ABSTRACT
Retinol-binding protein 4 (RBP4) was controversially associated with type 2 diabetes mellitus (T2DM). This meta-analysis aimed at evaluating the association between RBP4 level and T2DM risk. MEDLINE and EMBASE were searched to identify relevant studies up to 3 December 2022. Random effects model was used to pool multivariate-adjusted odds ratios (ORs) and 95% confidence intervals (CIs). Publication bias was estimated by Funnel plot and Egger’s test, it was considered to be significant when P < 0.05. Eight studies including 8087 participants were finally included. Compared to those with the lowest level, subjects with the highest level of RBP4 have a higher risk of T2DM (OR = 1.47, 95% CI: 1.16–1.78, P < 0.001, I2 = 86.9%). No publication bias among the included studies was found (t = 0.94, P = 0.377). This meta-analysis indicated that high RBP4 level was associated with increasing risk of T2DM.
Introduction
Diabetes is a systemic syndrome that can increase the risks of retinopathy, neuropathy, and nephropathy, and reduce the quality of life, shorten people’s life expectancy, and bring huge economic pressure to both individuals and society (Cho et al. Citation2018). Now the prevalence of diabetes is growing at an alarming rate, and it has become a global public health issue. The International Diabetes Federation reported that approximately 463 million people worldwide suffered from diabetes in 2019, and about 1.9 million people died directly from diabetes (Saeedi et al. Citation2019). Also, type 2 diabetes mellitus (T2DM) will affect an estimated 700 million people in 2045 worldwide (Saeedi et al. Citation2020). The pathophysiological mechanism of diabetes is related to insulin deficiency and insulin resistance. Some in-depth studies have found that multiple adipokines play important roles in the pathogenesis of diabetes (Sun et al. Citation2010; Wang et al. Citation2020). A scientometric review reported that biomarker discovery on diabetic retinopathy (DR) has become a trend (Ramin et al. Citation2015).
Retinol-binding protein 4 (RBP4) was initially considered as a hormone secreted by the liver and a transporter that transports retinol from the liver to peripheral tissues (Quadro et al. Citation2003). However, a meta-analysis reported that nonalcoholic fatty liver disease (NAFLD), non-alcoholic steatohepatitis (NASH), and simple steatosis (SS) patients have not altered RBP4 levels, although there was substantial unexplained between-study heterogeneity (Zhou et al. Citation2017). Recent research has found that adipocyte is another important source of RBP4, RBP4 was therefore considered as an adipokine (Newcomer and Ong Citation2000). Animal experiments have proven that RBP4 could change insulin sensitivity. Transgenic overexpression of human RBP4 or injection of purified RBP4 can lead to insulin resistance in mice (Yang et al. Citation2005). Meanwhile, an increased level of serum RBP4 level was reported to induce the expression of the gluconeogenic enzyme phosphoenol pyruvate carboxykinase and impaire insulin signal transmission in muscles and liver of mouse (Yang et al. Citation2005), that is the characteristic of diabetes. Conversely, some studies have also observed an opposite correlation between RBP4 and insulin sensitivity in humans (Suh et al. Citation2010; Meisinger et al. Citation2011). In addition to insulin resistance, insulin deficiency is also one of the mechanisms leading to diabetes, and islet β cell dysfunction is the main reason for insulin secretion reduction. Researches have reported that RBP4 is related to β cell dysfunction (Broch et al. Citation2007; Ribel-Madsen et al. Citation2009). A systematic review and meta-analysis indicated that the levels of circulating RBP4 of T2DM patients with micro/macroalbuminuria or declined estimated glomerular filtration rate (eGFR) were significantly higher, and the levels of circulating RBP4 were positively correlated with albumin-to-createnine ratio (ACR) but negatively related to eGFR (Zhang et al. Citation2020). Furthermore, epidemiological studies have indicated that elevated RBP4 is positively correlated to T2DM incidence (Jagannathan et al. Citation2015; Fan et al. Citation2019; Cho et al. Citation2020). In contrast, some cross-sectional studies showed that there was no association between RBP4 and T2DM (Chavez et al. Citation2009; Ulgen et al. Citation2010; Kaess et al. Citation2012). Therefore, conclusions about the relationship between RBP4 levels and the risk of T2DM are inconclusive.
Given the controversy of the results of these studies which regarding the relationship between RBP4 and the risk of T2DM, we conducted this meta-analysis to evaluate the correlation between RBP4 level and risk of type 2 diabetes.
