Abstract
Knowing the true levels of nutrients and dietary bioactives in fruit juices at the point of consumption is key to properly understand their potential health benefits. The objective was to characterise the vitamin C and flavanone content in commercial orange juices consumed in Europe, compared with fresh-squeezed juices. Commercial juices were a rich source of vitamin C (>30% of the Nutrient Reference Value). Vitamin C in fresh-squeezed juices, at the end of their shelf-life, remained 33% higher than the levels found in the commercial juices. Flavanones had similar values from both commercial and fresh juices, except for fresh samples stored for 48 h, where fresh juices had higher values (22.36 mg/100 mL). Thus, orange juices preserve their bioactive compounds during storage, with very little influence of the brand, country, industrial process or storage conditions. Main bioactive compounds in commercial juices are present at nutritionally significant levels to the freshly-squeezed ones.
Introduction
Citrus is one of the main cultivated fruits worldwide. Orange production has increased in recent years to almost 80 million tons produced worldwide per annum in 2021, with the 10 main orange producers being Brazil (16,214,982 tons), India (10,270,000 tons), China (7,550,000 tons), Mexico (4,595,128 tons), USA (4,015,200 tons), Spain (3,604,800 tons), Egypt (3,000,000 tons), Indonesia (2,513,860 tons), Iran (2,139,912 tons), and Italy (1,770,910 tons) (FAOSTAT Citation2022).
Citrus fruits are a source of bioactive compounds, such as flavanones, phenolic acids, carotenoids, vitamin C, minerals and fibre (Gironés-Vilaplana et al. Citation2014). Vitamin C is essential for human health since it cannot be synthesised by the body (due to a lack of the enzyme gulonolactone oxidase) and is involved in many biochemical functions including neutralisation of free radicals, iron absorption, synthesis of collagen, cholesterol metabolism, and bone formation (Dasgupta and Klein Citation2014; Kumar et al. Citation2022). Vitamin C also plays a fundamental role in some inflammatory diseases related to oxidative stress such as obesity, cancer, and type II diabetes (Grosso et al. Citation2013; Gironés-Vilaplana et al. Citation2014). The recommended daily intake of vitamin C varies depending on the country. In the EU, the Average Requirement has been set at 90 mg for men and 80 mg for women, while the Average Requirement for children ranges from 15–85 mg per day according to age (EFSA Citation2013). For labelling purposes, in the EU and UK, a Nutrient Reference Value of 80 mg has been set in law (R1169/Citation2011).
Other phytochemicals have been identified in citrus fruits, such as flavanones, which may have beneficial effects on human health including anti-inflammatory, anti-allergic, cardioprotective, anti-hyperglycaemic, neuroprotective, hepatoprotective and antioxidant effects (Fraga et al. Citation2023; Singh et al. Citation2023). In addition, the consumption of foods rich in flavonoids has been associated with a lower incidence of chronic diseases (type II diabetes, cardiovascular disease, and dyslipidaemias) (Kang et al. Citation2020; Fraga et al. Citation2023). Several studies have demonstrated the bioavailability of the flavanones present in orange juice (hesperidin and narirutin, mainly), which are absorbed as aglycones in the small intestine or metabolised by the gut microbiota at colonic level before absorption, to be subsequently metabolised by phase II enzymes (Agulló et al. Citation2020; Agulló et al. Citation2021; Pereira-Caro et al. Citation2023).
Fruit intake is associated with superior diet quality and better overall health (Dicklin et al. Citation2022) and food-based dietary guidelines from around the world recommend a minimum fruit and vegetable consumption, in many cases five daily servings (Cámara et al. Citation2021). Fruit juice is defined in European law as the fermentable but unfermented product obtained from the edible part of fruit and is made by juicing whole fruits (D2012/12/EU). 100% fruit juice can complement whole fruit intake by providing vitamins, minerals and phytochemicals, and has been positively associated with diet quality and several preventive features in human health (Bellisle et al. Citation2018; D’Elia et al. Citation2021; Liu et al. Citation2023; Rossi et al. Citation2023).
