Abstract
Purpose
Self-management for patients with bronchiectasis has been identified as an important component that could potentially empower patients to manage their condition and improve their quality of life. Evidence was reviewed to investigate what self-management programmes work, why and in what circumstances.
Methods
A systematic review and realist synthesis were conducted. A comprehensive database search was performed on seven databases for evidence published up to July 2021. Leading candidate self-management programmes identified from the systematic review became the focus of the realist synthesis. A realist logic of analysis was applied to produce explanatory context-mechanism-outcome configurations. These explanations were consolidated into programme theories drawing on health behaviour change theory.
Results
By synthesising the data from eight eligible articles, programme theories articulated how three different self-management programmes work that included: (i) education and action planning, (ii) education and airway clearance techniques (ACT) and, (iii) education, exercise and ACT. Patient characteristics and collaborative partnership between healthcare professionals and patients were identified as important contexts that influenced the improvement in self-efficacy, health-related quality of life, and exercise capacity.
Conclusions
This review contributes to a better understanding of how the complex interaction between contexts and mechanisms can improve outcomes of clinical interest.
This evidence synthesis has identified potentially important combinations of interventions to be considered in self-management programmes for adults with bronchiectasis.
Collaborative partnership between patient and healthcare professionals should be considered to improve short-term self-efficacy.
Targeting self-management programmes to increase short-term health-related quality of life and exercise capacity should consider the context of patient characteristics.
IMPLICATIONS FOR REHABILITATION
Introduction
Bronchiectasis is a long-term respiratory condition characterised by abnormal and permanent dilation of the bronchi [Citation1] with the diagnosis being established through clinical history and confirmed with a computed tomography (CT) scan [Citation1]. The burden of bronchiectasis to patients can be debilitating, leading to symptoms of breathlessness, wheezing, cough and chest pain, often resulting in a poorer quality of life [Citation2,Citation3] and clinical fatigue [Citation4].
The economic burden of bronchiectasis to society is significant with hospitalisation as the major driver of costs, especially in patients with frequent exacerbations [Citation5]. Exacerbations are a key feature of patients’ disease burden with almost 75% of patients (n = 1403) from the BronchUK registry reporting exacerbations in a 12-month period [Citation6]. Adherence to treatment and prescribed medication, however, may be as low as 20% in up to 50% of bronchiectasis patients [Citation7]. Reasons for low levels of adherence are diverse with research highlighting age and beliefs about negative consequences of therapy as primary factors [Citation8]. It is therefore paramount that patients are supported to use self-management interventions to improve adherence and learn to take control over the management of their condition.
Self-management is advocated by the World Health Organisation [Citation9] and can be defined as the ability to manage a chronic condition including its symptoms, treatment, physical and social ramifications, and lifestyle adjustments [Citation10]. There are well-established disease-specific self-management programmes for asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis [Citation11–13] but, despite a recommendation in Step 1 of the British Thoracic Society (BTS) guidelines, current evidence regarding self-management for bronchiectasis is very limited. Self-management in other chronic respiratory diseases consists of a variety of components and approaches such as exercise programmes and action plans with evidence showing that it can have significant improvement in health-related quality of life (HRQoL) and a decrease in healthcare utilisation [Citation14–16].
Common self-management interventions to most international guidelines include antibiotics, airway clearance techniques (ACT), and pulmonary rehabilitation [Citation1,Citation17]. The aim is to break the vicious recurrent cycle of infection, inflammation, impaired mucociliary clearance and structural lung damage. In addition to these interventions, treatment-seeking behaviour plans [Citation18] and an educational component are pivotal to facilitate patients with an understanding of the basic principles of disease management and recognition of an exacerbation to enable timely intervention [Citation19]. It is imperative therefore to investigate the efficacy and utility of self-management for patients with bronchiectasis; a research recommendation identified by the European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC) [Citation20], the Association of Respiratory Nurse Specialists UK [Citation21], and the BTS [Citation1].
A Cochrane review of randomised control trials found insufficient evidence to determine whether self-management interventions were effective in patients with bronchiectasis [Citation22]. Self-management programmes need to be developed to meet different contexts and needs of subpopulations of patients with bronchiectasis. A realist synthesis acknowledges the variation in self-management programme design and may offer practical insights to produce behaviour change by uncovering causal processes involved in patient behaviour. This review, therefore, extended the work of the Cochrane review by including all study designs. This systematic review and realist synthesis aimed to investigate what self-management programmes work, why and in what circumstances. We tested and refined an initial programme theory that posited: (i) the interventions that should be considered in self-management programmes, (ii) the mechanisms that facilitated delivery and uptake of self-management programmes, and (iii) the optimal outcomes for measuring the impact such programmes.
Method
Reporting and methods of the systematic review and realist synthesis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [Citation23] and Realist and Meta-Review Evidence Synthesis: Evolving Standards (RAMESES) guidelines [Citation24]. The full review protocol was registered on PROSPERO (CRD42018110103).
