Abstract
Background: Constipation is a common disorder in children.
Objective: The objective of this study is to assess the beneficial effects of a daily supplementation with Orafti® inulin‐type fructans in 2–5 year old constipated children.
Methods: Double‐blind, randomised, placebo‐controlled parallel group trial where constipated children received two doses of 2 g Orafti® inulin‐type fructans (OF:IN) or placebo (maltodextrin) for 6 weeks. Primary outcome was stool consistency. Secondary outcomes were stool frequency and gastrointestinal symptoms.
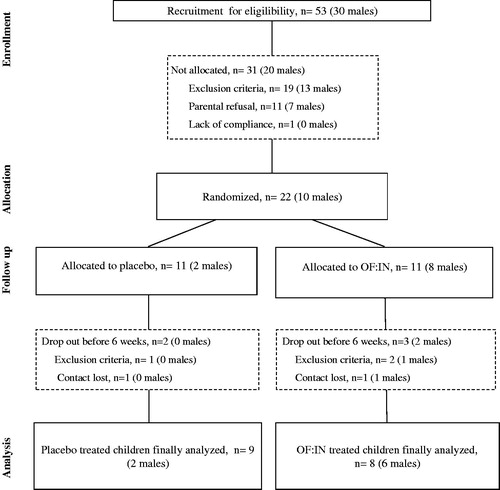
Results: Twenty-two children were included, 17 completed the study protocol (nine and eight for the control and the OF:IN group, respectively). Results showed that Orafti® inulin‐type fructans supplemented children had softer stools (p = .003). The longitudinal analysis showed no significant changes in controls, whereas supplemented children increased their stool consistency from 2.2 to 2.6 on the modified Bristol scale for children (five items instead of seven) (p = .040).
Conclusions: Prebiotic inulin‐type fructans supplementation improves stool consistency in constipated 2–5-year old children.
Clinicaltrials.gov, with number NCT02863848.
Introduction
Childhood constipation is considered as probably the most common gastrointestinal disorder in children (Tabbers Citation2011). The estimated prevalence ranges from 0.7 to 29.6%, with an average of 3% in the Western world (Mugie et al. Citation2011; Tabbers Citation2011). This ailment occurs in all paediatric age groups and its severity may vary from mild and short‐lived to severe and chronic, accompanied by faecal impaction (van den Berg et al. Citation2006) and requiring long term treatments (Pijpers et al. Citation2010). It causes distress to children and their families, worsening their quality of life (Bongers et al. Citation2009). The vast majority of constipated children (90–95%) suffer from functional constipation, which means that there is no obvious causal organic factor (Rubin & Dale Citation2006). Almost 40% of the children develop symptoms during the first year of life (Hyman et al. Citation2006), and more than 30% continue having problems beyond puberty (Bongers et al. Citation2010; Rubin & Dale Citation2006). Symptoms of functional constipation include infrequent depositions, possibly painful, and large and hard stools with or without faecal incontinence. Regarding therapeutic options, some evidence exists about prebiotic dietary fibre and their benefits on intestinal functions such as stools bulking, regularity and consistency of depositions (Sabater-Molina et al. Citation2009). However, current evidence on the use of dietary fibre supplements in the treatment of functional constipation specifically in 2–5-year old children is generally rather limited (Tabbers et al. Citation2014). The effectiveness of prebiotic fibres such as inulin-type fructans is due to their ability to resist digestion and to reach the large intestine, where they are completely fermented by the gut microbiota and are leading to a selective increase of beneficial bacteria (e.g. bifidobacteria and lactobacillus) (Roberfroid et al. Citation2010). The saccharolytic fermentation of prebiotics by the colonic microbiota is accompanied by an increase in the bacterial cell mass, a higher moisture content of digest (Cummings et al. Citation2001) and faeces (Gibson et al. Citation1995). A softer stool consistency and the increase in bowel content mechanically stimulate peristalsis (Roberfroid Citation1993) by what excretion is facilitated (Cherbut Citation2002). Moreover, fermentation leads to the generation of fermentation end products such as short chain fatty acids (SCFA) which can directly influence intestinal motility by stimulating peristaltic reflexes similar to that induced by mechanical stimulation (Grider & Piland Citation2007).