Worldwide, orange juice represents more than 40% of juices consumed (Neves et al. Citation2020). Orange juice may be freshly prepared at home or purchased as a commercially juiced product. For juices made at home, storage may lead to a deterioration in the nutritional composition, while the pasteurisation processes used by manufacturers to reduce microbial spoilage hence improving food safety and extending shelf-life can negatively affect the nutritional, organoleptic and functional properties of juices (Galaverna and Dall’Asta Citation2014; Chanson-Rolle et al. Citation2016; Salar et al. Citation2022). Therefore, the aim of the present study was to characterise the composition of the main commercial orange juices in Europe (France, United Kingdom, Germany, and Spain), in terms of vitamin C and flavanone content, and to compare it with fresh “Navelina” orange juice and up to 48 h of store refrigeration to evaluate how commercial juices compare to fresh samples.
Materials and methods
Beverages and plant materials
Market data were studied in order to select the commercial orange juices with the largest market share in each of the four countries of interest: France, UK, Germany, and Spain. Both chilled “not from concentrate” orange juice (Ch-NFC-OJ) and ambient concentrated orange juice (A-C-OJ) were included. Five different samples per country (6 from UK) were purchased from supermarkets in these countries () and sent via courier (3 bottles per sample) – chilled or ambient depending on the original product storage - to LabFAS, CEBAS-CSIC in Murcia, Spain. As a comparator, freshly squeezed “Navelina” oranges were obtained from 3 Spanish companies (AMC group, Murcia; Riverbend, Murcia; and ZUVAMESA (ZVM), Valencia) for juicing under laboratory conditions with an electric citrus juicer (Orbegozo EP 2210, Murcia, Spain). Fresh oranges (20 kg) from each company were used to prepare freshly squeezed juices (FSJ) and, in order to determine their shelf-life, samples were taken for analysis of vitamin C and flavanones at 0, 12, 24, and 48 h post-juicing (three batches of samples were prepared for each shelf-life time; FSJ1, FSJ3 and FSJ4). This was selected to represent the typical timeframe that a fresh home squeezed orange juice would be kept at home in the fridge. In addition, a further 20 kg of AMC oranges were obtained 1 week after the initial sampling, in order to compare different batches from the same company (FSJ1 for the initial sampling and FSJ2 for 1 week after).
Table 1. Commercial orange juices purchased from four European countries, each sample from a different company.
Chemicals and reagents
The following substances, chemicals and reagents were obtained for the nutritional analysis: hesperidin (Merck, Darmstadt, Germany); formic acid, methanol, acetonitrile and ethylenediaminetetraacetic acid disodium salt 2-hydrate (EDTA) (Panreac, Barcelona, Spain); and L-ascorbic (AA) and dehydroascorbic (DHAA) acids (Sigma-Aldrich, St. Louis, MO, USA). All solutions were prepared with ultrapure water from a Milli-Q Advantage A10 ultrapure water purification system (Millipore, USA).
Analysis of vitamin C: ascorbic acid (AA) and dehydroascorbic acid (DHAA)
The analysis of vitamin C was carried out according to Baenas et al. (Citation2019). Briefly, samples were extracted with EDTA 0.05% (v/v or w/v) in order to avoid degradation of the target compounds. The juices (3 mL per aliquot) were mixed with 10 mL of EDTA 0.05% for 2 min. Later, samples were centrifuged for 10 min at 3000 rpm at room temperature (21 ± 2 °C). The supernatants were filtered through a Sep-Pack classic cartridge C18 (Waters, Milford, MA, USA) and through 0.22 μm PVDF Millipore filter (Merck Millipore, Carrigtwohill, Ireland) before analysis. Filtered samples were diluted with EDTA (1:100) before UHPLC-QqQ-MS/MS analysis. Determination of vitamin C (AA and DHAA) was performed using a UHPLC coupled with a 6460 triple quadrupole-MS/MS (Agilent Technologies, Waldbronn, Germany), using a Pursuit XRs Diphenyl Pursuit USP L11 A6021100X030 column (3.0 × 100 mm, 3.0 μm particle size) supplied by Agilent Technologies (Amstelveen, The Netherland). The column temperature was set up at 30 °C. The mobile phases consisted of 0.1% formic acid in deionised Milli-Q-water (LC-MS grade) (Solvent A) and acetonitrile (Solvent B). The flow rate and injection volume were 0.4 mL/min and 20 μL, respectively. Both AA and DHAA were analysed upon the optimised gradient for 5.5 min (time: %B) (0: 0.05%), (2: 0.05%), (2.5: 90%), (3: 90%), (4: 0.05%), and (5.5: 0.05%, for column stabilisation).
