ABSTRACT
Memory consolidation during sleep is assumed to rely on the repeated reactivation of newly encoded memories particularly during slow wave sleep (SWS). It has been proposed that reactivated memories during sleep – like during wakefulness – undergo a labilisation process, enabling the strengthening and integration of new memories into pre-existing networks. Here, we tested this idea by introducing interference directly during sleep in the reactivation/consolidation phase. We predicted that cueing interfering memories during sleep would impair the consolidation of recently learned memories. Participants learned a visuo-spatial memory task before they were allowed to sleep for 40 min. During sleep, and particularly during SWS, subjects were presented with interference via odour cueing (compared to a no interference vehicle condition). In contrast to our hypothesis, cueing of the interference during sleep did not impair consolidation of the newly learned memories: odour and vehicle conditions did not differ in memory recall after sleep. On the contrary, subjects even displayed significantly fewer intrusions from the interference during memory recall when the odour was presented during sleep. These findings suggest that interference presentation during sleep does not disrupt endogenous memory consolidation, but might even facilitate pattern separation and memory stabilisation through generalisation processes.
Sleep is well known to support the consolidation and stabilisation of newly encoded memories (Diekelmann & Born, Citation2010; Walker, Citation2009). A common model assumes that the consolidation of memories during sleep relies on the repeated reactivation (“replay”) of the underlying neuronal connections leading to a strengthening of the memory traces as well as an integration of the new representations into the pre-existing network of long-term memories (Lewis & Durrant, Citation2011; Rasch & Born, Citation2013; Stickgold & Walker, Citation2013). This reactivation of memories occurs spontaneously during sleep, especially during slow wave sleep (SWS; Ji & Wilson, Citation2007; Lee & Wilson, Citation2002; Nadasdy, Hirase, Czurko, Csicsvari, & Buzsaki, Citation1999), and can be triggered by learning-associated cues such as odours and sounds (Antony, Gobel, O’Hare, Reber, & Paller, Citation2012; Cairney, Durrant, Hulleman, & Lewis, Citation2014; Oudiette & Paller, Citation2013; Rasch, Buchel, Gais, & Born, Citation2007; Rudoy, Voss, Westerberg, & Paller, Citation2009; Schonauer, Geisler, & Gais, Citation2014).
We have previously proposed that the reactivation of memories during sleep might go along with a transient labilisation of the memory traces enabling the integration of new contents into the pre-existing memory networks (Rasch & Born, Citation2007). In the wake state, the reactivation of memories labilises the memory traces making them susceptible to interference learning and disruption, with the reactivated memories requiring re-stabilisation (“reconsolidation”) in order to persist (Dudai, Karni, & Born, Citation2015; Hupbach, Gomez, Hardt, & Nadel, Citation2007; Lee, Citation2009; Nader & Hardt, Citation2009; Sara, Citation2000). To test whether similar processes of labilisation-restabilisation occur upon reactivation during sleep, in a previous study we asked subjects to learn a two-dimensional (2D) object location task in the presence of an odour and reactivated the learning material during subsequent SWS by presenting the odour again (Diekelmann, Buchel, Born, & Rasch, Citation2011). Immediately following the odour reactivation, subjects were awakened and asked to learn an interference task. We hypothesised that the odour reactivation during sleep would labilise the memories leading to a stronger impairment through subsequent interference learning. Contrary to this hypothesis, subjects were not impaired by interference learning but showed even better memory performance after odour presentation during sleep, suggesting that odour reactivation did not labilise but immediately stabilised the memories (Diekelmann et al., Citation2011). However, there is an important distinction to make between the process and the outcome of reactivation during sleep. While our previous study indicates that memory traces are more stable after sleep reactivation, the processes during sleep reactivation that lead to these more stable memory traces are unclear. According to the reconsolidation theory, in the wake state the outcome of reactivation-labilisation processes can be two-fold (Spiers & Bendor, Citation2014): (1) the memory can be disrupted or updated if interference is introduced when the reactivated memory is in a labile state (Exton-McGuinness, Lee, & Reichelt, Citation2015; Forcato, Rodriguez, Pedreira, & Maldonado, Citation2010; Hupbach, Gomez, & Nadel, Citation2009) and yet (2) the memory can be strengthened or stabilised if there is no interference in the labile state (Forcato, Fernandez, & Pedreira, Citation2014; Forcato, Fernandez, & Pedreira, Citation2013; Forcato, Rodriguez, & Pedreira, Citation2011). Similar processes, particularly with regard to the latter case, might be underlying sleep-dependent memory reactivation. Indeed, sleep would be an ideal state for reactivation-labilisation-strengthening processes, considering that interference is reduced to a minimum during sleep. Thus, a memory being more stable after sleep could still be the result of that very same memory being labilised (and subsequently re-stabilised and strengthened) during sleep. In keeping with our original idea, one possible explanation for the finding of our previous study – a more stable memory after reactivation during SWS (Diekelmann et al., Citation2011) – could be that the interference was introduced too late, i.e., after awakening. Other than in the wake state, the labilisation during sleep might be followed by a very rapid re-stabilisation either during sleep itself or upon awakening; or the labilised memories might be actively protected from interference upon awakening (Levy, Levitan, & Susswein, Citation2016).