In contrast to the age group of 2–5-year old children, there is considerable evidence for beneficial effects in infants on bowel function parameters. A meta-analysis including four studies performed in infants concluded that prebiotic supplemented infant formulas increased significantly the stools frequency compared to a placebo formula (Mugambi et al. Citation2012). Moreover, some studies have shown significant softer stools among those infants fed with a prebiotic supplemented formula compared with those fed with control (Ziegler et al. Citation2007; Fanaro et al. Citation2009). In accordance, we recently published an RCT where a combination of long-chain inulin and oligofructose (Orafti®Synergy1) supplemented infant formula improved the deposition pattern in infants, inducing more frequent depositions and softer stools, compared to placebo, and closer to the deposition pattern of breastfed children (Closa-Monasterolo et al. Citation2013). These changes were accompanied by a bifidogenic effect of prebiotic supplemented formula, as it was shown by bacterial counts in the stool samples (Closa-Monasterolo et al. Citation2013).
We hypothesised that inulin-type fructans may be beneficial for improvement of bowel function in functionally constipated children considering a target population of 2–5-years old children when this gastrointestinal disorder is more relevant.
The present work reports the results from a pilot study aimed to develop a feasible and suitable study design in order to investigate possible benefits of supplementing the daily diet with inulin‐type fructans in 2–5 years old-constipated children.
Methods
Study design
The study is a double‐blind, randomised, placebo‐controlled parallel group trial, where 2–5 years old-constipated children received inulin‐type fructans or the same amount of placebo (maltodextrin) during 6 weeks. We recruited children from pre-school age, so under 6 years, that were potty trained (to avoid the use of diaper) so older than 2 years of age. The study lasted 8 weeks (± 2 days) for each subject consisting of a 2 weeks baseline period (run in period) during which gastrointestinal symptoms were assessed followed by a 6 weeks intervention (intervention period).
Participants
We recruited subjects at the University Hospital Sant Joan de Reus (Spain) and primary health care centres from the Hospital reference area. The monitoring was carried out in the hospital. We included those children whose mothers reported in the anamnesis that their children fulfilled the Rome III diagnostic criteria for functional constipation in children up to 4 years (Hyman et al. Citation2006). Briefly, the Diagnostic Criteria for Functional Constipation in the absence of organic pathology were to fulfil at least two of the following for at least 1 month:
(1) ≤ 2 defecations per week, (2) at least one episode of incontinence per week after the acquisition of toileting skills, (3) history of excessive stool retention, (4) history of painful or hard bowel movements, (5) presence of a large faecal mass in the rectum and (6) history of large-diameter stools that may obstruct the toilet. Accompanying symptoms may include irritability, decreased appetite and/or early satiety. The accompanying symptoms disappear immediately following passage of a large stool.
Exclusion criteria were having any organic cause of gastrointestinal disorders, drug consumption (e.g. antibiotics) or labelled pre‐ and probiotics products influencing gastrointestinal function during 4 weeks prior to the baseline period, or receiving laxative treatment 2 weeks prior to the baseline period. Before final inclusion, a gastroenterologist evaluated the initial faecal impaction and the inclusion/exclusion criteria. If children had faecal impaction, a large lump of dry, hard stool that stays stuck in the rectum, a cleansing enema was administered. In this case, the start of the intervention was delayed for 2 weeks. A blood analysis was performed to exclude some organic cause of constipation.
Study intervention
The test product was a mixture of inulin-type fructans derived from chicory (70:30 combination of Orafti®P95 oligofructose and Orafti®GR inulin (OF: IN) produced and provided by BENEO GmbH, Germany). The daily dosage of inulin‐type fructans or placebo (digestible maltodextrin) was 4 g/d consumed as two doses of 2 g mixed into a dairy product (yogurt or fresh cheese without labelled pre- or probiotics). The vehicle could be chosen by the parents and had to be maintained throughout the study. The company provided both study products as coded sachets (labelled as product A or B). These were re-labelled with successive randomisation numbers according to a random list, created using the EPIDAT 3.1 programme (available at: http://www.paho.org/spanish/sha/epidat.htm) by a member of the study team who was not involved in the study visits or recruitment. This procedure guaranteed the double blind design throughout the study period. All children received some general recommendations to increase the intake of dietary fibre and fluids at the first visit.
