Abstract
Purpose
The aim of this prospective cohort study was to evaluate the level of physical activity, self-efficacy and health-related quality of life in patients with chronic pain, at baseline and one year after physiotherapy rehabilitation at a specialist pain clinic.
Materials and methods
All patients who underwent rehabilitation at the physiotherapy unit at the Pain Centre at Sahlgrenska University Hospital/Östra in Gothenburg during a nine-month period were asked to participate in the study. The participants were evaluated regarding self-efficacy, health-related quality of life (HRQoL) and physical activity during physiotherapy treatment and one year later. Physical activity was measured both subjectively (self-reported physical activity) and objectively (accelerometer).
Results
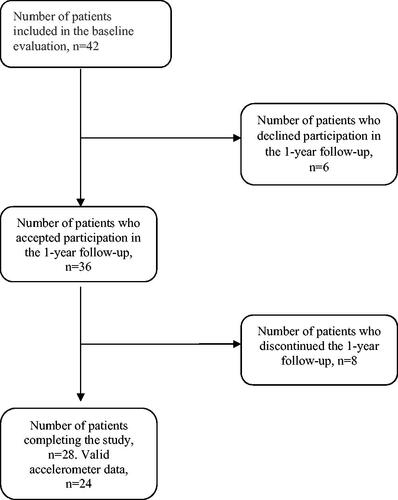
Out of 42 patients who participated in the baseline evaluation, 28 (19 women and nine men) were included in the one-year follow-up. The patients had increased levels of vigorous physical activity at one-year follow-up, without deterioration of pain. There were no significant changes regarding self-efficacy and HRQoL. Levels of physical activity and perceived physical function may be associated to levels of physical activity 1 year after rehabilitation.
Conclusion
Patients with chronic pain can increase their level of vigorous physical activity after a period of rehabilitation without deterioration of pain.
Physical activity is an important part of rehabilitation for chronic pain patients, but many patients expect more pain after exercise, which they fear may affect performance and maintenance of physical activity.
Patients with chronic pain at a specialist clinic increased their level of vigorous physical activity one year after physiotherapist led rehabilitation without deterioration of pain.
Levels of physical activity and perceived physical function during rehabilitation may predict levels of physical activity 1 year after rehabilitation.
Physiotherapist led rehabilitation seems to be beneficial for long-term improved physical activity in patients with chronic pain
IMPLICATIONS FOR REHABILIATION
Introduction
Chronic pain is defined as pain that lasts or recurs for longer than 3 months [Citation1]. In Europe, approximately 20% of adults suffer from moderate-to-severe chronic pain [Citation2]. For many patients, pain severely affects daily life and leads to impairment and disability, resulting in poor health-related quality of life (HRQoL) [Citation3]. Furthermore, chronic pain is associated with anxiety, depression, sleeping disorders and cardiovascular diseases (CVDs) [Citation4,Citation5]. The socio-economic costs for chronic pain are high and of the top six most common causes of sick leave or early retirement in Sweden, three of the diagnoses are linked to chronic pain conditions [Citation6].
There is strong evidence indicating that the level of chronic systemic diseases and depression is lower in adults who are physically active [Citation7]. Furthermore, exercise has been shown to have a positive effect on physical function, mental health and sleep [Citation4]. The current guidelines for physical activity (PA) are that healthy adults are recommended to spend at least 150 min/week in moderate-intensity aerobic PA or at least 75 min/week in vigorous intensity aerobic PA or an equivalent combination of moderate- and vigorous intensity activity [Citation7–9]. Every minute spent in PA may be included in the total volume of PA accumulated per day [Citation8].
Physical activity has many important and positive effects in patients with chronic pain, including reducing the consequences of pain for the individual. It also decreases the risk of diseases related to inactivity – CVDs, diabetes, obesity, and cancer – as well as improving HRQoL [Citation10]. However, being active often comes with considerable obstacles and barriers (physical as well as behavioural and mental) for patients with chronic pain. Depending on the pain condition, the response to exercise can vary between hypoalgesia, reduced hypoalgesia, and hyperalgesia in patients with chronic pain [Citation11], which can constitute one of the barriers of doing exercise.
