Abstract
Purpose
The study explored the acceptability of high repetition arm training as part of a randomised controlled trial, early after stroke, when fatigue levels and emotional strain are often high.
Materials and methods
36 sub-acute stroke survivors (61 years+/-15) attended for assessment sessions at 3, 6, and 12 weeks after stroke. Individuals were randomised to receive 6 high repetition arm training sessions between 3 and 6 weeks (intervention) or the control group. Semi-structured interviews were conducted at trial completion. Interview transcripts were analysed through framework analysis conducted independently by 2 researchers.
Results
Stroke survivors participated despite high levels of fatigue because they hoped for personal benefit or to potentially benefit future patients. Benefits reported from participation included physical improvements, psychological benefit, improved understanding of their condition as well as a feeling of hope and distraction. The arm training at three weeks after stroke, aiming for 420 movement repetitions was not considered to be too intensive or too early, and most individuals felt lucky to have been, or would have preferred to be in the early training group.
Conclusion
High repetition arm training early after stroke was acceptable to participants. Study participation was generally viewed as a positive experience, suggesting that early intervention may not only be physically beneficial but also psychologically.
Stroke survivors report that high repetition arm training early after stroke is acceptable.
Participation in rehabilitation research early after stroke provides stroke survivors with hope and meaning despite the high prevalence of fatigue.
Complex information needs to be repeated and provided in a number of formats early after stroke.
Implications for rehabilitation
Introduction
Arm recovery after stroke is often incomplete, leaving a third of all stroke survivors unable to use their arm in functional activity [Citation1,Citation2]. Most recovery occurs early after stroke during a period of increased neuroplasticity [Citation3] and the most effective interventions are exercise [Citation4,Citation5] and intensive practice of functional tasks [Citation6,Citation7]. This has led to calls for changes to practice so that patients receive much more intensive therapy during sub-acute stroke, and for trials to assess its effectiveness [Citation2,Citation8,Citation9]. However, the emotional strain at this time after a stroke is severe [Citation10] and the prevalence and severity of fatigue is increasingly recognised [Citation11]. Therefore the prospect of participating in a trial which includes high repetition training, may feel daunting [Citation12]. Anxiety about potential side-effects and the stigma of being used as “a guinea-pig” can also be off-putting [Citation13].
Randomised clinical trials are the gold standard for testing the effects of rehabilitation interventions however, stroke rehabilitation involves complex interventions which are difficult to implement in trials and in clinical practice [Citation14,Citation15]. An essential element of implementation success, whether during a trial or in clinical practice, is the feasibility and acceptability of the trial and the intervention for patients. These factors impact particularly on recruitment, retention and adherence to the trial but also uptake of the interventions in clinical practice. Although health care professionals’ views and influence on the decision-making processes have been considered during trials of sub-acute stroke rehabilitation [Citation12,Citation16,Citation17], the views and experiences of stroke survivors has received less attention.
The study reported here explores the views and experiences of stroke survivors regarding their participation in a trial to investigate the effect of high repetition arm training in sub-acute stroke (ISRCTN 81668376).
Materials and methods
Stroke survivors admitted to three in-patient stroke services in Northwest England were approached for recruitment during their acute admission and enrolled at 3 weeks after their stroke. Prior to participation, the study procedure including randomisation was explained to the participant and informed consent obtained in accordance with the Declaration of Helsinki. This parallel-randomised (1:1 allocation) study was approved by the NRES Committee Northwest - Greater Manchester West (REC: 15/NW/0703 & IRAS: 184096).