Search strategy
We searched the following sources: MEDLINE (Ovid), EMBASE (Ovid), CINAHL (EBSCO), AMED (Ovid), Web of Science Core Collection using six journal citation indexes (Science Citation Index Expanded [1970-present], Social Sciences Citation Index [1970-present], Arts & Humanities Citation Index [1975-present], Conference Proceedings Citation Index-Science [1990-present], Conference Proceedings Citation Index-Social Science & Humanities [1990-present], Emerging Sources Citation Index [2015-present]), Cochrane CENTRAL, and the clinical trials registries (clinicaltrials.gov and ICTRP, https://www.who.int/ictrp/en/). Searches were conducted from the inception of each database up to July 2021 and were limited to the English language. Details of the search strategy are available in an online Supplementary file (Tables S1-S3). Handsearching of grey literature was conducted on OpenGrey (http://www.opengrey.eu), Grey Literature Report (http://www.greylit.org), and the World Health Organisation (http://who.int/en).
Database searching was supplemented with CLUSTER (Citations, Lead Authors, Unpublished materials, Scholar searches, Theories, Early examples, and Related projects) searching [Citation25]. CLUSTER is one of the first models of searching for evidence for systematic reviews of complex interventions and it is particularly useful for identifying relevant theory [Citation26]. This maximised identification of evidence either instrumentally linked (i.e., “sibling studies”) or theoretically associated (i.e., “kinship studies”) with eligible studies [Citation27]. We undertook the identification of the most influential studies (i.e., key "pearl" citations) and performed a forward citation search on the key pearl citations to identify all related data (Step 5 of CLUSTER). We manually searched reference and publication lists of lead authors and co-authors as appropriate (Steps 2–4) and made contact with authors for relevant unpublished data (Step 7). Finally, we conducted searches by project name/identifier where applicable for other relevant projects (Steps 6, 12 and 13).
Study selection
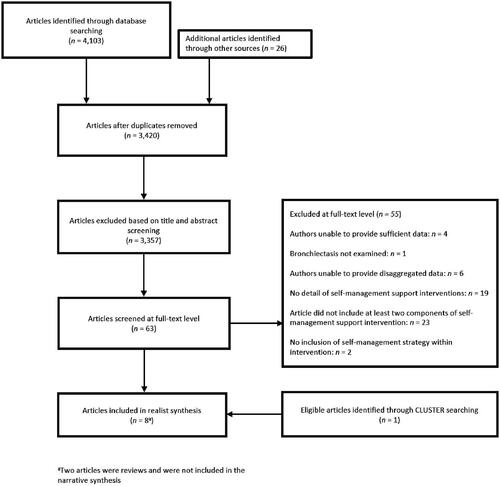
Self-management was defined as structured support interventions for individuals with bronchiectasis designed to improve self-health behaviours and self-management skills [Citation13]. As this review was an extension of a recent Cochrane review [Citation22], we defined a self-management programme as one that consists of two or more interventions as illustrated in , along with the full eligibility criteria following the PICO framework [Citation28]. Search results were compiled using Mendeley (v. 1.19.1). Following the removal of duplicates, two reviewers (AT and CK) independently screened titles and abstracts for inclusion and screened the full-text of retrieved articles for eligibility. Reasons for exclusion were recorded. Disagreements were resolved by consensus or discussion with a third reviewer (DL).
Table 1. Study selection criteria.
Data extraction and quality assessment
Study characteristics including participants, methods, interventions, and outcomes were extracted by one reviewer (AT) and verified by a second reviewer (CK). There were no disagreements regarding data extraction. Additionally, relevant sections of texts relating to contexts, mechanisms and/or their relationships to outcomes were tabulated and organised in an evidence table to enable thematising of emerging patterns of context-mechanism-outcome configurations (CMOCs).
The methodological quality of eligible articles were independently assessed by two reviewers (AT and HM). The risk of bias in randomised controlled trials (RCTs) were assessed using the seven domains comprising the risk of bias tool in the Cochrane Handbook of Systematic Reviews [Citation29], with each source of bias rated as low, high, or unclear. The Critical Appraisal Skills Programme (CASP) Qualitative Study Checklist [Citation30] was used to appraise qualitative studies. The quality of retrospective studies were assessed using the National Institutes for Health tool for studies with no control group [Citation31]. Grey literature was appraised using the Accuracy, Coverage, Objectivity, Date, Significance (AACODS) checklist [Citation32]. We resolved disagreements by consulting with a third reviewer (CK).