Study outcomes
A diary was used throughout the study to record the stool frequency, stool consistency (according to the Modified Bristol Stool Form Scale for children, Lane et al. Citation2011), gastrointestinal symptoms (i.e. abdominal pain) and medication. Pain during defaecation was evaluated using the Wong–Baker FACES Pain Rating Scale (Hockenberry Citation2009). Stool frequency (n stools/week) was calculated using data from the daily diary for each study period (baseline and intervention). Stool consistency as well as pain during defaecation was calculated in order to obtain the mean consistency and the pain during defaecation of the total stools reported in the diary.
Dietary intake was assessed via 3‐day food diaries (Cheng et al. Citation2013), once during the baseline period and once at the end of the intervention. A trained nutritionist calculated the consumed amounts of all foods following a Standard Operation Procedure (SOP). The SOP included instructions for interpretation of cooking methods, food portions and standard recipes written in the food diaries as well as for the data processing. Consumed foods were transformed into nutrients using dedicated software (Programa de Calculo Nutricional CESNID 1.0. Edicions Universitat de Barcelona, McGraw-Hill-interamericana de España, S.A.U. 2003). This programme included the food composition databases from the CESNID (Centre d’Ensenyament Superior de Nutrició i Dietètica). The mean nutrients intake of the 3 d was calculated. Nutrient intake outcomes were energy (kcal/day kg), protein, lipids and carbohydrates (in g/day kg and % of kcal/day), total fibre intake (mg/day kg) and water consumption (ml/day kg).
Ethics
The study has been conducted following the Helsinki declaration as revised in 1983 and guidelines for ethical conduct of medical research involving children. The study protocol was approved by the Ethical Committee of the University Hospital Sant Joan de Reus, Spain. All families received written information and signed informed consent to participate in the study. The pilot trial has been registered at Clinicaltrials.gov as NCT02863848.
Statistics
The statistical analysis was performed using IBM SPSS Statistics for Windows, Version 20.0 (IBM Corp., Armonk, NY). Kolmogorov–Smirnov test was performed in continuous variables to test the normal distribution. Skewed data, if any, were evaluated by non-parametric statistical test. Otherwise, parametric statistical tests were applied. Data were presented as mean ± SD or median (interquartile ranges (IQR)) for normal and skewed variables, respectively. Categorical variables were presented as absolute (n) and relative frequencies (%). The distribution of categorical variables between groups was analysed by Chi-square test.
We performed a cross-sectional analysis comparing both study groups at individual time periods (baseline and intervention separately) as well as a longitudinal analysis comparing changes between groups along the study periods (in order to integrate in the analyses the baseline status of every child). Cross-sectional differences in quantitative variables between groups in each study period were analysed by Student’s T test or Mann–Whitney U test as appropriate. The longitudinal analysis was performed applying a two-way ANOVA (general linear model) for repeated measures. In this model, time point (baseline versus intervention) and group were analysed together as the two factors influencing the outcomes of interest. The p for interaction between the two factors showed the probability of a different longitudinal evolution between groups. A multivariate analysis was performed to evaluate the combined effect of significant variables on stool consistency after 6 weeks of treatment by linear regression models. Statistical significance was accepted at the level of 0.05. Sample size calculations for a bigger study were performed using the GRANMO 7.12 software (IMIM, Barcelona, Spain).
Results
Study sample and baseline characteristics
From June 2012 to June 2013, 53 constipated children from 2 to 5 years‐old were assessed for eligibility. From those, 22 were allocated for randomisation and 31 were not allocated because of exclusion criteria (19 children), parental refusal (11 children) or lack of compliance (one children) (). During the study period, one of the randomised children was excluded because of cow’s milk allergy diagnose and two other children were excluded because of other exclusion criteria interfering with the study outcome (e.g. laxative treatment, antibiotic use during intervention). Two children did not fulfil the whole study protocol and, therefore, 17 children were included in the longitudinal analyses (). Both study groups showed similar values for the baseline characteristics as age, body weight, stools frequency and consistency, pain during defaecation, family history of constipation, initial faecal impaction as well as accomplishing the Rome III inclusion criteria. We found differences in gender distribution, showing higher proportion of males among the OF:IN group (p = .030). Moreover, in the placebo group, we found a non-significant trend towards a higher stool frequency prior to the intervention (p = .068) ().