Participants and study procedure
All patients met the following inclusion criteria: (i) sub-acute stroke survivors (∼3 weeks post stroke) with (ii) upper limb weakness (≤4 Medical Research Council [MRC]) of either triceps or anterior deltoid muscles, (iii) able to perform a reaching movement of ≥15 cm with the weight of the arm fully supported (which was the training task, ) and (iv) engaging in therapy sessions. We excluded individuals with (i) history of previous stroke or other concomitant neurological or musculoskeletal disease, (ii) contra-indications to transcranial magnetic stimulation, i.e., history of epilepsy, metal implant in head or neck etc. [Citation18], (iii) cerebellar stroke, (iv) proximal upper limb hypertonus ≥3 on Modified Ashworth scale (MAS), (v) severe sensory impairment (<6/12 Fugl-Meyer Sensory scale), (vi) shoulder pain ≥3/10 on self-rated continuous visual analogue scale, (vii) new self-reported uncorrected visual impairment, (viii) hemi-spatial neglect established by the Star Cancellation Task and (ix) cognitive and language impairment preventing the ability to perform the reaching task. All participants performed this research in addition to their normal care provided by NHS therapy teams either as in-patients or by the Early Supported Discharge team after discharge. 69% of participants were hospital in-patients at the onset of therapy and 14% at the time of the 12 week assessment. Individuals who had been discharged travelled to the lab for training sessions and assessments by transport organised by the research team or by their own means of transport (normally driven by partner) if they preferred.
Figure 1. Training set-up. Participants were seated with forehead, trunk and shoulder support. The weight of the arm was fully supported and the hand was strapped to the handle if required. Participants were unable to see their hand but were provided with feedback of the hand location and the reaching target by an image projected on a screen.

After the initial assessment, the randomisation process was again explained to the participants. Individuals were then randomised to high repetition arm training in addition to usual care (training group) or to only usual care (control group) between 3 and 6 weeks after their stroke. Participants in the control group were offered the same high repetition arm training after the main assessment period was complete (12 weeks after their stroke). The arm training consisted of six sessions in which participants performed up to 420 gravity – eliminated 20 cm reaching movement towards projected targets while vision of their arm was occluded [Citation19]. The effects of gravity were eliminated using a SAEBO MAS device (). The arm training performed in this research project was specifically designed to allow recruitment of individuals with a more severely affected arm because knowledge of sub-acute recovery processes in this patient group is limited [Citation8]. Therefore the paradigm only required proximal upper limb movements.
All participants attended the research laboratory at baseline (∼3 weeks post-stroke); end of intervention (∼6 weeks post-stroke) and follow up (∼6 weeks after the end of the intervention) to assess reaching accuracy, functional measures of arm movements and corticospinal connectivity. Connectivity from both the affected and unaffected hemisphere to the weak arm muscles was established by single pulse transcranial magnetic stimulation of up to 100% maximum stimulator output.
Semi-structured interviews regarding the participants’ views and experience of the high repetition arm training and participating in research early after stroke were conducted during the final assessment (∼12 weeks after stroke). Individuals in the control group, that elected to perform the arm training after the final assessment (n = 7), had an additional interview about their perceptions of the arm training after its completion at ∼15 weeks.
Data collection
Clinical Research Network practitioners trained to perform the interviews, but blinded to the participants’ intervention group, conducted most of the interviews using a topic guide. However, due to staffing levels the primary researcher (UH), trained in qualitative interview skills by the senior researcher (ST), conducted four (14%) of the interviews. For participants with significant communication difficulties (n = 4, 14%), UH, who delivered the intervention and therefore knew the stroke survivors well, sat in on the interviews to assist with interpretation and took field notes regarding supplementary non-verbal communication (e.g., pointing, nodding etc.) during the interview. The interviews were conducted in a quiet laboratory using an interview guide () that had been tested with a stroke survivor advisor. Stroke survivors’ partners/companions, when attending, stayed in the room if the participant preferred but were asked not to assist in answering any questions. The interviewer encouraged participants to share their experiences of participating in the research and of undertaking high repetition arm training early after their stroke using probing techniques and prompts to achieve further in-depth reflection. Interviews were audio recorded and field notes were taken.
Table 1. Interview guide – questions and prompts used to guide interviews of all participants.
