Abstract
Purpose
This systematic review explored how virtual reality (VR) has been used to rehabilitate aphasia.
Materials and methods
Empirical studies were included where VR was used to target language, well-being, or quality of life in adults with acquired language impairment. Degenerative communication disabilities were excluded. Seven health databases were searched in October 2021. Risk of Bias was assessed using published checklists and completeness of intervention reporting evaluated. Narrative synthesis described forms of VR, rationales given, outcome measures, communication functions targeted, characteristics of interventions, and outcomes achieved within the framework of impairment, activity, and participation.
Results
Fourteen studies, involving 229 participants, met the criteria. The studies employed four forms of VR with various rationales given. Interventions used published and novel protocols. Primary outcomes targeted language impairment (12/14), activity (1/14), and well-being (1/14) and achieved positive outcomes in impairment and activity. All studies were exploratory. Risk of bias was high. Findings are discussed in the context of gains achieved by VR in other health contexts and the multi-user gaming literature.
Conclusions
Uses of VR in aphasia rehabilitation described in the literature are limited. Most applications target the remediation of language impairments. Opportunities to address activity, participation, and wider aspects of well-being are rare.
Research documenting the use of virtual reality (VR) to rehabilitate aphasia is limited and exploratory, so does not yet offer clear guidance for clinicians.
Many of the identified studies have used known published protocols (e.g., naming therapy or scripts therapy) delivered through the novel VR format and focus on language impairment outcomes.
VR offers clinicians a unique opportunity to address communication activity and participation through the use of multi-user virtual worlds, but this has only been explored by only two research teams.
IMPLICATIONS FOR REHABILITATION
Introduction
Aphasia is a neurological condition that affects a person’s ability to use language [Citation1]. The most common cause of aphasia is a stroke. At least a quarter of those who survive a stroke will experience aphasia [Citation2]. It affects 0.1–0.4% of the population worldwide [Citation3] and 35 000 people in the UK [Citation4]. As stroke survival rates improve, more people are living with this lifelong disability [Citation5].
Aphasia has a negative impact on people’s lives. People lose their friends [Citation6] with negative emotional effects [Citation7]. Far-reaching consequences have been reported for social inclusion, social connectedness, access to information and services, equal rights, and well-being in family, community, and culture [Citation1]. Social isolation is linked to premature death, and poorer well-being [Citation8–10]. For these reasons, it has been argued that aphasia is a public health concern [Citation11]. There is a need for therapies that address both the aphasia and its impact on people’s lives.
Treatments for aphasia can focus on all levels of the International Classification of Functioning and Disability (ICF) framework [Citation12] and go beyond the ICF to focus on well-being and Quality of Life (QOL). The language impairment (the body structure and function domain) has been targeted in treatments for words, sentences, or narratives (for reviews see [Citation13–15]). Communication activity has been targeted in functional approaches [Citation16] as has societal participation [Citation17,Citation18]. Aphasia is known to have a particularly negative impact on well-being, leading to depression [Citation19,Citation20] and reduced QOL [Citation21–23]. Therefore, these constructs should form part of the focus of aphasia rehabilitation. The ICF framework, with the addition of well-being and QOL (hereafter referred to as ICF+) provides a structure for describing a wide range of potential rehabilitation outcomes in this review.
A key priority for people with aphasia is to improve communication in activities [Citation24]. Using communication in a conversational context has been described as “situated language use” [Citation25] and is key to this aspect of rehabilitation. It places the language functions (naming, syntax, narrative structure) in the context of the environment, the number of people in the conversation, interpersonal history, and the multimodal (facial expression, gesture, tone) nature of conversations [Citation26]. Multiple people and multiple environmental settings can be difficult to recreate in speech and language therapy sessions. There is a need for treatments that address this communication in context.
Virtual reality (VR) is the technology that allows one or many users to experience a three-dimensional space on a computer [Citation27]. Multi-user virtual environments may be uniquely placed to treat communication in context. The potential to create faithful, simulated experiences has been harnessed for learning in a range of contexts. Examples include an island where you interact only in German [Citation28] and recreations of surgical procedures for the training of medics [Citation29]. The simulation allows for practice with minimal risk. The safe practice space that VR offers has been explored in other communication disabilities, notably autism [Citation30]. This review will outline the ways the opportunity for simulated context has been used in aphasia rehabilitation.
The opportunities to interact with multiple users of VR may bestow social and emotional benefits. Indeed, such benefits have been reported in the gaming community where a sense of belonging and warm relationships are cited [Citation31]. Multi-user gaming has been embraced by people with disabilities. Interviews exploring the value of gaming with this group have highlighted why gaming was important to them (see Box 1). In addition to benefits cited by the general gaming community, people with disabilities highlighted the benefits of a space where they can be on an equal footing with other users and practice skills and showed an appreciation for the creativity in design and storytelling [Citation32].
Box 1. Why gaming is important to players with disabilities [Citation32].
VR can replicate real-world spaces or create novel environments. Some parts of the gaming community have embraced the development of novel creative spaces. There can be dream-like spaces (https://youtu.be/21FaS_bxReo) and worlds where the graphics are inspired by famous artists [Citation33]. Experiences of fun and diversion [Citation32] may have positive implications for mood and well-being. The potential for social and emotional benefits is notable in the context of the negative consequences of aphasia.
In stroke rehabilitation, there is a growing evidence base for the benefits of VR interventions in upper limb rehabilitation [Citation34,Citation35], balance and gait [Citation36], cognitive function, and activities of daily living [Citation37]. There is even some evidence that physical gains following VR rehabilitation may be accompanied by cortical changes [Citation38]. Several reviews have examined the use of VR to improve motor outcomes after stroke using the ICF framework [Citation39–42]. The most recent review identified 34 trials with impairment level outcomes, 17 trials with activity outcomes, and eight trials with a focus on participation [Citation42]. This illustrates that VR is used in physical rehabilitation to address all levels of the ICF, with the most emphasis on impairment.
Synthesis studies of VR in aphasia rehabilitation have been published since 2020 [Citation43–45]. In 2020, Repetto et al. investigated what innovative technologies (smartphones, tablets, and VR) were effective in post-stroke aphasia [Citation45]. This systematic review included three studies that used VR. They were Marshall et al. [Citation46] with EVA Park and Grechuta et al. [Citation47,Citation48] with the Rehabilitation Gaming System (RGS). Outcomes were descriptive with effect sizes reported for one study [Citation46]. The authors concluded that the field was in its infancy.
Picano et al. carried out a review that sought to understand “existing unconventional approaches” [Citation42, p. 2] to aphasia rehabilitation in 2021 [Citation44]. They included eight studies that used VR. The review gave a narrative description of EVA Park [Citation46,Citation49–51], RGS [Citation52,Citation53], the Virtual Reality Rehabilitation System (VRRS) tablet [Citation54], and Giachero et al.’s use of VR for functional communication situations [Citation55]. The authors concluded that VR has the potential to increase treatment dose, maximise sensorimotor stimulation and, overall, improve ecological validity of aphasia treatment [Citation44].
Cao et al. carried out a systematic review and meta-analysis of the effects of VR on post-stroke aphasia in 2021 [Citation43]. They explored whether VR interventions had an effect on communication activity and language function compared to a control condition. The five studies included were EVA Park [Citation46], RGS [Citation48], the VRRS tablet [Citation54], VR for communication situations [Citation55], and a conference paper exploring a virtual reality panoramic helmet [Citation56]. The review found a borderline effect of reducing language severity and no difference between VR and control for communication activity, word finding, or repetition outcomes. The control conditions were both an alternative SLT treatment and no treatment. The conclusions of this review were supported by meta-analyses; however, these were based on limited data (two studies per meta-analysis) and combined studies that employed different treatments (e.g., naming therapy combined with a conversation therapy) and different outcome measures (Communication Activities of Daily Living combined with the Communication Activities Log).
The current review updates and broadens the scope of these previous reviews. Firstly, it places greater emphasis on the rationales for using VR, the therapy goals, how they were measured and how VR was employed to enhance the therapy experience. The quality of reporting is also explored. Secondly, this review is not restricted to post-stroke aphasia. Aphasia can be caused by other brain pathology, e.g., a brain injury, tumour, or surgery. We did not restrict the underlying cause of aphasia. Moreover, given that VR use is an emerging field in this area we broadened the scope of the review to acquired non-progressive language disorders. This review sought to identify the ways in which VR has been used to support language and communication rehabilitation, particularly in reference to the domains of the ICF. Previous reviews highlight that VR has particular value in providing ecological validity, aligning with the activity domain of the ICF [Citation44]. However, the use of VR in rehabilitation is a recent innovation, meaning that applications in aphasia were likely to be limited. The authors were therefore interested in innovations in related disorders that could inform the development of VR for aphasia rehabilitation. Thus, we included cognitive communication disorder, a related disorder where the communication deficit is due to impaired cognitive functions rather than language [Citation57].