Table 1. Baseline characteristics by study groups.
Effect of intervention on gastrointestinal symptoms and stool characteristics
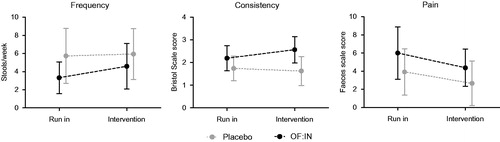
Stools during the intervention period were softer among children receiving OF: IN (1.63 ± 0.64 versus 2.57 ± 0.58 for placebo and OF:IN, respectively, p = .003) whereas there were no statistical differences between OF:IN and placebo groups for any of the other gastrointestinal outcomes. The results from the longitudinal analyses showed that stools frequency increased during the intervention period by 1.3 ± 0.6 stools/week among children having received OF:IN and 0.21 ± 1.9 stools/week among children in the placebo group (). Despite this pronounced difference of about one additional bowel movement per week, the difference in changes over time did not reach the level of statistical significance in this pilot study, neither between supplemented and non-supplemented group (interaction p value = .328) nor between time periods (run in and intervention) (p = .179). Results for stool consistency showed a progressive softening along the study period of 0.4 ± 0.65 units on the Bristol Stool Scale in the OF:IN group, whereas in the placebo, the consistency showed a hardening along the study (−0.11 ± 0.33 units on the Bristol Stool Scale). Differences in stool consistency between groups after intervention were borderline significant (p value = .053). Post hoc comparisons between time periods and group showed that whereas no changes were produced by the intervention within the placebo group (1.74 versus 1.63, p = .487), the OF:IN group increased the mean of the stools consistency score (what means softening) from 2.2 to 2.6 (p = .040). Comparison of the longitudinal patterns for each group was also borderline significant in a two-way ANOVA analysis (p = .051). Pain during defaecation clearly decreased during the intervention period (p = .014) in the two groups, and this effect was independent of the treatment (interaction p value = .736) (). We did not found any increase in distension or flatulence in the OF:IN group during the intervention (data not shown).
Dietary intake analyses
Similar intakes of energy, proteins, lipids, carbohydrates and fibre were observed in both study groups during the baseline and the intervention period (Supplemental Table 1). No significant differences in cross-sectional or longitudinal analysis were found between the groups.
Multivariate analyses: effect on stool consistency
In order to integrate all the results and variables that could modulate the stool consistency during intervention period, we performed linear regression analyses with different independent variables. The simple linear regression models showed that none of the inclusion criteria, nor initial impactation, nor family history of constipation, nor glycerine use were modulating stool consistency during the intervention (Supplementary Table 2). Conversely, gender (p = .018), baseline stool consistency and frequency (p = .002 and p = .036, respectively) or baseline pain during deposition (p = .011) as well as OF:IN supplementation (p = .003) were modulating the stool consistency during the intervention (Supplemental Table 2). None of dietary intake assessed variables, neither at the run in period nor after the intervention, were able to modulate the stools consistency after the intervention in the simple linear regression models (Supplemental Table 3). Variables with a significant effect on stools consistency after the intervention in simple regression models were introduced in a multiple linear regression model. The multiple linear regression model showed that the OF:IN supplementation and the baseline stools consistency (during the run in period) were able to significantly predict up to 60.8% of intervention stool consistency after the intervention ().
Table 2. Combined effect of different variables modulating intervention stool consistency.