Data analysis
Interviews were anonymised and transcribed verbatim by a professional transcription agency but not returned to participants for comments and/or corrections. A framework analysis approach was used to capture key messages under the themes dictated by the interview [Citation20–22]. Framework analysis follows a five-step process: familiarisation, identifying a thematic framework, indexing, charting and mapping and interpretation [Citation20,Citation21]. The main subsections highlighted in the topic guide were used as a framework for the analysis. Following familiarisation of the transcripts by reading them several times; the transcripts were indexed, charted and mapped against the framework independently by two of the authors (UH and MH) using an excel spreadsheet. They then discussed their emerging interpretation iteratively to reach a consensus. Another author (ST), who was independent of delivery of intervention and collection, then reviewed the analysis and interpretation and the emerging synthesis was refined following discussion with UH and MH. The thematic framework analysis allows researcher to start analysis before all data is collected and revise the interview guide as necessary [Citation21] however, we did not change the transcript during data collection. Analysis was initiated during data collection and continued as further interviews were transcribed. The analysis was performed by the authors UH, MH and ST and reviewed by two study participants for accuracy and completeness. UH (PhD) is a neurological physiotherapist by background with an interest in the mechanism (neurophysiology and kinematics) of recovery. She was the grant holder and delivered the intervention and was not involved in the delivery of day-to-day clinical service. ST (PhD) is a neurological physiotherapist by background with a long track record of mixed methods trials in delivery of stroke physiotherapy and MH a research associate. Both ST and MH were blinded to data collection and independent of the delivery of the intervention.
A fatigue assessment was conducted at base-line and follow up and analysed the percent of people that reported that they felt fatigued (i.e., scored >36 in the Fatigue Severity Scale [Citation23]). In addition participants gave quantitative and Likert style answers regarding their satisfaction in taking part in the research and whether they were concerned about the brain stimulation.
Results
Participants
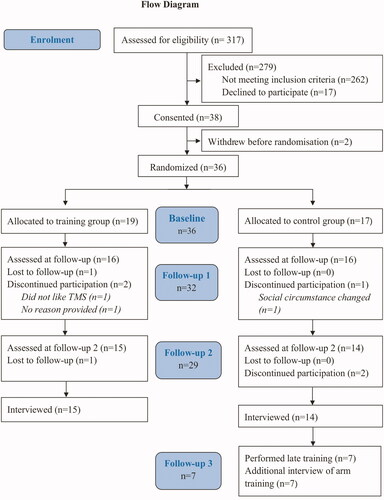
38 stroke survivors participated in the main trial and 29 completed the trial and participated in this interview study ( Flowchart). Interviews lasted between 6 and 16 min.
The demographics are summarised in . The groups were evenly matched in age, impairment and fatigue levels but not in gender. A larger proportion of women were presented in the control (57%) vs the training group (36%).
Table 2. Participant demographics and baseline measures (median and SD except if stated otherwise).
Fatigue was prevalent when performing a Fatigue Severity Scale (FSS) assessment. Two-thirds (63%) of participants were fatigued at baseline (FSS >36 [Citation23,Citation24]), and this was unchanged at the end of the intervention (12 weeks after stroke, 59.3%). Both the training and control) group reported fatigue at baseline (FSS – training mean = 37.4 ± 18.7 and control =37.3 ± 18.6) and at follow-up (training mean = 35.9 ± 17.9 and control mean = 37.6 SD = 18.1) (), without a difference between groups (t-test p = 0.867, 95% CI −16.59, 14.06).
Participant were asked to indicate their satisfaction of taking part in the study on a 5 point Likert scale where 1 = very satisfied and 5 = very dissatisfied. A greater number of individuals in the training group in comparison to the control group indicated that they were very satisfied with participating in the study (93.3 vs. 78.6%). All participants were somewhat or very satisfied with participation.
Overview of themes
4 main themes emerged, each with two to three subthemes that encapsulated the prominent messages of each theme. We believe that data saturation was achieved as no new themes were identified in later interviews. A summary of themes and subthemes is presented in .
Table 3: Summary of the themes related to individuals’ experiences of participating in rehabilitation research early after stroke.
Theme 1: Feelings about research participation early after stroke
Interesting and enjoyable
Participants found the research interesting because they gained a greater understanding of their condition. The visualisation of muscle responses by brain stimulation and physically seeing progress of arm movement gave useful feedback, which would not have been gleaned without the research study. “…finding out which area of your brain is affected and when you compare the affected side with the unaffected side, the comparison in the amount of (transcranial magnetic) stimulation that you had to do. Seeing the connection; the nervous connection between…you know, the muscle and the brain, that was interesting” (B32 age < 2 participant mean, Fugl-Meyer Upper Limb score (FM)>15 mean, FSS > 3 mean). Most participants found the training task enjoyable, seeing it as a game or challenge to be overcome. “Yes, I thoroughly enjoyed it. I was disappointed when it came to an end. I got quite addicted to that. I thought, well, next time, when I go, I’ll be able to probably hit the bull’s-eye” (R37 age > 33 mean, FM < 12 mean, FSS < 28 mean). While others found that it empowering to be actively working towards their recovery: “I had something else to do then I felt a bit useful, whereas I’ve sat there every day feeling useless and like nothing is working” (F18 age < 8 mean, FM < 23 mean, FSS > 16 mean). Participants were asked whether a more practical activity, such as making a cup of tea using virtual reality would be preferable to the reaching movement to a set target used in this study. None of the participants expressed that functional activity would be preferred in this research trial, which was an add-on to their normal therapy.
