Abstract
Purpose
To compare demographics, self-reported symptom burden, Health-Related Quality of Life (HRQL), and Self-Efficacy for Exercise (SEE) between participants and non-participants of Rock Steady Boxing (RSB), a non-contact boxing program for individuals with Parkinson’s disease (PD) that focuses on agility, balance, and speed training.
Materials and methods
Adults with PD who had heard of RSB completed a 20 min, 61-question electronic survey including the Parkinson’s Disease Questionnaire-39 (PDQ-39) and the Self-Efficacy for Exercise (SEE) scale. Differences between participants and never-participants were analyzed using chi-squared test, fisher’s exact test and Wilcoxon test.
Results
Of 2054 individuals enrolled in the survey, 1709 were eligible for analysis. 1333 were current participants, 166 previous-participants, and 210 never-participants. RSB participants were median age 69, 59% male, and 97% Caucasian. The majority of current participants reported that RSB improved their social life (70%), fatigue (63%), fear of falling (62%), depression (60%), and anxiety (59%). Compared to previous and never-participants, current participants had better median PDQ-39 scores (36 and 32 vs 25, p < 0.01) and SEE scores (43 and 48 vs 54, p < 0.01).
Conclusions
This is the largest survey of RSB use in PD. RSB participants report improvement in non-motor impairments and have significantly better HRQL and ESE compared to never-participants.
Parkinson’s disease (PD) is a slowly progressive neurodegenerative condition that affects motor function and subsequently, quality of life.
Exercise is increasingly recognized as an important treatment for motor and non-motor symptoms of PD.
Rock Steady Boxing (RSB) is a specific non-contact boxing program for PD that is growing and increasing in popularity, though there is limited data on its effect on PD symptoms and quality of life.
IMPLICATIONS FOR REHABILITATION
Introduction
Parkinson’s disease (PD) is the second most common neurodegenerative disorder, with an estimated United States prevalence of 572 per 100 000 and projected affected population of 930 000 individuals in 2020 [Citation1]. PD is characterized by the classic motor impairments of tremor, bradykinesia, rigidity, and postural instability. Common non-motor manifestation of PD include fatigue, apathy, anxiety, and depression [Citation2]. Non-motor impairments in particular can be disabling and lead to a decreased quality of life. Pharmacologic interventions are notoriously insufficient at addressing non-motor impairments of PD [Citation22]. A growing body of literature supports the benefits of exercise on motor and non-motor features of PD. Studies of aerobic exercise [Citation3], strength training [Citation4], yoga [Citation5], Tai Chi [Citation6], and Tango dancing [Citation7], have all reported a positive impact on gait, balance, and over-all mobility. Importantly, exercise may also benefit the non-motor impairments, including mood, cognition, and sleep [Citation8–11].
Rock Steady Boxing (RSB), a 501(c)(3) nonprofit organization, is a specific non-contact boxing group fitness program designed for people living with all levels of PD. Developed for people with Parkinson’s (PwP), RSB classes are “PD-specific” in that they include multi-modal exercises aimed to improve both fine and large motor impairments of PD (aerobic, strength training, core stability training, balance and flexibility exercises), and encourage loud vocalizations to improve participants’ speech. As a group exercise class, sessions foster socialization and empowerment with partner and team exercises that promote inter-partner encouragement. The ninety-minute classes are taught by “RSB Coaches” who are trained at the RSB headquarters. RSB participants can choose the number of classes they participate in per week and how long they participate in the program. The cost of attending RSB classes varies per site. The RSB program is growing rapidly and broadly. Currently, there are estimated to be 43 500 participants at approximately 900 RSB sites internationally.
