1109. doi:10.1164/rccm.201107-1190OC.
- EFSA Panel on Biological Hazards (BIOHAZ). 2016. Risks for public health related to the presence of Bacillus cereus and other Bacillus spp. including Bacillus thuringiensis in foodstuffs. EFSA Journal. 14(7):e04524. doi:10.2903/j.efsa.2016.4524.
- Ekhaise FO, Ighosewe OU, Ajakpovi OD. 2008. Hospital indoor airborne microflora in private and government owned hospitals in Benin City, Nigeria. 5.
- Farraia M, Paciência I, Ribeiro AI, Moreira A, Cavaleiro Rufo J 2019. Indoor air quality in hospitals: how is the situation in Portugal? In: Arezes PM, Baptista JS, Barroso MP, Carneiro P, Cordeiro P, Costa N, Melo RB, Miguel A and Perestrelo G, editors Occupational and environmental safety and health. In: Cham: Springer International Publishing; pp. 303–311. doi:10.1007/978-3-030-14730-3_33
- Fekadu S, Getachewu B. 2015. Microbiological assessment of indoor air of teaching hospital wards: a case of Jimma university specialized hospital. Ethiop J Health Sci. 25(2):117–122. doi:10.4314/ejhs.v25i2.3.
- Fu Shaw L, Chen IH, Chen CS, Wu HH, Lai LS, Chen YY, Wang FD. 2018. Factors influencing microbial colonies in the air of operating rooms. BMC Infect Dis. 18(1):4. doi:10.1186/s12879-017-2928-1.
- Gao X, Wei J, Lei H, Xu P, Cowling BJ, Li Y, Shaman J. 2016. Building ventilation as an effective disease intervention strategy in a dense indoor contact network in an Ideal City. PLoS One. 11(9):e0162481. doi:10.1371/journal.pone.0162481.
- Gizaw Z, Gebrehiwot M, Yenew C. 2016. High bacterial load of indoor air in hospital wards: the case of university of Gondar teaching hospital, Northwest Ethiopia. Multidiscip Respir Med. 11(1):24. doi:10.1186/s40248-016-0061-4.
- Haig CW, Mackay WG, Walker JT, Williams C. 2016. Bioaerosol sampling: sampling mechanisms, bioefficiency and field studies. J Hosp Infect. 93(3):242–255. doi:10.1016/j.jhin.2016.03.017.
- Hara S, Yamamoto H, Kawabata A, Azuma T, Ishii S, Okumura N, Ito Y. 2016. Airborne transmission from a neonate with Netherton syndrome during an outbreak of MRSA. Pediatr Int. 58(6):518–520. doi:10.1111/ped.12841.
- Hayleeyesus SF, Manaye AM. 2014. Microbiological quality of indoor air in University Libraries. Asian Pac J Trop Biomed. 4:S312–S317. doi:10.12980/APJTB.4.2014C807.
- Hemati S, Mobini GR, Heidari M, Rahmani F, Soleymani Babadi A, Farhadkhani M, Nourmoradi H, Raeisi A, Ahmadi A, Khodabakhshi A, et al. 2021. Simultaneous monitoring of SARS-CoV-2, bacteria, and fungi in indoor air of hospital: a study on hajar hospital in Shahrekord, Iran. Environ Sci Pollut Res. 28(32):43792–43802. doi:10.1007/s11356-021-13628-9.
- Horve PF, Lloyd S, Mhuireach GA, Dietz L, Fretz M, MacCrone G, Van Den Wymelenberg K, Ishaq SL. 2020. Building upon current knowledge and techniques of indoor microbiology to construct the next era of theory into microorganisms, health, and the built environment. J Expos Sci Environ Epidemiol. 30(2):219–235. doi:10.1038/s41370-019-0157-y.
- Hoseinzadeh E, Samarghandie M, Ghiasian S, Alikhani M, Roshanaie G. 2013. Evaluation of bioaerosols in five educational hospitals wards air in Hamedan, during 2011-2012. Jundishapur J Microbiol. 6(6). doi:10.5812/jjm.10704.