Feelings about timing of the intervention
Most participants felt that the intervention and research approach was not too early, and felt the study had come at a good time as it gave them something positive to focus on during a difficult period. Some participants reported some concerns about the timing when they were first approached about participation but in retrospect they felt the timing was right and that it was a positive experience at that stage post-stroke: “I don’t think you could do it much sooner because you’ve…I don’t think you’ve got your head round it yourself. No. I think it’s probably about right to be honest “(G13 age < 4 mean, FM = 7 mean, FSS > 6 mean). For three individuals, their concerns continued and they felt the intervention and trial was too early, “Because [one’s] mind was still a whizz” (F29, age = mean, FM < 18 mean, FSS > 25 mean).
Preference for early group
When asked, all experimental group participants were glad that they had been randomised to do the early training. “I was glad I was put in the early one. Yeah. Not the later one because I think the earlier you do it the better you’ll be able to do it” (C19 age > 3 mean, FM = mean, FSS > 11 mean). Some control group participants reported that they felt upset they had not been able to do the training in the early group. “When I was put in the last group I think it was a little bit…there was a bit of disappointment there” (F18). A few participants spoke of the pros and cons of being in either group, and only one participant concluded that later training was preferable.
Limited awareness of randomisation
A few participants reported that they were not aware of the randomisation process, or that they did not understand how it had worked or affected them. One participant was under the impression that participants were placed into groups based on ability: “You choose whichever person is the best for the job” (L20 age < 2 mean, FM < 8 mean, FSS < 28 mean). Whilst others were unaware of which group they had been allocated to, or why.
Theme 2: Benefits of high repetition arm training
Perceived potential to increase recovery
Many people reported that they felt improved movement and coordination following the arm training. “Well, when I came here the first time, I didn’t realise I was moving my own arm…because I’d just…you know, it was just a passenger, but now it’s not ….I’m better than I would have been. I feel my arm’s better now than it would have been if I hadn’t come here” (W09 age > 10 mean, FM < 12 mean, FSS > 17 mean).
Theme 3: Negative aspects of research participation
Fatigue
Fatigue was frequently reported as a side effect of the high repetition arm training however it did not interfere with participation as most participants found that it improved with time, and that some days were more difficult than others. “I felt tired the first few times and then it’s gone easier and easier but I am still tired afterwards, yeah” (W24 age > 10 mean, FM< 8mean, FSS > 3 mean).
Limited improvement
Although most perceived improvement in the movement of their arm, some felt that progress was limited. This was often expressed as the fault of the participant rather than external factors “Well it’s interesting but I…somehow I feel I should have made more progress than I did. But, as I say, it’s quite hard to do, you know” (S22 age > 19 mean, FM < 12 mean, FSS > 9 mean).
Boredom
Some participants found the arm training exercises boring. This was due to the repetitive nature of the task and the long duration of performing it. ”No, I…each day I came, I…very boring if you do…repetitive. But then it…I just made a game of it, like, trying to beat my own score and things like that” (F05 age <15 mean, FM > 30 mean, FSS > 26).
Concerns about brain stimulation
Participants initially had concerns about the safety of brain stimulation. This was generally attributed to the brain stimulation being a device, which the participant had not seen or used before. However, the same participants, went on to say that they found the stimulation to be fine once the initial assessment had been completed. “I was really scared at the beginning but … because I knew there would have to be some kind of pulse, or electric electrodes, or whatever …. But when I realised, one, it doesn’t hurt and, two, I could see what was happening it was fine. When I realised it wasn’t going to do any damage …” (F18). Participants were asked whether they initially had concerns about having brain stimulation performed (Yes, No, Not sure). 31% of the participating stroke survivors indicated that they did have concerns.