Materials and methods
Search strategy
A comprehensive literature research was performed using MEDLINE and EMBASE until 3 December 2022. The following key terms were used: “type 2 diabetes” or “T2DM” and “RBP4” or “retinol-binding protein 4” or “retinol-binding protein”. Specific search strategies are provided in the supplementary materials. The reference lists of relevant review articles were also scanned to identify more potential related researches. Two investigators independently reviewed all identified studies (Xm T and HZ). Any differences were resolved by a senior author (Xx L).
Eligibility criteria
Articles meeting the following criteria were included: (1) published in English language; (2) investigated the relationship between RBP4 and the risk of T2DM; (3) cohort study or case–control study; (4) reported the estimated Hazard Ratio/Risk Ratio/Odds Ratio (HR/RR/OR) with 95% CI or the indexes could be reckoned; (5) participants were restricted into adults. If two or more studies used the same datasets, the one with the most comprehensive information or the longest follow-up period would be included in the meta-analysis.
The exclusion criteria are (1) abstracts, reviews, unpublished studies, or repeated publications; (2) researches based on animals or cell; (3) HR/RR/OR with 95% CI could not be extracted or calculated from the articles.
Data extraction
Two researchers (Lm L, YC) extracted the following information independently: (1) first author’s surname; (2) publication year; (3) country and geographic location; (4) study design (cohort study or case–control study); (5) sources of control, number of cases and participants; (6) the sources of RBP4; (7) measurement methods of RBP4; (8) RR, HR, or OR and 95% CI of the highest level versus the lowest level of RBP4; (9) range of RBP4 levels, and (10) adjusted covariates in each study.
Quality assessment
The methodological quality of the selected studies was evaluated according to Newcastle-Ottawa scale (Hartling et al. Citation2013). This step was also conducted by two researchers (Ll C and Hh Z), and any disagreement was resolved by the third author (ZG). The full score of Newcastle-Ottawa scale was 9, and those studies with scores higher than 6 were considered as high quality. A reliability analysis among raters was also carried out.
Statistical methods
We performed meta-analyses of observational studies using the Mantel–Haenszel method to evaluate the association between RBP4 and the risk of T2DM. The pooled ORs together with its 95%CIs were obtained through the analysis. Heterogeneity among these studies was quantified using Q statistic (P < 0.05 was considered heterogeneous) and I2 statistic (I2>50% was considered to have high heterogeneity). The random effect model would be adopted to compute the pooled effect size if the heterogeneity among studies is large, otherwise we would choose the fixed-effect model to compute the pooled effect size. Besides, the leave-one-out sensitive analysis was used to assess the pivotal contributor which has a substantial impact on between-study heterogeneity, in case no significant covariates were found to be heterogeneous. Subgroup analyses were applied based on the study period and the source of RBP4. Egger’s test and Funnel plots were conducted to evaluate the potential publication bias, and P > 0.05 was considered to be not significant. Since there were only two studies conducted in North America, we also performed an analysis which excluded these two studies as a sensitivity analysis. STATA software (version 12.0; Stata Corporation, College Station, TX, USA) was used for all statistical analyses. Although this study was not pre-registered on PROSPERO, it was carried out in strict accordance with the process prescribed by PROSPERO.
Results
Literature search and study characteristics
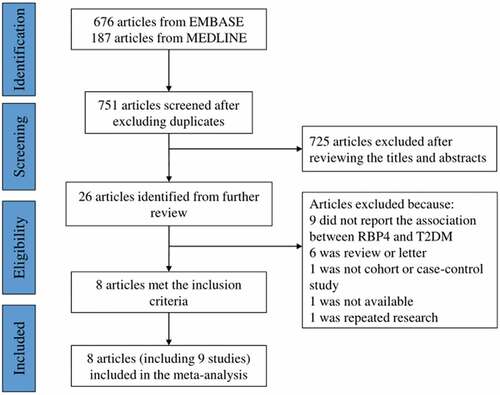
illustrates the specific process of retrieving and screening articles. Briefly, we retrieved 374 articles from PubMed and 714 from Web of Science, among which, 718 duplicates were identified. Only 13 articles were finally perceived necessary to read the full text after reading titles and abstracts of these articles. Of those, two reported a connection between RBP4 and prediabetes, two reported about RBP4 and metabolic syndrome, and one was a same population. After reading the full text, 8 articles (Xu et al. Citation2009; Luft et al. Citation2013; Sun et al. Citation2014; Jagannathan et al. Citation2015; Pandey et al. Citation2015; Fan et al. Citation2019; Wang et al. Citation2019; Cho et al. Citation2020), including 9 studies, were finally included in this meta-analysis.