As RSB is reaching more PwP, there is a need for more specific data regarding the effect of RSB on PD impairments [Citation12]. To date, research on the benefits of non-contacting boxing for PD has been limited to small studies. Participating in 90 min of RSB approximately one time per week was associated with improvements in gait velocity, mobility, quality of life, and Unified Parkinson’s Disease Rate Scale (UPDRS) motor scores [Citation13,Citation14]. Compared to traditional exercise, a randomized controlled trial of 31 PwP demonstrated that RSB was associated with greater improvements in gait velocity, while both groups significantly improved balance, mobility, and quality of life [Citation15]. Qualitative data from 20 people in one city suggests that the RSB program’s message of empowerment and community-building leads to inspiration, anxiety relief, camaraderie, and maintaining a sense of identity [Citation16]. We aimed to further study patient-reported motor and nonmotor impairments, quality of life, apathy, and self-efficacy through a large survey. The objectives of this study were to (1) understand the demographics and disease profiles of PwP who participate in RSB compared to those who do not, (2) compare their health-related quality of life (HRQL), self-efficacy for exercise (SEE), apathy level, and self-perceived improvement in motor and non-motor impairments, (3) determine aspects of RSB that contribute to continued participation, and (4) identify barriers to RSB participation between PwP who participate in RSB and PwP who do not participate in RSB.
Methods
Study design
A 20 min, 61-item electronic survey was administered online. Participants were recruited during regular clinic visits at the Northwestern University PD and Movement Disorders Center (NUPDMDC) by clinicians who shared an Internal Review Board (IRB)-approved flyer explaining the study and including the survey weblink. Recruitment e-mails containing the survey weblink were sent to existing NUPDMDC patients through a pre-existing list-serve, and the survey weblink was shared on RSB Inc. and the Parkinson Foundation websites. The survey was open for recruitment and participation from July 2018 through February 2019. Eligible participants were adults, aged 18 years or older, with physician-diagnosed PD, who had to have heard of RSB. Prior to participation in the online survey, participants reviewed and signed an electronic consent form informing participants that their participation was voluntary and would not affect their clinical care. While care partners could not consent for or complete the survey on behalf of participants, care partner assistance was permitted for technical support in survey completion. Participants were excluded from data analysis if they did not complete all survey questions. All information obtained during the survey was anonymous, as no personal identifying information was obtained. The study was approved by the Northwestern University IRB.
Survey design and measures
An electronic survey was designed by the study team using the online survey platform, SurveyMonkey®. Survey respondents were first asked if they have heard of RSB, and only individuals responding yes were included in analysis. Next, survey respondents were asked to identify themselves as belonging to one of three groups: current RSB participants, previous participants, or never-participants. All respondents were asked 12 demographic questions and 13 questions about PD disease characteristics. Demographic data included age, sex, employment status, income, and living environment. PD characteristics included years since diagnosis, use of PD medications and/or Deep Brain Stimulation, and details of PD management including use of ancillary services. All respondents answered 16 questions about exercise habits, RSB practices, attitudes towards RSB classes, and barriers to participation in RSB. Previous and current RSB participants were asked what effect RSB had on 4 motor impairments (tremor, medication wearing-off, freezing of gait, and falls) and 8 non-motor impairments (fear of falls, depression, anxiety, sleep, memory loss or other cognitive impairments, fatigue, dizziness or lightheadedness, and social life), with response options of “improved,” “no effect,” or “worsened.”
The survey included three validated patient-reported outcome measures to measure HRQL, SEE, and apathy of participants in all three groups:
The Parkinson’s Disease Questionnaire-39 (PDQ-39) is a 39-item scale of 8 domains. Each item is rated on a 5-point Likert scale with response categories ranging from “never” to “always.” Total scores are scaled from 0 to 100; higher scores indicate lower HRQL. Internal consistency (Cronbach's α = 0.84) has been demonstrated [Citation17]. The Minimal clinically important difference (MCID) for the PDQ-39 is estimated to be −4.72 and +4.22 for clinically relevant improvement and worsening, respectively [Citation18].
The Self-Efficacy for Exercise Scale (SEE) is a 9-item scale that asks individuals to report their confidence in their ability to continue exercise despite the presence of specific barriers, from 0 ("not confident") to 10 ("very confident"). The total score is calculated by summing the responses to each question, with a range of total scores from 0 to 90. A higher score indicates higher self-efficacy for exercise. The reliability, validity, and internal consistency (Cronbach’s α = 0.92) of the SEE has been established in the older adult population [Citation19]. It is primarily used as a screening tool, with limited data on responsiveness to change.