Sample size calculation
As a pilot study, one of the objectives was obtaining results to perform a sample size calculation for a further study. Considering the stool consistency as the primary output, we calculated the sample size needed to detect a desired significant effect. Stool consistency during the baseline period from the 20 children from those we have data were 2.1 ± 0.6. The correlation coefficient, using all the available data (excluding the child with cow’s milk intolerance), for stool consistency between baseline and end of intervention was 0.705 (p = .001). Using these data, we calculated the necessary sample size to detect changes in consistency during the intervention of around 0.5 SD (approximately 0.3 units from the Bristol scale as obtained with the pilot study). The most used method in Randomised Clinical Trials to detect differences between groups, because it takes into account changes in the placebo, is the approach that analyse differences between effects obtained by the intervention in the OF:IN and the placebo group. The sample size pairs obtained for a further study, considering this approach with a power of 80% and a 95% confidence interval and a correlation coefficient of 0.7, was 38 patients per group.
Discussion
Low-fibre intake has been associated with constipation in childhood (Kranz et al. Citation2012). The results of our study reinforce the possible beneficial effects of the use of inulin-type fructans as fully fermentable dietary fibre from chicory roots for counteracting constipation in young children and supporting normal bowel habits. This has important consequences in daily life because functional constipation causes a poor quality of life (Bongers et al. Citation2009). Current therapeutic approaches include education and dietary advice. Although dietary fibre is associated with lower constipation prevalence (Kranz et al. Citation2012), there is limited evidence from controlled intervention studies that additional fibre improves constipation compared with a placebo in children (Tabbers et al. Citation2014). Nevertheless, previous studies demonstrated certain dietary fibre-induced benefits on functional constipation in children. Castillejo et al. (Citation2006) showed a reduction of the colonic transit time in children receiving a cocoa husk supplement during 4 weeks. Similarly, Loening–Baucke showed an improvement in constipated children after 4 weeks of glucomannan supplementation (Loening-Baucke et al. Citation2004). However, since in 2010, Pijpers et al. (Citation2010) recommended in their systematic review to design new RCTs in order to investigate the potential of dietary treatments for constipation, no specific trials assessing the effect of dietary fibre supplementation, such as inulin-type fructans, in children have been published.
Alternative laxative treatments have been shown to be effective as a maintenance treatment, but those treatments require long-term use and potential unexpected side effects cannot be excluded. Therefore, achieving any improvement in symptoms associated with functional constipation (i.e. especially in younger children) by the use of a prebiotic fibre could be of great interest, especially considering the need of a long-term supplementation required. In this context, inducing softer stools, as observed in the OF:IN group in this study, could improve the success of constipation treatment and could reduce the use of laxatives. Our results in young children are in line with previous studies having reported softer stools after a supplementation with prebiotics in adults (Sabater-Molina et al. Citation2009) and in infants (Ziegler et al. Citation2007; Fanaro et al. Citation2009).
In our clinical trial, the supplementation with prebiotic had no relation on the subjects’ reasons for dropping out the study and the number of excluded infants were similar between both groups. Five infants were excluded during the study period, three of them in the supplementation group and two in the placebo group.
Despite the pilot study nature of our work (with a small sample size), OF:IN and placebo groups were similar regarding major baseline characteristics, except for significant differences in gender distribution and in the percentage of children who had previously suffered from excessive retention. However, history of faecal withholding behaviour did not significantly influence primary outcomes of this study as reported by regression analyses. Conversely, cross-sectional and longitudinal analyses showed that two daily doses of 2 g OF:IN were able to improve stool consistency as compared with children having received the placebo. Similarly, we detected a trend to increase the frequency of stools by supplementation with OF:IN. A sample size of 38 patients per group would allow detecting significant differences in stool consistency change throughout intervention of about 0.3 units on the Bristol scale; as well as detecting differences in the frequency of stools between groups of about 1 deposition/week (considering a power of 80% and a 95% confidence interval). Other parameters