Theme 4: Factors influencing participants’ decision to take part in the research project
Research participation as an opportunity to improve recovery or benefit future stroke survivors
Although it was explained that the study might provide no individual benefit, participants reported a sense of opportunity when taking part. This often took the form of “doing anything to get right” (C19), at a time when the participant was feeling low or unstimulated. The opportunity to have access to treatment that participants otherwise “probably wouldn’t have done” (W09) was an important motivator. “You think there’s no hope, and when somebody hands you a lifeline, even if it’s new, you’re going to take it. I thought, no matter what, it can only help” (W09). The opportunity to participate in a study that may benefit other stroke survivors was another motivator “we’re benefitting from what people have learnt historically so why would I not want to help someone in the future” (G13).
Participating to learn more about their condition
Participants were able to learn more about their condition “I think it was for more understanding of what had happened to me because it was a bolt out of the blue and it was, like, oh, I really don’t know anything about this…. So I wanted to know more about it” (H10 age < 12 mean, FM > 21 mean, FSS > 19 mean).
Family and care-provider were frequently consulted and their encouragement was important
The decision to participate was often performed in consultation with family and care-provider who reassured or encouraged participation. “I talked to my husband, […]” and he said “go for it,” just straightaway, “just go for it, go and see, it’s okay.” So that’s why I’ve done it” (F29) “Well there was X, one of the physios, and he thought I’d benefit from it, which I have done…he made me think about doing it, yeah” (W24).
Discussion
The stroke survivors that participated in this study were keen to be involved in this research project early after stroke. The major drivers for participation were the opportunity to receive extra treatment that may be beneficial for their recovery and that the findings may help fellow stroke survivors in the future. Consequently stroke survivors participated in the research despite high levels of fatigue and initial concerns about the safety of brain stimulation. This is contrary to some commonly held beliefs that high repetition training is too challenging for stroke survivors early after stroke [Citation25], when they may be too fatigued and overwhelmed by the life changing event [Citation26,Citation27]. The participants’ enthusiasm to receive more training and arm therapy in the first weeks after stroke may reflect the low intensity of arm therapy, which is currently provided [Citation28,Citation29]. It could also be due to the boredom and frustration reported in previous studies of in-patient stroke therapy [Citation16,Citation30]. Our findings indicate that high repetition arm training can be acceptable early after stroke. The evidence supporting the use of arm training in the first 4 weeks after stroke are increasing [Citation7], however our findings are not a “carte blanche” for intensive training very early after stroke. Our participants gave some indication that if the research and intervention had commenced earlier than three weeks post stroke, they may have found this too overwhelming. Several upper limb exercise and functional training interventions have been found to be effective [Citation31–35], but most trials involve patients in the sub-acute and chronic stages of stroke recovery. Further research is needed to establish the optimal time to initiate training, the most appropriate dosage and how this should be stratified for different levels of impairment and activity limitation, and how this can be implemented most effectively.
Recruitment to research projects early after stroke is notoriously challenging: fear of experimental procedures and potential harm or side-effects, feeling overwhelmed by the emotional strain of a life-changing event, fatigue and cognitive impairments associated with sub-acute stroke, and the possible stigma of being a “guinea-pig” have been highlighted as possible deterrents to participation [Citation13,Citation17,Citation36]. The notion of being a “guinea-pig” was not expressed in the current study but a fear of experimental procedures, namely brain stimulation was common. This further highlights the need to provide clear, consistent information to potential participants. Health care professionals involved in recruitment need to have sufficient knowledge of the research project to accurately and clearly answer all participants’ potential questions in detail [Citation12,Citation17]. Our findings also highlight the important role that “significant others” and treating clinicians play in stroke survivors’ decision-making to participate in research studies. It is important to provide information and other resources to inform and support both the stroke survivor and their relatives in this role [Citation37].