Identification and quantification of the bioactives were performed by mass spectrometry operated in the multiple reaction monitoring (MRM) and negative mode, recording information from preferential MRM transitions (quantification and confirmation) for the corresponding analytes. Data acquisition and processing were performed by MassHunter software version B.04.00 (Agilent Technologies, Walbronn, Germany). The concentration of vitamin C (AA + DHAA) was calculated by comparison with freshly prepared AA and DHAA standard curves. The results were expressed as mg per 100 mL of juice.
Analysis of flavanones
For the identification and quantification of flavanones, samples were analysed following the method described by Salar et al. (Citation2021) by HPLC-DAD, with a Luna 5 μm C18(2)100 Å column (250 × 4.6 mm), using Security Guard Cartridges PFD C18 4 × 3.0 mm, both supplied by Phenomenex (Torrance, CA, USA). Chromatographic separation was performed using 5% formic acid (solvent A) and methanol (solvent B), upon a linear gradient (time, %B) (0, 15%); (20, 30%); (30, 40%); (35, 60%); (40, 90%); (44, 90%), and back to initial conditions, allowing 10 min for column stabilisation. The quantification was carried out using an Agilent Technologies 1220 Infinity Liquid Chromatograph, equipped with an auto-injector (G1313, Agilent Technologies, Santa Clara, CA, USA) and a Diode Array Detector (1260, Agilent Technologies). The flow rate was 0.9 mL/min. Chromatograms were recorded at 280 nm and processed on an Agilent ChemStation for LC 3D systems. Flavanones were identified by comparison with authentic standards of analytical grade. Flavanones were quantified as hesperidin at 280 nm. The concentration of phenolic compounds was expressed as mg per 100 mL of juice.
Statistical analysis
The statistical analysis was carried out using the XLSTAT software (2016.02.27444 version, Addinsoft, Paris, France). One-way analysis of variance (ANOVA) and Tukey’s multiple range test were used to compare experimental data. The level of significance was set at p < 0.05. All analyses were carried out in triplicate.
Results and discussion
Vitamin C
Significant differences in vitamin C (AA + DHAA) content among commercial orange juices evaluated within the same country were found, while the average content of vitamin C in commercial orange juices between the countries was not significantly different (). The variability was lower than 5% in vitamin C analysis. FJ1 sample of Ch-NFC-OJ (France) had the lowest vitamin C concentration (19.54 ± 0.46 mg/100 mL) while the highest vitamin C concentrations were seen in GJ5 sample of A-C-OJ (70.95 ± 0.85 mg/100 mL; Germany) and SJ3 sample of Ch-NFC-OJ (94.53 ± 1.65 mg/100 mL; Spain). Thermal treatments carried out in industries have the advantage of eliminating microbial contamination and reaching high levels of enzyme inactivation (Petruzzi et al. Citation2017), but they are also mainly responsible for loss of vitamin C during processing (Martí et al. Citation2009; Aschoff et al. Citation2015). These heat treatments are not carried out in the same way in all industries, which may be one of the factors that influence the final amount of vitamin C in commercial orange juices.
Table 2. Concentration of ascorbic acid, dehydroascorbic acid, and vitamin C (mg/100 mL) in commercial orange juice samples from four different countries.
