 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Purpose
Ankle-foot orthoses (AFOs) are used to improve physical performance measures of physical function (PF) post-stroke; however, the perception of improved PF of this population has not been described. The purpose of this study was to identify the predictors of self-reported PF of individuals seeking orthotic intervention post-stroke.
Materials and Methods
A retrospective analysis of 237 patients at a nationwide orthotic services provider in the United States was conducted to characterize PF using the Patient-Reported Outcome Measures Information System®. A backward stepwise multiple regression was conducted to identify demographic characteristics predictive of self-reported PF.
Results
The mean T-score of PF of the sample was 30.8 (6.5), two standard deviations below the US general population mean, indicating significant impairment. The regression model explained approximately 15% (R = 0.411) of the variance in PF of the sample. Self-reported PF was worse for individuals requiring more supportive assistive devices (
= 0.270, p = 0.001), those with more recent ankle problems (
= −0.167, p = 0.035), and those with greater living assistance (
= −0.139, p = 0.089).
Conclusions
These results improve understanding of the factors that contribute to impaired self-reported PF of stroke survivors in need of AFO intervention.
Ankle-foot orthoses (AFOs) are often used to improve physical performance measures of physical performance (PF) during stroke rehabilitation.
Our data indicate that the self-reported PF of AFO users is severely impaired.
Level of assistance, time since ankle and foot problems began, and living assistance status are important clinical characteristics to consider when planning AFO intervention for this population.
Implications for rehabilitation
Introduction
Stroke has a negative impact on an individual’s physical function (PF) and, depending on the severity of the stroke, has the potential to limit the ability to walk, balance, and maintain a level of independence conducive to the high quality of life [Citation1]. Treatments are often targeted at improved PF with the aim to regain as much functional independence as possible. Ankle-foot orthoses (AFOs) have been shown to facilitate recovery of PF such as walking speed, balance, and energy efficiency [Citation2–5]. These improvements are notably reflected within the laboratory or clinic-based assessments. The degree to which the individual experiences similar improvements outside the research or clinical setting while using the AFO is not fully understood; however, it is the ultimate clinical aim of rehabilitation intervention [Citation6]. Patient-reported outcome measures (PROMs) of physical function provide clinicians the ability to monitor and assess the impact of rehabilitation, including post-stroke, beyond a controlled setting and are influenced by more global experiences including day-to-day variability in ability and activities of daily living (ADLs) [Citation7].
Several instruments have been developed and studied in the stroke population as the need for valid and reliable PROMs has grown, and most assess PF as an important domain of self-reported health. The Medical Outcomes Study 36-item Short-Form Health Survey (SF-36) became a widely used PROM in clinical trials [Citation8]. A primary benefit of the SF-36 is its generic application, allowing for the comparison of self-reported health across a wide range of diagnoses, including the US general population. The SF-36 however was found to have limitations in the post-stroke population [Citation9], including significant floor effects, making it difficult to measure self-reported health outcomes among those with more severe levels of impairment [Citation10]. Over time, the Stroke Impact Scale-16 (SIS-16), emerged as a preferred measure of PF as it captured underlying disability that had gone previously undetected by practitioner-reported outcome measures, even in patients with mild stroke [Citation11–Citation12]. While the SIS-16 may be a suitable candidate, there are a variety of conditions that necessitate AFO intervention, thus preventing the necessary comparison of outcomes outside the post-stroke population. Despite the strengths of the SIS, stroke-specific outcome measures of PF also do not allow for comparison to estimated function prior to stroke—or the general, non-affected population [Citation13].
Clinicians and researchers across a wide range of specialties encounter these issues in assessing impairment and PF in search of appropriate outcome measures [Citation14–16]. To meet the demand for valid and reliable self-reported outcome measures, the National Institutes of Health developed the Patient-Reported Outcome Measurement Information System (PROMIS®) [Citation17]. PROMIS® assessments are designed for use across multiple chronic conditions and are scaled to the US general population. Scaling provides the opportunity to compare scores within a specific health domain (e.g.s., fatigue, social participation, PF) to the average, non-affected individual [Citation17–20]. PROMIS® measures of PF have proven highly useful in the post-stroke population; they perform similarly to the SIS-16, are resistant to floor effects, have a good correlation with other commonly used functional scales [Citation21], and are sensitive to changes in PF over time [Citation22].