A large European survey reports that 50% of patients with chronic pain are limited in performing exercise and 23% are unable to exercise because of their pain. The survey also reports that 79% of patients with chronic pain experience that activities during the day cause increased pain [Citation2]. Several studies indicate that levels of PA are lower in patients with chronic low back pain compared with healthy controls [Citation12–14] and that chronic pain often leads to behavioural changes, with reduced PA being a common consequence [Citation15,Citation16].
The term “self-efficacy” describes the “confidence a person has in her or his own ability to achieve a desired outcome in a given situation” [Citation17]. Studies have shown that a higher level of self-efficacy is associated with a lower level of pain and disability [Citation18–21]. Furthermore, self-efficacy seems to be more important than pain intensity and pain duration in determining disability [Citation22]. It has also been demonstrated that higher self-efficacy correlates to better physical functioning, better health and work status and fewer depressive symptoms [Citation20]. Results from previous studies indicate that high self-efficacy regarding physical exercise in healthy adults correlates with physical exercise level. In these studies, patients with high self-efficacy conducted more physical exercise [Citation23].
Consequently, PA is now part of treatment for patients with chronic pain. However, the exact nature of PA (dose, duration) and the long-term effects of performing PA are insufficiently known. It would be of added value to predict which patients are more likely to be more active after rehabilitation.
There is limited knowledge about whether patients continue to be physically active after rehabilitation with a focus on exercise. A study evaluating supervised physiotherapy in patients with fibromyalgia showed that the patients improved their muscle function and health status, had better management of daily activities and had reduced pain intensity during the ongoing rehabilitation programme. However, in the long-term follow-up, the effects had decreased back to baseline values [Citation24]. This indicates that patients may have difficulties maintaining PA without professional support.
Therefore, the aim of this follow-up study was to evaluate the level of PA, self-efficacy and HRQoL in patients with chronic pain, during and one year after physiotherapy rehabilitation at a specialist pain clinic. A secondary aim was to study whether the patients achieved the recommended level of PA, and whether the level of PA after one year was related to any of the measures at baseline (self-efficacy, HRQoL, level of PA).
Materials and methods
Study design
The present study is a prospective cohort study. The study is a one-year follow-up based on an earlier research study evaluating an instrument to evaluate self-efficacy described elsewhere, which constitutes the baseline [Citation25].
Patients and recruitment
During ninth month, all patients who underwent rehabilitation at the physiotherapy unit at the Pain Centre at Sahlgrenska University Hospital/Östra in Gothenburg, Sweden, were consecutively invited to participate in a study, which constitutes the baseline of this study [Citation25]. The physiotherapy rehabilitation included a thorough examination from a biopsychosocial perspective. Thereafter, an individually tailored treatment plan focused on improving physical function was created. The patients received an exercise programme and were encouraged to exercise regularly at home or at the clinic. The treatment period lasted for at least six months, but the treatment period varied between the patients in accordance with individual goals and needs. Inclusion criteria were age over 18 years and ability to understand Swedish. The patients had a variety of chronic pain diagnoses including nociceptive pain, neuropathic pain and nociplastic pain. The present study included a follow-up one year after the baseline evaluation, which was during the rehabilitation period. All patients, who participated in the first study, were invited via a letter to participate in the follow-up study. After receiving the letter, the patients were contacted by telephone and asked about participation. All patients received written study information, and those who agreed to participate signed an informed consent.
Procedure
All patients who participated in this follow-up study had already completed questionnaires about personal characteristics (age and employment status), as well as number of years with pain, self-efficacy, HRQoL and level of PA, during the baseline measurements described above. To complement the questionnaires, they also wore an accelerometer, for seven days, to measure level of PA. One year after the first evaluation, they were sent the same questionnaires again together with an accelerometer to be used for seven days. The questionnaires and the accelerometer were returned by the patients via postal mail.