The Starkstein Apathy Scale (SAS), is a 14 questions scale. Each question is scored on a 4-point Likert scale, with response categories ranging from “not at all” to “a lot.” Each answer is assigned point value of 0–3; for questions 1–8, not at all = 3 points; slightly = 2 points; some = 1 point, a lot = 0 point and for questions 9–14, not at all = 0 points; slightly = 1 point; some = 2 points; a lot = 3 points. A higher total score (range 0–42) indicates more severe apathy, with a score greater than 14 or greater is indicative of clinical apathy [Citation20,Citation21]. The SAS has been validated for use in PD with adequate internal consistency (Cronbach's α = 0.69) [Citation22]. It is primarily used as a screening tool, with limited data on responsiveness to change.
Analysis
For demographic data, RSB participants (combined previous and current) were compared to never-participants. For participant-reported improvement in PD impairments, current participants’ responses were compared to previous participants’ responses to assess for statistical difference. Data on facilitators and barriers to RSB participation were compared between current participants, previous participants, and never participants as three separate groups, as well as between all participants (current and previous) and never participants. Scores on the three outcome scales of PDQ-39, SEE, and SAS were compared between the three groups of current participants, previous participants, and never-participants.
Descriptive statistics were calculated for all variables of interest. Categorical variables were summarized with frequencies and percentages, and continuous variables were summarized with medians and interquartile range. Wilcoxon Mann Whitney rank sum test, Kruskal–Wallis test, as appropriate for continuous variable or Chi-squared test, Fisher’s exact test, as appropriate for categorical variable, were used to examine the association between demographic variables, PDQ-39, SEE, SAS and RSB participation. Bar plots with p-value from Wilcoxon test were created to show the difference in median scores on PDQ-39, SEE, SAS between the three groups.
Results
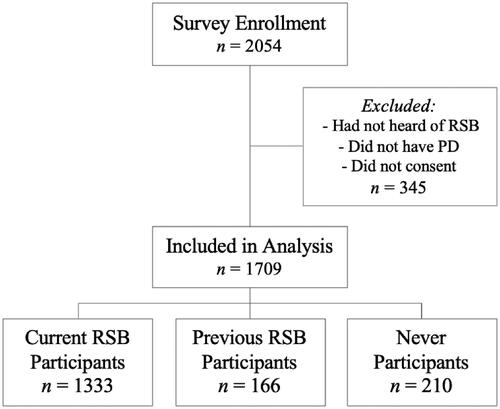
A total of 2054 respondents completed the survey. After 345 were excluded for incomplete data, not having a PD diagnosis, or not having heard of RSB, 1709 respondents qualified for analysis. 87.7% (n = 1499) were current or previous RSB participants; 78% (n = 1333) of these were currently boxing. 210 (12.3%) respondents had heard of but never participated in RSB ().
Participant and non-participant demographics
RSB participants were median age 69 (range 24–96), which differed with statistical significance from never-participants median age of 68 (p = 0.03) (). RSB participants were 59% male, and 97% Caucasian, which did not significantly differ from never-participants (57% male, and 96% Caucasian) (). The groups differed with statistical significance in employment status and marital status; compared to never-participants, a higher percentage of participants were retired (76 vs 66%, p < 0.01) and married/partnered (86 vs 80%, p = 0.03) (). An additional difference was that RSB participants were more likely than never-participants to be taking PD medications (p < 0.05) ().
Table 1. Survey participant demographics.
Table 2. Survey participant disease characteristics and exercise habits.
There were no significant between-group differences in years since PD diagnosis or movement disorders specialist use (67% vs 62% respectively).
Objective measures
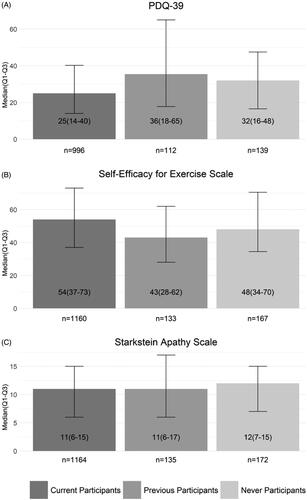
Compared to previous and never-participants, current participants had better median PDQ-39 scores (36 and 32 vs 25, p < 0.01) and better SEE scores (43 and 48 vs 54, p < 0.01), indicating better HRQL and exercise self-efficacy respectively. SAS scores did not significantly differ between the three groups ().