Regarding the content of vitamin C during the shelf-life study (), freshly squeezed juices retained 85–92% of their initial vitamin C content after 48 h of storage at 4 °C. Furthermore, there was wide variation within the different samples at initial content (t0). FSJ3 had the highest values (91.80 ± 1.45 mg/100 mL), followed by FSJ2 (88.28 ± 1.00 mg/100 mL) and, with the lowest content for FSJ1 and FSJ4 (74.63 ± 1.07 and 74.62 ± 0.92 mg/100 mL, respectively). Differences were also found between samples from the same company FSJ1 and FSJ2 so, therefore, there were not only differences in terms of processing among companies, but also in the concentrations associated with the different batches of oranges that arrive within the same company. These differences in vitamin C between juices made from the same company could be due to the state of ripeness of the oranges at the time of processing as well as to differences in the growing area or the agricultural practices followed. Some authors have shown that a higher ripening stage reduces the vitamin C content in citrus (Sites and Reitz Citation1950). In addition, these differences could derive from the amount of albedo that passes to the juice, since this fruit part has 19% of the total vitamin C content in citrus (Martí et al. Citation2009). This finding implies that the type of apparatus used to squeeze oranges in the home could influence the vitamin C content.
Table 3. Concentration of ascorbic acid, dehydroascorbic acid, and vitamin C (mg/100 mL) in freshly squeezed orange juice samples at different timepoints (t0, t12, t24 and t48 h).
The content of vitamin C in the fresh juices, after the storage period, remained significantly higher than that found in the commercial juices studied (73.73 ± 7.74 versus 50.13 ± 14.30 mg/100 mL, respectively). Commercial samples had the lowest values in AA (47.33 ± 14.10 mg/100 mL) and total vitamin C (50.13 ± 14.30 mg/100 mL) but did not present differences in DHAA (, ). Commercial samples likely had lost 30–40% of vitamin C compared with fresh juices. This is not surprising as vitamin C is highly thermolabile, so heat treatments cause a loss of its content, bioactivity and healthy characteristics (Patras et al. Citation2010; Brenes et al. Citation2022). Therefore, differences in vitamin C content between commercial and fresh juices are likely to be due to the pasteurisation processes used in the manufacture of commercial juices. Based on the data obtained in the shelf-life experiment, a vitamin C degradation equation was developed () to predict the equivalent time in which freshly squeezed samples would reach the average vitamin C concentration seen in commercial samples. This time was estimated to be at 176 h (7.3 days) of shelf-life.
Figure 1. Box plots for the commercial and freshly squeezed orange juice samples (0, 12, 24 and 48 h). The red cross is the mean value, the Middle line is the median.
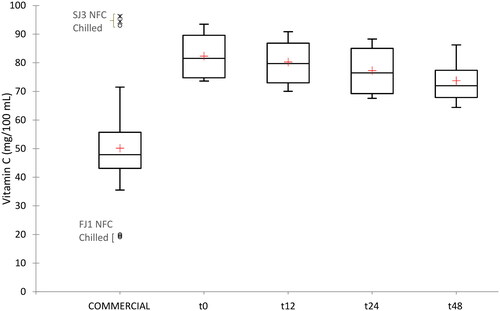
Figure 2. Prediction time of the vitamin C degradation in freshly squeezed orange juice samples. The mean value of commercial samples is highlighted in orange colour.
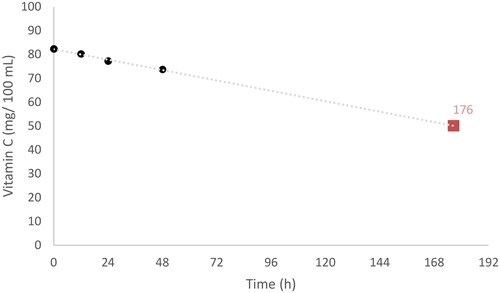
Table 4. Concentration of ascorbic acid, dehydroascorbic acid and total vitamin C (mg/100 mL) in freshly squeezed orange juice samples at different timepoints (t0, t12, t24 and t48 h, mean of all companies) and commercial juices.