assessed remained similar and were not influenced by the intervention. Our results are in line with another recent study that showed improvements in consistency without changes in frequency in constipated infants consuming an infant formula with prebiotics and palmitic acid (Bongers et al. Citation2007). In adults suffering from functional constipation, Fateh et al. (Citation2011) showed an improvement in frequency and consistency after supplementation with a mixture of probiotics and prebiotics. However, as far as we know, this is the first RCT in children of this age group that evaluated the effect of a single prebiotic source, namely inulin-type fructans, on functional constipation. It is worth commenting that we observed an improvement of the pain reported during defaecation independently of the study group. This finding may be an evidence of the highly suggestive nature of constipation in those young children. Another cause to explain the reduction of reported pain in the OF:IN as well as the placebo group may be the dietary advice provided to all children. In fact, in some cases, constipation is improved by a higher consumption of fibre and fluids (Bardisa-Ezcurra et al. Citation2010), and dietary advice is intended to promote these habits. However, it does not seem to be the case in our study as we found an unexpected reduction of the fibre intake during the intervention, compared with the intake at baseline, in both study groups of children. We did not find a clear explanation for this behaviour change but other authors have reported the difficulty to improve fibre intake in children despite the dietary advice (McClung et al. Citation1995). We assessed also the child’s dietary intake to exclude any impact of this variable on the observed results. None of the intake variables was able to modulate stool consistency during the intervention, as observed by linear regression models. The sum of the study product and the baseline stool consistency were able to predict up to the 60.8% of stool consistency during intervention whereas baseline pain and frequency were not statistically modulating stool consistency during intervention (). Even though multivariate analyses would be more conclusive with a bigger sample, these results reinforce the possible benefits of the use of OF:IN in constipated children.
Supplementing the daily diet with a rather low dose of inulin-type fructans, a fully fermentable fibre source, seems to be promising for treating functional constipation in young children. Hence, functional constipation seems to be a relevant target for larger and confirmative studies focussing on digestive health benefits of inulin-type fructans in children of this particular age group.
Supplemental tables 1-3
Download PDF (139.6 KB)Acknowledgements
The authors gratefully acknowledge participants families for their support to the study.
Disclosure statement
Stephan Theis is the Head of Nutrition Science of the Suedzucker/BENEO Group. He participated in the study design and critical reading of the manuscript. There was no further involvement in the conduct, statistical evaluation and reporting of the study. The other authors, independently from the company, have no conflicts of interest to declare.
Additional information
Funding
References
- Bardisa-Ezcurra L, Ullman R, Gordon J. 2010. Diagnosis and management of idiopathic childhood constipation: summary of NICE guidance. BMJ. 340:c2585.
- van den Berg MM, Benninga MA, Di Lorenzo C. 2006. Epidemiology of childhood constipation: a systematic review. Am J Gastroenterol. 101:2401–2409.
- Bongers MEJ, Benninga MA, Maurice-Stam H, Grootenhuis MA. 2009. Health-related quality of life in young adults with symptoms of constipation continuing from childhood into adulthood. Health Qual Life Outcomes. 7:20.
- Bongers MEJ, van Wijk MP, Reitsma JB, Benninga MA. 2010. Long-term prognosis for childhood constipation: clinical outcomes in adulthood. Pediatrics. 126:e156–e162.
- Bongers MEJ, de Lorijn F, Reitsma JB, Groeneweg M, Taminiau JAJM, Benninga MA. 2007. The clinical effect of a new infant formula in term infants with constipation: a double-blind, randomized cross-over trial. Nutr J. 6:8.
- Castillejo G, Bulló M, Anguera A, Escribano J, Salas-Salvadó J. 2006. A controlled, randomized, double-blind trial to evaluate the effect of a supplement of cocoa husk that is rich in dietary fiber on colonic transit in constipated pediatric patients. Pediatrics. 118:e641–e648.
- Cheng G, Hilbig A, Drossard C, Alexy U, Kersting M. 2013. Relative validity of a 3 d estimated food record in German toddlers. Public Health Nutr. 16:645–652.
- Cherbut C. 2002. Inulin and oligofructose in the dietary fibre concept. Br J Nutr. 87:S159–S162.
- Closa-Monasterolo R, Gispert-Llaurado M, Luque V, Ferre N, Rubio-Torrents C, Zaragoza-Jordana M, Escribano J. 2013. Safety and efficacy of inulin and oligofructose supplementation in infant formula: results from a randomized clinical trial. Clin Nutr. 32:918–927.
- Cummings JH, Macfarlane GT, Englyst HN. 2001. Prebiotic digestion and fermentation. Am J Clin Nutr. 73:415S–420S.