Assessments
Physical activity
Physical activity was both objectively measured with an accelerometer and self-reported using a questionnaire. The participants wore the Axivity AX3 accelerometer (Axivity Ltd, Newcastle upon Tyne, UK) over the right hip in an elastic belt around the waist. Accelerometers in this position provide an accurate measure of overall PA intensity [Citation26]. The participants were instructed to wear the accelerometer 24 h a day during seven consecutive days but to take it off during water activities. The accelerometer was set to collect triaxial data with a sampling frequency of 50 Hz. The downloaded raw accelerometer data were processed into 60-s epoch ActiGraph counts for each of the three axes (x, y, z) using validated open-source algorithms [Citation26]. Non-wear time was defined as 60 min of continuous zero counts and excluded, and a valid day included at least 10 h of wear-time. Participants with at least four valid days were included in further analyses.
The vector magnitude (VM) of the counts from the three axes was calculated (VM = √[x2 + y2 + z2]) and used to determine the time spent in moderate PA (MPA) (2,690–6,166 counts per minute) and vigorous PA (VPA) (≥6,167 counts per minute), applying the cut points by Sasaki et al. [Citation27]. Time in MPA and VPA was summed for each day, where MPA was provided a weight of 1 and VPA had a weight of 2 (MVPA = MPA + 2*VPA) [Citation28].
This calculation is in accordance with how the two intensity levels are weighted in international recommendations of PA [Citation7]. Two definitions of compliance were applied: a total of at least 150 min of MVPA (the less strict definition), and accumulation of at least 30 min per day of MVPA on at least 5 days of the week, that is, most days (the stricter definition) [Citation8]. In cases where participants had fewer than seven valid days, time spent in MVPA/VPA and compliance with the PA recommendations was estimated by extrapolating from the valid days to seven days.
Self-reported PA was assessed with questions from Haskell et al. [Citation29], formulated as follows: “How many times a week do you devote yourself to 30 min of moderate exercise?” and “How many times per week do you devote yourself to 20 min of vigorous physical activity?” Each question has eight possible answers, from zero times a week to seven times a week, and scores between 0 and 18.9 points can be calculated. Higher scores indicate higher level of PA. A mean of 5 points or more corresponds to complying with the PA recommendation of at least 30 min/day of MVPA on at least 5 days of the week.
Self-efficacy
Self-efficacy was evaluated using the Arthritis Self-efficacy Scale (ASES) and Self-efficacy for Exercise Scale (SEE). The ASES is a self-assessment questionnaire originally designed for patients with arthritis, using three subdomains: pain, perceived function, and other symptoms [Citation30]. The Swedish version of the ASES has been tested for reliability and validity [Citation31–33]. The SEE is a questionnaire that assesses self-efficacy especially for exercise [Citation34]. It has been evaluated for various patient groups and recently it was evaluated for patients with chronic pain [Citation25,Citation34].
Health-related quality of life
Health-related quality of life was measured using the RAND-36 and EuroQol Five Dimensions (EQ-5D) visual analogue scale (VAS). The RAND-36 is a modified version of the 36-item Short Form Health Survey (SF-36), which is a generic HRQoL questionnaire [Citation35]. The Swedish version has been evaluated for reliability and validity [Citation35,Citation36]. The EQ-5D VAS is a health barometer that records patients’ self-rated health. Scores range from 0 to 100. Zero corresponds to the worst possible health state and 100 to the best possible health state. The instrument has been evaluated for reliability and validity in patients with chronic pain [Citation37].
