Abstract
Purpose: Exercise therapy is an effective intervention in a variety of chronic diseases. The prescription of exercise therapy is usually directed toward an index disease. The presence of comorbidity may require adaptations to the exercise program as intended for the index disease. This paper aims to structure the clinical reasoning process of health professionals when prescribing exercise therapy for the individual patient with an index disease and comorbidity.
Methods: We adapted the previously published strategy for developing guidelines and protocols on comorbidity-adapted exercise to a version that can be used for individual exercise prescription.
Results: Essential steps and considerations involved in prescribing an exercise program to an individual patient with comorbidity are described. A case description is used as an example of how the proposed strategy leads to clinical decisions.
Conclusions: The proposed strategy may have a role in educational and professional development. The advanced clinical expertise needed for safe and effective exercise therapy in patients with a complex health status is emphasized.
The presence of comorbidity may require adaptations to exercise therapy.
We describe the essential steps and considerations involved in prescribing an exercise program to an individual patient with an index disease and comorbidity.
The proposed strategy can be used to structure the clinical reasoning process of health professionals.
Implications for Rehabilitation
Introduction
Exercise therapy is an important intervention for a wide range of chronic health conditions, as it improves physical functioning and reduces morbidity and mortality [Citation1–3]. The prescription of exercise therapy is usually directed toward an index disease (mostly the disease for which the person seeks care, e.g., knee complaints due to osteoarthritis). Specific frequency, intensity, type and time (FITT) factors are required to achieve optimal outcome, based on best available evidence [Citation4]. However, coexisting conditions (referred to as comorbidity) are highly prevalent in most chronic diseases and may impact on the ability to exercise (e.g., because of reduced exercise tolerance in case of comorbid diabetes). Adaptations to the exercise program may therefore be needed [Citation5,Citation6]. To provide an effective and safe exercise program for patients with multiple morbidity, advanced clinical reasoning of health professionals (HPs) is required.
Clinical reasoning is the process leading to clinical decisions. It has been defined as “an inferential process used by practitioners to collect and evaluate data and to make judgments about the diagnosis and management of patient problems” [Citation7]. The process of clinical reasoning involves interaction of the HP with a patient, collecting information, generating and testing hypotheses, and determining optimal diagnosis and treatment [Citation7]. Clinical expertise of the HP is essential in this process. Clinical expertise includes the general basic knowledge and skills of clinical practice, as well as the experience of the individual practitioner [Citation8]. For optimal results, the patient’s clinical state and circumstances, relevant research evidence, and the patient’s preferences and actions should be integrated, as described in the evidence based practice model [Citation8,Citation9] (see ).
Figure 1. Evidence-based practice model for clinical decisions [Citation8].
![Figure 1. Evidence-based practice model for clinical decisions [Citation8].](/cms/asset/04f53c65-f42b-4eeb-b6dd-6876ade16ebe/idre_a_1527953_f0001_b.jpg)
Individual HPs often have expertise related to a particular cluster of diseases (e.g., musculoskeletal diseases), while expertise on the comorbid disease (e.g., cardiovascular disease (CVD)) may not have been sufficiently developed. Exercise guidelines provide limited support to fill this gap, since these guidelines are developed for a single disease and usually do not take comorbidity into account [Citation10]. Therefore, we previously developed recommendations for exercise adaptations to comorbidity in patients with knee osteoarthritis [Citation5] and with breast cancer [Citation6]. Although these recommendations aim to support HPs in their clinical decision making, they cannot substitute clinical reasoning, which is still needed to apply the recommendations to an individual patient.
Structuring the process of clinical reasoning has a role in educational and professional development. It can be used to teach clinical reasoning skills in novices, and also facilitates a critical appraisal of why and how decisions are made by more experienced professionals [Citation11]. A commonly used model for clinical reasoning by physiotherapists is the Hypothesis Oriented Algorithm for Clinicians (HOAC)-II. The HOAC-II model, described by Rothstein et al. [Citation12], provides guidance related to clinical reasoning during patient management. Another clinical reasoning model is the Rehab-CYCLE, with the Rehabilitation Problem-Solving Form (RPS-Form) as a central clinical tool in physiotherapy and rehabilitation medicine [Citation13]. The RPS-form is based on the International Classification of Functioning, Disability and Health (ICF), and can be used to identify specific and relevant target problems, discern factors that cause or contribute to these problems, plan the most appropriate interventions and facilitate communication [Citation13]. The HOACII as well as the Rehab-CYCLE are hypothesis-oriented models as they assist the health care professional generating testable hypotheses. Both models are open to every treatment, including exercise therapy.