Figure 2. RSB participants’ and never-participants’ self-reported outcome measures. Current, previous, and never participants completed the three scales displayed in A, B, and C. Scale scores are displayed as median (Q1, Q3). PDQ-39 and the SEE Scale score differences were statistically significant between the three groups (p < 0.01).
Self-reported symptom improvement
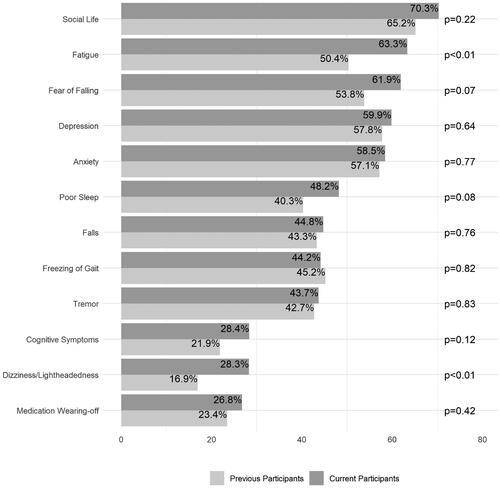
When asked “what effect did RSB have on the following impairments,” the majority of current RSB participants reported improvement in the non-motor impairments of social life (70.3% of participants), fatigue (63.3%), fear of falling (61.9%), depression (59.9%), and anxiety (58.5%) (). Though not the majority, a notable proportion of current RSB participants reported improvement in select motor impairments: tremor (43.7%), falls (44.8%), freezing of gait (44.2%), and medication wearing-off (26.8%) (). Comparing current and previous participants’ responses, there was only a statistically significant difference in percentage reporting improvement in fatigue and lightheadedness/dizziness ().
Figure 3. Participant-reported improvement in PD symptoms. Previous and current RSB participants were asked what effect RSB had on PD symptoms; response options “improved,” “no effect,” or “worsened.” Percentages indicate participants who selected “improved” for the corresponding symptom. Comparison was significant (p < 0.01) for fatigue and dizziness/lightheadedness.
RSB participation: motivation and barriers
Participants and never-participants reported hearing about RSB from various sources including from their physician (26% and 21%), a friend or relative (34% and 29%), their support group (21% for both groups), news (27% and 30%), website (14% and 30%), or pamphlet (6% and 9%). The most common reasons for participating in RSB was the perceived PD-specific nature (83%) and group structure (52%). Regarding group difference in general exercise habits, RSB participants reported more hours of moderate and vigorous exercise (of any modality) per week than never-participants (p < 0.01) ().
The majority of previous participants stopped because of an illness/hospitalization (44%) or inconvenient RSB location (37%). Compared to the 25% of never-participants who stated high cost as a barrier, only 13% of previous participants cited high cost of RSB classes as an impediment.
Among the 12% who never participated, difficult access to RSB sites was the most common reason (37%), followed by high cost (25%). However, the same proportion of never-participants and current participants were current drivers (88% and 87%), with similar proportions relying on others to drive (22% vs 21%, respectively). Additionally, the cost per month for RSB participation was similar between previous and current participants: 63.2% and 56% spending <$100 per month, 27% and 41% spending $100-$200 per month, and less than 2% for each group spending $200–$300 or greater than $300 per month. Few never-participants cited exercise-specific variables as reasons to not participate in RSB: 3% had no interest in non-contact boxing, 2% had no interest in PD-specific exercise, 3% preferred a different form of exercise, and 7% felt the exercises may be too difficult.
Of previous participants, 90% felt that the benefits of RSB were worth the price, similar to the proportion of current participants (99%). Notably, 99% of current and 94% of previous participants would recommend RSB to others with PD.
Discussion
This is the largest survey of RSB participant characteristics and perceived benefits related to participation in the RSB program. This survey revealed high satisfaction with the program, in addition to self-reported benefits on quality of life, depression, anxiety, and fatigue. Previous participants who stopped attending RSB largely did not do so because of dissatisfaction, but rather because of illness or hospitalization. Social life improved in 70% of participants, aligning with the anecdotal suggestion that PwPs are drawn to the social support provided by the class. An attestation of participant loyalty to the RSB program is the fact that the overwhelming majority of participants recommend RSB, including those who no longer participate (94–99%).