Figure 1. Flow chart of literature search and study selection. RBP4, retinol-binding protein 4. T2DM, type 2 diabetes mellitus.
provides an overview of the characteristics of the included studies and participants. A total of 8087 participants were included in this study. Half of the researches were case–control studies, and the remaining half were cohort studies or cross-sectional studies, and the sample sizes varied from 147 to 2091. Six studies were carried out in Asia and the other two in the United States. All participants were from the community population. All the studies detected RBP4 by enzyme-linked immunosorbent assay, and half of the studies examined plasma RBP4 and half examined serum RBP4.
Table 1. Characteristics of the included studies of RBP4 and risk of diabetes.
Quality assessment
The results of quality assessment are presented in Supplementary Table S1, and all the studies included scored more than 6. They could therefore be reckoned as high quality.
Meta-analysis of RBP4 level and risk of T2DM
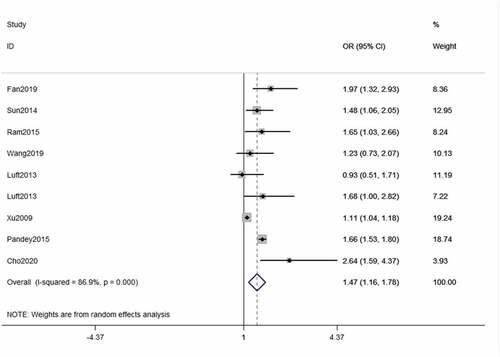
The pooled OR value of the highest versus the lowest categories of RBP4 level was calculated, and we found that RBP4 level is strongly associated with the increased risk of T2DM (OR = 1.47, 95% CI = 1.16–1.78, ).
Sensitivity analysis and publication bias
The results demonstrated that there was a high heterogeneity across the included studies (I2 = 89.5%, P < 0.001, ). In addition to finding out the sources of heterogeneity, we conducted leave-one-out sensitive analysis, and found that every pooled OR value showed no significant change when removing any single study in turn and evaluating the pooled ORs of the remaining studies, which confirmed the robustness of the findings in this study. Meanwhile, we also found Xu’s study had the most dominant impact on heterogeneity. After excluding his research, the I-squared decreased to 28.9% while P = 0.443. In addition, we found that the association between RBP4 level and T2DM risk still remains significant after removing the studies conducted in North America (OR = 1.53, 95%CI = 1.18–1.88, Supplementary Figure S1).
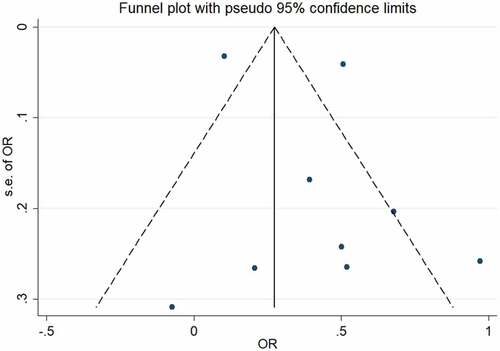
The results obtained from funnel plot and Egger’s test applied to estimate publication bias displayed in . No publication bias among the included studies was observed through the funnel plot () and Egger’s test (t = 0.94, P = 0.377). Yet, since we have limited primary included papers to English, the possibility of publication bias still exists.
Subgroup analysis
Subgroup analysis was conducted based on the following aspects regarding the association between RBP4 level and risk of T2DM: study period (before 2015 and after 2015) (Supplementary Figure S2) and source of RBP4 (serum and plasma) (Supplementary Figure S3). Through subgroup analysis, we found that the pooled OR before 2015 and after 2015 subgroups were 1.66 (95% CI 1.52–1.81, P = 0.398) and 1.17 (95%CI 0.96–1.39, P = 0.267), respectively, which indicated that the studies before 2015 group enhanced the association between RBP4 level and T2DM. Subgroup-analysis for the source of RBP4 demonstrated that the pooled OR was 1.60 (95% CI 1.17–2.04, P < 0.001) in serum group, and 1.30 (95% CI 0.99–1.61, P = 0.444) in plasma group. Among three subgroups analysis, significant heterogeneity was not found only in the study period subgroup.