- Jalili D, Dehghani M, Fadaei A, Alimohammadi M, Ssempebwa J. 2021. Assessment of airborne bacterial and fungal communities in Shahrekord hospitals. J Environ Public Health. 2021:1–7. doi:10.1155/2021/8864051.
- Jayaprakash B, Adams RI, Kirjavainen P, Karvonen A, Vepsäläinen A, Valkonen M, Järvi K, Sulyok M, Pekkanen J, Hyvärinen A et al. 2017. Indoor microbiota in severely moisture damaged homes and the impact of interventions. Microbiome. 5(1):138. doi:10.1186/s40168-017-0356-5.
- Kassambara A. 2020. Ggpubr: “ggplot2” based publication ready plots. [ place unknown]. h ttp s://CRA N.R-proje ct.or g/packa ge=ggp ubr\}.
- Kozajda A, Jeżak K, Kapsa A. 2019. Airborne staphylococcus aureus in different environments-a review. Environ Sci Pollut Res Int. 26(34):34741–34753. doi:10.1007/s11356-019-06557-1.
- Kummer V, Thiel WR. 2008. Bioaerosols – Sources and control measures. Int J Hyg Environ Health. 211(3–4):299–307. doi:10.1016/j.ijheh.2007.06.006.
- Kunwar A, Tamrakar S, Poudel S, Sharma S, Parajuli P. 2019. Bacteriological assessment of the indoor air of different hospitals of Kathmandu District. In: Falkinham J, editor. International Journal of Microbiologyp. 5320807. doi:10.1155/2019/5320807
- Lakhundi S, Zhang K. 2018. Methicillin-Resistant staphylococcus aureus: molecular characterization, evolution, and epidemiology. Clin Microbiol Rev. 31(4):e00020–18. doi:10.1128/CMR.00020-18.
- Landry KG, Ascension NM, Armelle DCI, Hortense GK, François-Xavier E. 2018. Assessment of indoor microbial quality of library’s premise: case of Central Library of the university of Yaoundé I. Open J Prev Med. 8(4):109–120. doi:10.4236/ojpm.2018.84011.
- León MG F-D, Duarte-Escalante E, Calderón-Ezquerro MDCC, Jiménez-Martínez MDC, Acosta-Altamirano G, Moreno-Eutimio MA, Zúñiga G, García-González R, Ramírez-Pérez M, Reyes-Montes MDR. 2016. Diversity and characterization of airborne bacteria at two health institutions. Aerobiologia. 32(2):187–198. doi:10.1007/s10453-015-9389-z.
- Melymuk L, Bohlin-Nizzetto P, Prokeš R, Kukučka P, Klánová J. 2016. Sampling artifacts in active air sampling of semivolatile organic contaminants: comparing theoretical and measured artifacts and evaluating implications for monitoring networks. Environ Pollut. 217:97–106. doi:10.1016/j.envpol.2015.12.015
- Mirhoseini SH, Nikaeen M, Khanahmad H, Hatamzadeh M, Hassanzadeh A. 2015. Monitoring of airborne bacteria and aerosols in different wards of hospitals - particle counting usefulness in investigation of airborne bacteria. Ann Agri Environ Med. 22(4):670–973. doi:10.5604/12321966.1185772.
- Mirhoseini SH, Nikaeen M, Shamsizadeh Z, Khanahmad H. 2016. Hospital air: a potential route for transmission of infections caused by β-lactam-resistant bacteria. Am J Infect Control. 44(8):898–904. doi:10.1016/j.ajic.2016.01.041.
- Mirhoseini SH, Nikaeen M, Shamsizadeh Z. 2019. Occurrence of airborne methicillin-resistant staphylococcus aureus in different hospital wards. EnvironmentAsia. 12(3):121–128. doi:10.14456/ea.2019.52.
- Mirhoseini SH, Didehdar M, Akbari M, Moradzadeh R, Jamshidi R, Torabi S. 2020. Indoor exposure to airborne bacteria and fungi in sensitive wards of an academic pediatric hospital. Aerobiologia. 36(2):225–232. doi:10.1007/s10453-020-09624-0.