Limitations
It has to be noted that only participants that completed the study were interviewed. Therefore feedback from individuals who withdrew, or who declined to undertake the high repetition training was not captured. Interviewing these individuals would indicate the barriers for implementing these new approaches into clinical practice and provide a more balanced approach. They would also provide valuable insight into the timeliness and acceptability of the intervention. However, the dropout rate during the study was low. Therefore, our findings demonstrate that there is a group of stroke survivors, with severe arm weakness, who welcome the opportunity to participate in research and high repetition arm training early after stroke. Further research to investigate the views and experiences of stroke survivors who decline to participate or drop out of early training studies is warranted.
Bias could have been introduced in the findings as the researcher conducted, or was present in some interviews, which may have led to a social desirability bias. However, participants raised positive points as well as negative aspects of their participation, which indicates that we achieved the necessary rapport. Furthermore, we encouraged participants to be frank in the interviews as we wanted to use their views to develop practice and to improve future trials.
In our study we recruited individuals with a wide variety of impairments including language and communication difficulties. This clearly limited some individuals’ ability to express all their views and experiences and led to frustration in some. Additionally we realised that the randomisation process was sometimes poorly understood although this was explained before recruitment and again after baseline measurements when individuals were informed of their group allocation. This could be due to some language difficulties, memory deficits or difficulty taking in complex information early after stroke. This replicates findings in other health conditions [Citation38] and highlights the need to explore more effective ways to provide complex information and enable participants to make truly informed choices [Citation39].
ISSM_COREQ_Checklist_UH.pdf
Download PDF (480.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. The Lancet. 2011;377(9778):1693–1702.
- Krakauer JW, Carmichael ST, Corbett D, et al. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair. 2012;26(8):923–931.
- Cortes JC, Goldsmith J, Harran MD, et al. A short and distinct time window for recovery of arm motor control early after stroke revealed with a global measure of trajectory kinematics. Neurorehabil Neural Repair. 2017;31(6):552–560.
- Corti M, McGuirk TE, Wu SS, et al. Differential effects of power training versus functional task practice on compensation and restoration of arm function after stroke. Neurorehabil Neural Repair. 2012;26(7):842–854.
- Kitago T, Liang J, Huang VS, et al. Improvement after constraint-induced movement therapy: recovery of normal motor control or task-specific compensation? Neurorehabil Neural Repair. 2013;27(2):99–109.
- Pollock A, Farmer SE, Brady MC, et al. Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev. 2014;(11):CD010820.
- Wattchow KA, McDonnell MN, Hillier SL. Rehabilitation interventions for upper limb function in the first four weeks following stroke: a systematic review and meta-analysis of the evidence. Arch Phys Med Rehabil. 2018;99(2):367–382.
- Stinear C, Ackerley S, Byblow W. Rehabilitation is initiated early after stroke, but most motor rehabilitation trials are not: a systematic review. Stroke. 2013;44(7):2039–2045.
- Corbett D, Jeffers M, Nguemeni C, et al. Lost in translation: rethinking approaches to stroke recovery. Prog Brain Res. 2015;218:413–434.
- Fure B, Wyller TB, Engedal K, et al. Emotional symptoms in acute ischemic stroke. Int J Geriat Psychiatry. 2006;21(4):382–387.
- Hawkins L, Lincoln NB, Sprigg N, et al. The Nottingham Fatigue After Stroke (NotFAST) study: results from follow-up six months after stroke. Top Stroke Rehabil. 2017;24(8):592–596.
- Thomas N, Plant S, Woodward-Nutt K, et al. Health care professionals' views of the factors influencing the decision to refer patients to a stroke rehabilitation trial. Trials. 2015;16:577.
- Quinn GP, Bell BA, Bell MY, et al. The guinea pig syndrome: improving clinical trial participation among thoracic patients. J Thorac Oncol. 2007;2(3):191–196.
- Campbell NC, Murray E, Darbyshire J, et al. Designing and evaluating complex interventions to improve health care. BMJ. 2007;334(7591):455–459.
- Luker JA, Craig LE, Bennett L, Ellery F, et al. Implementing a complex rehabilitation intervention in a stroke trial: a qualitative process evaluation of AVERT. BMC Med Res Methodol. 2016;16:52.
- Horne M, Thomas N, Vail A, et al. Staff's views on delivering patient-led therapy during inpatient stroke rehabilitation: a focus group study with lessons for trial fidelity. Trials. 2015;16:137.