In general, apart from FJ1 sample of Ch-NFC-OJ (France), 200 mL of both commercial and fresh juices contained sufficient vitamin C to reach the EU adequate intake of 80 or 90 mg/day for women and men respectively (EFSA Citation2013) and hence would be considered to contain nutritionally significant levels of vitamin C. In addition, since regular juice consumption is associated with a higher intake of fruits and vegetables, this dietary pattern is likely to ensure that juice consumers reach the daily recommendation for vitamin C (Bellisle et al. Citation2018; Murphy et al. Citation2020; Dicklin et al. Citation2022).
In addition and for marketing and labelling purpose, all orange juices analysed (with the exception of sample FJ1 NFC Chilled) can be labelled as “high content in Vitamin C”, as they showed a minimum content of 15% nutrient reference value (R1924/Citation2006 and R1169/Citation2011).
Flavanones
Relative to the flavanones in orange juices (), the most abundant were narirutin (naringenin 7-O-rutinoside) and hesperidin (hesperetin 7-O-rutinoside). In commercial samples, in general, juices from Germany and UK had the highest total flavanone content (18.58 ± 3.28 and 17.96 ± 2.30 mg/100 mL, respectively), compared with France and Spain (15.51 ± 1.60 and 15.32 ± 3.42 mg/100 mL, respectively). The variability was lower than 2% from flavanones analysis. These differences may be due to the ripening stage of the oranges used to make the juice, since the flavonoid content depends on the ripening stage and the variety of the fruit (Vandercook and Tisserat Citation1989).
Table 5. Content of total and individual flavanones (mg/100 mL) of commercial orange juice samples from four different countries.
Regarding the shelf-life of fresh juices, after 48h, results indicated that total flavanones slightly increased from time 0, something that was also reported by Salar et al. (Citation2021) which could be due to cell lysis. Nevertheless, differences between t0 (19.01 ± 3.46 mg/100 mL) and t48 (22.36 ± 5.77 mg/100 mL), are unlikely to have any biological significance ().
Table 6. Content of total and individual flavanones (mg/100 mL) of freshly squeezed orange juice samples at different timepoints (t0, t12, t24 and t48 h).
Comparing shelf-life samples of fresh juices with commercial juices (), O-triglycosyl naringenin presented its highest values in the commercial samples (1.48 ± 0.35 mg/100 mL). On the other hand, the commercial samples had values similar to fresh juices at time 0 for hesperetin 7-O-rutinoside (8.89 ± 1.07 and 11.11 ± 3.19 mg/100 mL, respectively), but lower at times 12, 24 and 48 h (11.62 ± 3.19, 13.03 ± 4.27 and 14.23 ± 4.65 mg/100 mL, respectively). For total flavanones, the commercial samples had values similar to those obtained from fresh juices, except at t48 hours where fresh juices had higher values (22.36 ± 5.77 mg/100 mL). These results were in agreement with those obtained by Chanson-Rolle et al. (Citation2016) due to the hesperidin, which was present in the highest concentration and is known to be stable to thermal treatments, like pasteurisation. Interestingly, Silveira et al. (Citation2014) found that industrial squeezing processes led to a higher concentration of phenolic compounds (4-fold and 2.8-fold more of hesperidin, respectively) compared with home-made juices. This finding was mainly due to the major content of flavanone in the cloud fraction in commercially squeezed juices (compared with the soluble fraction), as commercial squeezing methods extract higher amounts of flavanones from the albedo than home-made squeezing (Tomás-Barberán and Clifford Citation2000), allowing greater availability for enzymatic actions in the gastrointestinal tract (Gil-Izquierdo et al. Citation2003).
Table 7. Concentration of total and individual flavanones (mg/100 mL) in freshly squeezed orange juice samples at different timepoints (t0, t12, t24 and t48 h, mean of all companies) and commercial juices.