This review aimed to find out how VR has been used in the rehabilitation of acquired communication disorders. Specifically, it explored the following research questions:
What forms of VR were used?
What rationale(s) were given for the use of VR?
What outcome measures were used?
What communication functions were targeted?
What were the characteristics of the interventions?
What outcomes were achieved?
Methods
The reporting of this review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. The protocol was prospectively registered with PROSPERO (CRD42020196285).
Eligibility criteria
Studies were eligible if VR was used in an intervention study targeting any of the following for people with aphasia or acquired cognitive communication disorder: language, communication activity, participation, well-being, and quality of life. There were no language restrictions and dates were the earliest available within each database.
The population was defined as adults (>18 years) with aphasia and/or cognitive communication disorders following a stroke or traumatic brain injury. Mixed population studies were only included if outcomes were reported separately for people with aphasia or cognitive communication disorder. Studies of motor speech disorders were excluded. Degenerative language disorders, such as dementia, progressive neurological conditions, and primary progressive aphasia were excluded. In degenerative conditions the aims and methods of rehabilitation are different, and it would not be valid to conflate progressive and non-progressive participants.
For this review, we defined virtual reality as a set of images and sounds produced by a computer that seems to represent a real or imagined place or situation that a person can take part [Citation27]. Both immersive (using equipment, such as a head-mounted display) and non-immersive (interacting with an image on a screen) 3D environments were included. 2D applications were excluded. Studies had to report on empirical data with experimental controls and be published in a peer-reviewed journal. Beyond this, there were no constraints on study design, i.e., experimental single case and case series designs were included. New data had to be reported, so review papers were excluded. Box 2 summarises the Population, Intervention, Comparison, and Outcome of interest (PICO) in this systematic review.
Box 2. Population, intervention, comparison and outcome (PICO) framework.
Information sources
Seven electronic databases were searched following consultation with the subject librarian: CINAHL, Communication source, MEDLINE, Academic Search Complete, PsycINFO, Embase, and Ovid Emcare. Citation tracking from eligible articles was carried out using Scopus. Searches were run on 30 June–3 July 2020 and repeated on 19, 20, and 21 October 2021.
Search strategy
Search terms were variations on three concepts: acquired language impairments, rehabilitation, and virtual worlds. Search strings varied slightly depending on the MESH terms within each database. Truncation (*) was used to capture variations in terms, e.g., aphasia/aphasic. Box 3 illustrates an example search string. Full searches are available in Appendix 1.
Box 3. Search string example.
Study selection
Screening on title and abstract used the following hierarchy:
Participants were adults with aphasia or acquired cognitive communication disorder following a stroke or other acquired brain injury.
Virtual reality was used.
Intervention studies to remediate language impairment, communication activity, participation, or quality of life were reported.
Full-text articles were included if:
Participants had aphasia or acquired cognitive communication disorder.
Intervention targeted language impairment, communication activity, participation, or quality of life.
Empirical data was reported with an experimental control, e.g., across time or a comparator group.
Immersive or non-immersive virtual reality was used.
The publication was peer-reviewed.
Data collection process
Search results were double screened on title and abstract, and full-text articles were double screened independently by the first (ND) and fourth author (AR). A data extraction table was developed in Microsoft Excel to cover study characteristics, participants, intervention, outcomes, and VR aspects. Data were extracted independently by ND and AR and any discrepancies were resolved by discussion between reviewers. If consensus could not be reached, a third senior researcher (KH) had the deciding vote.
The aim of the intervention was determined by the primary outcome measure used. Outcome measures of selected studies were mapped onto the ICF + categories. For example, a language test as primary outcome measure (e.g., The Western Aphasia Battery–Revised [Citation58]) indicated the use of VR to change language impairment (ICF domain: impairment), whereas a communication test (e.g., The Communication Activities of Daily Living [Citation59]) indicated the use of VR to change activity (ICF: activity). All outcome measures (primary and secondary) and ICF + domains were independently mapped by two authors (ND and KH). These decisions were subsequently checked against the categorisation published by Wallace et al. [Citation60] and found to be in agreement, with the exception that Wallace collapsed activity and participation into one category. Secondary outcome measures were recorded to indicate intervention aims that targeted additional levels of the ICF. Discrepancies were resolved by discussion between reviewers. If consensus could not be reached, a third senior researcher (JM) had the deciding vote.
Data items
Data items described (i) the forms of virtual reality employed, (ii) the theoretical basis given by authors for employing virtual reality, (iii) the primary and secondary outcome measures used, (iv) the ICF domain targeted by these measures, (v) the intervention characteristics, and (vi) the outcomes achieved/changes reported on outcome measures. Additional variables collected were participant number, age, sex, aphasia type, time post-onset, study setting, and country. If data was missing it was indicated as not reported.
Risk of bias
Completeness of the intervention reporting was explored using the TIDieR framework [Citation61]. The framework outlines 12 items that should be reported. A complete TIDieR framework indicates a high quality of reporting that provides enough information for researchers and clinicians to replicate the intervention. Information from each study was extracted to complete the TIDieR framework by the first author (ND) with 35% (5/14 studies) independently extracted by the second author (NB). Discrepancies were resolved by discussion. Each study was given a point if the item was present in the report, to give each study a rating out of 12 for completeness of reporting.
The Physiotherapy Evidence Database (PEDro) scale [Citation62] was used to rate the methodological quality of randomised and non-randomised controlled trials. This is an 11-item checklist that gives a total score out of 10 (item 1 does not contribute to the total score). Quality is considered excellent if a study scores 9–10, good if a study scores 6–8, fair if a study scores 4–6, and poor if a study scores <4.
The Risk of Bias in N-of-1 Trials (RoBiNT) [Citation63] scale was used to assess single-case experimental designs. This 30-item checklist addresses the internal and external validity of studies. The RoBiNT authors subsequently published an algorithm to qualify the methodological rigor of the internal validity [Citation64]. A flow chart is followed to arrive at one of 10 grades from “very low” to “very high.” A point to note is that the RoBiNT tool was designed for studies that have dramatic “on-off” effects” [Citation65, p. 621] where a decline in performance is hypothesised when treatment is withdrawn. Conversely, in speech and language therapy intervention studies the very aim of treatment is for lasting effects. Participants are not expected to revert completely to pre-treatment levels when the stimulus (treatment) is removed. Nevertheless, this design is considered “non-withdrawal” and described as an AB + maintenance design in RoBiNT and scores 0 [Citation63]. If a study scores a 0 for design it can only score as “very low” for quality in the algorithm [Citation64, p. 12].
National Heart, Lung and Blood Institute (NIH) Quality Assessment tool for Before After studies with no control group was used to rate before-after studies with the condition rather than group control. This 12-item checklist addresses the internal validity of a study. Quality descriptions followed published guidance [Citation66], based on the number of items in the quality tool that was not present: a score of 0–3 N (N = not present) indicates a low risk of bias, a score of 4–8 N indicates a moderate risk of bias, and a score with 9–11 N indicates a high risk of bias.
Each study was rated for risk of bias independently by two authors (RoBiNT: ND, AR, KH, and JM; PEDro: ND, AR, and NB; NIH Quality Assessment tool: ND and AR). Allocation ensured that authors did not rate their own publications. Disagreements were resolved by discussion.
Summary measures and data synthesis
Descriptive statistics were used to summarise participants. Descriptive information and a narrative synthesis described the focus and detail of interventions, outcome measures used, VR used, and underlying theory. These were tabulated Microsoft Excel. The TIDieR was used to summarise completeness of reporting. Low quality studies are known to provide biased results [Citation67]. Therefore, only studies of adequate quality were included in the synthesis of outcomes. These were studies that scored 4 and above on the PEDro scale, graded as fair-to-very high on the RoBiNT scale, and moderate to low risk of bias on the NIH quality assessment tool. Where data permitted, effect sizes were calculated (d = 0.2, medium is d = 0.5, and large d = 0.8) [Citation68]. A meta-analysis was planned for eligible group-level studies that used the same outcome measures using standardised mean differences (SMD) and a fixed-effect model. However, heterogeneity in the outcome measures was high and a meta-analysis was not feasible.