Here, we tested this possibility by adapting our previous study design to allow for the presentation of the interference not after but directly during sleep. We introduced two important changes to the design: (1) while in our previous study, the first-learned 2D object location task was our target task and the second-learned task served as interference, we reversed this order in the present study, i.e., the first-learned task became the interference and the second-learned task was our target and (2) while in our previous study, we externally reactivated the target task with the odour to facilitate the labilisation of the target memories, in the present study we externally reactivated the interference task with the odour as a means of introducing interference to the target memories during sleep. Specifically, subjects learned the first 2D object location task in the presence of an odour on Day 1, with this task serving as interference later. On Day 2 (24 h later), subjects learned a second 2D object location task without the odour, with this task serving as target memory task. During subsequent sleep, when the second (target) task was assumed to be spontaneously reactivated and labilised, the first (interference) task from Day 1 was externally reactivated by presenting the odour again during SWS (). We hypothesised that, if the spontaneous endogenous reactivation of the second-learned target task labilised the underlying memory traces, then the odour-induced reactivation of the first-learned interference task would disrupt the labile target memories, leading to degraded memory retrieval of the second-learned target task.
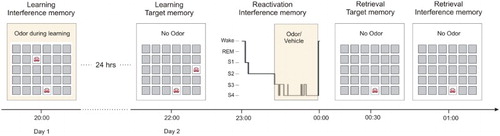
Figure 1. Experimental procedure. Each subject participated in two conditions – odour and vehicle – in a counterbalanced order. Participants learned a 2D object location task (interference memory task) on Day 1 in the presence of an odour. On Day 2, 24 h later, participants learned a similar 2D object location task with the same card pairs but the second card of each pair being presented at a different location (target memory task). Learning of the target memory task took place without odour. Following learning of the target memory task, participants were allowed to sleep for ∼40 min and received either odour stimulation (in the odour condition) or a vehicle (in the vehicle condition) for ∼20 min during SWS. After being awakened, participants were tested on their recall of the target memory task first, followed by a recall test of the interference memory task. Both retrieval tests took place without odour presentation.
Methods
Participants
A total of 25 healthy young adults participated in the study. One subject had to be excluded from the final analysis due to poor sleep quality (sleep latency > 50 min). Five more subjects were excluded from the final analysis as outliers because of unusually low or high memory performance: two subjects performed at chance level during recall of the interference memory task (<2 correct card locations) indicating that these subjects failed to encode the interference memory task, one subject performed very poorly during recall of the target memory task (<2 SD of the group mean, almost at chance level) indicating that this subject might not have followed task instructions or might have had a very hard time to remember the card locations, and two subjects displayed exceptionally good learning performance of the target memory task (100% correct card locations in the first trial) indicating that these subjects might have used specific learning strategies that could have influenced normal consolidation processes.
The 19 subjects included in the final analysis had a mean age of 22.05 ± 2.46 (range 18–25; 12 females). All subjects were non-smokers and reported regular sleep-wake cycles (≥6 h sleep per night), had no history of any neurological, psychiatric or endocrine disorder, did not take any medication, and had no nasal infections at the time of the experiments. Alcohol was prohibited for the entire duration of the experiment. Prior to the experimental nights, subjects spent an adaptation night in the sleep laboratory including electrode placement and application of the nasal mask. All subjects gave written informed consent and were paid for participation. The study followed the principles of the Declaration of Helsinki.