Self-reported PF has been identified as the most negatively impacted health domain following a stroke [Citation23]. Within the general stroke population, there is a spectrum of recovery; some survivors will make a complete recovery while others only partially regain their ability to walk. Those with permanent PF impairment in need of support through AFO intervention may represent a subset of the stroke population with impairment exceeding the average stroke survivor. To evaluate this, a nationwide orthotic services provider in the United States administered a PROM form for use during routine clinical care for individuals post-stroke seeking AFO intervention. The form was customized to capture demographic information, patient-reported factors, practitioner-reported factors, and eight items selected from the PROMIS® Item Bank v1.0 – Physical Function. The purpose of this manuscript was to describe the self-reported PF of individuals post-stroke seeking AFO intervention and to identify which patient- or practitioner-reported factors best predict PROMIS® measures of PF.
Materials and methods
AFO user outcome collection form
This multi-site retrospective analysis study was approved by the University of Houston’s Institutional Review Board. The AFO User Outcome Collection Form was developed by subject matter experts within the orthotic and prosthetic services provider in 2016 using items from the PROMIS® Item Bank v1.0 – Physical Function based on the items’ potential relatedness to AFO intervention. The custom short form was administered in clinics across the United States as a part of routine clinical care to monitor the PF of individuals seeking lower limb orthotic care. The form collects demographic and medical history items reported by the patient, clinical characteristics reported by the treating practitioner, and eight PROMIS® PF assessment questions: PFA56, PFA30, PFA9, PFC6r1, PFC38, PFA41, PFB50, PFA1.
Inclusion criteria
The data were collected through paper-based administration between January 2016 and June 2020. Patients over 18 years old with a diagnosis of stroke were included in the analysis. Only cases with PROMIS® outcome assessments at an initial evaluation appointment were included.
Data analysis
PF scores were calculated from the responses and standardized to the general US adult population using the online HealthMeasures Scoring Service provided through PROMIS®. Scores were calibrated to the default sample provided by the scoring service. The resultant scores are scaled such that a mean T-score for the US general population is 50.0 with a standard deviation of 10.0. Higher T-scores represent a higher level of PF. Lower T-scores represent more impaired PF. Responses were identified as outliers if the calculated T-score was
3 standard deviations beyond the data sample mean.
Backward stepwise regression was used to estimate the model that best predicts PF among AFO users. Hypothesized predictors of PF were selected a priori based on previous studies utilizing self-reported measures of PF [Citation24–31]. The six factors were: gender, age, pain, fall history, AFO history, and ankle mobility. A log transformation of pain level was applied to pain scores due to responses being positively skewed towards a low level of pain. The model containing the hypothesized predictors was simplified through backward elimination based on a threshold of p < 0.05.
A secondary analysis was also conducted to examine how each of the patient-reported variables predicted PF. The goal of this secondary analysis was to provide insight into novel factors of PF that have not previously been investigated in this population. The intent of this analysis is to provide a novel contribution to the evidence base regarding a larger potential set of self-reported measures of PF in a highly impaired sample. In the secondary analysis, a second backward elimination regression was conducted with all patient-reported factors entered into the regression: age, gender, body mass index (BMI), employment status, living status, the time at which they began to experience problems with their foot and ankle, frequency of brace wear, use of an assistive device, participation in physical therapy, stumble frequency, fall frequency, ability to walk 25 feet, and log-transformed pain level in the past seven days. BMI was calculated from self-reported height and weight.
Results
A total of 237 respondents met the inclusion criteria for the analysis. Respondent demographics can be found in and . Three outliers were excluded from the analysis due to residuals that were more than three standard deviations away from the mean. The mean T-score of PF was 30.8 (6.5) with a range from 15.3 to 47.7, data are shown in . The mean age of the respondents was 62.2 (
11.9) years. The mean BMI of the sample was 28.9 (
6.6) kg/m2. The mean pain level reported was 3.60 (
3.06) prior to log transformation. A majority of the respondents were male (59.7%), disabled or retired (88.7%), living with some form of assistance (62.4%), and began experiencing problems over two years ago (53.5%). Most respondents were receiving some form of physical therapy (61.8%) and 67.0% of respondents reported that they were able to walk 25 feet on a level surface.