Conclusions
The most practical conclusion is that the concentrations of flavanones and vitamin C are constant among countries and processing systems. It can also be concluded that orange juices preserve their bioactive compounds during a typical period of shelf-life and that, even if there are differences between raw oranges and commercial juices, the industrial treatment tends to minimise these differences. Due to this, when ingesting a commercial orange juice, the main bioactive compounds are present at nutritionally significant levels, as is the case for fresh juices. Moreover, it could be predicted that freshly squeezed orange juice would have a similar vitamin C content to commercial ones after 1 week, always over 30% of the Nutrient Reference Value of 80 mg.
Acknowledgements
P.S.-B. was supported by the grant for the recall of the Spanish university system for the training of young doctors (Margarita Salas, 04912/2021) funded by the European Union-Next Generation EU and the Ministry of Universities of Spain. F.J.-S. was supported by an FPU (FPU18/00332) grant of the Fellowship Program from the Spanish Ministry of Science, Innovation, and Universities (MICIU).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Agulló V, Domínguez-Perles R, García-Viguera C. 2021. Sweetener influences plasma concentration of flavonoids in humans after an acute intake of a new (poly)phenol-rich beverage. Nutr Metab Cardiovasc Dis. 31(3):930–938. doi:10.1016/j.numecd.2020.11.016.
- Agulló V, Domínguez-Perles R, Moreno DA, Zafrilla P, García-Viguera C. 2020. Alternative sweeteners modify the urinary excretion of flavanones metabolites ingested through a new maqui-berry beverage. Foods. 9(1):41. doi:10.3390/foods9010041.
- Aschoff JK, Kaufmann S, Kalkan O, Neidhart S, Carle R, Schweiggert RM. 2015. In vitro bioaccessibility of carotenoids, flavonoids, and vitamin C from differently processed oranges and orange juices [Citrus sinensis (L.) Osbeck]. J Agric Food Chem. 63(2):578–587. doi:10.1021/jf505297t.
- Baenas N, Salar FJ, Domínguez-Perles R, García-Viguera C. 2019. New UHPLC-QqQ-MS/MS method for the rapid and sensitive analysis of ascorbic and dehydroascorbic acids in plant foods. Molecules. 24(8):1632. doi:10.3390/molecules24081632.
- Bellisle F, Hébel P, Fourniret A, Sauvage E. 2018. Consumption of 100% pure fruit juice and dietary quality in french adults: analysis of a nationally representative survey in the context of the WHO recommended limitation of free sugars. Nutrients. 10(4):459. doi:10.3390/nu10040459.
- Brenes X, Guevara M, Wong E, Cortés C, Usaga J, Rojas-Garbanzo C. 2022. Effect of high intensity ultrasound on main bioactive compounds, antioxidant capacity and color in orange juice. Food Sci Technol Int. 28(8):694–702. doi:10.1177/10820132211050203.
- Cámara M, Giner RM, González-Fandos E, López-García E, Mañes J, Portillo MP, Rafecas M, Domínguez L, Martínez JA. 2021. Food-based dietary guidelines around the world: a comparative analysis to update AESAN scientific committee dietary recommendations. Nutrients. 13(9):3131. doi:10.3390/nu13093131.
- Chanson-Rolle A, Braesco V, Chupin J, Bouillot L. 2016. Nutritional composition of orange juice: a comparative study between French commercial and home-made juices. FNS. 07(04):252–261. doi:10.4236/fns.2016.74027.
- Dasgupta A, Klein K. 2014. Chapter 15—Antioxidant Vitamins and Minerals, in Antioxidants. Elsevier San Diego, CA, USA. p. 277–294.
- D’Elia L, Dinu M, Sofi F, Volpe M, Strazzullo P, SINU Working Group, Endorsed by SIPREC. 2021. 100% Fruit juice intake and cardiovascular risk: a systematic review and meta-analysis of prospective and randomised controlled studies. Eur J Nutr. 60(5):2449–2467. doi:10.1007/s00394-020-02426-7.
- Dicklin MR, Barron R, Goltz S, Warren J, Boileau T, Pigat S, Maki KC. 2022. Fibre and micronutrient intakes among fruit juice consumers and non-consumers in the UK and France: modelling the effects of consumption of an orange pomace juice product. J Hum Nutr Diet. 35(6):1230–1244. doi:10.1111/jhn.12995.