Statistical analyses
The data were analysed with non-parametric tests. Dichotomized variables were analysed with McNemar’s test, while Wilcoxon signed-rank test was used for continuous variables; both tests consider repeated measures within individuals. Spearman’s rank correlation coefficient (rs) was used for all correlations, which were interpreted according to Munro: 0.00–0.25 very weak or no correlation, 0.26–0.49 weak correlation, 0.50–0.69 moderate correlation, 0.70–0.89 strong correlation and 0.90–1.00 very strong correlation [Citation38]. All tests were two-tailed, and a significance level of 5% was used. Statistical analyses were performed using IBM SPSS software, version 25 (IBM, Armonk, NY, USA).
Ethical approval
The initial study was approved by the Regional Ethical Review Board in Gothenburg (Dnr: 1087-17). The Swedish Ethical Review Authority approved the supplementary application for the 1-year follow-up study (Dnr: 2019-01250). Before enrolling in the study all patients gave oral and written consent.
Results
Patients
A total of 42 patients, 30 women and 12 men, participated in the baseline evaluation. From this group, 36 agreed to participate in the one-year follow-up (). During the year, eight patients dropped out. In total, 19 women and nine men completed the one-year follow-up. In addition, four patients were excluded from the accelerometer analyses because of too short wear time. Baseline characteristics are presented in . There were no differences between baseline values for the patients who participated in the 1-year follow-up (n = 28) and all patients who were included in the baseline evaluation (n = 42) with regard to gender, age, self-assessed level of PA, self-efficacy (SEE and ASES) and HRQoL (EQ-5D VAS and RAND-36).
Table 1. Demographic data on the study population.
Patients meeting the recommendations regarding level of physical activity
At baseline, 79% of the participants achieved the recommendation of 150 min/week of MVPA, but only 29% were adherent when using the stricter criteria of 30 min/day of PA on at least 5 days of the week (). At one-year follow-up, the proportion of the patients meeting the recommendation of 150 min/week of PA was the same. However, the proportion of patients adhering to the stricter criteria increased from 29% to 54% (), although the difference was not statistically significant.
Table 2. Number of patients fulfilling the recommendations for level of physical activity, n = 24.
Level of physical activity, self-efficacy and quality of life during and 1 year after rehabilitation
No significant increase in time spent in MVPA was found at follow-up (median of 244 min/week at baseline and 321 min/week at follow-up, p = 0.130) (). However, there was a significant increase in time spent in VPA (4 min/week at baseline and 18 min/week at follow-up, p = 0.002). displays the differences in MVPA and VPA between baseline and follow-up for each participant. Fourteen participants (58%) increased their level of MVPA and 16 (67%) increased their VPA.
Figure 2. Individual differences regarding minutes per week in vigorous physical activity (VPA) and moderate and vigorous physical activity (MVPA) between baseline and follow-up. Positive numbers indicate an increase during follow-up compared to baseline. *MVPA is calculated using MPA + 2*VPA.
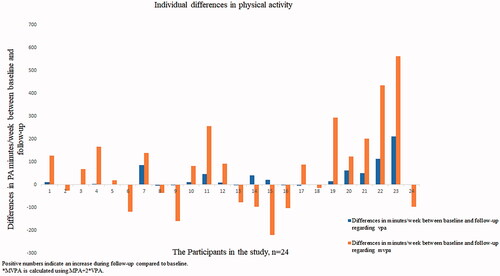
Table 3. Level of physical activity, self-efficacy and health-related quality of life at baseline and 1-year follow-up.
There were no significant changes regarding self-assessed level of PA, self-efficacy (SEE and ASES) or HRQoL, according to the RAND-36 or EQ-5D VAS, between baseline and one-year follow-up (). However, after one year, the patient group reported self-assessed level of PA >5, corresponding to the PA recommendation of at least 30 min/day of MPVA on at least 5 days of the week.
Correlations between level of physical activity during follow-up, and baseline values of physical activity, self-efficacy and health-related quality of life
There was a moderate correlation in minutes per week of accelerometer MVPA between baseline and the one-year follow-up (rs = 0.64), as well as in VPA between the two measurement points (rs = 0.55). This indicates that a higher level of PA may predict a higher level of PA 1 year later (). Self-reported level of PA at baseline also correlated with a higher level of both MVPA and VPA 1 year later. No correlations were seen between SEE score at baseline and level of PA one year later. However, the scores for the ASES subgroup perceived function and the RAND-36 subgroup physical functioning were moderately to strongly correlated to level of PA after 1 year (rs = 0.61, rs = 0.73).