To date, no guidance is available on the process of tailoring exercise therapy to individual patients with an index disease and comorbidity. Exercise therapy for these complex patients is not without risk and is likely effective only when comorbidity is taken into account [Citation14]. Therefore, as a further specification of current clinical reasoning models, the aim of this paper is to structure the clinical reasoning process of HPs when prescribing a safe and effective exercise program for individual patients with an index disease and comorbidity.
Individual exercise prescription based on the i3-S strategy
We previously developed the i3-S strategy for the development of guidelines and protocols on comorbidity-adapted exercise [Citation10]. According to this strategy, three inventories are made (i3 of i3-S), leading to a synthesis (S of i3-S). The i3S strategy can also be used, with some adaptations, for individual exercise prescription. Starting point is an indication for evidence-based exercise therapy to target impairments in functions and activity limitations related to the index disease, with goals that are relevant for the individual patient. Specific FITT factors are determined to reach the goals that are set. In parallel, a periodization model on the planned manipulation of the FITT factors over time is determined [Citation15]. The steps as described in the adapted version of the i3-S strategy guide the process of tailoring the intended exercise program to the comorbid conditions that are present in the individual patient (see ).
Figure 2. The adapted i3-S strategy for tailored exercise therapy in an individual patient with any index disease and comorbidity.
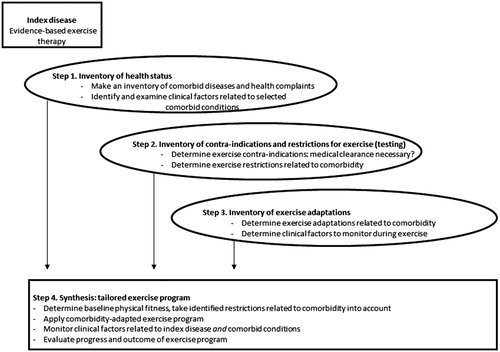
Step 1 of the adapted i3-S strategy involves the assessment of all health conditions that are present. First, comorbid diseases and general health complaints are identified. In order to make a standardized inventory of comorbidity, a measurement tool can be used. An example of such a tool is the Cumulative Illness Rating Scale (CIRS) [Citation16,Citation17]. The CIRS consists of 13 domains related to different body systems. Scoring on the different domains is weighted by the severity of the comorbid condition (scores range from 0 (none) to 4 (extremely severe)). From the conditions that affect daily functioning or require daily treatment (CIRS ≥2) clinical factors are identified during history taking and physical examination. Clinical factors include signs and symptoms, clinical parameters (such as blood cell counts, results from imaging, blood pressure values), medication and other treatments, and health devices. A rigorous health assessment may require extra time, as well the availability of appropriate testing materials such as an oxygen saturation meter in case of comorbid COPD or a sensibility testing kit in case of comorbid diabetes. HPs should consider whether they have sufficient expertise to perform relevant tests and to interpret signs, symptoms and test findings of the conditions that are present. Inter-collegial consultation or referral may be required, as well as consultation of the referring physician for further medical information.
In step 2, contra-indications and restrictions for the intended exercise program are determined. A contra-indication is any condition that precludes the application of the intended exercise (testing) [Citation5,Citation6]. Patients at high risk for exercise-related cardiovascular adverse events are identified from the inventory of health conditions in step 1. Patients at high risk include those with diagnosed CVD, metabolic disease (type I or type II diabetes) or renal disease, or signs or symptoms suggestive of these diseases. According to the updated ACSM’s recommendations, medical clearance is recommended for these patients [Citation18]. Medical clearance is defined as approval from a health care professional (usually the treating physician) to engage in exercise, based on the patient’s medical status, exercise history and the intended exercise intensity. On the basis of this evaluation, the health care provider can decide whether exercise testing or a specific medical examination is appropriate before the initiation of a moderate to high intensity exercise program [Citation18]. When there are no contra-indications, restrictions for exercising are determined. A restriction is defined as a condition limiting the performance of the intended exercise program, requiring adaptations [Citation5,Citation6]. A restriction could also limit the performance of recommended fitness tests for the index disease (to measure baseline physical fitness). Restrictions are derived from the clinical factors as collected in step 1, and/or from the exercise testing or specific medical examination results (if applicable).