Data synthesis
All data were synthesised narratively due to the descriptions of the interventions were too heterogeneous to allow for pooling. This involved textual descriptions of the articles and tabulation of data. The leading candidate self-management programmes from eligible articles became the focus for the realist synthesis. Realist synthesis fulfilled the needs of this particular review in which quantitative evidence were the main focus and qualitative evidence were used for their explanatory potential. A central element of this interpretative, theory-driven approach was the development of programme theories (i.e., the ideas and assumptions of how an intervention is expected to achieve particular outcomes) that described the link between context (e.g., characteristics of a self-management programme, condition of participants) in which self-management programmes work by identifying the mechanisms (i.e., underlying processes) such as the resources or response being offered by or embedded in a self-management programme would generate outcomes [Citation33]. The development of our initial programme theory was achieved through the extrapolation of a previous Cochrane Review [Citation22], evidence in other respiratory conditions [Citation14,Citation34,Citation35], and a clinical advisory group that included experts by experience. The methods, results, and discussion sections of all eligible articles were used to test and refine our initial programme theory. The overarching programme theory developed in this review attempted to explain how different self-management programmes work by applying a realist logic of analysis to develop CMOCs that supported or refuted the initial programme theory [Citation36]. The synthesis was an iterative process and substantive theory was sought to consolidate the programme theories by providing plausibility and coherence [Citation37].
Results
Description of included articles
A systematic search conducted from the inception of databases up to 2 July 2021 identified 3,420 unique articles. Following the screening of titles and abstracts, 3,357 articles were considered irrelevant. The full texts for the remaining 63 articles were obtained and eight articles met the full eligibility criteria. A summary of the article selection can be seen in . Two articles were not included in the narrative synthesis as they were reviews [Citation22,Citation38]. However, these reviews were used to refine CMOCs using the authors' interpretations. Six studies were descriptively summarised and synthesised, two of which were RCTs [Citation39,Citation40], two qualitative studies [Citation41,Citation42], one retrospective [Citation43], and one prospective cohort study [Citation44]. We contacted the authors of [Citation39] for disaggregated data for the subset of bronchiectasis participants that were reported in this review. Additionally, the author of [Citation44] was contacted for additional information that was used in this review. The characteristics of included studies are highlighted in .
Table 2. Characteristics of included studies.
A total of 288 patients with a mean age of 70 with a confirmed diagnosis of bronchiectasis were included in the review. There were 155 females and 133 males. The studies were conducted in four countries including England [Citation39,Citation41], Northern Ireland [Citation40,Citation42], Canada [Citation44], and Italy [Citation43]. All patients except in one study [Citation39] were in a stable state condition upon recruitment. The diagnostic criteria varied, four studies used a CT/high-resolution CT scan [Citation40–43] and two confirmed diagnoses via respiratory physician [Citation39,Citation40].
Description of self-management interventions
There were a total of four different types of interventions (education, exercise, action planning, and ACT) that were examined in four unique self-management programmes. One self-management programme included exercise, education, and ACT [Citation45], one included action planning, education, and ACT [Citation46], another included exercise and education [Citation42], and one included action planning and education [Citation44]. Education was the most prevalent intervention being included in all four self-management programmes whereas, exercise, action planning, and ACT were only included in two self-management programmes. Exercise, action planning, or ACT were included in all self-management programmes that had an education component. The patients in both qualitative studies had discussed exercise, education, and ACT. Uniquely, action plans were discussed by patients in one qualitative study [Citation41], whereas symptom monitoring was discussed in the other [Citation42].
Patient educational topics ranged from general health education (e.g., nutrition and self-management treatment strategies) [Citation40,Citation43,Citation44] to more disease-specific information such as dealing with the signs and symptoms of bronchiectasis and understanding of an exacerbation [Citation40,Citation44]. Exercise activities included daily supervised volitional strength and non-volitional techniques [Citation39], lower limb endurance training [Citation43], and upper body exercises [Citation43]. Action planning was part of an Expert Patient Programme (EPP) [Citation40] and a bespoke action plan was used in conjunction with the BTS action plan [Citation44] that provided patients with weekly goals of their choice (e.g., picking an activity) and were asked about their confidence level in achieving their goals. ACT ranged from pursed-lip breathing and exhalation on effort [Citation43] to the active cycle of breathing and the huff cough technique [Citation44].
The shortest to longest self-management intervention programmes ranged from 3 weeks [Citation43] to 8 weeks [Citation40]. One study is still ongoing [Citation44]. The intensity of the interventions ranged from daily sessions [Citation39] to weekly 2.5-h sessions [Citation40] to between 12 and 15 sessions of 2 to 3-h over three weeks [Citation43]. Four studies had either a physiotherapist and/or respiratory nurse to deliver and supervise the intervention [Citation39,Citation40,Citation43,Citation44]. Two studies delivered the intervention in groups [Citation40,Citation43]. One-to-one delivery of theprogrammes were used in two studies which later transitioned to telephone consultation [Citation39,Citation44].