- D2012/12/EU. Directive 2012/12/EU of the European Parliament and of the Council of 19 April 2012 amending Council Directive 2001/112/EC relating to fruit juices and certain similar products intended for human consumption. Consolidated version 27/04/ 2012.
- EFSA. 2013. Scientific opinion on dietary reference values for vitamin C. Consolidated version 01/01/2018. EFSA J. 11(11):3418.
- FAOSTAT. 2022. Food and Agriculture Organization of the United Nations database. [accessed 28 March 2023]. https://www.fao.org/faostat/es/#data/QCL.
- Fraga LN, Milenkovic D, Anacleto SL, Salemi M, Lajolo FM, Hassimotto NMA. 2023. Citrus flavanone metabolites significantly modulate global proteomic profile in pancreatic β-cells under high-glucose-induced metabolic stress. Biochim Biophys Acta Proteins Proteom. 1871(3):140898. doi:10.1016/j.bbapap.2023.140898.
- Galaverna G, Dall’Asta C. 2014. Chapter 21 - Production processes of orange juice and effects on antioxidant components. In: Preedy V, editor. Processing and Impact on Antioxidants in Beverages. San Diego: Academic Press; p. 203–214.
- Gil-Izquierdo A, Gil MI, Tomas-Barberan FA, Ferreres F. 2003. Influence of industrial processing on orange juice flavanone solubility and transformation to chalcones under gastrointestinal conditions. J Agric Food Chem. 51(10):3024–3028. doi:10.1021/jf020986r.
- Gironés-Vilaplana A, Moreno DA, García-Viguera C. 2014. Phytochemistry and biological activity of Spanish citrus fruits. Food Funct. 5(4):764–772. doi:10.1039/C3FO60700C.
- Grosso G, Bei R, Mistretta A, Marventano S, Calabrese G, Masuelli L, Giganti MG, Modesti A, Galvano F, Gazzolo D. 2013. Effects of vitamin C on health: a review of evidence. Front Biosci (Landmark Ed). 18(3):1017–1029. doi:10.2741/4160.
- Kang GG, Francis N, Hill R, Waters D, Blanchard C, Santhakumar AB. 2020. Dietary polyphenols and gene expression in molecular pathways associated with type 2 diabetes mellitus: a review. Int J Mol Sci. 21(1):140. doi:10.3390/ijms21010140.
- Kong Y, Wang X, Wu Z, Li Y, Xu F, Xie F. 2010. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Foods. 12(24):3–11. doi:10.1016/j.tifs.2009.07.004.
- Kumar M, Pratap V, Gour JK, Singh MK. 2022. Chapter 4.22 - Vitamin C. In: Nabavi SM, Silva AS, editors. Antioxidants Effects in Health. Cambridge: Elsevier; p. 535–546.
- Liu Q, Chiavaroli L, Ayoub-Charette S, Ahmed A, Khan TA, Au-Yeung F, Lee D, Cheung A, Zurbau A, Choo VL, et al. 2023. Fructose-containing food sources and blood pressure: a systematic review and meta-analysis of controlled feeding trials. PLoS One. 18(8):e0264802. doi:10.1371/journal.pone.0264802.
- Martí N, Mena P, Cánovas JA, Micol V, Saura D. 2009. Vitamin C and the role of citrus juices as functional food. Nat Prod Commun. 4(5):677–700. doi:10.1177/1934578X0900400506.
- Murphy MM, Barraj LM, Brisbois TD, Duncan AM. 2020. Frequency of fruit juice consumption and association with nutrient intakes among Canadians. Nutr Health. 26(4):277–283. doi:10.1177/0260106020944299.
- Neves MF, Trombin VG, Marques VN, Martinez LF. 2020. Global orange juice market: a 16-year summary and opportunities for creating value. Trop Plant Pathol. 45(3):166–174. doi:10.1007/s40858-020-00378-1.