Figure 1. Distribution of self-reported physical function T-score for the sample. Mean (SD) = 30.8 (6.5). Mean (SD) T-score for physical function for the general U.S. population = 50 (10). Higher T-scores indicate better physical function.
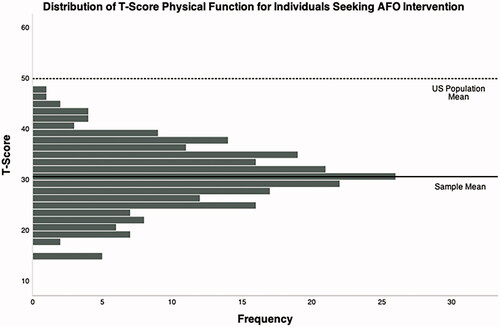
Table 1. Respondent demographics.
Table 2. Respondent device and fall history.
Most respondents reported the use of an assistive device (78.2% of total sample: cane 36.4%, two canes 2.4%, walker 18.2%, Hemi walker 5.5%, wheelchair 15.8%). Among those who had previously used an AFO (50.4%), 77.2% (88 out of 114) reported wearing their AFO on a daily basis. Some of the respondents reported experiencing a fall in the previous 4 weeks (46.5%). Among fallers, 43.8% (46 out of 105) reported falling three or more times. Fifty-four percent (54%) of respondents experienced a stumble in the previous 4 weeks. Among those who stumbled, 72.8% (83 out of 114) reported stumbling more than three times.
Despite prior reports of specific predictors within the evidence base, none of the predictors chosen for the primary analysis (gender, age, pain, fall history, AFO history, and ankle mobility) were significant predictors of self-reported PF in this sample. The results of the secondary analysis revealed that three of the thirteen patient-reported factors were significant predictors of PF among individuals post-stroke seeking AFO intervention (F(3,144) = 9.733, p < 0.001) (). Assistive device type emerged as the strongest predictor of PF ( = −0.270, p = 0.001, VIF = 1.201), followed by problem onset (
= −0.167, p = 0.035, VIF = 1.060), and living status (
= −0.139, p = 0.089, VIF = 1.137). Overall, the model explained approximately 15% (R = 0.411) of the variance in PF of individuals post-stroke seeking AFO intervention.
Table 3. Regression analysis summary for patient-reported variables predicting physical function.
Discussion
This retrospective analysis was conducted to identify which patient- and practitioner-reported factors best predicted PROMIS® measures of PF for individuals post-stroke seeking AFO intervention. Though supported by existing literature, our initially hypothesized predictors (gender, age, pain, fall history, AFO history, and ankle mobility) were not supported by the results. Although the regression model only explained 15.1% of the variance in PF, assistive device type, time since problems with the foot and ankle began, and living status emerged as significant predictors of PF for the sample. PF was lower for those who required more support from an assistive device (e.g., none vs. single point cane vs. walker), those who more recently began having foot and ankle problems, and those who require more living assistance (e.g., living at home independently vs. at home with assistance vs. skilled nursing facility). The results suggest that these clinical characteristics (including type of assistive device, time since problem onset, and living status) may be important to consider when developing treatment goals for this population. We also acknowledge that living status contributed to the significance of the secondary analysis while itself presenting at p = 0.089 within the model; indicating that this factor should be considered in conjunction with other measures of PF, not independently.
The mean PF T-score of the sample was 30.7 ( 6.5), indicating that the impairment of PF in individuals post-stroke seeking AFO intervention is significantly lower than the general US population. T-scores below 30.0, which is two standard deviations below the US general population mean of 50.0, are considered to reflect the “severe impairment” of PF per the PROMIS® system. All T-scores reported in the sample were significantly below the general population reference value and were significantly lower than previous reports of PROMIS® PF T-score in the post-stroke population. A retrospective analysis on a large clinical sample of individuals post-stroke reported a mean PROMIS® PF T-score of 41.4 (
11.5), approximately one standard deviation below the US general population [Citation21].
One potential explanation for the significantly lower scores in the present study is the condition under which an individual needs lower limb orthotic intervention post-stroke. Unmet care needs related to rehabilitation have been shown to influence self-reported health in the post-stroke population [Citation32] and those seeking AFO intervention may cross a threshold of PF impairment that requires above-average treatment and resources to improve. Despite most of the respondents currently using an assistive device and receiving physical therapy, the severity of PF impairment was such that additional support from an AFO was required.