Outcome measures
All four interventional studies reported health-related quality of life (HRQoL) using either St George's Respiratory Questionnaire [Citation39,Citation40], the EuroQol Visual Analogue Scale component of the EuroQol-5 dimensions scale [Citation43] or Quality of Life-Bronchiectasis and Bronchiectasis Health Questinonaire [Citation44]. Exercise capacity was reported in two studies using either incremental shuttle walk test and endurance shuttle walk test [Citation39] or six minute walk test [Citation43]. One study reported an unplanned readmission rate at 12 months, number of hospital stays, and mortality [Citation39].
Other outcomes unique to individual studies include measuring self-efficacy using the Chronic Disease Self-efficacy [Citation40], coping as measured by Revised Illness Perception Questionnaire and two EPP questionnaires were used to assess other factors such as self-rated health, ability to manage the condition, and self-rated health care use [Citation40]. One study reported fatigue using the Borg Scale and dyspnoea was assessed by the Transition Dyspnoea Index [Citation43]. The number of clinic visits and the number of patients using an oscillating positive expiratory pressure (OPEP) device was assessed in one study [Citation44].
Quality assessment
There was consistent agreement (86%) between reviewers for study quality. The qualitative studies addressed all ten CASP criteria. The unpublished study was considered low quality based on our assessment of the materials provided and available to us using the AACODS checklist. The RCTs and the retrospective study were rated as low risk of bias as they were well reported and of high methodological quality. Further details pertaining to the quality assessment are provided in Table S4.
Programme theory and CMOCs
Similar to previous realist syntheses [Citation45,Citation46], ours considered a multiplicity of different interventions rather than a single intervention. Due to the heterogenous nature of self-management programmes that can involve a combination of several interventions, the development of the initial programme theory can be described as rough. We postulated that self-management programmes should include at least two interventions (e.g., action planning and exercise) that would empower patients to seek care in a timely manner leading to improved HRQoL, reduced exacerbation frequency and severity, and are cost-effective.
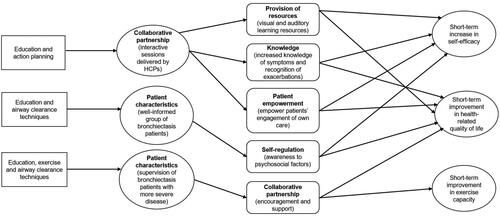
The following will provide a narrative overview of the programme theories for self-management programmes for adult patients with bronchiectasis. The narrative presents the programme theories that emerged following analysis of the data: (i) self-management programme consisting of education and action planning; (ii) self-management programme consisting of education and ACT; and iii) self-management programme consisting of education, exercise, and ACT. The programme theories underpinned by explanatory CMOCs were substantiated by drawing on the different underlying constructs of the integrated theory of health behaviour model [Citation47] to enhance the plausibility and coherence of the synthesis. The fostering and enhancing of “knowledge and beliefs” impacts behaviour-specific self-efficacy and influences health behaviour change was linked with the first programme theory. The construct of “self-regulation” explaining the regulation of negative emotions was associated with successful management of chronic conditions was linked with the second programme theory. The “social facilitation” construct mapped onto the third programme theory to support the idea of supervising the patient via a collaborative partnership between the healthcare professional (HCP) and the patient. provides an overarching realist programme theory that consolidated the relationships between the 10 explanations (i.e., CMOCs) that emerged from the data organised around the programme theories. The full list of 10 CMOCs along with illustrative data excerpts from the articles can be found in Table S5.
Self-management programme consisting of education and action planning
Three mechanisms were identified through which a self-management programme consisting of education and action planning leads to short-term increase in self-efficacy and HRQoL (CMOCs 1–6 in Table S5). Interactive sessions delivered by HCPs provided visual and auditory learning resources that enabled different ways to acknowledge new information (CMOCs 1 and 2 in Table S5). Evidence of posters and picture handouts depicting a pulmonary exacerbation were found [Citation42,Citation44] and the provision of YouTube videos showing the use of an OPEP device [Citation44] was one of the pathways for patients to learn about bronchiectasis. We also identified patterns in the interactive sessions being taught involved educational topics about signs and symptoms, goals of treatment, problem-solving, and general health promotion (e.g., nutrition). This increased patient knowledge of symptoms and their ability to recognise exacerbations, including the commencement of a home supply of prescribed antibiotics may explain the increase in self-efficacy and HRQoL (CMOCs 3 and 4 in Table S5).
The interactive sessions provided by HCPs facilitated patients to actively take part in planning their goals empowered patients’ engagement of own care (CMOCs 5 and 6 in Table S5). The evidence found indicated patients actively participated in sessions by completing a worksheet about their usual symptoms of bronchiectasis, how they manage them on a daily basis, and triggers that make the usual symptoms worse [Citation40]. Patterns of patients taking responsibility for the status of their bronchiectasis and making their own decisions through weekly action planning and goal setting were also found. Action plans served as a treatment-seeking support tool that gave patients the opportunity to set goals that they wish to achieve in a given week (e.g., perform a particular ACT or exercise), how they intended to achieve it (e.g., how often they wish to perform their chosen activity) and they would discuss and feed back in the following week. Patients increased engagement in their own self-management would be recorded to gauge their confidence [Citation42,Citation44] and motivational levels [Citation44] that could explain the positive outcomes.