- Patras A, Brunton NP, O’Donnell C, Tiwari BK. 2010. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends in Food Science & Technology. 21(1):3–11. doi:10.1016/j.tifs.2009.07.004.
- Pereira-Caro G, Almutairi TM, Cáceres-Jiménez S, Moreno-Rojas JM, Malkova D, García AL, Crozier A. 2023. Bioavailability of orange juice (poly)phenols: β-glucan-rich oat bran decreases urinary excretion of flavanone phase II metabolites and enhances excretion of microbiota-derived phenolic catabolites. Free Radic Biol Med. 199:34–43. doi:10.1016/j.freeradbiomed.2023.02.002.
- Petruzzi L, Campaniello D, Speranza B, Corbo MR, Sinigaglia M, Bevilacqua A. 2017. Thermal treatments for fruit and vegetable juices and beverages: a literature overview. Compr Rev Food Sci Food Saf. 16(4):668–691. doi:10.1111/1541-4337.12270.
- R1924/ 2006. Regulation (EC) No 1924/2006 of the European Parliament and of the Council of 20 December 2006 on nutrition and health claims made on foods. Consolidated version: 13/12/2014
- R1169/ 2011. European Union Regulation (EU) No 1169/2011. Regulation (EU) No 1169/2011 of the European Parliament and of the Council of 25 October 2011 on the provision of food information to consumers, amending Regulations (EC) No 1924/2006 and (EC) No 1925/2006 of the European Parliament and of the Council, and repealing Commission Directive 87/250/EEC, Council Directive 90/496/EEC, Commission Directive 1999/10/EC, Directive 2000/13/EC of the European Parliament and of the Council, Commission Directives 2002/67/EC and 2008/5/EC and Commission Regulation (EC) No 608/2004.
- Rossi I, Mignogna C, Del Rio D, Mena P. 2023. Health effects of 100% fruit and vegetable juices: evidence from human intervention studies. Nutr Res Rev. 1–45. doi:10.1017/S095442242300015X.
- Salar FJ, Domínguez-Perles R, García-Viguera C, Fernández PS. 2022. Ifs and buts of non-thermal processing technologies for plant-based drinks’ bioactive compounds. Food Sci Technol Int. 29(5):445–479. doi:10.1177/10820132221094724.
- Salar FJ, Periago PM, Agulló V, García-Viguera C, Fernández PS. 2021. High hydrostatic pressure vs. thermal pasteurization: the effect on the bioactive compound profile of a citrus maqui beverage. Foods. 10(10):2416. doi:10.3390/foods10102416.
- Silveira JQ, Cesar TB, Manthey JA, Baldwin EA, Bai J, Raithore S. 2014. Pharmacokinetics of flavanone glycosides after ingestion of single doses of fresh-squeezed orange juice versus commercially processed orange juice in healthy humans. J Agric Food Chem. 62(52):12576–12584. doi:10.1021/jf5038163.
- Singh S, Maurya AK, Meena A, Mishra N, Luqman S. 2023. Narirutin. A flavonoid found in citrus fruits modulates cell cycle phases and inhibits the proliferation of hormone-refractory prostate cancer cells by targeting hyaluronidase. Food Chem Toxicol. 174:113638. doi:10.1016/j.fct.2023.113638.
- Sites JW, Reitz HJ. 1950. The variation in individual Valencia oranges from different locations of the tree as a guide to sampling methods and spot-picking for quality. Part II. Titratable Acid and the Soluble Solids/Titratable Acid Ratio of the Juice. Proc Am Soc Hort Sci. 55:73–80.
- Tomás-Barberán FA, Clifford MN. 2000. Flavanones, chalcones and dihydrochalcones – nature, occurrence and dietary burden. J Sci Food Agric. 80(7):1073–1080. doi:10.1002/(SICI)1097-0010(20000515)80:7<1073::AID-JSFA568>3.0.CO;2-B.
- Vandercook CE, Tisserat B. 1989. Flavonoid changes in developing lemons grown in vivo and in vitro. Phytochemistry. 28(3):799–803. doi:10.1016/0031-9422(89)80118-9.