Results
Study selection
The search of seven databases identified 639 articles with a further 13 identified through citation tracking on Scopus. All 652 records were imported into the evidence synthesis software, EPPI Reviewer [Citation69]. The software removed 232 duplicates. The title and abstract of the remaining 420 articles were screened against the inclusion criteria by ND and AR. 392 were excluded from the review because they did not involve adults with aphasia or acquired cognitive communication disorder (n = 244), did not use virtual reality (n = 68) or they were not intervention studies (n = 78) and two further duplicates were found by reviewers. Full-text articles were retrieved for the remaining 28 records. Two were not available as they were abstracts from conference proceedings. The full text was reviewed of the remaining 26 articles by ND and AR. 12 more were excluded at this stage as they did not meet the inclusion criteria (see ). Fourteen articles met the criteria and were included in the review.
Figure 1. PRISMA flow diagram of study selection process.
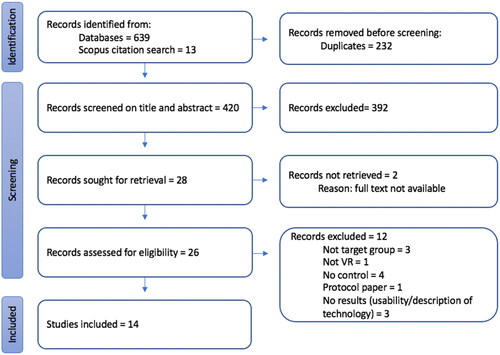
The review process had a good agreement between raters, with 95% agreement on title and abstract decisions and 89% agreement on full text (25/28). Disagreements were resolved with discussion.
Study characteristics
Fourteen articles were included in the review. These articles represent 14 different studies investigating seven different examples of virtual reality in aphasia rehabilitation. They represent the work of seven research teams, working in the UK (n = 1) (EVA Park) including a collaboration with Australia, Spain (n = 1) [Rehabilitation Gaming Software (RGS)], Italy (n = 2) [NeuroVR 2.0 and Virtual Reality Rehabilitation System (VRRS-tablet)], and the USA (n = 3) (Sentactics, AphasiaScripts/Web ORLA and the Virtual Human toolkit [Citation70]). No articles were found on empirical research investigating the use of VR to rehabilitate the language of people with the acquired cognitive-communication disorder.
Seven studies were randomised controlled trials, five were single case studies and two were before-after studies with no control group, where the experimental control was different conditions within the group, e.g., two different cuing methods. All studies were described as exploratory: they described feasibility, pilot, or efficacy studies or reported a sample size too small to be a definitive effectiveness study. Study characteristics are summarised in .
Table 1. Study (n = 14) and participant (n = 229) characteristics.
Participant characteristics
The fourteen studies reported on a total of 229 adults with aphasia, 95 female and 134 males. Almost all were in the chronic stage post-stroke (>6 m), with only one participant reported as <6 months post-stroke. Where mean age was reported (nine studies), all means were younger than 60 years old and ages ranged from 40 to 71 years. Where reported (11 studies), participants were predominantly people with non-fluent aphasia, n = 119 vs. n = 26 with fluent aphasia. The studies were carried out in three settings: community, hospital, and research laboratory. summarises the studies and study participants.
Risk of bias
The seven randomised control trials were quality assessed using the PEDro scale, see . Two studies were rated as good quality (6–8/10) [Citation48,Citation73], three studies were rated as fair (4–5/10) [Citation46,Citation51,Citation55] and two were rated poor (<4/10) [Citation54,Citation76].
Table 2. Randomised controlled trials rated using the PEDro.
The five single case studies were rated using the Risk-of-Bias in N-of-1 trials (RoBiNT) tool, see . These studies received scores ranging from 7 to 18/30 on the RoBiNT 30-item scale, with four studies scoring 15–18/30 and one 7/30. All five studies scored higher on external validity than internal validity. As expected, all five studies were scored as very low quality, when the RoBiNT algorithm was applied, despite the variability in scores. A score for blinding in the intervention was given for one study only where stimuli were computer delivered [Citation75]. None of the studies received points for replication.
Table 3. Single case designs rated using the RoBiNT.
The final two studies were assessed for bias using the NIH Quality Assessment tool for Before After studies with no control group, see . Both studies were judged to have a moderate risk of bias. Neither study reported whether all participants who were eligible were enrolled, gave a rationale for the sample size, blinded assessors, or reported follow-up rate.
Table 4. NIH quality assessment tool for before after studies with no control group.
Completeness of intervention reporting was assessed using the TIDieR checklist [Citation61], see . Complete reporting allows for the replication of interventions for research and clinical practice. The 14 studies scored from 6 to 10 on the 12-point scale. The rationale, materials, and procedures, mode of delivery, schedule, and dose (items 2, 3, 4, 6, and 8) were most consistently reported. Tailoring, modification, and treatment fidelity (items 9–12) were rarely reported.
Table 5. TIDieR ratings of intervention description in studies (n = 14).
Forms of VR used
Various forms of VR were used in a range of different ways. The treatment was delivered by the computer program or a therapist, the user was represented in the VR or not, the VR was single user or multi-user, the navigation was user-controlled or not, and finally, the VR environment was of a virtual clinician, a scenario, or a virtual world. These are summarised in and described in this section.
Table 6. How VR is being used in the included studies.
In six of the studies reported, the treatment was delivered by the computer program [Citation54,Citation72–76]. These include the programs using a virtual clinician; AphasiaScripts, Web ORLA, Sentactics, the Virtual Human toolkit, as well as the VRRS-tablet which uses virtual scenarios. The Web ORLA/AphasiaScripts and Sentactics VR applications are based on the virtual agent software from the Centre for Spoken Language Research at the University of Colorado [Citation76]. The VR depicts the moving head and shoulders of a clinician. The virtual clinician is pre-programmed to deliver the treatment. Her lips move in a naturalistic way to give visuomotor prompts. Snell et al. [Citation75] used the Virtual Human toolkit software [Citation77]. The VR shows the full body of a virtual clinician avatar standing outside a café. In the five programs using a virtual clinician, the user is not represented in the virtual world. These five programs are for a single user. In the studies, the navigation of the program was controlled by the participant [Citation73,Citation76], a physically present researcher who moved the script forward [Citation72], or selected a pre-programmed response [Citation74,Citation75]. In two studies this was arranged using a “wizard of oz” paradigm where the researcher controlled the virtual clinician from behind a curtain to give the illusion of independent use [Citation74,Citation75].
The VR element of the Virtual Reality Rehabilitation System (VRRS-tablet) intervention was delivered by the computer program [Citation54]. VRRS was single-use and independently navigated by the user. It is not reported if users are represented in the VRRS 3D module. The paper also reports that “the linguistic module with 2D was mainly used” (p4) and the content of the 3D module was not further explained.
The four EVA Park studies used a multi-user virtual world [Citation46,Citation49–51]. Virtual worlds are defined as “shared, simulated spaces which are inhabited and shaped by their inhabitants who are represented as avatars” [Citation78, p. 1099]. Users had EVA Park set up on a laptop in their own homes. Users were represented by personalised avatars. Users viewed the world from a third-person viewpoint just behind their avatar’s head. They could see and move around the EVA Park island and see and speak to the avatars of other users. Users could independently navigate and click on objects within the island to interact with them, e.g., they could click on the diving board and the avatar dived into the lake. The island was made up of a town square, two houses, a disco, a lakeside picnic area, and a tiki bar on a smaller island, with all areas linked by green spaces. Interventions were delivered in real-time by a therapist, communication support worker, or group coordinator who was also represented in the world by an avatar.
The Rehabilitation Gaming System (RGS) technology provided a representation of the physical room in which the users sat [Citation47,Citation48,Citation52,Citation53]. Two users were positioned facing each other at the same physical table with two monitors between them. The monitor showed a virtual representation of the user’s arms, a virtual table, and a virtual peer across the table. The representation of the arms on the screen corresponded to the movement of the user’s arms through the use of Microsoft Kinect technology. Users watched themselves select and pass virtual objects that had been requested. A rehabilitation assistant was present in the room to resolve technical or communication difficulties.
The virtual space in NeuroVR 2.0 [Citation55] represented functional scenarios, such as a station, a hotel, or a supermarket. Three participants with aphasia sat with the SLT in the same room with a large 50” curved screen showing the virtual scene. Participants had tasks within each scenario. For example, they bought a train ticket, checked the platform, and responded to an unexpected request for help from someone who had been mugged. Participants indicated their choice verbally to the SLT, and the SLT controlled and navigated the technology.