The prevalence of falls and stumbles in this sample could be driving these individuals to seek out further PF treatment options, such as an AFO: 46.5% of the sample reported at least one fall in the previous month and 20.4% reported three or more falls. Fifty-four percent (54%) of the sample reported at least one stumble in the previous month, and 39.3% reported three or more stumbles. These findings support the notion that patients who need AFO intervention may have needs distinct from the general stroke population.
The possibility that individuals seeking AFO intervention may represent a unique subgroup of the post-stroke population worthy of further attention is supported by previous work. A previous study demonstrated that PROMIS® PF scores are also correlated with the modified Rankin Scale (mRS) [Citation21], a widely accepted measure of functional independence after stroke [Citation33]. The mRS categorizes global disability by the level of assistance a person requires in carrying out activities of daily living post-stroke. The ability to walk without assistance in part distinguishes “moderate disability” (able to walk without assistance) from “moderately severe disability” (unable to walk without assistance), and “severe” disability on the mRS is characterized by the need for constant nursing care and attention. In the present study, both living status and assistive device type were predictive of PROMIS® PF and reflect similar group distinctions found in the mRS; 78.2% of the respondents reported the use of an assistive device and 62.4% were living with the assistance of some form. The large proportion of respondents utilizing some form of assistance in the present study may explain why the initially hypothesized predictors of PF were not supported by the results. Predictors of self-reported health in the post-stroke population are different among those who are ADL-dependent versus ADL-independent [Citation32]; therefore, previously established predictors of PF (e.g., age and pain) may be less important for individuals with severe PF impairment, as seen in the current study sample.
Interestingly, PF T-scores were higher for individuals who had been dealing with foot and ankle problems for a longer period of time. Because problems with the foot and ankle are likely associated with the timing of the stroke in this population, it is possible that these individuals have worked to overcome PF limitations over time and have greater acceptance of their “new normal”. Conversely, those whose stroke symptoms began more recently may still be coping with the acceptance of their post-stroke disability, as their pre-stroke levels of PF are easier to recall for comparison. Though this explanation is in support of previous findings [Citation34], the current study did not measure disability acceptance. Future longitudinal studies should consider how disability acceptance impacts patient-reported outcome measures over time, particularly for disability secondary to an acute traumatic event such as stroke, brain injury, or spinal cord injury, among others. Controlling for time since stroke and disability acceptance can help future studies distinguish improvements in PF secondary to targeted therapies from improvements that may have naturally occurred with time.
AFO history (i.e., whether the respondent was currently using an AFO) did not emerge as a significant predictor of PF in this study as originally hypothesized. However, of those who were currently using an AFO, 77.2% (88 out of 114) reported wearing their AFO daily. It is reasonable to believe that an individual would not use an orthosis every day if there was no perceived benefit; therefore, the questions included in the custom short form may not be sensitive to the impact of AFOs on self-reported PF, particularly for those with severe PF impairment. This finding raises the question of how PF of daily AFO users might be affected if the AFOs were discontinued, however, the retrospective approach of this study could not address this question.
Given how low self-reported PF was in the current study, the questions included from the PROMIS® item bank reference activities that could be considered highly demanding for the post-stroke population such as, walking 100 meters, walking at a normal speed, stepping up and down curbs, squatting and getting up, and the ability to participate in vigorous activities. It is possible that the inclusion of items that capture more basic PF, such as standing unsupported, standing from a toilet, or completing a transfer without assistance would enhance the sensitivity of the measure to AFO intervention. However, self-reported PF scores were normally distributed, supporting previous findings that PROMIS® items are resistant to floor effects [Citation21]. Alternatively, AFO intervention may influence factors more indirectly related to PF, such as balance confidence [Citation35]. It is common for post-stroke AFO intervention studies to investigate the most direct mechanism of action for improved PF from AFOs. For example, the translation of dorsiflexion assistance (body/physical function treatment) into improved walking performance is a typical study done in this area. Though these are important treatment theories to establish, there is a need for future investigations on the effects of AFO treatment that are more difficult to directly measure such as social participation or global health.