- Mirzaei R, Shahriary E, Qureshi MI, Rakhshkhorshid A, Khammary A, Mohammadi M. 2014. Quantitative and qualitative evaluation of bio-aerosols in surgery rooms and emergency department of an educational hospital. Jundishapur J Microbiol. 7(8):e11688. doi:10.5812/jjm.11688.
- Mirzaii M, Emaneini M, Jabalameli F, Halimi S, Taherikalani M. 2015. Molecular investigation of staphylococcus aureus isolated from the patients, personnel, air and environment of an ICU in a hospital in Tehran. J Infect Public Health. 8(2):202–206. doi:10.1016/j.jiph.2014.09.002.
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. 2009. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 6(7):1–6. doi:10.1371/journal.pmed.1000097.
- Montazeri A, Zandi H, Teymouri F, Soltanianzadeh Z, Jambarsang S, Mokhtari M. 2020. Microbiological analysis of bacterial and fungal bioaerosols from burn hospital of Yazd (Iran) in 2019. J Environ Health Sci Eng. 18(2):1121–1130. doi:10.1007/s40201-020-00531-7.
- Monteiro A, Cabo Verde S. 2017. Bacterial bioburden in hospital environment. In: Viegas C; Viegas S; Gomes A; Täubel M and Sabino R, editors. Exposure to microbiological agents in indoor and occupational environments. Cham: Springer International Publishing; pp. 321–328. [accessed 2021 Nov 8]. 10.1007/978-3-319-61688-9_15.
- Monteiro A, Almeida B, Paciência I, Cavaleiro Rufo J, Ribeiro E, Carolino E, Viegas C, Uva AS, Verde SC. 2021. Bacterial contamination in health care centers: differences between urban and rural settings. Atmosphere. 12(4):450. doi:10.3390/atmos12040450.
- Moussa M, Abou Chakra M, Dellis A, Moussa Y, Papatsoris A. 2020. Pharmacotherapeutic advances for recurrent urinary tract infections in women. Expert Opin Pharmacother. 21(16):2011–2026. doi:10.1080/14656566.2020.1795128.
- Napoli C, Marcotrigiano V, Montagna MT. 2012. Air sampling procedures to evaluate microbial contamination: a comparison between active and passive methods in operating theatres. BMC Public Health. 12(1):594. doi:10.1186/1471-2458-12-594.
- Nasiri N, Gholipour S, Akbari H, Koolivand A, Abtahi H, Didehdar M, Rezaei A, Mirzaei N. 2021. Contamination of obsterics and gynecology hospital air by bacterial and fungal aerosols associated with nosocomial infections. J Environ Health Sci Eng. 19(1):663–670. doi:10.1007/s40201-021-00637-6.
- Nguyen M, Holmes EC, Angenent LT, Dunea D. 2021. The short-term effect of residential home energy retrofits on indoor air quality and microbial exposure: a case-control study. PLoS One. 16(9):e0230700. doi:10.1371/journal.pone.0230700.
- Nunes Z, Martins A, Altoe A, Nishikawa M, Leite M, Aguiar P, Fracalanzza S. 2005. Indoor air microbiological evaluation of offices, hospitals, industries, and shopping centers. Memórias Do Instituto Oswaldo Cruz. 100(4):351–357. doi:10.1590/S0074-02762005000400003.
- Osman ME, Ibrahim HY, Yousef FA, Elnasr AAA, Saeed Y, Hameed AAA. 2018. A study on microbiological contamination on air quality in hospitals in Egypt. Indoor and Built Environment. 27(7):953–968. doi:10.1177/1420326X17698193.
- Pasquarella C, Pitzurra O, Savino A. 2000. The index of microbial air contamination. J Hosp Infect. 46(4):241–256. doi:10.1053/jhin.2000.0820.
- Qudiesat K, Abu-Elteen K, Elkarmi A, Hamad M, Abussaud M. 2009. Assessment of airborne pathogens in healthcare settings. Afr J Microbiol Res. 3.
- R Core Team RC. 2011. R: a language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing. http://www.r-project.org/.