What rationale(s) were given for the use of VR
Researchers cited a variety of reasons for employing VR, which are summarised in and mapped out for ease of comparison in Table A (Supplementary Material). In Australia, where large, sparsely populated geographical regions make accessibility of services particularly pertinent, the accessibility of a remote online telehealth platform has been highlighted [Citation49]. Cherney et al. [Citation72] argued that a computer-delivered intervention removed human variation and therefore increased treatment integrity. She also argued for more efficiency as human clinicians could potentially “detract from critical treatment time” [Citation73]. Two studies [Citation73,Citation76] additionally argued that computer program-delivered treatments allowed for an increased dose without the additional cost of the therapist’s time. Giachero et al. [Citation55] and Marshall et al. [Citation46,Citation50,Citation51] highlighted the ecological validity of setting treatments in simulated real word situations. Settings are considered ecologically valid if they reflect how people behave in a real-world setting. Giachero et al. [Citation55] also cited embodied theory—the idea that semantics and language are multimodal [Citation79] and delivering language therapy in a virtual simulation creates a multimodal learning environment. Grechuta et al. [Citation48] similarly discussed the value of delivering a “socially embedded” protocol by using peer interactions (p.1). Six studies [Citation46–48,Citation54,Citation73,Citation74] cited the opportunity for increased intensity as a rationale for VR treatments.
What outcome measures were used
The 14 studies used 14 different primary outcome measures, see . Even when studies targeted the same impairment, e.g., object naming, the outcome measures used were different which made the data too heterogeneous for a meta-analysis. For example, Grechuta et al. [Citation47,Citation53] reported a bespoke naming latency measure to demonstrate improved naming while Marshall et al. [Citation50] reported naming correctness, as measured by the Action and Object Naming Battery [Citation81].
Table 7. Outcome measures used in included studies and ICF + classification.
Outcome measures used to measure a change in the language impairment were the Aachen Aphasia Test (AAT) [Citation82], the Boston Diagnostic Aphasia Examination (BDAE) [Citation83], the Token Test [Citation84], the Naming and Oral Reading for Language in Aphasia 6-point scale, NORLA-6 [Citation85], content of narratives [Citation80], script accuracy, and therapy specific noun naming and verbal fluency tests [Citation46,Citation53].
Outcome measures used to measure communication activity and participation were the Communication Activities of Daily Living–2nd Edition, (CADL-2) [Citation59], the Conversation Analysis Profile for People with Aphasia [Citation86], and the Psychosocial Impact of Assistive Devices Scale (PIADS) [Citation87], the quantity and quality of information in a dialogue [Citation74], and the Communication Activities Log [Citation88].
In terms of ICF + outcomes, well-being was measured using the Warwick-Edinburgh Mental Well-being Scale (WEMWBS) [Citation89], self-esteem was measured using the Visual Analogue Self Esteem Scale (VASES) [Citation90], confidence was measured using the Communication Confidence Rating Scale for Aphasia (CCRSA) [Citation91], depression was measured using the Aphasic Depression Rating Scale (ADRS) [Citation92] and quality of life was measured using the World Health Organisation Quality of Life Scale (WHOQOL) [Citation93] the Euro-Qol-5D (EQ5D) [Citation94], the PIADS [Citation87] and The Stroke and Aphasia Quality Of Life Scale-39 generic stroke version (SAQOL-39g) [Citation95].
The robustness of the measures used was variable. Eight studies used well-validated measures as a primary outcome measure [Citation46–48,Citation50,Citation51,Citation53–55,Citation73,Citation76]. Three studies used measures that required rater reliability checks [Citation49,Citation74,Citation75], with two reporting the interrater reliability agreement [Citation49,Citation74]. In one study, the computer automatically recorded the primary outcome [Citation47] and two studies presented their own specially developed outcome measures as their primary measure [Citation53,Citation72].
What communication functions were targeted
This review explored how VR was used to treat language and communication within the framework of the ICF+, as determined by the primary outcome measure, see . The majority of studies (n = 11) were primarily influencing the language impairment in aphasia [Citation48–50,Citation52–55,Citation72,Citation73,Citation75,Citation76]. In two studies, the primary outcome was communication activity [Citation46,Citation74], and one aimed to improve well-being [Citation51]. Secondary outcome measures had a broader spread, in that they addressed impairment and activity/participation domains of the ICF as well as well-being, depression, self-esteem, confidence, and QOL.
Characteristics of the interventions
The VR interventions addressed word finding (four studies), sentence structure (one study), narratives (one study), communication activity (four studies), script training (one study), oral reading (one study), comprehension (one study), and social support (one study). A summary of the intervention characteristics is in .
Table 8. Outcomes of VR interventions in included studies.
Some studies took existing protocols into the novel VR environment and some studies created a new protocol for the new environment. Nine studies used published intervention protocols. One naming therapy study [Citation50] used the protocols of Woolf [Citation96] and Edmonds [Citation97]. The RGS papers [Citation47,Citation48,Citation53] used the principles of Intensive Language Action Therapy [Citation98]. The sentence treatment followed the Treatment of Underlying Forms (TUF) protocol [Citation76]. The narrative treatment is described as an adaptation of the Interactive Storytelling Therapy [Citation71]. The script training [Citation72] used the script protocol from the team’s previous research [Citation99], and WebORLA used the protocol from Oral Reading for Language in Aphasia [Citation100]. Marshall et al. [Citation51] developed a group social support intervention that drew on elements of several published approaches [Citation101–104].
Two studies developed novel interventions that exploited the potential of simulated real-world environments available in VR technology [Citation46,Citation55]. Giachero et al. created functional scenarios for communication activity practice, e.g., check-in to the hotel, decide how long to stay, and book breakfast. There were additional, unexpected events to navigate, e.g., a forgotten suitcase. Marshall et al. [Citation46] used the EVA Park virtual world to address participant-led communication activity goals, e.g., requesting a haircut in the barbers, ordering dinner in a restaurant, and sharing biographical stories.
Of the remaining three studies, two described exploratory work with a view to developing a novel protocol for dialogue practice tools [Citation74,Citation75], and the final one described an “experimental linguistic treatment” (p. 3) that was delivered using paper and pencil for the control group and via the VRRS-tablet for the experimental group [Citation54].
Total treatment hours (dose) ranged from <1 to 100 h [Citation54] with a mean treatment dose of 19.59 h, and both a median and mode of 20 h. The duration of treatment ranged from one session to six months [Citation51,Citation54,Citation55]. The frequency of the interventions (sessions per week) was mostly once a day, with 4 or 5 sessions per week [Citation46–50,Citation53–55,Citation76] with one study delivering one session every fortnight [Citation51]. Treatments were delivered by qualified SLTs, professionals in aphasia support services (e.g., aphasia group co-ordinators), and computer-delivered. Treatments were delivered in one to one, peer interaction activities and group contexts. The VR interventions were set in hospitals, research laboratories, and community settings, e.g., participant’s home.
What outcomes were achieved
Changes demonstrated in the outcome measures for all studies are presented in . Only studies rated as fair quality or above are included in this section (n = 7) and are highlighted in grey in [Citation46,Citation48,Citation51,Citation53,Citation55,Citation72,Citation73].
As reflects the preliminary nature of the research, two trials included feasibility outcomes. They compared VR to a no treatment control and were rated as fair on the PEDro quality measure [Citation46,Citation51]. They demonstrated that virtual worlds showed promise for delivering a communication activity intervention [Citation46] and that online social support groups of up to eight people with aphasia with four additional support staff, each logging in from their own home, were feasible [Citation51].
Five studies employed a measure of language impairment as their primary outcome with all five reporting significant treatment-induced improvement [Citation48,Citation53,Citation55,Citation72,Citation73]. Three studies explored change in communication activity either as a primary or secondary measure, and all three reported positive change [Citation46,Citation53,Citation55]. Two studies [Citation51,Citation55] explored the quality of life as a secondary outcome with one reporting positive change [Citation55]. One study explored change in activity/participation as a secondary measure and reported positive change [Citation48]. One study employed a measure of well-being as their primary outcome measure and reported no significant changes [Citation51].
Four trials compared VR with face-to-face speech and language therapy [Citation48,Citation54,Citation55,Citation76]. Only two of these studies were rated fair or good quality [Citation48,Citation55]. They both demonstrated no difference between the groups on the primary outcome measures. These findings offer preliminary evidence of equivalence between VR and face-to-face therapy.