One of the primary strengths of this study was the unique population studied and the use of validated questions from the PROMIS® item bank for PF. The severe PF impairment reported in this study is novel, as previous investigations of self-reported PF in the post-stroke population reported mild to moderate levels of impairment [Citation10,Citation11,Citation21]. In addition, the responses were collected during routine clinical care procedures, rather than during the rehabilitation phase. This likely provided a more representative sample of the post-stroke population which included individuals unlikely to meet inclusion criteria or not motivated for laboratory-based studies on PF. The multi-site nature of the study also considers the different rehabilitative approaches that can occur in different regions of the country. The results should be interpreted with caution, however, as the overall variance explained by the three predictors was low, at 15.1%. It remains largely unknown which factors influence the self-reported physical function of patients who need an AFO after stroke. One limitation of this study is that cognitive function was not measured and could therefore have influenced the results. In addition, no instructions were provided to the respondents as to the definition of a stumble or a fall.
Conclusion
Individuals post-stroke seeking AFO intervention report severe PF impairment, supported by the current data set. Self-reported PF of the sample in this study well below previous reports of PF in the post-stroke population as well as the general, unimpaired population. Most of the sample presented within the current data set reported having received what is considered standard levels of care; a majority of respondents reported the use of an assistive device, had previously received AFO intervention and were currently participating in physical therapy. Assistive device type, time since problems with the foot and ankle began, and living status emerged as significant predictors of PF; however, these factors only explained 15% of the variance in self-reported PF of the sample. Future studies are needed to identify more influential predictors of self-reported PF, to examine the sensitivity of PROMIS® outcome measures to AFO intervention, and the long-term impact of orthotic treatment on self-reported PF in individuals post-stroke.
Acknowledgements
The authors would like to thank Dweisha England, Mandi Laurie, and Akshay Tolani for their efforts with data input.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Schneider S, Taba N, Saapar M, et al. Determinants of long-term health-related quality of life in young ischemic stroke patients. J Stroke Cerebrovasc Dis. 2021;30(2):105499.
- Tyson SF, Kent RM. Effects of an ankle-foot orthosis on balance and walking after stroke: a systematic review and pooled meta-analysis. Arch Phys Med Rehabil. 2013;94(7):1377–1385.
- Tyson SF, Sadeghi-Demneh E, Nester CJ. A systematic review and meta-analysis of the effect of an ankle-foot orthosis on gait biomechanics after stroke. Clin Rehabil. 2013;27(10):879–891.
- Daryabor A, Yamamoto S, Orendurff M, et al. Effect of types of ankle-foot orthoses on energy expenditure metrics during walking in individuals with stroke: a systematic review. Disabil Rehabil. 2020. DOI:10.1080/09638288.2020.1762767.
- Daryabor A, Arazpour M, Aminian G. Effect of different designs of ankle-foot orthoses on gait in patients with stroke: a systematic review. Gait Posture. 2018;62:268–279.
- Whyte J. Contributions of treatment theory and enablement theory to rehabilitation research and practice. Arch Phys Med Rehabil. 2014;95(1 Suppl):S17–23 e2.
- Salinas J, Sprinkhuizen SM, Ackerson T, et al. An international standard set of patient-centered outcome measures after stroke. Stroke. 2016;47(1):180–186.
- Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: scoping review. SAGE Open Med. 2016;4:2050312116671725.
- Hobart JC, Williams LS, Moran K, et al. Quality of life measurement after stroke: uses and abuses of the SF-36. Stroke. 2002;33(5):1348–1356.
- Lai SM, Perera S, Duncan PW, et al. Physical and social functioning after stroke: comparison of the stroke impact scale and short form-36. Stroke. 2003;34(2):488–493.
- Lai SM, Studenski S, Duncan PW, et al. Persisting consequences of stroke measured by the stroke impact scale. Stroke. 2002;33(7):1840–1844.
- Kasner SE. Clinical interpretation and use of stroke scales. Lancet Neurol. 2006;5(7):603–612.
- Reeves M, Lisabeth L, Williams L, et al. Patient-reported outcome measures (PROMs) for acute stroke: rationale, methods and future directions. Stroke. 2018;49(6):1549–1556.
- Brehm M, Bus SA, Harlaar J, et al. A candidate core set of outcome measures based on the international classification of functioning, disability and health for clinical studies on lower limb orthoses. Prosthet Orthot Int. 2011;35(3):269–277.
- Fatone S, Jerousek S, Slater BCS, et al. Identifying instruments to assess care quality for individuals with custom ankle foot orthoses: a scoping review. Arch Phys Med Rehabil. 2021;102(4):709–734.