- Rida RH, Al Laham NA, Elmanama AA. 2018. Carbapenem resistance among clinical and environmental gram-negative isolates recovered from hospitals in Gaza strip, Palestine. Germs. 8(3):147–154.
- Sajjadi SA, Shakeri H, Haghighi MHM, Mohammadzade A. 2016. Microbial indoor air quality of public places in a semi-dry city in Iran. Int J Trop Med. 11(4):102–107.
- Sandle T. 2010. Selection of active air samplers. Eur J Parent Pharm Sci. 15:119–124.
- Siegel JD, Rhinehart E, Jackson M, Chiarello L, Health Care Infection Control Practices Advisory Committee. 2007. Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 35(10 Suppl 2):S65–164. doi:10.1016/j.ajic.2007.10.007.
- Soleimani Z, Parhizgari N, Dehdari Rad H, Akhoond MR, Kermani M, Marzouni MB, Goudarzi H, Goudarzi G. 2015. Normal and dusty days comparison of culturable indoor airborne bacteria in Ahvaz, Iran. Aerobiologia. 31(2):127–141. doi:10.1007/s10453-014-9352-4.
- Srikanth P, Sudharsanam S, Steinberg R. 2008. Bio-Aerosols in indoor environment: composition, health effects and analysis. Indian J Med Microbiol. 26(4):302–312. doi:10.1016/S0255-0857(21)01805-3.
- Stauning MA, Bediako-Bowan A, Bjerrum S, Andersen LP, Andreu-Sánchez S, Labi A-K, Kurtzhals JAL, Marvig RL, Opintan JA. 2020. Genetic relationship between bacteria isolated from intraoperative air samples and surgical site infections at a major teaching hospital in Ghana. J Hosp Infect. 104(3):309–320. doi:10.1016/j.jhin.2019.11.007.
- Stockwell E, Ballard E, O’-Rourke P, Knibbs L, Morawska L, Bell S. 2019. Indoor hospital air and the impact of ventilation on bioaerosols: a systematic review. J Hosp Infect. 103. doi:10.1016/j.jhin.2019.06.016
- Taha MPM, Drew GH, Longhurst PJ, Smith R, Pollard SJT. 2006. Bioaerosol releases from compost facilities: evaluating passive and active source terms at a green waste facility for improved risk assessments. Atmos Environ. 40(6):1159–1169. doi:10.1016/j.atmosenv.2005.11.010.
- Taylor TA, Unakal CG. 2022. Staphylococcus aureus. In: StatPearls. Treasure Island (FL): StatPearls Publishing. accessed 2022 Apr 14. http://www.ncbi.nlm.nih.gov/books/NBK441868/.
- Verhoeff AP, van Wijnen JH, Boleij JSM, Brunekreef B, van Reenen‐hoekstra ES, Samson RA, Wijnen JH. 1990. Enumeration and identification of airborne viable mould propagules in houses: a field comparison of selected techniques. Allergy. 45(4):275–284. doi:10.1111/j.1398-9995.1990.tb00496.x.
- Wickham H 2016. Ggplot2: elegant graphics for data analysis. [place unknown]: Springer-Verlag New York. h ttp s://gg plot2.tid yverse.o rg\}.
- Wu B, Qi C, Wang L, Yang W, Zhou D, Wang M, Dong Y, Weng H, Li C, Hou X, et al. 2020. Detection of microbial aerosols in hospital wards and molecular identification and dissemination of drug resistance of Escherichia coli. Environ Int. 137:105479. doi:10.1016/j.envint.2020.105479
- Yan W, FangXia S, MaoSheng Y. 2010. Use of gelatin filter and BioSampler in detecting airborne H5N1 nucleotides, bacteria and allergens. J Aerosol Sci. 41(9):869–879.
- Yao M, Mainelis G. 2006. Effect of physical and biological parameters on enumeration of bioaerosols by portable microbial impactors. J Aerosol Sci. 37(11):1467–1483. doi:10.1016/j.jaerosci.2006.06.005.
- Yassin MF, Almouqatea S. 2010. Assessment of airborne bacteria and fungi in an indoor and outdoor environment. Int J Environ Sci Technol. 7(3):535–544. doi:10.1007/BF03326162.