- Tyson SF, Thomas N, Vail A, et al. Recruiting to inpatient-based rehabilitation trials: lessons learned. Trials. 2015;16:75.
- Rossi S, Hallett M, Rossini PM, Safety of TMS Consensus Group, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120(12):2008–2039.
- Hammerbeck U, Yousif N, Hoad D, et al. Chronic stroke survivors improve reaching accuracy by reducing movement variability at the trained movement speed. Neurorehabil Neural Repair. 2017;31(6):499–508.
- Tong A, Sainsbury P, Craig J. Consolidated criteria for reporting qualitative research (COREQ): a 32-item checklist for interviews and focus groups. Int J Qual Health Care. 2007;19(6):349–357.
- Gale NK, Heath G, Cameron E, et al. Using the framework method for the analysis of qualitative data in multi-disciplinary health research. BMC Med Res Methodol. 2013;13:117.
- Thorarinsdottir K, Kristjansson K. Patients' perspectives on person-centred participation in healthcare: a framework analysis. Nurs Ethics. 2014;21(2):129–147.
- Lerdal A, Bakken LN, Rasmussen EF, et al. Physical impairment, depressive symptoms and pre-stroke fatigue are related to fatigue in the acute phase after stroke. Disabil Rehab. 2011;33(4):334–342.
- Drummond A, Hawkins L, Sprigg N, et al. The Nottingham Fatigue after Stroke (NotFAST) study: factors associated with severity of fatigue in stroke patients without depression. Clin Rehabil. 2017;31(10):1406–1415.
- Tyson SF, Woodward-Nutt K, Plant S. How are balance and mobility problems after stroke treated in England? An observational study of the content, dose and context of physiotherapy. Clin Rehabil. 2018;32(8):1145–1152.
- Jeong BO, Kang HJ, Bae KY, et al. Determinants of quality of life in the acute stage following stroke. Psychiatry Investig. 2012;9(2):127–133.
- Jones F, Riazi A. Self-efficacy and self-management after stroke: a systematic review. Disabil Rehabil. 2011;33(10):797–810.
- Lang CE, MacDonald JR, Reisman DS, et al. Observation of amounts of movement practice provided during stroke rehabilitation. Arch Phys Med Rehabil. 2009;90(10):1692–1698.
- Hayward KS, Brauer SG. Dose of arm activity training during acute and subacute rehabilitation post stroke: a systematic review of the literature. Clin Rehabil. 2015;29(12):1234–1243.
- Kenah K, Bernhardt J, Cumming T, et al. Boredom in patients with acquired brain injuries during inpatient rehabilitation: a scoping review. Disabil Rehabil. 2018;40(22):2713–2710.
- Harris JE, Eng JJ. Strength training improves upper-limb function in individuals with stroke: a meta-analysis. Stroke. 2010;41(1):136–140.
- van der Lee JH, Snels IA, Beckerman H, et al. Exercise therapy for arm function in stroke patients: a systematic review of randomized controlled trials. Clin Rehabil. 2001;15(1):20–31.
- Mehrholz J, Pohl M, Platz T, et al. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev. 2015;(11):CD006876.
- French B, Thomas LH, Coupe J, et al. Repetitive task training for improving functional ability after stroke. Cochrane Database Syst Rev. 2016;(11):CD006073.
- Corbetta D, Sirtori V, Castellini G, et al. Constraint-induced movement therapy for upper extremities in people with stroke. Cochrane Database Syst Rev. 2015;(10):CD004433.
- Boxall L, Hemsley A, White N. Exploring recruitment issues in stroke research: a qualitative study of nurse researchers' experiences. Nurse Res. 2016;23(5):8–14.
- Mead G, Dennis M, Brady M. Developing easy access patient information booklets and consent forms for use in multicentre stroke trials. Trials. 2013;14(Suppl 1):P58.
- Featherstone K, Donovan JL. Random allocation or allocation at random? Patients' perspectives of participation in a randomised controlled trial. BMJ. 1998;317(7167):1177–1180.
- Busse M, Tyson SF. How many body locations need to be tested when assessing sensation after stroke? An investigation of redundancy in the Rivermead Assessment of Somatosensory Performance. Clin Rehabil. 2009;23(1):91–95.