- Robinson C, Fatone S. You’ve heard about outcome measures, so how do you use them? Integrating clinically relevant outcome measures in orthotic management of stroke. Prosthet Orthot Int. 2013;37(1):30–42.
- Rose M, Bjorner JB, Becker J, et al. Evaluation of a preliminary physical function item bank supported the expected advantages of the patient-reported outcomes measurement information system (PROMIS). J Clin Epidemiol. 2008;61(1):17–33.
- Rose M, Bjorner JB, Gandek B, et al. The PROMIS physical function item bank was calibrated to a standardized metric and shown to improve measurement efficiency. J Clin Epidemiol. 2014;67(5):516–526.
- Cook KF, Jensen SE, Schalet BD, et al. PROMIS measures of pain, fatigue, negative affect, physical function, and social function demonstrated clinical validity across a range of chronic conditions. J Clin Epidemiol. 2016;73:89–102.
- Schalet BD, Hays RD, Jensen SE, et al. Validity of PROMIS physical function measured in diverse clinical samples. J Clin Epidemiol. 2016;73:112–118.
- Katzan IL, Fan Y, Uchino K, et al. The PROMIS physical function scale: a promising scale for use in patients with ischemic stroke. Neurology. 2016;86(19):1801–1807.
- Lapin B, Thompson NR, Schuster A, et al. Clinical utility of patient-reported outcome measurement information system domain scales. Circ Cardiovasc Qual Outcomes. 2019;12(1):e004753.
- Katzan IL, Thompson NR, Uchino K, et al. The most affected health domains after ischemic stroke. Neurology. 2018;90(16):e1364–e1371.
- Alschuler KN, Jensen MP, Sullivan-Singh SJ, et al. The association of age, pain, and fatigue with physical functioning and depressive symptoms in persons with spinal cord injury. J Spinal Cord Med. 2013;36(5):483–491.
- Bjerk M, Brovold T, Skelton DA, et al. Associations between health-related quality of life, physical function and fear of falling in older fallers receiving home care. BMC Geriatr. 2018;18(1):253.
- Smit EB, Bouwstra H, van der Wouden JC, et al. Development of a Patient-Reported outcomes measurement information system (PROMIS(R)) short form for measuring physical function in geriatric rehabilitation patients. Qual Life Res. 2020;29(9):2563–2572.
- Deyo RA, Katrina R, Buckley DI, et al. Performance of a patient reported outcomes measurement information system (PROMIS) short form in older adults with chronic musculoskeletal pain. Pain Med. 2016;17(2):314–324.
- Vanden Wyngaert K, Van Craenenbroeck AH, Eloot S, et al. Associations between the measures of physical function, risk of falls and the quality of life in haemodialysis patients: a cross-sectional study. BMC Nephrol. 2020;21(1):7.
- Queen RM, Grier AJ, Butler RJ, et al. The influence of concomitant triceps surae lengthening at the time of total ankle arthroplasty on postoperative outcomes. Foot Ankle Int. 2014;35(9):863–870.
- Phisitkul P, Rungprai C, Femino JE, et al. Endoscopic gastrocnemius recession for the treatment of isolated gastrocnemius contracture: a prospective study on 320 consecutive patients. Foot Ankle Int. 2014;35(8):747–756.
- Gianakos A, Yasui Y, Murawski CD, et al. Effects of gastrocnemius recession on ankle motion, strength, and functional outcomes: a systematic review and national healthcare database analysis. Knee Surg Sports Traumatol Arthrosc. 2016;24(4):1355–1364.
- Bjalkefur K, Nasic S, Bertholds E, et al. Self-rated health over the first five years after stroke. BMC Neurol. 2020;20(1):389.
- Banks JL, Marotta CA. Outcomes validity and reliability of the modified rankin scale: implications for stroke clinical trials: a literature review and synthesis. Stroke. 2007;38(3):1091–1096.
- Chiu SY, Livneh H, Tsao LL, et al. Acceptance of disability and its predictors among stroke patients in Taiwan. BMC Neurol. 2013;13:175.
- Zissimopoulos A, Fatone S, Gard S. The effect of ankle-foot orthoses on self-reported balance confidence in persons with chronic poststroke hemiplegia. Prosthet Orthot Int. 2014;38(2):148–154.