In step 3, the adaptations to exercise (testing) are determined, to account for the identified restrictions in step 2. Exercise adaptations can be physiological (e.g., adapting FITT factors to accommodate to impaired physiological response due to comorbid heart failure), behavioral (e.g., adding pain coping skills training to the exercise program in case of chronic pain), or environmental (e.g., applying a hygiene protocol in case of low white blood cells as a response to chemotherapy) [Citation5,Citation6]. Adaptations can be derived from guidelines on exercise for specific comorbid diseases. For exercise in knee OA and in breast cancer, possible exercise adaptations to common comorbidities and side effects of medical treatment have been proposed previously [Citation5,Citation6]. Also, the signs, symptoms and clinical parameters that need to be monitored during exercise are determined. For example, blood glucose values should be measured before and after every training session in case of comorbid insulin-dependent diabetes.
Step 4 involves the synthesis of the information obtained in the first three steps, leading to the application of a tailored exercise program. Physical fitness is measured to define the patients’ baseline level (e.g., aerobic capacity, muscle strength) and to estimate starting values for exercise. Tests should be selected that are valid, feasible, and safe for the individual patient given the presence of comorbidity. Safety precautions (e.g., presence of an automated external defibrillator and other first aid procedures) should be taken. An evidence-based exercise program for the index disease, targeting the patients’ functional problems, remains the starting point. This program is applied if the identified comorbid conditions do not pose restrictions. If restrictions are identified, an adapted program is provided to accommodate these restrictions, while maintaining an adequate stimulus for physiological and behavioral adaptation. Consultation with other HPs should be explicitly considered, e.g., with a dietician or a psychologist. During the exercise program, signs, symptoms, and clinical parameters of the index disease and the comorbid disease(s) are monitored regularly because of the often changing health condition of a patient. This may result in further adaptation of the exercise program prescribed, for example, in adapting FITT factors or the model of periodization. Also, progress in physical fitness and personal goals should be evaluated at regular, predetermined intervals during the program. For example, muscle strength testing should be repeated every four weeks to adequately prescribe strength training intensity by ensuring sufficient overload.
At the end of the exercise program, the outcome of the intervention is evaluated. Exercise behavior should be continued after the program has ended to maintain or further improve physical fitness and to sustain the acquired health benefits. This topic should be explicitly addressed, preferably at the start of the program, and appropriate behavioral strategies should be used during the program to optimize exercise self-efficacy and intention [Citation4,Citation6].
As an example of how the strategy can be used, a case description is provided. presents a case of a woman with knee osteoarthritis, with the intended exercise program to increase physical fitness and to target symptoms and activity limitations related to her knee symptoms [Citation4,Citation14]. Subsequently, the steps to tailor the intended exercise program to the presence of comorbidity, following the adapted i3-S strategy, are described. The exercise adaptations are based on previous recommendations on comorbidity-adapted exercise therapy in knee OA [Citation5].
Table 1. Case description: adapting an exercise program for an individual with knee OA-related symptoms and activity limitations to the presence of comorbid conditions, following the i3-S strategy.
Discussion
We proposed a strategy to facilitate and structure the clinical reasoning process of HPs to prescribe a safe and effective exercise program for individuals with an index disease and comorbidity. The steps and considerations involved are described explicitly and may help HPs in developing consistent clinical processes. The i3-S strategy has previously shown its use on a more aggregated level [Citation10]. The steps of the adapted version of the i3-S strategy, as presented in the present paper, can be integrated within the process of individual patient management according to the principles of evidence-based practice [Citation8,Citation9].