Table 4. Correlations between moderate and vigorous physical activity (MVPA), vigorous physical activity (VPA), self-efficacy and health-related quality of life (HRQoL) at baseline, and accelerator-measured level of MVPA and VPA at follow-up.
Discussion
The main finding of this prospective study was that patients with chronic pain who had undergone physiotherapy rehabilitation had increased levels of VPA one year after rehabilitation, without deterioration of pain (ASES, RAND-36). This has clinical value as many patients with chronic pain referred to multidisciplinary pain treatment expect more pain after exercise and describe that exercise increases their pain at the beginning of an exercise programme [Citation11]. As vigorous PA may confer the greatest health benefits in CVD-healthy individuals, this finding has clear clinical relevance. The finding is also important because behavioural changes such as long-term compliance to exercise programmes are difficult to achieve.
At an individual level, some patients did decrease their level of physical activity during follow-up though. This may be due to the specific week that was measured; one year after the first measurement. Activity level can vary between the weeks. Though, the significant results on a group level indicate that it is possible for this group of patients to maintain and even increase level of PA without deterioration of pain, even though the rehabilitation period is over.
A higher level of PA, bodily function and self-efficacy regarding perceived function was correlated to the level of PA, both to level of MVPA and to level of VPA, one year after rehabilitation. From a clinical perspective, this indicates that the patients’ physical function and self-efficacy seem important in maintaining or increasing the level of PA. The relevance of these results for clinical practice would be that it may be of importance to evaluate these parameters before the end of the rehabilitation period and provide extra support to patients with lower PA in order to maintain or increase their PA. Results from a previous study that investigated PA on prescription (PAP) in primary health care settings, with 298 patients participating at a 6 months’ follow-up, reported a significant change in self-reported physical exercise, with patients performing more physical exercise, measured using four different questionnaires, as well as increased HRQoL, after the intervention [Citation39]. It would be interesting to investigate whether PAP can increase or maintain levels of PA in patients with chronic pain after completing physiotherapy rehabilitation, especially those with low PA [Citation40].
In contrast to results shown previously, self-efficacy for exercise in this study was not correlated to level of PA [Citation23]. In this patient group, self-efficacy regarding perceived function may be more important than self-efficacy for exercise in predicting level of PA, both on a moderate and on a vigorous level, after one year.
Regarding the degree of fulfilment of the current recommendations for PA, 79% of the patients in the study met the recommendations both at baseline and during follow-up. Using the stricter recommendation for PA (30 min/day on 5 days of the week), 29% of the patients met the recommendations at baseline and 54% did during follow-up. This finding may be interpreted as a positive change in activity patterns, where the participants distributed their activity over the week. This is also in line with the patients’ self-reported PA, as the patients on average reported that they met these recommendations.
Since the current recommendations for PA are based on studies using self-reported data, our use of objective measurements should be interpreted with caution. Self-reported PA and objectively assessed PA generally show low to moderate agreement [Citation41]. Nonetheless fewer than half of our patients reported adherence to the recommendations. This highlights the importance of improving the level of PA in patients with chronic pain to reduce the risk of both morbidity and mortality from many chronic diseases [Citation10], and also to reduce secondary effects of pain such as depression, anxiety and sleeping disturbance [Citation4]. In addition, exercise therapy over 8–12 weeks may induce clinically relevant reductions in pain in patients with osteoarthritis [Citation42]. Further, a single session of exercise might induce hypoalgesia in persons without pain (exercise-induced hypoalgesia). In patients with chronic pain, the response can vary between hypoalgesia, reduced hypoalgesia, and, the opposite effect, hyperalgesia (i.e., increased sensitivity to pain), depending on the pain condition [Citation11]. This may also explain why the evidence is inconsistent regarding the effects of improved pain severity after a period of exercise in chronic pain conditions [Citation4].