Self-management programme consisting of education and ACT
In the context of a well-informed population of patients regarding their disease, a self-management programme consisting of education and ACT can raise their awareness of psychosocial factors (e.g., relationships with people and levels of anxiety and depression) that leads to a short-term increase in self-efficacy and HRQoL (CMOCs 7 and 8 in Table S5). A lack of competence and confidence are perceived psychological factors associated with performing ACT correctly [Citation42]. HCPs can help address these perceived obstacles by providing relevant educational material that would encourage patients to be more independent with further aspects of their care [Citation42]. Furthermore, feedback from patients has suggested that learning how to deal with bronchiectasis socially and psychologically as part of patient education can be of great benefit in addressing these potential issues [Citation22,Citation40].
Perceived emotional issues such as worry, fear and embarrassment may be associated with their condition, particularly when an exacerbation is experienced [Citation42]. The management of these negative emotional states may be ameliorated through education by learning how to differentiate symptoms between stable and exacerbation phases to increase self-efficacy (CMOC 7 in Table S5). There is some evidence of health-related behaviour from patients that is influenced by their attitudes and social expectations. For example, patients follow advice from family members given to them as they believed it to be important [Citation42]. Additionally, family members can have a role in providing emotional support and assisting with performing active cycle of breathing techniques and using the OPEP device correctly to improve HRQoL by reducing the frequency of exacerbations [Citation44] (CMOC 8 in Table S5).
Self-management programme consisting of education, exercise, and ACT
Supervision of patients with more severe disease that undertake a self-management programme consisting of education, exercise, and ACT provided a collaborative partnership leading to short-term HRQoL and short-term exercise capacity (CMOCs 9 and 10 in Table S5). A structured programme of exercise, education, and psychosocial support in the form of the SPACE manual [Citation48] have been implemented for those with bronchiectasis [Citation39]. Self-management strategies that can be learnt from the manual include improving problem-solving skills, behavioural management, and decision making [Citation48].
Patients with more severe cases of bronchiectasis in terms of the level of airflow obstruction and the number of exacerbations experienced in the previous year before being enrolled on a self-management programme [Citation43] may have had an effect on the outcomes [Citation38]. This particular subset of patients may be perceived as more deconditioned, therefore having a higher magnitude for improvement in HRQoL compared to patients with more preserved lung functioning (CMOC 9 in Table S5).
The improvement in exercise capacity was achieved when exercise sessions were individualised and supervised by HCPs (CMOC 10 in Table S5). The evidence indicated that the intensity and duration of exercise sessions were individually tailored based on patient tolerance to accommodate patients’ disease severity. Exercise activities included super volitional and non-volitional techniques [Citation39] and lower and upper limb training [Citation43]. Pursed lip breathing, exhalation on effort, and forward lean position were examples of ACT performed in relation to patients’ needs [Citation43]. When face-to-face supervision was not provided (e.g., during the home segments of a self-management programme), telephone support was offered as an alternative [Citation39].
Discussion
Summary of findings and comparison with existing literature
This review contributes to the literature that acknowledges the wider contextual circumstances and attempts to explain how and why different self-management programmes work for adult patients with bronchiectasis. In doing this, the review does not produce evidence of the effectiveness or advantages of self-management programmes. Instead, it elicits potential underlying processes why these programmes work generating particular outcomes.
The overarching programme theory of this review explains how and why three leading candidate self-management programmes work in certain contexts to increase self-efficacy, HRQoL, and exercise capacity in the short term (the most common outcomes identified in the data). These outcomes result from complex interactions between the contexts in which patient characteristics (e.g., patients with more severe disease) and collaborative partnership between HCP and patient and the mechanisms (i.e., provision of resources, knowledge, patient empowerment, self-regulation, and collaborative partnership)triggered in these contexts. The integrated theory of health behaviour change was used as a substantive theory to enhance the plausibility and coherence of the programme theories [Citation47].
The findings in this review in part answer the call for further research needed in self-management for adult patients with bronchiectasis [Citation1]. In particular, this review has provided explanatory accounts of the potentially most useful interventions in self-management programmes for different subsets of patients. This is in line with the assertion that interventions of self-management programmes may need to accommodate condition-specific patients [Citation49]. For instance, education about the disease may be less useful for a well-informed group of patients and could benefit from learning how to deal with the psychosocial factors of bronchiectasis instead.