RSB participants report improvement in difficult-to-treat nonmotor PD impairments, including fatigue, anxiety, depression, and fear of falling, as well as improvement in their social life. Compared to current participants, previous participants had lower HRQL and exercise self-efficacy, but their self-reported symptom improvements from RSB were similar. This finding possibly suggests that individuals did not stop RSB classes because of a failure to see positive change in their PD impairments. However, this lack of significance may have been due to a small sample of previous participants.
Fatigue is one of the most common non-motor impairments of PD, with reported prevalence between 33% and 58% of PwPs [Citation23]. Fatigue is closely related to impaired HRQL with one of the highest negative effects of non-motor impairments [Citation24]. There is limited evidence of efficacy for any pharmacologic intervention for fatigue in PwPs [Citation23], and a recent systematic review confirmed “low” evidence for exercise treating fatigue in PD [Citation25]. To our knowledge, self-reported fatigue improvement by 63% of RSB participants is an unprecedented level of improvement ascribed to a single intervention.
Similar to fatigue, depression and anxiety are relatively common in PD with prevalence of 20–40%, and 40% respectively [Citation26]. Mood impairments are difficult to treat pharmacologically [Citation26], and there is limited evidence on the effect of exercise modalities [Citation9]. As 60% and 59% of current RSB participants reported improvement in depression and anxiety, respectively, we argue that future randomized controlled trials should investigate the effect of RSB on specific nonmotor symptoms of PD. Clinicians may consider RSB as an adjunct intervention to address these nonmotor impairments.
The prevalence of fear of falling (FOF) in PwPs is estimated to be between 37% and 59% [Citation27,Citation28]. FOF causes activity limitations in 70% of PwPs, and is associated with poor HRQL [Citation28,Citation29]. As 61.9% of RSB participants reported improvement in FOF due to RSB participation, RSB has the potential to decrease activity limitations and improve HRQL by decreasing FOF. While neither RSB participants nor non-participants reported a high frequency of actual falls (87.4% and 81.3% reporting rarely or never falling, respectively), fall frequency was significantly different in participants compared to non-participants (). Given the cross-sectional nature of this survey study, it remains unclear if a lower fall frequency enabled RSB participation, resulted from it, or both.
Higher levels of self-reported physical functioning, social functioning, and vitality have been correlated with better HRQL and greater “preference for current health” in PwP [Citation30]. This survey’s identification of a positive change in social life associated with RSB participation (70.3% of RSB participants reported improvement in this impairment) adds to the limited literature on a specific exercise’s impact on this domain of HRQL [Citation31].
Exercise is an important determinant of HRQL in PD; it has been shown that amongst PwP, consistent exercisers have a smaller decline in HRQL over time than non-exercisers [Citation32]. Studies have shown that specific exercise modalities improve PD-HRQL including resistance exercise [Citation4], treadmill training [Citation33], Tai Chi [Citation10], and Tango [Citation34]. Though the cross-sectional nature of our survey prohibits the establishment of causality, RSB participants did have better HRQL compared to never-participants, exceeding clinically meaningful values [Citation20], suggesting it may be a quality-of-life-affecting exercise modality. Similarly, while RSB participants had higher levels of SEE than never-participants, it is unclear if greater SEE led to RSB participation, resulted from it, or both. Regardless of cause or effect, self-efficacy is more strongly associated with exercise in PwP compared to disability [Citation35] and thus should be considered in future studies. As previously suggested by Afshari et al., the association of higher SEE and increased participation in exercise amongst PwP may represent a feedback loop that is reinforced by activity in the brain’s reward circuitry during exercise [Citation36,Citation37]. However, additional psychometric research is needed to better understand whether SEE is modifiable and responsive to behavioral or exercise interventions.