Discussion
In order to enrich the clinical predictors of T2DM and explore its possible pathological pathways, many studies have investigated the relationship between RBP4 level and risk of T2DM, but their results are contradictory. Therefore, we conducted the current meta-analysis based on observational studies to make the results accurate and reliable. Our results showed that RBP4 level was positively correlated with the risk of type 2 diabetes, and there was no publication bias in this meta-analysis.
There are several mechanisms which might explain the positive correlation between RBP4 level and risk of T2DM. Firstly, RBP4 can increase glucose production by inducing liver to express phosphoenolpyruvate kinase, a key gluconeogenic enzyme (Yang et al. Citation2005). Secondly, RBP4 reduces the activity of insulin receptor PI3K and affects insulin signaling conduction in the muscle (Yang et al. Citation2005). Thirdly, it can also induce the expression of pro-inflammatory cytokines in macrophages, promote the development of inflammatory state in adipose tissue, and inhibit the transmission of insulin signaling (Norseen et al. Citation2012). Finally, RBP4 can activate JAK2/STAT5 signal to inhibit the transmission of insulin signal in adipocytes directly (Berry et al. Citation2011). These findings indicated that RBP4 can induce insulin resistance in skeletal muscle, liver, and adipose tissue. Notably, insulin resistance is one of the causes of diabetes. However, several population-based studies included in the current research have shown that the positive correlation between RBP4 level and the risk of T2DM still exists even if homeostasis model assessment for insulin resistance (HOMA-IR) was adjusted (Pandey et al. Citation2015; Fan et al. Citation2019), which indicates that RBP4 may increase the risk of diabetes through a pathway that does not completely overlap with insulin resistance.
In addition, plenty of studies have reported that the decrease in insulin secretion caused by dysfunction and apoptosis of β cells is the core pathological cause of diabetes (Palmer et al. Citation2019; Veelen et al. Citation2020). Interestingly, evidence showed that RBP4 level is negatively related to β cell function. Craig et al. have found that the mutations of non-coding regions in or near RBP4 caused the reduction of insulin secretion and increased the risk of T2DM in white people (Craig et al. Citation2007), but the mechanism is unclear. Therefore, more research is needed to explore the mechanism in which RBP4 causes β cell dysfunction. Although other studies have reported that there is no correlation between RBP4 level and risk of diabetes (Chavez et al. Citation2009; Ulgen et al. Citation2010; Kaess et al. Citation2012), most of them are experimental studies or cross-sectional studies carried out in a small number of people, which may be not sufficient to detect and prove the effect of RBP4. Based on the available evidences, we conducted this meta-analysis and found that there is an association between RBP4 level and T2DM.
It is crucial to find the source of heterogeneity in this meta-analysis. Although sensitivity analysis showed that our results are robust, we found that the heterogeneity decreased from 86.9% to 28.9% when Xu’s research (Xu et al. Citation2009) was eliminated by leave-one-out method, and the pooled OR had no substantial change (OR = 1.56, 95% CI: 1.32–1.79). This indicated that Xu’s research may be a major source of heterogeneity. Therefore, we compared this study with other studies and found that the case group in this study included not only newly diagnosed diabetes patients but also the people with impaired glucose regulation who had not been diagnosed with diabetes. We thought it may overestimate the relationship between RBP4 level and the risk of T2DM, and may also be the source of heterogeneity.
Besides, subgroup analyses indicated that the period (before or after 2015) in which the study was conducted may be another source of heterogeneity. This phenomenon may be caused by the development of sample collection and measuring method. Graham et al. reported that compared with quantitative western blot, various sources of ELISA kits overestimated the RBP4 concentration in normal blood glucose participants and underestimated the RBP4 level in impaired glucose tolerance participants (Graham et al. Citation2007). ELISA kits were used to detect the concentration of RBP4 in most of the studies included in the current meta-analysis, so the relationship between RBP4 level and T2DM risk may be weakened. Hence, effective tools and methods should be applied to generate more accurate data in the future research. On the other hand, we guess that as people’s living standards gradually improve, so does the public awareness of health care. Most people will focus on a balanced diet and early prevention of disease, which may also reduce the occurrence of excessive RBP4.