Analysis of the included data elicited a clearer understanding of the mechanisms that are triggered from different contextual factors. Increasing patient engagement in their own care and increasing their knowledge of symptoms are some of the identified underlying processes that can cause a behavioural change in patients. These findings build on existing evidence that suggests patients become more confident at managing their own health [Citation50] through empowerment provided by self-management strategies [Citation51–53]. Furthermore, one of the important contexts identified in this review reflected the importance of the clinician-patient relationship, where interactive sessions that involve action planning can lead to an improvement in self-efficacy via activation of patient empowerment. A recent study has evaluated the use of a novel action plan, the Bronchiectasis Empowerment Tool [Citation54] to facilitate appropriate treatment-seeking behaviour in patients. Their findings indicated a high demand for such intervention but according to the authors it required extensive modification due to the arduous nature of the tool for users. This suggests the development of treatment-seeking tools needs to be suitable for patients and not contribute to the burden of the disease.
Methodological limitations and implications for further research
One important caveat is the strength of the evidence was limited by a lack of studies and reporting limitations of the specificity of interventions included in self-management programmes. However, we contacted authors where possible for additional information to overcome this shortcoming. There were studies that could have further refined the programme theories but were excluded due to ineligibility. Noteworthy studies that were omitted included one multi-faceted qualitative study [Citation55] that explored the factors that influenced adherence to self-management strategies and three studies that only evaluated a single intervention [Citation54,Citation56,Citation57]. The overall findings should be viewed as provisional and non-exhaustive due to limited iterative searching resulting from a lack of resources.
Psychological aspects and the role of family members were alluded to in the data; however, the absence of evidence did not allow us to identify specific generative causal mechanisms. Recent evidence has demonstrated an association between anxiety and depression in patients with bronchiectasis [Citation58], which highlights an issue that should be addressed. The importance of family members can help combat negative emotional factors associated with chronic conditions [Citation59]. There are assertions that positive social support is associated with improved health outcomes in COPD patients [Citation60] and better disease management behaviours [Citation61].
The impact of limiting the inclusion criteria to the English language only may explain the absence of data for self-management interventions relating to symptom monitoring and adherence to treatment. However, there is not an international consensus on the definition of self-management for bronchiectasis. The current definition is largely derived from a recently published review [Citation22]. It is therefore important to continue to research and develop programmes tailoring to different subsets of adult patients with bronchiectasis with an emphasis on the collaborative partnership between patient and provider [Citation62]. There was also a clear lack of sociodemographic data for patients that participated in self-management programmes, which may limit the transferability of our findings. There was no data in any of the reviewed studies regarding patients’ lifestyles or health literacy. Future carefully designed studies and clinical trials should aim to clearly define and justify the specific nature of self-management, outline sociodemographic data including length of diagnosis of patients (e.g., less than a year) as this information will help further elicit a better understanding of which self-management programmes works, for whom, and in what circumstances.
Conclusion
Given the magnitude of the global burden of bronchiectasis and the complexity of self-management programmes, it is vital to identify the interplay between contexts and mechanisms that result in outcomes of clinical interest. There is limited scope for improvement if the contexts in which different self-management programmes are implemented remain unacknowledged. This provisional realist synthesis of the evidence contributes to our understanding of how patient characteristics and collaborative partnership between HCP and patient can influence outcomes (albeit short-term) in different self-management programmes. Further research including a realist evaluation could provide data to scrutinise the provisional programme theories developed in this review.
Author contributions
AT, SS and CK contributed to the conception and design of the review. SS and CK shaped the formal search strategies. AT carried out all search strategies. AT, CK and DL carried out screening and article selection processes. AT, HM and CK conducted quality assessment. AT performed data extraction and CK carried out consistency checks. CK facilitated the clinical advisory group for the review. AT contributed to the development and refinement of the programme theory in consultation with CK. AT applied a realist logic of analysis to the data and identified the theoretical idea for the overarching programme theory. AT wrote the manuscript with input from SS. All authors read and approved the final manuscript.
Supplemental Material
Download PDF (277.8 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Hill AT, Sullivan AL, Chalmers JD, et al. British thoracic society guideline for bronchiectasis in adults. Thorax. 2019;74(1):1–69.
- O’Leary CJ, Wilson CB, Hansell DM, et al. Relationship between psychological well-being and lung health status in patients with bronchiectasis. Respir Med. 2002;96(9):686–692.
- Chang AB, Bell SC, Torzillo PJ, et al. Chronic suppurative lung disease and bronchiectasis in children and adults in Australia and New Zealand thoracic society of Australia and New Zealand guidelines. Med J Aust. 2015;202(3):130.
- Hester KLM, Macfarlane JG, Tedd H, et al. Fatigue in bronchiectasis. QJM. 2012;105(3):235–240.
- Goeminne PC, Hernandez F, Diel R, et al. The economic burden of bronchiectasis - known and unknown: a systematic review. BMC Pulm Med. 2019;19(1):54.
- Brown J, Bradley J, Copeland F, et al. M27 bronchiectasis multicentre cohort; baseline demographics from BRONCHUK. Thorax. 2019;74:A249.