Discussion
The use of VR in the rehabilitation of aphasia is in the exploratory stages of research. In this review, four forms of VR were seen, none of which were immersive. Rationales for employing VR varied and despite the ecological validity offered by multi-user virtual environments, VR interventions predominantly targeted language impairments and used language impairment measures as the primary outcome. Most interventions used previously published protocols with two technologies making use of the simulated real-world environments available in VR. In terms of outcomes, improvements in language impairment (n = 5 studies) and communication activity (n = 3 studies) were achieved through the use of VR for aphasia rehabilitation. When compared to face-to-face therapy there was a suggestion that VR interventions achieve equivalent outcomes to face to face therapy (n = 2 studies), with one study reporting added benefits to communication activity/participation.
Four forms of VR have been used in aphasia therapy research to date. They sit on a spectrum that ranges from a constrained, pre-programmed task with a static view to an open virtual space that can be used by multiple people for multiple activities. The computer-programmed virtual clinician, often only the clinician’s head, is a static view with computer-delivered tasks [Citation72–76]. The replicated table with objects from RGS widens the lens to the table, the arms, and the peer sitting opposite but the view is still static [Citation47,Citation48,Citation53]. In the virtual scenarios of everyday communication situations, a whole environment is represented, e.g., a train station. The view is moveable and unexpected communication tasks are presented [Citation55]. Finally, EVA Park presents a whole island environment where some elements of the environment are interactive. Participants are represented by a personalised avatar. They moved around the island, chose how they would be represented in the virtual space, and meet multiple other users. Images 1–4 (Supplementary Material) depict examples of the forms of VR used in aphasia rehabilitation.
Recently, a framework for describing situated language has been proposed [Citation26]. It defines language use as (1) interactive, (2) multimodal, and (3) contextual. As the spectrum of VR spaces opens up to encompass multi-users within rich environments, they offer an opportunity for embedding situated language use into aphasia rehabilitation. When VR is selected as the mode of treatment delivery, the possibilities are wide-ranging. VR is not bound by the constraints of geography, physical laws (e.g., in EVA Park you can fly), or physical impairments. There is a potential to develop novel, creative multi-user environments that address the particular issues pertinent to aphasia rehabilitation: how to improve situated language use, mitigate the loss of social networks, and support the renegotiation of identity [Citation105]. This review has demonstrated that this potential is not yet being fully realised. With the exception of EVA Park, where experiences outside of the bounds of reality are possible (e.g., avatars can ride on a turtle underwater), uses of VR in aphasia rehabilitation currently replicate reality or even the constraints of the aphasia clinic room.
Researchers cited a variety of rationales for the use of VR. Some aligned with the rationale for 2D online remote delivery; that it is accessible and provides increased intensity and dose [Citation106,Citation107]. A number of the studies use VR to replicate the clinical context and deliver treatments either remotely and/or independently of therapist input [Citation47,Citation48,Citation53,Citation72,Citation73,Citation76]. Receiving interventions at your home via your computer with the option to practice an unlimited amount in your own time has been shown to be acceptable [Citation108,Citation109]. Other rationales were cited that relate to the unique properties of VR, such as the opportunity to situate practice within multimodal simulations of the real world, and these were cited by seven studies. The rationale for the unexpected nature of fantasy elements in EVA Park was that it motivated genuine conversational exchange.
Some rationales were specific to multi-user VR. Gaming research shows users play multi-user games because of (1) warm relationships with others, (2) a sense of accomplishment, (3) a sense of belonging, and (4) fun and enjoyment [Citation31]. These align with speech and language therapy aims to improve social networks and social connectedness (see [Citation51]). Participant views on the multi-user EVA Park interventions similarly included fun, humour, and warm relationships [Citation110,Citation111]. Seven of the studies in this review could be described as multi-user. In one study the interaction with the technology was as a single user but the therapeutic experience was in a group of three [Citation55]. Perhaps unsurprisingly, the studies with multi-user technology were those that cited socially motivated rationales, e.g., ecological validity, socially embedded, and social networks.
It is interesting to reflect on the rationales that were not cited in the papers. A theme in gaming literature is the agency of the user. The degree to which the user can effect change in the virtual space is considered important [Citation112] because the agency is said to support users in feeling like they are really there; a concept called immersion [Citation113]. There is a suggestion that immersion is a potential mechanism of change in VR for health. For example, in virtual reality exposure therapy, it is proposed that the treatment works because the virtual representation is real enough to elicit the anxiety [Citation114]. Seven studies in this review reported that the user was represented in the world. In the four EVA Park studies the users have agency of movement in the virtual space. Additionally, users in EVA Park can interact with objects in the virtual space and can effect some limited changes. None of the studies alluded to concepts of immersion or presence in their rationales. Similarly, none referred to the experiences of other disability groups with VR. In summary, only some studies are citing rationales that are unique to VR environments. There is a potential that has not yet been explored.
In relation to outcome measures, all studies but one reported multiple outcome measures. In the study that reported a single score, the WAB LQ [Citation73], the score is calculated from multiple language tasks (reading, writing, speech, comprehension, repetition, and naming). The outcome measures covered all domains of the ICF with language impairment most represented. Additionally, QoL, confidence communicating, well-being, self-esteem, and depression were measured. The wide range of measures used has implications for evaluating the efficacy of these treatments. For meta-synthesis to be carried out, the measurement of outcomes needs to be rationalised. A core outcome set (COS) for aphasia research was agreed upon in 2018 [Citation115]. Eight studies from this review were published after 2018 and yet only one measure from the COS was used in the included studies, the SAQOL-39g [Citation51]. The Scenario Test was recently named as the measure of communication activity in the core outcome set for aphasia [Citation116]. The Scenario Test was not used by any of the studies in this review.
With regards to the communication functions targeted, VR in aphasia rehabilitation has been used predominantly to rehabilitate language impairments (12/14 studies reviewed). This finding is consistent with the physiotherapy literature [Citation42]. Communication activity is cited as a priority for both people with aphasia and clinicians [Citation24,Citation117] and multi-user simulated environments are uniquely placed to target this [Citation44]. This could have driven a rise in the use of VR for communication activity, however, communication activity was the primary outcome in only one study in this review.
The interventions often used familiar therapies delivered in the novel format of VR. This was the goal in some treatments. For example, virtual clinicians freed up therapist time while increasing patient dose. It is interesting that, to date, VR has not taken aphasia therapy in radical new directions. In some cases [Citation46,Citation55], there was an attempt to exploit the virtual environment to promote generalisation of skills or to address multiple levels of the ICF, but this is not possible on all platforms or not attempted in all studies.
The completeness of the intervention description in this review was comparable to an umbrella review of intervention descriptions in aphasia [Citation118]. The umbrella review, 50% of studies scored 8 out of a possible 12 items, and in this review 9/14 (64%) studies scored 8 or more out of the possible 12 items. The location of the intervention, the fidelity plan, and adherence were the missing items in both reviews. This makes it difficult to replicate therapies, with implications for developing the evidence base and implementing therapies in clinical practice.
Positive language impairment outcomes have been achieved in VR-based interventions. When these outcomes were compared to face-to-face delivery, in two studies of “fair” and “good” quality, they were equivalent. This finding mirrors what is known about non-VR computer-delivered speech and language treatments [Citation96,Citation119] where online and face-to-face delivery were equally effective [Citation96,Citation119].
There was some preliminary evidence that VR-delivered therapy can achieve change in other dimensions of the ICF, with changes seen in communication activity (n = 3, fair or moderate quality) and participation (n = 1, good quality), and improved quality of life (n = 1, fair quality). Changes beyond the language impairment were seen only when interventions were delivered in multi-user virtual environments where there were opportunities to converse with peers with aphasia [Citation48,Citation53] and/or therapists [Citation46,Citation55]. This finding adds to the argument that therapy activities must target areas beyond impairment if we are to see these gains [Citation120].
Feasibility outcomes from the feasibility studies (n = 2, fair quality) in this review were positive [Citation46,Citation51]. This review was confined to experimental studies. However, qualitative investigations linked to these studies have explored the acceptability of VR studies to people with aphasia [Citation110,Citation111] and service providers [Citation121] show that VR interventions are acceptable.
Outcome evidence is only indicative but suggests that VR treatments are feasible and can achieve similar gains to those reported from face-to-face therapy. However, the strength of evidence is weak and many issues relating to the potential outcomes of VR therapy remain unexplored.