We did not observe a significant difference in apathy between RSB participation groups in this cross-sectional study. However, prior research has shown that greater baseline participation in physical activity was associated with significantly better apathy at a one-year follow-up, although the magnitude of the between-group differences was small (<3 points) [Citation38]. The median SAS score of our respondents (11–12) indicated that the majority of people taking this survey would not be diagnosed with clinical apathy using the proposed cut-off score of 14 [Citation20,Citation21], potentially reflecting that our web-based sample strategy was less likely to recruit apathetic participants. Further explaining the lack of between-group difference is that the SAS is primarily a screening tool; it has been demonstrated that the SAS does not reflect outcome changes from an intervention [Citation39]. Future research should prospectively evaluate the SAS as a potential outcome measurement rather than a screening tool.
The ability of RSB to improve PwP social life and difficult-to-treat symptoms of depression, anxiety, fatigue, and FOF, suggests that there are advantages of its relatively unique PD-specific group-based exercise style over other exercise forms, such as non-PD-specific non-contact boxing (though no direct comparison exists). However, geographical and financial barriers to RSB does limit its utilization, and need to be addressed in order to improve access.
Limitations
We recognize that our conclusions are limited due to the self-reported cross-sectional nature of our study. In addition, our generalizability is limited by the lack of objective disease severity and motor impairment outcome measurements, with survey questions reliant on participant recall without controlling for the potential confounder of cognitive impairment. Thus, we cannot conclude that there is a causal relationship between RSB participation, improvements in motor or nonmotor impairments, and better HRQL or SEE. Additionally confounding the nature of the relationship between RSB participation and outcomes is the fact that participants could have had concomitant medication or nonpharmacologic management changes contributing to symptom change. Furthermore, recruitment bias was inherent in the utilization of the Parkinson’s foundation and RSB Headquarters websites for recruitment, as never-participants were less likely to be recruited. There may be a respondent bias, with those more satisfied with the intervention being more motivated to respond to the survey. Of note, the majority of survey participants were male, Caucasian, and educated above a high school degree. It is unclear if this adequately represents the demographics of all RSB participants, is a reflection of recruitment bias by use of social media and foundation platforms, or both. This limits the generalizability of recommending RSB as a mode of exercise for all PwP. Further study and analysis of RSB participants’ demographics is necessary to determine participants’ demographic profile.
Conclusions
It is now a widely held position among healthcare providers that regular exercise is an essential component of PD care. PwP who do not exercise regularly have worse quality of life, physical function, and disease progression after controlling for demographics, disease duration and severity [Citation40]. While more providers know to recommend exercise for their PD patients, the method, quantity, and type of exercise intervention to prescribe for specific PD impairments or overall well-being is not clear.
Our study, the largest analysis of RSB use and its effects on PD impairments, helps to inform the Neurology and Movement Disorder community on recommendations for PD patients’ exercise participation. When counseling their patients about exercise, clinicians may benefit from this patient-reported data showing that RSB is associated with less fatigue, depression, anxiety, and fear of falling. Providers and patients should be aware that though not definitively causative, RSB participation is also associated with better HRQL and SEE.
Ethical compliance statement
This research study was approved by Northwestern University’s Internal Review Board.
All participants had to sign an electronic written consent in order to participate in the electronic survey. This electronic informed consent process was approved by the reviewing IRB.
All authors confirm that we have read the Journal’s position on issues involved in ethical publication and we affirm that this work is consistent with those guidelines.
Author contributions
DL: Research project conception, organization, execution; statistical analysis design, review and critique; manuscript: writing the first and subsequent drafts.
CY: Statistical Analysis design, review and critique; manuscript review and critique.
MR: Research project conception; manuscript: editing the manuscript, review and critique.
DB: Research project conception, organization; statistical analysis design, review and critique; manuscript: editing, review and critique.
Acknowledgments
Rock Steady Boxing Headquarters and the Parkinson’s Foundation were instrumental in recruiting RSB participants to our online survey.