The proposed strategy could have a role in educational and professional development. It can be used to teach clinical reasoning skills and may facilitate a critical appraisal of why and how decisions are made [Citation11]. Since mentoring and reflection are regarded as important instruments to facilitate the progression of clinical reasoning skills in HPs, several tools for guided mentorship and reflection have been developed to date [Citation11,Citation19,Citation20]. As far as we know, our strategy is the first that specifically addresses clinical reasoning related to exercise therapy in patients with a complex health status.
To develop clinical expertise in providing exercise therapy for patients with a complex health status, specific knowledge and skills are prerequisite. Topics that should be addressed in professional education include profound knowledge on pathologies and interaction of pathologies and medication in relation to exercise physiology, skills in inter-professional collaboration and incorporation of principles of chronic disease management (i.e., skills on promotion of patient self-management) [Citation21]. Also, dedicated time and effort are required to increase clinical expertise. HPs should reflect on their knowledge and skills since limited experience in treating these complex patients can lead to suboptimal or even counterproductive results.
Although the need for tailoring exercise therapy to comorbidity is clear from a clinical perspective, there is remarkably little attention to this topic in research. Exercise trials often exclude patients with (severe) comorbidity or, when included, provide limited information on how the exercise intervention is tailored to comorbidity. An important requirement for reports on exercise trials is that a detailed description of the goals and content of the exercise program is provided, to allow for interpretation and replication, and to facilitate implementation into clinical practice [Citation22,Citation23]. Beside information such as exercise dosage, compliance and mode of delivery, this should also include specific information on tailoring of the exercise program [Citation22]. In a recent study in knee OA, an exercise protocol with adaptations to common comorbid conditions was developed [Citation5] and tested on its effectiveness in a randomized controlled trial [Citation14]. The results showed that even in patients with knee OA and severe comorbidity tailored exercise therapy was safe and greatly improved physical functioning [Citation14]. There is a call for more research in this area, which may also inform updates of exercise guidelines.
Some issues need to be considered. As our strategy is the first to support HPs in tailoring exercise therapy to comorbidity, further development and external validation of the strategy is recommended. Furthermore, the proposed strategy to clinical reasoning for exercise therapy in complex cases is not exclusive. Other approaches, for example the clinical reasoning model by Elven et al. integrating behavioral change into physiotherapy, can be complementary [Citation24]. Behavior change strategies are an integral part of the application of exercise therapy. Specific goal setting and reviewing of goals, shaping knowledge, and promoting feedback and reinforcement are some examples of strategies for behavior change [Citation24]. Patients' ability to change their exercise behavior and adhere to an exercise regimen is highly dependent on psychological and social factors. Especially, the role of self-efficacy is mentioned in the literature as important in behavioral change [Citation25]. Goal setting is more realistic when comorbidity is taken into account, and therefore goals will be more achievable. This increases the chance of a successful experience, and with it, the self-efficacy of the patient. Finally, factors such as patients’ preferences, costs and adequate professional encouragement are important for a successful exercise program [Citation26].
In conclusion, we proposed a generic strategy to the process of clinical reasoning regarding exercise therapy for patients with an index disease and comorbidity. Adequate clinical decisions require advanced clinical expertise of HPs, which can be supported by a structured process of clinical reasoning.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Smidt N, de Vet HC, Bouter LM, et al. Effectiveness of exercise therapy: a best-evidence summary of systematic reviews. Aust J Physiother. 2005;51:71–85.
- Taylor NF, Dodd KJ, Damiano DL. Progressive resistance exercise in physical therapy: a summary of systematic reviews. Phys Ther. 2005;85:1208–1223.
- Naci H, Ioannidis JP. Comparative effectiveness of exercise and drug interventions on mortality outcomes: metaepidemiological study. BMJ. 2013;347:f5577.
- Pescatello LS, Arena R, Riebe D, et al. ACSM's guidelines for exercise testing and prescription (American College of Sports Medicine). 9th ed. Philadelphia (PA): Wolters Kluwer/Lippincott Williams & Wilkins; 2014.
- de Rooij M, van der Leeden M, Avezaat E, et al. Development of comorbidity-adapted exercise protocols for patients with knee osteoarthritis. Clin Interv Aging. 2014;9:829–842.