There are several strengths of the current work. In the first place, this is the first meta-analysis to systematically and quantitatively analyze the relationship between serum/plasma RBP4 level and T2DM risk. Although several reviews have been conducted to investigate the relationship between RBP4 and T2DM, none of them has carried out the quantitative analysis (von Eynatten and Humpet Citation2008; Kotnik et al. Citation2011; Christou et al. Citation2012; Olsen and Blomhoff Citation2020). Moreover, sensitivity analysis and subgroup analysis were also performed to explore the sources of heterogeneity in this study. In the second place, compared with those original studies, our result is more reliable because of the larger sample size and stronger statistical ability.
At the same time, we should also admit some limitations in this study. Firstly, some studies showed that the level of RBP4 in Whites is relatively higher than in Asians (Xu et al. Citation2009; Jagannathan et al. Citation2015), which suggests that the level of RBP4 may vary among different races. However, most of the studies we included were conducted among Asians, so the region outside Asia should pay attention to the ethnic variation when using our result. Secondly, the OR value we extracted from each study was the value after adjusting the potential confounding factors to the greatest extent, but the covariates included in each study are different, so the covariate adjustment may also affect the relationship between RBP4 and the risk of T2DM. Furthermore, the ELISA kit used in each study was different, which may introduce measurement bias into the study. Finally, half of the studies in this meta-analysis were case–control studies, so the recall bias is inevitable.
In conclusion, the current study illustrated that RBP4 level is positively correlated with the risk of T2DM. It may be a new predictor and research target of T2DM. Future studies should focus on the predictive efficacy of RBP4 on T2DM and its possible mechanisms and pathways.
Authors’ contributions
Study design: Zhao ZJ
Study research: Tan XM, Zhang H, and Liu XX
Data extraction: Liu LM and Chen Y
Quality assessment: Cui LL, Zhang HH, and Gao Z
Statistical analysis: Tan XM, Yu ZL, and Zhang H
Manuscript writing: Tan XM
Manuscript revision: Zhang HH and Cui LL
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Berry DC, Jin H, Majumdar A, Noy N. 2011. Signaling by vitamin a and retinol-binding protein regulates gene expression to inhibit insulin responses. P Natl Acad Sci USA. 108(11):4340–4345. English. doi:10.1073/pnas.1011115108.
- Broch M, Vendrell J, Ricart W, Richart C, Fernandez-Real JM. 2007. Circulating retinol-binding protein-4, insulin sensitivity, insulin secretion, and insulin disposition index in obese and nonobese subjects. Diabetes Care. 30(7):1802–1806. doi:10.2337/dc06-2034.
- Chavez AO, Coletta DK, Kamath S, Cromack DT, Monroy A, Folli F, DeFronzo RA, Tripathy D. 2009. Retinol-binding protein 4 is associated with impaired glucose tolerance but not with whole body or hepatic insulin resistance in Mexican Americans. Am J Physiol-Endoc M. 296(4):E758–764. English. doi:10.1152/ajpendo.90737.2008.
- Cho NH, Ku EJ, Jung KY, Oh TJ, Kwak SH, Moon JH, Park KS, Jang HC, Kim YJ, Choi SH. 2020. Estimated association between Cytokines and the progression to diabetes: 10-year follow-up from a community-based cohort. J Clin Endocrinol Metab. 105(3):e381–389. doi:10.1210/clinem/dgz171.
- Cho NH, Shaw JE, Karuranga S, Huang Y, Jd DRF, Ohlrogge AW, Malanda B. 2018. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pract. 138:271–281. doi:10.1016/j.diabres.2018.02.023.
- Christou GA, Tselepis AD, Kiortsis DN. 2012. The metabolic role of retinol binding protein 4: an update. Horm Metab Res. 44(1):6–14. English. doi:10.1055/s-0031-1295491.
- Craig RL, Chu WS, Elbein SC. 2007. Retinol binding protein 4 as a candidate gene for type 2 diabetes and prediabetic intermediate traits. Mol Genet Metab. 90(3):338–344. English. doi:10.1016/j.ymgme.2006.11.003.
- Fan J, Yin S, Lin D, Liu Y, Chen N, Bai X, Ke Q, Shen J, You L, Lin X, et al. 2019. Association of serum retinol-binding protein 4 levels and the risk of incident type 2 diabetes in subjects with prediabetes. Diabetes Care. 42(8):1574–1581. doi:10.2337/dc19-0265.