Limitations
A limited number of studies met the criteria for this review. Despite opening the criteria to include acquired cognitive communication disorder, no articles were found that reported the use of VR to remediate language in this area. This finding was similar to a recent review [Citation122] where authors expanded their search after finding no examples of designing VR for traumatic brain injury. None of the studies included in the review were definitive studies, demonstrating how new this field of research is. The criterion to include only intervention studies may have excluded some of the more creative and novel developments of VR. These are published as user testing or platform development articles. For example, a head-worn display that provides vocabulary cues in context [Citation123]. Qualitative literature about the experience of people with aphasia using VR was also beyond the scope of the review, but this literature points to good acceptability [Citation110,Citation111]. Moreover, only half of the studies were rated as of fair or higher quality with the risk of bias high amongst single case studies.
The participants were younger than is typical for the stroke population. The national average age for a first stroke is 68 years for males and 73 years for females [Citation124]. The relevance of age, e.g., with respect to technology uptake, is not clear-cut [Citation125]. Nevertheless, it would be desirable to test VR treatments with participants who more closely reflect the age of typical stroke survivors. Wider demographic data were not always available and were not extracted from the studies. It would be important to explore such variables, again to ensure that participants reflect the intended user population.
Another problem for this review was the disparate primary outcomes. This meant meta-analysis was not a sensible option. A previous review carried out a meta-analysis of a maximum of two studies [Citation43].
Directions for future research
VR has the potential to create novel, multi-user spaces that engender fun and a feeling of belonging. They can mimic the real world or extend into fantasy. Such spaces may help to address aphasia and its negative social and emotional consequences. There is a need for well-designed empirical studies that explore the impact of multi-user VR interventions on the ICF domains of activity and participation and beyond. Potential variables to be explored are (a) the impact of immersion and presence, to investigate whether this is a mechanism for change in aphasia rehabilitation as it is suggested in the VR exposure therapy literature, (b) the impact of autonomy/agency in using VR and/or creating within VR, e.g., personalising avatars, and (c) the impact of receiving treatments in beautiful, playful spaces. Collaborations between the gaming community, human-computer interaction design, and speech and language therapy may achieve this potential.
There is a need for larger studies and more studies with a low risk of bias to provide definitive evidence. Designs should also explore a wider range of questions, such as whether VR shows equivalence to face-to-face therapy and whether there are added benefits of VR, for example with respect to generalisation and maintenance of change. Additional outcome issues (e.g., cost-effectiveness) could also be explored.
Consistent use of the Core Outcome Set for aphasia rehabilitation [Citation115] will support future meta-analyses in this field.
Conclusion
VR for the rehabilitation of aphasia is being used for predominantly impairment-level interventions with non-definitive evidence of positive outcomes. The rationales for using VR vary across studies, from releasing SLT time to creating ecologically valid environments. There is a need for future studies to strengthen the evidence and explore the particular benefits of VR over other technologies. The opportunity to create novel multi-user spaces for communication activity gains has not been exploited.
Supplementary Material.docx
Download MS Word (57.1 KB)Supplementary Material.docx
Download MS Word (1.9 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Berg K, Isaksen J, Wallace SJ, et al. Establishing consensus on a definition of aphasia: an e-Delphi study of international aphasia researchers. Aphasiology. 2022;36(4):385–400.
- Ali M, Lyden P, Brady M, et al. Aphasia and dysarthria in acute stroke: recovery and functional outcome. Int J Stroke. 2015;10(3):400–406.
- Code C, Petheram B. Delivering for aphasia. Int J Speech Lang Pathol. 2011;13(1):3–10.
- Stroke Association. State of the nation: stroke statistics [cited 2019 Oct 26]. Available from: https://www.stroke.org.uk/resources/state-nation-stroke-statistics
- Royal College of Physicians. Sentinel Stroke National Audit Programme (SSNAP) post-acute organisational audit; 2015. Available from: https://www.strokeaudit.org/Documents/National/PostAcuteOrg/2015/2015-PAOrgGenericReportPhase2.aspx
- Northcott S, Hilari K. Why do people lose their friends after a stroke?: friendship loss post stroke. Int J Lang Commun Disord. 2011;46(5):524–534.
- Worrall L, Ryan B, Hudson K, et al. Reducing the psychosocial impact of aphasia on mood and quality of life in people with aphasia and the impact of caregiving in family members through the aphasia action success knowledge (aphasia ASK) program: study protocol for a randomized controlled trial. Trials. 2016;17:153.
- Cornwell EY, Waite LJ. Social disconnectedness, perceived isolation, and health among older adults. J Health Soc Behav. 2009;50(1):31–48.
- House JS. Social isolation kills, but how and why? Psychosom Med. 2001;63(2):273–274.
- Brummett BH, Barefoot JC, Siegler IC, et al. Characteristics of socially isolated patients with coronary artery disease who are at elevated risk for mortality. Psychosom Med. 2001;63(2):267–272.
- Simmons-Mackie N, Cherney LR. Aphasia in North america: highlights of a white paper. Arch Phys Med Rehabil. 2018;99(10):e117.
- World Health Organization. International classification of functioning, disability and health: ICF. Geneva: World Health Organization; 2001. Available from: https://apps.who.int/iris/handle/10665/42407
- Wisenburn B, Mahoney K. A meta-analysis of word-finding treatments for aphasia. Aphasiology. 2009;23(11):1338–1352.
- Mehri A, Jalaie S. A systematic review on methods of evaluate sentence production deficits in agrammatic aphasia patients: validity and reliability issues. J Res Med Sci. 2014;19(9):885–898.
- Dipper L, Marshall J, Boyle M, et al. Treatment for improving discourse in aphasia: a systematic review and synthesis of the evidence base. Aphasiology. 2021;35(9):1125–1167.
- Wilkinson R, Wielaert S. Rehabilitation targeted at everyday communication: can we change the talk of people with aphasia and their significant others within conversation? Arch Phys Med Rehabil. 2012;93(1 Suppl):70.
- Horton S, Lane K, Shiggins C. Supporting communication for people with aphasia in stroke rehabilitation: transfer of training in a multidisciplinary stroke team. Aphasiology. 2016;30(5):629–656.
- Shrubsole K, Lin T, Burton C, et al. Delivering an iterative communication partner training programme to multidisciplinary healthcare professionals: a pilot implementation study and process evaluation. Int J Lang Commun Disord. 2021;56(3):620–636.
- Kauhanen M, Korpelainen JT, Hiltunen P, et al. Domains and determinants of quality of life after stroke caused by brain infarction. Arch Phys Med Rehabil. 2000;81(12):1541–1546.
- Hilari K, Northcott S, Roy P, et al. Psychological distress after stroke and aphasia: the first six months. Clin Rehabil. 2010;24(2):181–90.
- Hilari K, Needle JJ, Harrison KL. What are the important factors in health-related quality of life for people with aphasia? A systematic review. Arch Phys Med Rehabil. 2012;93(1):S86–S95.e4.
- Hilari K. The impact of stroke: are people with aphasia different to those without? Disabil Rehabil. 2011;33(3):211–218.
- Lam JMC, Wodchis WP. The relationship of 60 disease diagnoses and 15 conditions to preference-based health-related quality of life in Ontario hospital-based long-term care residents. Med Care. 2010;48(4):380–387.
- Worrall L, Sherratt S, Rogers P, et al. What people with aphasia want: their goals according to the ICF. Aphasiology. 2011;25(3):309–322.
- Doedens W, Meteyard L. The importance of situated language use for aphasia rehabilitation. PsyArXiv. 2018.
- Doedens WJ, Meteyard L. What is functional communication? A theoretical framework for real-world communication applied to aphasia rehabilitation. Neuropsychol Rev. 2022.
- Cambridge University Press. Cambridge Dictionary [cited 2021 Apr 13]. Available from: https://dictionary.cambridge.org/dictionary/english/virtual-reality
- Thomas M, Cinganotto L, Heike P. Digital game-based language learning in 3D immersive environments: the GUINEVERE project. Innovation in Language Learning, International Conference; 2018.
- Sutherland LM, Middleton PF, Anthony A, et al. Surgical simulation: a systematic review. Ann Surg. 2006;243(3):291–300.
- Bryant L, Brunner M, Hemsley B. A review of virtual reality technologies in the field of communication disability: implications for practice and research. Disabil Rehabil Assist Technol. 2020;15(4):365–372.
- Lin Y, Lin H, Yang Y. Players’ value structure in digital games. Games Cult. 2017;12(1):72–99.
- Cairns P, Power C, Barlet M, et al. Enabled players: the value of accessible digital games. Games Cult. 2021;16(2):262–282.