Procedure
Each subject participated in two conditions, an odour condition and a vehicle condition, in counterbalanced order separated by at least two weeks (). In each of the two conditions, participants reported to the laboratory at 19:00 on Day 1. After filling in standard questionnaires and performing on control tasks, subjects performed on an odour detection test with an 80% pass threshold to ensure olfactory sensitivity. The learning phase then started, consisting of the first visual-spatial 2D object location task (from now on called “interference memory task”). During this task, subjects were presented with the experimental odour. After learning, subjects left the laboratory for a night of sleep at home and returned to the lab on Day 2 at 21:00, i.e., on the next day about 24 h after the first learning session. Following placement of the electrodes for polysomnographic recordings, subjects filled in the standard questionnaires and performed on the control tasks again before the second learning session started at around 22:00. This second learning session was identical to the first learning session on Day 1, except that subjects learned a slightly different (but closely related) version of the 2D object location task (from now on called “target memory task” see below for details on the task) without presentation of the odour. Participants went to bed at around 23:00 and were allowed to sleep for approximately 40 min. During this sleep period, and specifically during periods of SWS, either the odour (in the odour condition) or an odourless vehicle (in the vehicle condition) was applied for around 20 min. Odour/vehicle presentation was double-blind and followed a 30-s on/30-s off scheme to prevent habituation. Presentation of the odour/vehicle started as soon as online polysomnographic recordings indicated SWS and was halted whenever signs of arousals, awakening or changes in sleep stages appeared. Depending on the stability of the SWS-period odour/vehicle stimulation could be extended (to a maximum of 30 min) or reduced (to a minimum of 15 min) within a maximum of 90 min of sleep. After the end of the stimulation (at around 00:00), participants were awakened and the electrodes were removed. The retrieval session started 30 min after awakening to allow for recovery from sleep inertia. During the retrieval session, subjects were tested on their recall of the target memory task first, followed by the control tasks and recall of the interference memory task. Retrieval testing took place without odour presentation.
2D object location task
The 2D object location task resembles the game “concentration” and has been applied successfully in previous studies (Diekelmann et al., Citation2011; Rasch et al., Citation2007). In this study, participants were required to learn two different versions of the task: the interference memory task and the target memory task (). The interference memory task was learned on Day 1. The task required learning the location of 15 card pairs showing coloured pictures of different animals and everyday objects presented on a computer screen. At learning, each card pair was presented by showing the first card alone, followed by presentation of both cards. The whole set of card pairs was presented twice in different orders. Immediately after these two runs, recall of the spatial locations was tested using a cued recall procedure, i.e., the first card of each pair was presented and the subject had to indicate the location of the second card with a computer mouse. Feedback was given immediately by presenting the correct location of the second card for re-encoding of the correct location independent of whether the participant’s response was correct or not. The cued recall procedure was repeated until the subject reached a criterion of 60% correct responses (subjects who did not reach the criterion within five trials were sent home and the experiment was discontinued). During the entire learning session, the odour was delivered in a stimulus-locked way, starting with the onset of the presentation of the first card of each pair and stopping when presentation of both cards ended.
The target memory task was learned on Day 2 and was basically identical to the interference memory task with two important differences: (1) the same 15 card pairs were shown but the second card of each pair was presented at a different location (resembling an A–B, A–C interference learning paradigm with A, B and C representing the locations) and (2) no odour was presented during any part of the learning session. Retrieval testing for both task versions took place after sleep by means of a cued recall procedure that was identical to the immediate cued recall test after learning, but without feedback, without odour presentation and consisting of only one recall trial. For the two experimental conditions (odour and vehicle) two parallel versions of the 2D object location task were used in counterbalanced order with different sets of pictures and locations.
Control tasks, sleep recordings and odour presentation
To control for general alertness and sleepiness, subjects performed on a vigilance task and rated their subjective sleepiness before learning of the interference memory task on Day 1, before learning of the target memory task on Day 2 and after the final recall on Day 2. Vigilance was measured as reaction times in a task that required subjects to press the left or right button as fast as possible whenever a dot appeared on the left or right side of the screen. Subjective sleepiness was assessed with the Stanford Sleepiness Scale ranging from 1 (“feeling active, vital, alert, or wide awake”) to 7 (“no longer fighting sleep, sleep onset soon; having dream-like thoughts”). Standard polysomnographic recordings included electroencephalogram (at C3 and C4), electrooculogram and electromyogram. Recordings were visually scored online as well as offline according to standard criteria (Rechtschaffen & Kales, Citation1968).