- Graham TE, Wason CJ, Bluher M, Kahn BB. 2007. Shortcomings in methodology complicate measurements of serum retinol binding protein (RBP4) in insulin-resistant human subjects. Diabetologia. 50(4):814–823. English. doi:10.1007/s00125-006-0557-0.
- Hartling L, Milne A, Hamm MP, Vandermeer B, Ansari M, Tsertsvadze A, Dryden DM. 2013. Testing the Newcastle Ottawa Scale showed low reliability between individual reviewers. J Clin Epidemiol. 66(9):982–993. English. doi:10.1016/j.jclinepi.2013.03.003.
- Jagannathan R, Snehalatha C, Selvam S, Nanditha A, Shetty AS, Godsland IF, Johnston DG, Ramachandran A. 2015. Retinol binding protein-4 predicts incident diabetes in Asian Indian men with prediabetes. Biofactors. 41(3):160–165. doi:10.1002/biof.1209.
- Kaess BM, Enserro DM, McManus DD, Xanthakis V, Chen MH, Sullivan LM, Ingram C, O’donnell CJ, Keaney JF, Vasan RS, et al. 2012. Cardiometabolic correlates and heritability of fetuin-A, retinol-binding protein 4, and fatty-acid binding protein 4 in the Framingham heart study. J Clin Endocrinol Metab. 97(10):E1943–1947. doi:10.1210/jc.2012-1458.
- Kotnik P, Fischer-Posovszky P, Wabitsch M. 2011. RBP4: a controversial adipokine. Eur J Endocrinol. 165(5):703–711. English. doi:10.1530/EJE-11-0431.
- Luft VC, Pereira M, Pankow JS, Ballantyne C, Couper D, Heiss G, Duncan BB, Investigators A. 2013. Retinol binding protein 4 and incident diabetes–the atherosclerosis risk in communities study (ARIC Study). Rev Bras Epidemiol = Braz J Epidemiol. 16(2):388–397. doi:10.1590/S1415-790X2013000200014.
- Meisinger C, Ruckert IM, Rathmann W, Doring A, Thorand B, Huth C, Kowall B, Koenig W. 2011. Retinol-binding protein 4 is associated with prediabetes in adults from the general population the cooperative health research in the region of Augsburg (KORA) F4 study. Diabetes Care. 34(7):1648–1650. English. doi:10.2337/dc11-0118.
- Newcomer ME, Ong DE. 2000. Plasma retinol binding protein: structure and function of the prototypic lipocalin. Biochim Biophys Acta. 1482(1–2):57–64. doi:10.1016/S0167-4838(00)00150-3.
- Norseen J, Hosooka T, Hammarstedt A, Yore MM, Kant S, Aryal P, Kiernan UA, Phillips DA, Maruyama H, Kraus BJ, et al. 2012. Retinol-binding protein 4 inhibits insulin signaling in adipocytes by inducing proinflammatory Cytokines in Macrophages through a c-Jun N-Terminal Kinase- and toll-like receptor 4-dependent and retinol-independent mechanism. Mol Cell Biol. 32(10):2010–2019. English. doi:10.1128/MCB.06193-11.
- Olsen T, Blomhoff R. 2020. Retinol, Retinoic Acid, and Retinol-Binding Protein 4 are differentially associated with Cardiovascular disease, type 2 diabetes, and obesity: an overview of human studies. Adv Nutr. 11(3):644–666. English. doi:10.1093/advances/nmz131.
- Palmer AK, Gustafson B, Kirkland JL, Smith U. 2019. Cellular senescence: at the nexus between ageing and diabetes. Diabetologia. 62(10):1835–1841. English. doi:10.1007/s00125-019-4934-x.
- Pandey GK, Balasubramanyam J, Balakumar M, Deepa M, Anjana RM, Abhijit S, Kaviya A, Velmurugan K, Miranda P, Balasubramanyam M, et al. 2015. Altered circulating levels of retinol binding protein 4 and transthyretin in relation to insulin resistance, obesity, and glucose intolerance in Asian Indians. Endocr Pract. 21(8):861–869. doi:10.4158/EP14558.OR.
- Quadro L, Hamberger L, Colantuoni V, Gottesman ME, Blaner WS. 2003. Understanding the physiological role of retinol-binding protein in vitamin a metabolism using transgenic and knockout mouse models. Mol Aspects Med. 24(6):421–430. English. doi:10.1016/S0098-2997(03)00038-4.