- Yarwood J. The most beautiful video games inspired by famous artists [cited 2021 Dec 17]. Available from: https://www.vice.com/en/article/qbxm9d/the-most-beautiful-video-games-inspired-by-famous-artists-211
- Mekbib DB, Han J, Zhang L, et al. Virtual reality therapy for upper limb rehabilitation in patients with stroke: a meta-analysis of randomized clinical trials. Brain Inj. 2020;34(4):456–465.
- Karamians R, Proffitt R, Kline D, et al. Effectiveness of virtual reality- and gaming-based interventions for upper extremity rehabilitation poststroke: a meta-analysis. Arch Phys Med Rehabil. 2020;101(5):885–896.
- Ghai S, Ghai I, Lamontagne A. Virtual reality training enhances gait poststroke: a systematic review and meta‐analysis. Ann N Y Acad Sci. 2020;1478(1):18–42.
- Chen X, Liu F, Lin S, et al. Effects of virtual reality rehabilitation training on cognitive function and activities of daily living of patients with poststroke cognitive impairment: a systematic review and meta-analysis. Arch Phys Med Rehabil. 2022;103(7):1422–1435.
- You SH, Jang SH, Kim Y-H, et al. Virtual reality–induced cortical reorganization and associated locomotor recovery in chronic stroke. Stroke. 2005;36(6):1166–1171.
- Lohse KR, Hilderman CGE, Cheung KL, et al. Virtual reality therapy for adults post-stroke: a systematic review and meta-analysis exploring virtual environments and commercial games in therapy. PLOS One. 2014;9(3):e93318.
- Aminov A, Rogers JM, Middleton S, et al. What do randomized controlled trials say about virtual rehabilitation in stroke? A systematic literature review and meta-analysis of upper-limb and cognitive outcomes. J Neuroeng Rehabil. 2018;15(1):29.
- Alt Murphy M, Resteghini C, Feys P, et al. An overview of systematic reviews on upper extremity outcome measures after stroke. BMC Neurol. 2015;15(1):29.
- Palma G, Freitas T, Bonuzzi G, et al. Effects of virtual reality for stroke individuals based on the international classification of functioning and health: a systematic review. Top Stroke Rehabil. 2017;24(4):269–278.
- Cao Y, Huang X, Zhang B, et al. Effects of virtual reality in post-stroke aphasia: a systematic review and meta-analysis. Neurol Sci. 2021;42(12):5249–5259.
- Picano C, Quadrini A, Pisano F, et al. Adjunctive approaches to aphasia rehabilitation: a review on efficacy and safety. Brain Sci. 2021;11(1):41.
- Repetto C, Paolillo MP, Tuena C, et al. Innovative technology-based interventions in aphasia rehabilitation: a systematic review. Aphasiology. 2020;35:12, 1623–1646.
- Marshall J, Booth T, Devane N, et al. Evaluating the benefits of aphasia intervention delivered in virtual reality: results of a quasi-randomised study. PLOS ONE. 2016;11(8):e0160381.
- Grechuta K, Bellaster BR, Munne RE, et al. The effects of silent visuomotor cueing on word retrieval in Broca’s aphasies: a pilot study. IEEE Int Conf Rehabil Robot. 2017;2017:193–199.
- Grechuta K, Ballester B, Munne R, et al. Augmented dyadic therapy boosts recovery of language function in patients with nonfluent aphasia. Stroke. 2019;50(5):1270–1274.
- Carragher M, Steel G, Talbot R, et al. Adapting therapy for a new world: storytelling therapy in EVA park. Aphasiology. 2021;35(5):704–729.
- Marshall J, Devane N, Edmonds L, et al. Delivering word retrieval therapies for people with aphasia in a virtual communication environment. Aphasiology. 2018;32(9):1054–1074.
- Marshall J, Devane N, Talbot R, et al. A randomised trial of social support group intervention for people with aphasia: a novel application of virtual reality. PLOS One. 2020;15(9):e0239715.
- Grechuta K, Rubio B, Duff A, et al. Intensive language-action therapy in virtual reality for a rehabilitation gaming system. J Pain Manage. 2016;9(3):243–254.
- Grechuta K, Rubio Ballester B, Espín Munné R, et al. Multisensory cueing facilitates naming in aphasia. J NeuroEngineering Rehabil. 2020;17(1):122.
- Maresca G, Maggio MG, Latella D, et al. Toward improving poststroke aphasia: a pilot study on the growing use of telerehabilitation for the continuity of care. J Stoke Cerebrovasc Dis. 2019;28(10):104303.
- Giachero A, Calati M, Pia L, et al. Conversational therapy through semi-immersive virtual reality environments for language recovery and psychological well-being in post stroke aphasia. Behav Neurol. 2020;2020:2846046.
- Zhang Y, Chen P, Li X, et al. Clinical research on therapeutic effect of virtual reality technology on Broca aphasia patients. In: 2nd International Conference on Information Technology (INCIT); 2017 November. p. 1–5.
- Togher L, Wiseman-Hakes C, Douglas J, et al. INCOG recommendations for management of cognition following traumatic brain injury, part IV: cognitive communication. J Head Trauma Rehabil. 2014;29(4):353–368.
- Kertesz A. The western aphasia battery–revised (WAB-R). Pearson; 2007. doi: 10.1037/t15168-000.
- Holland A, Frattali C, Fromm D. Communication activities of daily living-2. Austin (TX): Pro-Ed; 1999.
- Wallace SJ, Worrall L, Le Dorze G, et al. Many ways of measuring: a scoping review of measurement instruments for use with people with aphasia. Aphasiology. 2022;36(4):401–466.
- Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687.
- Moseley AM, Szikszay TM, Lin C-C, et al. A systematic review of the measurement properties and usage of the physiotherapy evidence database (PEDRO) scale. Physiotherapy. 2015;101:e1043.
- Tate RL, Rosenkoetter U, Wakim D, et al. Risk of bias in N-of-1 trials (RoBiNT) scale: an expanded manual for the critical appraisal of single-case reports. Sydney: PsycBITE; 2015.
- Perdices M, Tate RL, Rosenkoetter U. An algorithm to evaluate methodological rigor and risk of bias in single-case studies. Behav Modif. 2019:014544551986303.
- Tate RL, Perdices M, Rosenkoetter U, et al. Revision of a method quality rating scale for single-case experimental designs and n-of-1 trials: the 15-item risk of bias in N-of-1 trials (RoBiNT) scale. Neuropsychol Rehabil. 2013;23(5):619–638.
- American Occupational Therapy Association. Guidelines for systematic reviews [cited 2022 Jan 14]. Available from: https://ajot.submit2aota.org/journals/ajot/forms/systematic_reviews.pdf
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: assessing the quality of controlled clinical trials. BMJ. 2001;323(7303):42–46.
- Cohen J. Statistical power analysis for the behavioral sciences. New York: Routledge; 2013.
- Thomas J, Graziosi S, Brunton J, et al. EPPI-reviewer: advanced software for systematic reviews, maps and evidence synthesis. EPPI-Centre software. London: UCL Social Research Institute; 2020.
- Hartholt A, Traum D, Marsella S, et al. All together now, introducing the virtual human toolkit. Conference: Intelligent Virtual Agents; 2013. p. 368.
- Carragher M, Sage K, Conroy P. Preliminary analysis from a novel treatment targeting the exchange of new information within storytelling for people with nonfluent aphasia and their partners. Aphasiology. 2015;29(11):1383–1408.
- Cherney LR, Braun EJ, Lee JB, et al. Optimising recovery in aphasia: learning following exposure to a single dose of computer-based script training. Int J Speech Lang Pathol. 2019;21(5):448–458.
- Cherney LR, Lee JB, Kim KA, et al. Web-based oral reading for language in aphasia (Web ORLA®): a pilot randomized control trial. Clin Rehabil. 2021;35(7):976–987.
- Kalinyak-Fliszar M, Martin N, Keshner E, et al. Using virtual technology to promote functional communication in aphasia: preliminary evidence from interactive dialogues with human and virtual clinicians. Am J Speech Lang Pathol. 2015;24(4):S974–S989.
- Snell S, Martin N, Keshner EA. Engagement with a virtual clinician encourages gesture usage in speakers with aphasia. International Conference on Virtual Rehabilitation; 2017. p. 1–5.
- Thompson C, Choy JJ, Holland A, et al. Sentactics®: computer-automated treatment of underlying forms. Aphasiology. 2010;24(10):1242–1266.
- Hartholt A, Traum D, Marsella S, et al. All together now, introducing the virtual human toolkit. Conference: Intelligent Virtual Agents; 2013.