- McCullough AR, Tunney MM, Quittner AL, et al. Treatment adherence and health outcomes in patients with bronchiectasis. BMC Pulm Med. 2014;14(1):14.
- McCullough AR, Tunney MM, Elborn JS, et al. Predictors of adherence to treatment in bronchiectasis. Respir Med. 2015;109(7):838–845.
- Epping-Jordan JE, Pruitt SD, Bengoa R, et al. Improving the quality of health care for chronic conditions. Qual Saf Health Care. 2004;13(4):299–305.
- Jordan RE, Majothi S, Heneghan NR, et al. Supported self-management for patients with moderate to severe chronic obstructive pulmonary disease (COPD): an evidence synthesis and economic analysis. Health Technol Assess. 2015;19(36):1–516.
- Gibson PG, Powell H, Coughlan J, et al. Self-management education and regular practitioner review for adults with asthma. Cochrane Database Syst Rev. 2003;(1):CD001117.
- Monninkhof E, van der Valk P, van der Palen J, et al. Self-management education for patients with chronic obstructive pulmonary disease: a systematic review. Thorax. 2003;58(5):394–398.
- Effing TW, Bourbeau J, Vercoulen J, et al. Self-management programmes for COPD: moving forward. Chron Respir Dis. 2012;9(1):27–35.
- Lenferink A, Brusse-Keizer M, van der Valk PD, et al. Self-management interventions including action plans for exacerbations versus usual care in patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017;(8):CD011682.
- Zwerink M, Brusse-Keizer M, van der Valk P, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2014;(3):CD002990.
- Pinnock H, Parke HL, Panagioti M, et al. Systematic meta-review of supported self-management for asthma: a healthcare perspective. BMC Med. 2017;15(1):64.
- Polverino E, Goeminne PC, McDonnell MJ, et al. European respiratory society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1–23.
- Walters JA, Turnock AC, Walters EH, et al. Action plans with limited patient education only for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2010;(5):CD005074.
- Hester KLM, Newton J, Rapley T, et al. Patient information, education and self-management in bronchiectasis: facilitating improvements to optimise health outcomes. BMC Pulm Med. 2018;18(1):80.
- Chalmers JD, Crichton M, Goeminne PC, et al. The European Multicentre Bronchiectasis Audit and Research Collaboration (EMBARC): experiences from a successful ERS clinical research collaboration. Breathe. 2017;13(3):180–192.
- Yorke J, Prigmore S, Hodson M, et al. Evaluation of the current landscape of respiratory nurse specialists in the UK: planning for the future needs of patients. BMJ Open Respir Res. 2017;4(1):e000210.
- Kelly C, Grundy S, Lynes D, et al. Self-management for bronchiectasis. Cochrane Database Syst Rev. 2018;(2):CD012528.
- Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097.
- Wong G, Greenhalgh T, Westhorp G, et al. RAMESES publication standards: realist syntheses. BMC Med. 2013;11:21.
- Booth A, Harris J, Croot E, et al. Towards a methodology for cluster searching to provide conceptual and contextual “richness” for systematic reviews of complex interventions: case study (CLUSTER). BMC Med Res Methodol. 2013;13:118.
- Tsang A, Maden M. CLUSTER searching approach to inform evidence syntheses: a methodological review. Res Synth Methods. 2021;12(5):576–589.
- Booth A. Searching for qualitative research for inclusion in systematic reviews: a structured methodological review. Syst Rev. 2016;5:74.
- Schardt C, Adams MB, Owens T, et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007;7(1):16.
- Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- CASP. Critical Appraisal Skills Programme. 10 questions to help you make sense of qualitative research [Internet]. 2013 [cited 2021 Jul 20]. Available from: https://casp-uk.net/casp-tools-checklists/
- NIH. Quality assessment tool for before-after (pre-post) studies with no control group [Internet]. Bethesda (MD): National Institutes of Health; 2014 [cited 2021 Jul 20]. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools
- Tyndall J. ACCODS Checklist [Internet]. Clovelly Park (Australia): Flinders University; 2010 [cited 2021 Jul 20]. Available from: https://canberra.libguides.com/c.php?g=599348&p=4148869.
- Dalkin SM, Greenhalgh J, Jones D, et al. What’s in a mechanism? Development of a key concept in realist evaluation. Implement Sci. 2015;10:49
- Peytremann-Bridevaux I, Arditi C, Gex G, et al. Chronic disease management programmes for adults with asthma. Cochrane Database Syst Rev. 2015;(5):CD007988.
- Savage E, Beirne PV, Ni Chroinin M, et al. Self-management education for cystic fibrosis. Cochrane Database Syst Rev. 2011;(9):CD007641.
- Pawson R. Evidence-based policy: a realist perspective. London (UK): Sage; 2006.
- Merton R. On sociological theories of the Middle-range. On theoretical sociology: Five essays, old and new. New York (NY): Free Press; 1967. p. 39–72.