Strengths and limitations
A strength of this study is that all patients who underwent rehabilitation at the physiotherapy unit were asked to participate in the study. We therefore believe that the study population can be considered a representative sample of patients with chronic pain undergoing physiotherapy at a specialist clinic. The heterogeneity of the diagnosis can be questioned though. The patients had different diagnoses of chronic pain including nociceptive pain, neuropathic pain and nociplastic pain. The results may have differed if the diagnoses were separated, but from a clinical perspective, the consequences of chronic pain on physical activity can be seen regardless of the origin of pain. It is also shown in the literature that physical activity can be affected in different pain conditions [Citation16,Citation43,Citation44]. Therefore, we chose to include all kinds of chronic pain.
The results must be interpreted with caution, since this is a small study and that 14 out of 42 patients either did not participate in or did not complete the one-year follow-up, which may have affected the results. Patients interested in PA may have been more interested in study participation, which must be taken into consideration. However, there were no differences between baseline values for these patients and the patients who participated in the one-year follow-up. There are several possible explanations for the dropout phenomenon; one is that the accelerometer had to be worn for seven consecutive days, which may be difficult for some patients. If the follow-up would have taken place at the hospital setting, the compliance may have been better. However, this was not possible for practical reasons. During the initial phone interview, several patients mentioned that they were experiencing a lot of stress and that it was too stressful to complete all the questionnaires and to wear the accelerometer for seven days.
Furthermore, the study population was relatively small. It is important to recognize this as it is difficult to detect significant changes in small groups. There is also a higher risk that the study sample is unrepresentative of the whole population when a study sample is small. In further research, a larger sample size may be needed.
An additional factor that might have affected the results was that the patients had individual treatment programmes with different rehabilitation progress and duration. Some of the patients had recently completed their treatment programme at 1-year follow-up and therefore may have had a higher level of PA and self-efficacy compared with the patients with faster rehabilitation progress who had completed their treatment programme earlier, a long time before the one-year follow-up. This variation is inevitable in clinical practice.
Future research
Further research in larger patient populations with an even longer follow-up is needed to investigate the level of PA after completed physiotherapy rehabilitation. Furthermore, more research is needed to examine the correlation between self-efficacy and level of PA and investigate whether self-efficacy can predict the level of PA at follow-up.
Moreover, it would be interesting to study level of PA in patients with chronic pain before, during and 1 year after physiotherapy rehabilitation, to assess levels of PA, self-efficacy and HRQoL and analyse possible predictive factors for increased PA.
Conclusions
Patients with chronic pain can increase their level of vigorous PA after physiotherapy rehabilitation without deterioration of pain. These findings are of clinical importance, as health benefits may be greater from vigorous PA. Level of PA and perceived physical function may predict level of PA 1 year after rehabilitation. Thus, physiotherapist led rehabilitation seems to be beneficial for long-term improved physical activity in in patients with chronic pain.
Acknowledgements
We would like to thank physiotherapist Eva-Lotte Karlsson for assisting in data collection. Our thanks also go to the participating patients whose work has made this study possible.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain. 2019;160(1):19–27.
- Breivik H, Collett B, Ventafridda V, et al. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333.
- Lerman SF, Rudich Z, Brill S, et al. Longitudinal associations between depression, anxiety, pain, and pain-related disability in chronic pain patients. Psychosom Med. 2015;77(3):333–341.
- Geneen LJ, Moore RA, Clarke C, et al. Physical activity and exercise for chronic pain in adults: an overview of cochrane reviews. Cochrane Database Syst Rev. 2017;4(4):CD011279.
- Fayaz A, Ayis S, Panesar SS, et al. Assessing the relationship between chronic pain and cardiovascular disease: a systematic review and meta-analysis. Scand J Pain. 2016;13:76–90.