Disclosure statement
All authors: no financial disclosures or conflicts of interest concerning the research related to the manuscript. Financial disclosures of all authors for the preceding 12 months: DL has received grant support from the Parkinson Foundation and the Huntington Disease Society of America; CY has no financial disclosures; MR has received honoraria for Parkinson's Foundation Parkinson's Outcomes Project Steering Committee membership and speaking for the Institute for Knowledge Translation. She has received grant support from the Parkinson's Foundation; National Institute on Disability, Independent Living and Rehabilitation Research; Davis Phinney Foundation; Academy of Neurologic Physical Therapy; and Foundation for Physical Therapy; DB serves as a speaker for Acorda Therapeutics, Teva Pharmaceuticals, Adamas Pharmaceuticals, and Neurocrine Biosciences. He has served as a consultant for Acadia Pharmaceuticals, Genentech, Amneal Pharmaceuticals, Biogen Pharmaceuticals, Gerson Lehrman Group, Guidepoint, and L.E.K. Consulting. He is on the editorial board for Annals of Clinical & Translational Neurology. He has received grant support from the Parkinson Foundation and the Huntington Disease Society of America.
References
- Marras C, Beck JC, Bower JH, et al. Prevalence of Parkinson’s disease across North America. NPJ Parkinson's Dis. 2018;4(1):1–7.
- Jankovic J. Parkinson's disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry. 2008;79(4):368–376.
- Shu H-F, Yang T, Yu S-X, et al. Aerobic exercise for Parkinson’s disease: a systematic review and Meta-analysis of randomized controlled trials. PLoS One. 2014;9(7):e100503.
- Corcos DM, Robichaud JA, David FJ, Leurgans SE, et al. A two-year randomized controlled trial of progressive resistance exercise for Parkinson’s disease. Mov Disord. 2013;28(9):1230–1240.
- Van Puymbroech M, Walter A, Hawkins BL, et al. Functional improvements in Parkinson’s disease following a randomized trial of yoga. eCAM. 2018;2018:1–8.
- Li F, Harmer P, Fitzgerald K, et al. Tai chi and postural stability in patients with Parkinson’s disease. NEJM. 2013;9:511–519.
- Lotzke D, Ostermann T, Bussing A. A systematic review and meta-analysis on the effectiveness of tango Argentino in Parkinson’s disease. EuJIM. 2015;7:14–14.
- Reynolds GO, Otto MW, Ellis TD, et al. The therapeutic potential of exercise to improve mood, cognition, and sleep in Parkinson's disease. Mov Disord. 2016;31(1):23–38.
- Kowk J, Kwan J, Auyeung M, et al. Effects of mindfulness yoga vs stretching and resistance training exercises on anxiety and depression for people with Parkinson disease: a randomized clinical trial. JAMA Neurol. 2019;76(7):75.
- Li F, Harmer P, Liu Y, et al. A randomized controlled trial of patient-reported outcomes with tai chi exercise in Parkinson's disease. Mov Disord. 2014;29(4):539–545.
- McNeely ME, Duncan RP, Earhart GM. Impacts of dance on non-motor symptoms, participation, and quality of life in Parkinson disease and healthy older adults. Maturitas. 2015;82(4):336–341.
- Morris ME, Ellis TD, Jazayeri D, et al. Boxing for Parkinson's disease: Has implementation accelerated beyond current evidence? Front Neurol. 2019;10:1222.
- Coombs SA, Diehl MD, Staples WH, et al. Boxing training for patients with Parkinson disease: a case series. Phys Ther. 2001;91(1):132–142.
- Coombs SA, Diehl MD, Chrzastowski C, et al. Community-based group exercise for persons with Parkinson disease: a randomized controlled trial. Neurorehabilitation. 2013;32(1):117–124.
- Larson D, Bega D. Effects of Rock Steady Boxing on activities of daily living and motor symptoms of Parkinson’s disease. AAN Annual Meeting, Los Angeles, CA, April 2018.
- Sheehy TL, McDonough MH, Zauber SE. Social comparisons, social support, and Self-Perceptions in group exercise for people with Parkinson's disease. J Appl Sport Psychol. 2017;29(3):285–303.
- Jenkinson C, Fitzpatrick R, Peto V, et al. The Parkinson's Disease Questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age Ageing. 1997;26(5):353–357.
- Horvath K, Aschermann Z, Kovács M, et al. Changes in quality of life in Parkinson’s disease: how large must they be to be relevant? Neuroepidemiology. 2017;48(1–2):1–8.