- Lee AL, Hill CJ, McDonald CF, et al. Pulmonary rehabilitation in individuals with non-cystic fibrosis bronchiectasis: a systematic review. Arch Physi Med Rehabil. 2017;98:774.
- Greening NJ, Williams JEA, Hussain SF, et al. An early rehabilitation intervention to enhance recovery during hospital admission for an exacerbation of chronic respiratory disease: randomised controlled trial. BMJ. 2014;349:g4315.
- Lavery KA, O’Neill B, Parker M, et al. Expert patient self-management program versus usual care in bronchiectasis: a randomized controlled trial. Arch Psys Med Rehabil. 2011;92(8):1194–1201.
- Kelly CA, Tsang A, Lynes D, et al. ‘It’s not one size fits all’: a qualitative study of patients’ and healthcare professionals’ views of self-management for bronchiectasis. BMJ Open Respir Res. 2021;8:e000862.
- Lavery K, O’Neill B, Elborn JS, et al. Self-management in bronchiectasis: the patients’ perspective. Eur Respir J. 2007;29(3):541–547.
- Zanini A, Aiello M, Adamo D, et al. Effects of pulmonary rehabilitation in patients with non-cystic fibrosis bronchiectasis: a retrospective analysis of clinical and functional predictors of efficacy. Respiration. 2015;89(6):525–533.
- LeClerc I, Muggah E. Bronchiectasis self-management education starts in primary care: a pilot study. Can J Respir Crit Care Sleep Med. 2019;11:11.
- Sholl S, Ajjawi R, Allbutt H, et al. Balancing health care education and patient care in the UK workplace: a realist synthesis. Med Educ. 2017;51(8):787–801.
- Kehoe A, McLachlan J, Metcalf J, et al. Supporting international medical graduates’ transition to their host-country: realist synthesis. Med Educ. 2016;50(10):1015–1032.
- Ryan P. Integrated theory of health behavior change: background and intervention development. Clin Nurse Spec. 2009;23(3):161–172.
- Apps LD, Mitchell KE, Harrison SL, et al. The development and pilot testing of the self-management programme of activity, coping and education for chronic obstructive pulmonary disease (SPACE for COPD). Int J Chronic Obstruct Pulmon Dis. 2013;8:317–327.
- Ong KC, Wong WP, Jailani AR, et al. Effects of a pulmonary rehabilitation programme on physiologic and psychosocial outcomes in patients with chronic respiratory disorders. Annals Acad Med. 2001;30:15–21.
- van Zeller M, Mota PC, Amorim A, et al. Pulmonary rehabilitation in patients with bronchiectasis: pulmonary function, arterial blood gases, and the 6-minute walk test. J Cardiopulm Rehabil Prev. 2012;32(5):278–283.
- Kennedy A, Bower P, Reeves D, et al. Implementation of self management support for long term conditions in routine primary care settings: cluster randomised controlled trial. BMJ. 2013;346:f2882.
- McCorkle R, Ercolano E, Lazenby M, et al. Self-management: enabling and empowering patients living with cancer as a chronic illness. CA Cancer J Clin. 2011;61(1):50–62.
- Alpay L, van der Boog P, Dumaij A. An empowerment-based approach to developing innovative e-health tools for self-management. Health Informatics J. 2011;17(4):247–255.
- Brockwell C, Stockl A, Clark A. Randomised controlled trial of the effect, cost and acceptability of a bronchiectasis self-management intervention. Chronic Respir Dis. 2020;17:1–11.
- McCullough AR, Ryan C, O’Neill B, et al. Defining the content of a theory-based intervention to change AdhereNce to treatment in BonchiEctasis (CAN-BE): a systematic approach using the theoretical domains framework and behavioural change techniques. Int J Pharm Pract. 2015;23:30.
- Al Moamary MS. Impact of a pulmonary rehabilitation programme on respiratory parameters and health care utilization in patients with chronic lung diseases other than COPD. East Mediterr Health J. 2012;18(2):120–126.
- Hester K, Ryan V, Newton J, et al. Bronchiectasis information and education: a randomised, controlled feasibility trial. Trials. 2020;21(1):331.
- Niknafs A, Blakney RA, Goldstein D, et al. Prevalence and clinical characteristics of anxiety and depression in patients with non-CF bronchiectasis: a single-center, cross-sectional study. ATS Conf. 2021;203:A1565.
- Grady PA, Gough LL. Self-management: a comprehensive approach to management of chronic conditions. Am J Public Health. 2014;104(8):e25–e31.
- Chen Z, Fan VS, Belza B, et al. Association between social support and Self-Care behaviors in adults with chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2017;14(9):1419–1427.
- DiNicola G, Julian L, Gregorich SE, et al. The role of social support in anxiety for persons with COPD. J Psychosom Res. 2013;74(2):110–115.
- Lorig KR, Holman H. Self-management education: history, definition, outcomes, and mechanisms. Ann Behav Med. 2003;26(1):1–7.