- Gustavsson A, Bjorkman J, Ljungcrantz C, et al. Socio-economic burden of patients with a diagnosis related to chronic pain-register data of 840,000 Swedish patients. Eur J Pain. 2012;16(2):289–299.
- Bull FC, Al-Ansari SS, Biddle S, et al. World health organization 2020 guidelines on physical activity and sedentary behaviour. Br J Sports Med. 2020;54(24):1451–1462.
- health.gov. [internet] Office of Disease Prevention and Health Promotion; Physical Activity Guidelines Advisory Committee. 2018. Physical Activity Guidelines Advisory Committee Scientific Report 2018 [cited 20201201]. Available from: 2018 Physical Activity Guidelines Advisory Committee Scientific Report (health.gov.)
- Piepoli MF, ESC Scientific Document Group, Hoes AW, Agewall S, et al. 2016 European guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–2381.
- health.gov. [Internet] Washington (DC): Department of Health and Human Services; Physical Activity Guidelines for Americans 2018. [cited 20201201]. Available from: https://health.gov/sites/default/files/2019-09/Physical_Activity_Guidelines_2nd_edition.pdf.
- Vaegter HB, Jones MD. Exercise-induced hypoalgesia after acute and regular exercise: experimental and clinical manifestations and possible mechanisms in individuals with and without pain. Pain Rep. 2020;5(5):e823.
- Ryan CG, Grant PM, Dall PM, et al. Individuals with chronic low back pain have a lower level, and an altered pattern, of physical activity compared with matched controls: an observational study. Aust J Physiother. 2009;55(1):53–58.
- Spenkelink CD, Hutten MMR, Hermens HJ, et al. Assessment of activities of daily living with an ambulatory monitoring system: a comparative study in patients with chronic low back pain and nonsymptomatic controls. Clin Rehabil. 2002;16(1):16–26.
- Al-Obaidi SM, Al-Zoabi B, Al-Shuwaie N, et al. The influence of pain and pain-related fear and disability beliefs on walking velocity in chronic low back pain. Int J Rehabil Res. 2003;26(2):101–108.
- Ellingson LD, Shields MR, Stegner AJ, et al. Physical activity, sustained sedentary behavior, and pain modulation in women with fibromyalgia. J Pain. 2012;13(2):195–206.
- Weering MGH, Vollenbroek-Hutten MMR, Tönis TM, et al. Daily physical activities in chronic lower back pain patients assessed with accelerometry. Eur J Pain. 2009;13(6):649–654.
- Bandura A. Self-efficacy: the exercise of control. New York: W.H. Freeman and company; 1997.
- Costa LDCM, Maher CG, McAuley JH, et al. Self-efficacy is more important than fear of movement in mediating the relationship between pain and disability in chronic low back pain. Eur J Pain. 2011;15(2):213–219.
- Woby SR, Urmston M, Watson PJ. Self-efficacy mediates the relation between pain-related fear and outcome in chronic low back pain patients. Eur J Pain. 2007;11(7):711–718.
- Martinez-Calderon J, Zamora-Campos C, Navarro-Ledesma S, et al. The role of self-efficacy on the prognosis of chronic musculoskeletal pain: a systematic review. J Pain. 2018;19(1):10–34.
- Jackson T, Wang Y, Wang Y, et al. Self-efficacy and chronic pain outcomes: a meta-analytic review. J Pain. 2014;15(8):800–814.
- Denison E, Asenlof P, Lindberg P. Self-efficacy, fear avoidance, and pain intensity as predictors of disability in subacute and chronic musculoskeletal pain patients in primary health care. Pain. 2004;111(3):245–252.
- Sherwood NE, Jeffery RW. The behavioral determinants of exercise: implications for physical activity interventions. Annu Rev Nutr. 2000;20:21–44.