- Ramin S, Gharebaghi R, Heidary F. 2015. Scientometric analysis and mapping of scientific articles on diabetic retinopathy. Med Hypothesis Discov Innov Ophthalmol. 4(3):81–100.
- Ribel-Madsen R, Friedrichsen M, Vaag A, Poulsen P. 2009. Retinol-binding protein 4 in twins: regulatory mechanisms and impact of circulating and tissue expression levels on insulin secretion and action. Diabetes. 58(1):54–60. doi:10.2337/db08-1019.
- Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, et al. 2019. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas 9(th) Diabetes Res Clin Pract. 157: 107843. 10.1016/j.diabres.2019.107843
- Saeedi P, Salpea P, Karuranga S, Petersohn I, Malanda B, Gregg EW, Unwin N, Wild SH, Williams R 2020. Mortality attributable to diabetes in 20-79 years old adults, 2019 estimates: results from the international diabetes federation diabetes atlas. 9(th) Diabetes Res Clin Pract. 162: 108086. 10.1016/j.diabres.2020.108086
- Suh JB, Kim SM, Cho GJ, Choi KM, Han JH, Taek Geun H. 2010. Elevated serum retinol-binding protein 4 is associated with insulin resistance in older women. Metabolism. 59(1):118–122. doi:10.1016/j.metabol.2009.06.025.
- Sun L, Qi Q, Zong G, Ye X, Li H, Liu X, Zheng H, Hu FB, Liu Y, Lin X. 2014. Elevated plasma retinol-binding protein 4 is associated with increased risk of type 2 diabetes in middle-aged and elderly Chinese adults. J Nutr. 144(5):722–728. doi:10.3945/jn.113.189860.
- Sun Q, Rm VD, Meigs JB, Franco OH, Mantzoros CS, Hu FB. 2010. Leptin and soluble leptin receptor levels in plasma and risk of type 2 diabetes in U.S. women: a prospective study. Diabetes. 59(3):611–618. doi:10.2337/db09-1343.
- Ulgen F, Herder C, Kuhn MC, Willenberg HS, Schott M, Scherbaum WA, Schinner S. 2010. Association of serum levels of retinol-binding protein 4 with male sex but not with insulin resistance in obese patients. Arch Physiol Biochem. 116(2):57–62. doi:10.3109/13813451003631421.
- Veelen A, Tapia EE, Oscarsson J, Schrauwen P. 2020. Type 2 diabetes subgroups and potential medication strategies in relation to effects on insulin resistance and beta-cell function: a step towards personalized diabetes treatment? Mol Metab. 46:101158. doi:10.1016/j.molmet.2020.101158.
- von Eynatten M, Humpet PM. 2008. Retinol-binding protein-4 in experimental and clinical metabolic disease. Expert Rev Mol Diagn. 8(3):289–299. English. doi:10.1586/14737159.8.3.289.
- Wang Y, Koh WP, Sim X, Yuan JM, Pan A. 2020. Multiple biomarkers improved prediction for the Risk of Type 2 diabetes mellitus in Singapore Chinese men and women. Diabetes Metab J. 44(2):295–306. doi:10.4093/dmj.2019.0020.
- Wang Y, Sun L, Lin X, Yuan J-M, Koh W-P, Pan A. 2019. Retinol binding protein 4 and risk of type 2 diabetes in Singapore Chinese men and women: a nested case-control study. Nutr Metab. 16(1): doi:10.1186/s12986-018-0329-0.
- Xu M, Li XY, Wang JG, Wang XJ, Huang Y, Cheng Q, Huang HE, Li R, Xiang J, Tan JR, et al. 2009. Retinol-binding protein 4 is associated with impaired glucose regulation and microalbuminuria in a Chinese population. Diabetologia. 52(8):1511–1519. doi:10.1007/s00125-009-1386-8.
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. 2005. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 436(7049):356–362. doi:10.1038/nature03711.
- Zhang L, Cheng YL, Xue S, Xu ZG. 2020. The role of circulating RBP4 in the Type 2 diabetes patients with kidney diseases: a systematic review and meta-analysis [review]. Dis Markers. 2020:8830471. eCollection 8832020. doi:10.1155/2020/8830471.
- Zhou Z, Chen H, Ju H, Sun M. 2017. Circulating retinol binding protein 4 levels in nonalcoholic fatty liver disease: a systematic review and meta-analysis [meta-analysis review systematic review]. Lipids Health Dis. 16(1):180. doi:10.1186/s12944-017-0566-7.