- van der Leeden M, Huijsmans RJ, Geleijn E, et al. Tailoring exercise interventions to comorbidities and treatment-induced adverse effects in patients with early stage breast cancer undergoing chemotherapy: a framework to support clinical decisions. Disabil Rehabil. 2018;40:486–496.
- Higgs JJM. Clinical decision making and multiple problem spaces. In: Higgs JJM, Loftus S, Christensen N, editors. Clinical reasoning in the health professions. 3rd ed. Boston, USA: Butterworth-Heinemann; 2008. p. 4–19.
- Haynes RB, Devereaux PJ, Guyatt GH. Clinical expertise in the era of evidence-based medicine and patient choice. ACP J Club. 2002;136:A11–A14.
- Guyatt G, Cook D, Haynes B. Evidence based medicine has come a long way. BMJ. 2004;329:990–991.
- Dekker J, de Rooij M, van der Leeden M. Exercise and comorbidity: the i3-S strategy for developing comorbidity-related adaptations to exercise therapy. Disabil Rehabil. 2016;38:905–909.
- Atkinson HL, Nixon-Cave K. A tool for clinical reasoning and reflection using the international classification of functioning, disability and health (ICF) framework and patient management model. Phys Ther. 2011;91:416–430.
- Rothstein JM, Echternach JL, Riddle DL. The Hypothesis-Oriented Algorithm for Clinicians II (HOAC II): a guide for patient management. Phys Ther. 2003;83:455–470.
- Steiner WA, Ryser L, Huber E, et al. Use of the ICF model as a clinical problem-solving tool in physical therapy and rehabilitation medicine. Phys Ther. 2002;82:1098–1107.
- de Rooij M, van der Leeden M, Cheung J, et al. Efficacy of tailored exercise therapy on physical functioning in patients with knee osteoarthritis and comorbidity: a randomized controlled trial. Arthritis Care Res (Hoboken). 2017;69:807–816.
- Buford TW, Rossi SJ, Smith DB, et al. A comparison of periodization models during nine weeks with equated volume and intensity for strength. J Strength Condition Res. 2007;21:1245–1250.
- Hudon C, Fortin M, Soubhi H. Abbreviated guidelines for scoring the Cumulative Illness Rating Scale (CIRS) in family practice. J Clin Epidemiol. 2007;60:212.
- Hudon C, Fortin M, Vanasse A. Cumulative Illness Rating Scale was a reliable and valid index in a family practice context. J Clin Epidemiol. 2005;58:603–608.
- Riebe D, Franklin BA, Thompson PD, et al. Updating ACSM's recommendations for exercise preparticipation health screening. Med Sci Sports Exerc. 2015;47:2473–2479.
- Baker SE, Painter EE, Morgan BC, et al. Systematic Clinical Reasoning in Physical Therapy (SCRIPT): tool for the purposeful practice of clinical reasoning in orthopedic manual physical therapy. Phys Ther. 2017;97:61–70.
- Donaghy M, Morss K. An evaluation of a framework for facilitating and assessing physiotherapy students' reflection on practice. Physiother Theory Pract. 2007;23:83–94.
- Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet. 2012;380:7–9.
- Slade SC, Dionne CE, Underwood M, et al. Consensus on Exercise Reporting Template (CERT): modified Delphi study. Phys Ther. 2016;96:1514–1524.
- Van der Leeden MSB, Beekman E, Hendriks E, et al. Development of a framework to describe goals and content of exercise interventions in physical therapy: a mixed method approach including a systematic review. Phys Ther Rev. 2014;19:1–14.
- Elven M, Hochwalder J, Dean E, et al. A clinical reasoning model focused on clients' behaviour change with reference to physiotherapists: its multiphase development and validation. Physiother Theory Pract. 2015;31:231–243.
- Artinian NT, Fletcher GF, Mozaffarian D, et al. Interventions to promote physical activity and dietary lifestyle changes for cardiovascular risk factor reduction in adults: a scientific statement from the American Heart Association. Circulation. 2010;122:406–441.
- Kampshoff CS, Jansen F, van Mechelen W, et al. Determinants of exercise adherence and maintenance among cancer survivors: a systematic review. Int J Behav Nutr Phys Act. 2014;11:80.