- Larsson A, Palstam A, Lofgren M, et al. Resistance exercise improves muscle strength, health status and pain intensity in fibromyalgia-a randomized controlled trial. Arthritis Res Ther. 2015;17:161.
- Dahlbäck A, Andréll P, Varkey E. Reliability and aspects of validity of the swedish version of Self-Efficacy for exercise scale for patients with chronic pain. 2020; [Manuscript submitted for publication].
- Brønd JC, Andersen LB, Arvidsson D. Generating ActiGraph counts from raw acceleration recorded by an alternative monitor. Med Sci Sports Exerc. 2017;49(11):2351–2360.
- Sasaki JE, John D, Freedson PS. Validation and comparison of ActiGraph activity monitors. J Sci Med Sport. 2011;14(5):411–416.
- Arvidsson D, Leijon M, Sundquist J, et al. Cross-cultural validation of a simple self-report instrument of physical activity in immigrants from the Middle east and native swedes. Scand J Public Health. 2014;42(3):255–262.
- Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American college of sports medicine and the American heart association. Med Sci Sports Exerc. 2007;39(8):1423–1434.
- Shoor SM, Holman HR. Development of an instrument to explore psychological mediators of outcome in chronic arthritis. Trans Assoc Am Physicians. 1984;97:325–331.
- Lomi C. Evaluation of a Swedish version of the arthritis self-efficacy scale. Scand J Caring Sci. 1992;6(3):131–138.
- Lomi C, Burckhardt C, Nordholm L, et al. Evaluation of a Swedish version of the arthritis self-efficacy scale in people with fibromyalgia. Scand J Rheumatol. 1995;24(5):282–287.
- Lomi C, Nordholm LA. Validation of a Swedish version of the arthritis self-efficacy scale. Scand J Rheumatol. 1992;21(5):231–237.
- Rydwik E, Hovmoller F, Bostrom C. Aspects of reliability and validity of the Swedish version of the self-efficacy for exercise scale for older people. Physiother Theory Pract. 2014;30(2):131–137.
- Sullivan M, Karlsson J. The Swedish SF-36 health survey III. Evaluation of criterion-based validity: results from normative population. J Clin Epidemiol. 1998;51(11):1105–1113.
- Orwelius L, Nilsson M, Nilsson E, et al. The Swedish RAND-36 health survey – reliability and responsiveness assessed in patient populations using Svensson's method for paired ordinal data. J Patient Rep Outcomes. 2017;2(1):4.
- Linde L, Sørensen J, Ostergaard M, et al. Health-related quality of life: validity, reliability, and responsiveness of SF-36, 15D, EQ-5D [corrected] RAQoL, and HAQ in patients with rheumatoid arthritis. J Rheumatol. 2008;35(8):1528–1537.
- Munro B. Statistical methods for health care research. 3rd ed. New York: Lippincott Williams & Wilkins; 1997.
- Kallings LV, Leijon M, Hellenius ML, et al. Physical activity on prescription in primary health care: a follow-up of physical activity level and quality of life. Scand J Med Sci Sports. 2007;18(2):154–161.
- Lundqvist S, Börjesson M, Cider Å, et al. Long-term physical activity on prescription intervention for patients with insufficient physical activity level-a randomized controlled trial. Trials. 2020;21(1):793.
- Prince SA, Adamo KB, Hamel ME, et al. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act. 2008;5:56.
- Skou ST, Roos EM. Physical therapy for patients with knee and hip osteoarthritis: supervised, active treatment is current best practice. Clin Exp Rheumatol. 2019;37(5):112–117.
- Kichline T, Cushing CC, Ortega A, et al. Associations between physical activity and chronic pain severity in youth with chronic abdominal pain. Clin J Pain. 2019;35(7):618–624.
- Segura-Jiménez V, Álvarez-Gallardo IC, Estévez-López F, et al. Differences in sedentary time and physical activity between female patients with fibromyalgia and healthy controls: the al-Ándalus project. Arthritis Rheumatol. 2015;67(11):3047–3057.