- Fanaro S, Marten B, Bagna R, Vigi V, Fabris C, Peña-Quintana L, Argüelles F, Scholz-Ahrens KE, Sawatzki G, Zelenka R, et al. 2009. Galacto-oligosaccharides are bifidogenic and safe at weaning: a double-blind randomized multicenter study. J Pediatr Gastroenterol Nutr. 48:82–88.
- Fateh R, Iravani S, Frootan M, Rasouli MR, Saadat S. 2011. Synbiotic preparation in men suffering from functional constipation: a randomised controlled trial. Swiss Med Wkly. 141:w1323.
- Gibson GR, Beatty ER, Wang X, Cummings JH. 1995. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 108:975–982.
- Grider JR, Piland BE. 2007. The peristaltic reflex induced by short-chain fatty acids is mediated by sequential release of 5-HT and neuronal CGRP but not BDNF. Am J Physiol Gastrointest Liver Physiol. 292:G429–G437.
- Hockenberry MJWD. 2009. Wong’s essentials of pediatric nursing. 8th ed. St. Loius: Mostby.
- Hyman PE, Milla PJ, Benninga MA, Davidson GP, Fleisher DF, Taminiau J. 2006. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology. 130:1519–1526.
- Kranz S, Brauchla M, Slavin JL, Miller KB. 2012. What do we know about dietary fiber intake in children and health? The effects of fiber intake on constipation, obesity, and diabetes in children. Adv Nutr. 3:47–53.
- Lane MM, Czyzewski DI, Chumpitazi BP, Shulman RJ. 2011. Reliability and validity of a modified Bristol Stool Form Scale for children. J Pediatr. 159:437–441.
- Loening-Baucke V, Miele E, Staiano A. 2004. Fiber (glucomannan) is beneficial in the treatment of childhood constipation. Pediatrics. 113:e259–e264.
- McClung HJ, Boyne L, Heitlinger L. 1995. Constipation and dietary fiber intake in children. Pediatrics. 96:999–1000.
- Mugambi MN, Musekiwa A, Lombard M, Young T, Blaauw R. 2012. Synbiotics, probiotics or prebiotics in infant formula for full term infants: a systematic review. Nutr J. 11:81.
- Mugie SM, Benninga MA, Di Lorenzo C. 2011. Epidemiology of constipation in children and adults: a systematic review. Best Pract Res Clin Gastroenterol. 25:3–18.
- Pijpers MAM, Bongers MEJ, Benninga MA, Berger MY. 2010. Functional constipation in children: a systematic review on prognosis and predictive factors. J Pediatr Gastroenterol Nutr. 50:256–268.
- Roberfroid M. 1993. Dietary fiber, inulin, and oligofructose: a review comparing their physiological effects. Crit Rev Food Sci Nutr. 33:103–148.
- Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, et al. 2010. Prebiotic effects: metabolic and health benefits. Br J Nutr. 104:S1–S63.
- Rubin G, Dale A. 2006. Chronic constipation in children. BMJ. 333:1051–1055.
- Sabater-Molina M, Larqué E, Torrella F, Zamora S. 2009. Dietary fructooligosaccharides and potential benefits on health. J Physiol Biochem. 65:315–328.
- Tabbers MM. 2011. Clinical practice: diagnosis and treatment of functional constipation. Eur J Pediatr. 170:955–963.
- Tabbers MM, DiLorenzo C, Berger MY, Faure C, Langendam MW, Nurko S, Staiano A, Vandenplas Y, Benninga MA; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; North American Society for Pediatric Gastroenterology. 2014. Evaluation and treatment of functional constipation in infants and children: evidence-based recommendations from ESPGHAN and NASPGHAN. J Pediatr Gastroenterol Nutr. 58:258–274T.
- Ziegler E, Vanderhoof JA, Petschow B, Mitmesser SH, Stolz SI, Harris CL, Berseth CL. 2007. Term infants fed formula supplemented with selected blends of prebiotics grow normally and have soft stools similar to those reported for breast-fed infants. J Pediatr Gastroenterol Nutr. 44:359–364.