- Girvan C. What is a virtual world? definition and classification. Education Tech Research Dev. 2018;66(5):1087–1100.
- Fernandino L, Binder JR, Desai RH, et al. Concept representation reflects multimodal abstraction: a framework for embodied semantics. Cereb Cortex. 2016;26(5):2018–2034.
- Nicholas LE, Brookshire RH. A system for quantifying the informativeness and efficiency of the connected speech of adults with aphasia. J Speech Hear Res. 1993;36(2):338–350.
- Druks J, Masterson J. An object and action naming battery. Hove: Psychology Press; 2000.
- Huber W, Poeck K, Willmes K. The aachen aphasia test. Adv Neurol. 1984;42:291–303.
- Goodglass H, Kaplan E. The assessment of aphasia and related disorders. 2nd ed. Philadelphia (PA): Lea & Febiger; 1983.
- De Renzi E, Vignolo LA. The token test: a sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678.
- Pitts LL, Hurwitz R, Lee JB, et al. Validity, reliability and sensitivity of the NORLA-6: naming and oral reading for language in aphasia 6-point scale. Int J Speech Lang Pathol. 2018;20(2):274–283.
- Whitworth A, Perkins L. Conversation analysis profile for people with aphasia (CAPPA). London: Whurr Publishers Ltd; 1997.
- Jutai J, Day H. Psychosocial impact of assistive devices scale (PIADS). TAD. 2002;14(3):107–111.
- Pulvermüller F, Neininger B, Elbert T, et al. Constraint-induced therapy of chronic aphasia after stroke. Stroke. 2001;32(7):1621–1626.
- Tennant R, Hiller L, Fishwick R, et al. The Warwick Edinburgh Mental Well-Being Scale (WEMWBS): development and UK validation. Health Qual Life Outcomes. 2007;5(1):63.
- Brumfitt SM, Sheeran P. The development and validation of the Visual Analogue Self-Esteem Scale (VASES). Br J Clin Psychol. 1999;38(4):387–400.
- Cherney LR, Babbitt EM, Semik P, et al. Psychometric properties of the communication confidence rating scale for aphasia (CCRSA): phase 1. Top Stroke Rehabil. 2011;18(4):352–360.
- Benaim C, Cailly B, Perennou D, et al. Validation of the aphasic depression rating scale. Stroke. 2004;35(7):1692–1696.
- WHO. The world health organization quality of life (WHOQOL). Geneva: World Health Organisation; 2012. Available from: https://www.who.int/publications/i/item/WHO-HIS-HSI-Rev.2012.03
- Balestroni G, Bertolotti G. EuroQol-5D (EQ-5D): an instrument for measuring quality of life. Monaldi Arch Chest Dis. 2012;78(3):155–159.
- Hilari K, Byng S, Lamping DL, et al. Stroke and Aphasia Quality of Life Scale-39 (SAQOL-39): evaluation of acceptability, reliability, and validity. Stroke. 2003;34(8):1944–1950.
- Woolf C, Caute A, Haigh Z, et al. A comparison of remote therapy, face to face therapy and an attention control intervention for people with aphasia: a quasi-randomised controlled feasibility study. Clin Rehabil. 2016;30(4):359–373.
- Edmonds LA, Nadeau SE, Kiran S. Effect of verb network strengthening treatment (VNeST) on lexical retrieval of content words in sentences in persons with aphasia. Aphasiology. 2009;23(3):402–424.
- Difrancesco S, Pulvermüller F, Mohr B. Intensive language-action therapy (ILAT): the methods. Aphasiology. 2012;26(11):1317–1351.
- Cherney LR, Halper AS, Holland AL, et al. Computerized script training for aphasia: preliminary results. Am J Speech Lang Pathol. 2008;17(1):19–34.
- Cherney LR, Merbitz CT, Grip JC. Efficacy of oral reading in aphasia treatment outcome. Rehabil Lit. 1986;47(5–6):112–118.
- Shadden BB, Agan JP. Renegotiation of identity: the social context of aphasia support groups. Top Lang Disord. 2004;24(3):174–186.
- Holland AL, Nelson RL, Goldberg SA. Counselling in communication disorders: a wellness perspective. 2nd ed. San Diego (CA): Plural Publishing; 2014.
- Attard MC, Lanyon L, Togher L, et al. Consumer perspectives on community aphasia groups: a narrative literature review in the context of psychological well-being. Aphasiology. 2015;29(8):983–1019.
- Seligman MEP, Steen TA, Park N, et al. Positive psychology progress: empirical validation of interventions. Am Psychol. 2005;60(5):410–421.
- Konnerup U. Renegotiation of self after a brain injury using immersive virtual environments: a contribution to technology-mediated speech therapy. Denmark: Aalborg University; 2015.
- Hall N, Boisvert M, Steele R. Telepractice in the assessment and treatment of individuals with aphasia: a systematic review. Int J Telerehabil. 2013;5(1):27–38.
- Palmer R, Enderby P, Paterson G. Using computers to enable self-management of aphasia therapy exercises for word finding: the patient and carer perspective. Int J Lang Commun Disord. 2013;48(5):508–521.
- Palmer R, Dimairo M, Cooper C, et al. Self-managed, computerised speech and language therapy for patients with chronic aphasia post-stroke compared with usual care or attention control (big CACTUS): a multicentre, single-blinded, randomised controlled trial. Lancet Neurol. 2019;18(9):821–833.
- Des Roches CA, Kiran S. Technology-based rehabilitation to improve communication after acquired brain injury. Front Neurosci. 2017;11:382.
- Amaya A, Woolf C, Devane N, et al. Receiving aphasia intervention in a virtual environment: the participants’ perspective. Aphasiology. 2018;32(5):538–558.
- Galliers JR, Wilson S, Marshall J, et al. Experiencing EVA park, a multi-user virtual world for people with aphasia. New York: Association for Computing Machinery; 2017.
- Cole T, Gillies M. Thinking and doing: challenge, agency, and the eudaimonic experience in video games. Games Cult. 2021;16(2):187–207.
- Cairns P, Cox A, Nordin AI. Immersion in digital games: review of gaming experience research. In: Angelides MC, Angelides MC, Agius H, Agius H, editors. Handbook of digital games. 1st ed. Hoboken (NJ): Wiley; 2014. p. 337–361.
- Maples-Keller J, Bunnell BE, Kim S, et al. The use of virtual reality technology in the treatment of anxiety and other psychiatric disorders. Harv Rev Psychiatry. 2017;25(3):103–113.
- Wallace SJ, Worrall L, Rose T, et al. A core outcome set for aphasia treatment research: the ROMA consensus statement. Int J Stroke. 2019;14(2):180–185. doi: 10.1177/1747493018806200.
- Wallace SJ, Worrall L, Rose T, et al. Report from ROMA: an update on the development of a core outcome set for aphasia research. Aphasiology. 2018;32(sup1):241–242.
- Wallace SJ, Worrall L, Rose T, et al. Which treatment outcomes are most important to aphasia clinicians and managers? An international e-Delphi consensus study. Aphasiology. 2017;31(6):643–673.
- Dipper LT, Franklin S, De Aguiar V, et al. An umbrella review of aphasia intervention description in research: the AsPIRE project. Aphasiology. 2022;36(4):467–492.
- Spaccavento S, Falcone R, Cellamare F, et al. Effects of computer-based therapy versus therapist-mediated therapy in stroke-related aphasia: pilot non-inferiority study. J Commun Disord. 2021;94:106158.
- Webster J, Whitworth A, Morris J. Is it time to stop “fishing”? A review of generalisation following aphasia intervention. Aphasiology. 2015;29(11):1240–1264.
- Caute A, Cruice M, Devane N, et al. Delivering group support for people with aphasia in a virtual world: experiences of service providers. Disabil Rehabil. 2021;28:1–19.
- Brassel S, Power E, Campbell A, et al. Recommendations for the design and implementation of virtual reality for acquired brain injury rehabilitation: systematic review. J Med Internet Res. 2021;23(7):e26344.
- Williams K, Moffatt K, McCall D, et al. Designing conversation cues on a head-worn display to support persons with aphasia. In: CHI '15: Proceedings of the 33rd Annual ACM Conference on Human Factors in Computing Systems; 2015; ACM. p. 231–240.
- Public Health England. Briefing document: first incidence of stroke. London: PHE Publications; 2018. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/678444/Stroke_incidence_briefing_document_2018.pdf
- Menger F, Morris J, Salis C. The impact of aphasia on internet and technology use. Disabil Rehabil. 2020;42(21):2986–2996.