- Resnick B, Jenkins LS. Testing the reliability and validity of the self-efficacy for exercise scale. Nurs Res. 2000;49(3):154–159.
- Starkstein SE, Mayberg HS, Preziosi TJ, et al. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. J Neuropsychiatry Clin Neurosci. 1992;4(2):134–139.
- Starkstein SE, Merello M, Jorge R, et al. The syndromal validity and nosological position of apathy in Parkinson's disease. Mov Disord. 2009;24(8):1211–1216.
- Pedersen KF, Alves G, Larsen JP, et al. Psychometric properties of the Starkstein apathy scale in patients with early untreated Parkinson disease. Am J Geriatr Psychiatry. 2012;20(2):142–148.
- Herlofson K, Kluger B. Fatigue in Parkinson's disease. J Neurol Sci. 2017; 374:38–41.
- Friedman J, Friedman H. Fatigue in Parkinson's disease. Neurology. 1993;43(10):2016–2018.
- Khan F, Bhasker A. Management of fatigue in neurological disorders: implications for rehabilitation. JISPRM. 2018;1(2):9–36.
- Gallagher DA, Schrag A. Psychosis, apathy, depression and anxiety in Parkinson's disease. Neurobiol Dis. 2012;46(3):581–589.
- Lindholm B, Hagell P, Hansson O, et al. Factors associated with fear of falling in people with Parkinson's disease. BMC Neurol. 2014;14:19.
- Grimbergen YAM, Schrag A, Mazibrada G, et al. Impact of falls and fear of falling on health-related quality of life in patients with Parkinson's disease. J Parkinsons Dis. 2013;3(3):409–413.
- Bloem BR, Grimbergen YA, Cramer M, et al. Prospective assessment of falls in Parkinson's disease. J Neurol. 2001;248(11):950–958.
- Morimoto T, Shimbo T, Orav JE, et al. Impact of social functioning and vitality on preference for life in patients with Parkinson's disease. Mov Disord. 2003;18(2):171–175.
- Rodriques de P, et al. Impact of an exercise program on physical, emotional, and social aspects of quality of life in individuals with Parkinson’s disease. Mov Disord. 2006; 21(8):1073–1077.
- Rafferty MR, Schmidt PN, Luo ST, et al. Regular exercise, quality of life, and mobility in Parkinson’s. JPD. 2017;7(1):193–202.
- Harro C, Shoemaker M, Frey O, et al. The effects of speed-dependent treadmill training and rhythmic auditory-cued overground walking on balance function, fall incidence, and quality of life in individuals with idiopathic Parkinson's disease: a randomized controlled trial. Neurorehabilitation. 2014;34(3):541–556.
- Hackney M, Earhart G. Health-related quality of life and alternative forms of exercise in Parkinson disease. Parkinsonism Relat Disord. 2009;15(9):644–648.
- Ellis T, Cavanaugh JT, Earhart GM, et al. Factors associated with exercise behavior in people with Parkinson disease. Phys Ther. 2011;91(12):1838–1848.
- Afshari M, Yang A, Bega D. Motivators and barriers to exercise in Parkinson’s. JPD. 2017;7(4):703–711.
- Sacheli M, Murray D, Vafai N, et al. Exercise alters response of reward anticipation in the ventral striatum of subjects with Parkinson’s disease [abstract]. Mov Disord. 2017;32(suppl 2):1064–1066. http://www.mdsabstracts.org/abstract/exercise-alters-response-of-reward-anticipation-in-the-ventral-striatum-of-subjects-with-parkinsons-disease/
- Ng Y-E, eta al. Physical activity improves anxiety and apathy in early Parkinson’s disease: a longitudinal follow-up study. Front Neurol. 2021;11:1908.
- Wetmore JF, Arbelo JM, Catalan MJ, et al. Psychometric properties of the apathy scale in advanced Parkinson's disease. Parkinsons Dis. 2019;2019:1965394.
- Oguh O, Eisenstein A, Kwasny M, et al. Back to the basics: regular exercise matters in Parkinson's disease: results from the National Parkinson Foundation QII registry study. Parkinsonism Relat Disord. 2014;20(11):1221–1225.