The experimental odour was isobutyraldehyde diluted in the odourless mineral oil 1,2-propanediol at a concentration of 1:200 (Diekelmann et al., Citation2011; Rihm, Diekelmann, Born, & Rasch, Citation2014). Odour and vehicle (filtered room air) were presented via a computer-controlled olfactometer that allowed rapid odour onset/offset times of 300–500 ms. The olfactometer was placed in an adjacent room and was connected to the subject’s nasal mask via Teflon tubes that allowed normal breathing and constant stimulation without disturbing the participant.
Statistical analysis
Memory performance at retrieval was calculated as relative change of recall performance from learning performance, with learning performance set to 100%, for the target memory task and the interference memory task, respectively. Intrusions in the target memory task were assessed as the number of correct card locations of the interference memory task falsely indicated during retrieval of the target memory task, relative to the learning performance of the interference memory task. Similarly, intrusions in the interference memory task were assessed as the number of correct card locations of the target memory task falsely indicated during retrieval of the interference memory task, relative to the learning performance of the target memory task. All memory measures, sleep data and the number of stimulations were analysed with paired-samples t-tests. Analyses of variance for repeated measures with the factors “odour/vehicle” and “session” were applied for vigilance, subjective sleepiness and odour detection accuracy. Greenhouse-Geisser correction of degrees of freedom was applied where appropriate. Effect sizes are indicated as Cohen’s d. The level of significance was set to p = .05.
Results
2D object location task
Recall of the target memory task after sleep did not differ between the odour and vehicle conditions (). Following odour presentation, subjects correctly recalled 106.35 ± 4.72% of the target memory locations. In the vehicle condition, subjects correctly recalled 102.73 ± 4.39% of the target memory locations (t(18) = 0.59, p = .56; ). Despite no differences in target memory recall, following odour presentation subjects reported fewer intrusions from the interference memory task during recall of the target memory task. In the odour condition, subjects indicated 0.53 ± 0.63% intrusions, whereas in the vehicle condition intrusions amounted to 3.42 ± 1.47% (t(18) = 2.49, p = .023, d = 0.44; ). Although intrusion rates were generally very low, intrusions were significantly different from zero in the vehicle condition (p = .03) but not in the odour condition (p = .33). While in the vehicle condition five subjects indicated one or two intrusions during recall of the target memory task, only one subject reported one intrusion after odour administration. However, note that after including the five outliers that were excluded from the analyses due to unusually low or high performance (see methods section), the difference between conditions in intrusions fails to reach significance (p = .47; all other memory measures remain unchanged).
Figure 2. Performance on the target memory task after reactivation of the interference memory task during sleep. Recall performance (left) is indicated as per cent of correctly recalled card locations at retrieval relative to learning performance. The odour and vehicle conditions did not differ significantly. Intrusions (right) are indicated as per cent of card locations from the interference memory task falsely indicated during recall of the target memory task, relative to learning of the interference memory task. Participants displayed fewer intrusions following odour presentation compared to vehicle.* p < .05.
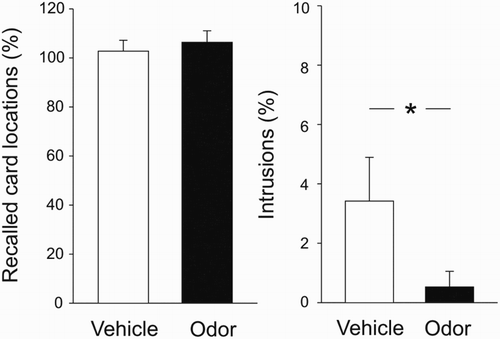
Table 1. Absolute memory performance.
Recall of the interference memory task was comparable between the odour and vehicle conditions (odour vs. vehicle: 45.61 ± 5.02% vs. 46.53 ± 4.58%; t(18) = 0.13, p = .90). Similarly, intrusions from the target memory task during recall of the interference memory task were overall higher but did not differ between conditions (odour vs. vehicle: 8.17 ± 1.81% vs. 10.27 ± 4.87%; t(18) = 0.40, p = .70). The odour and vehicle conditions were also comparable in their initial learning performance for the target memory task (number of learned card locations: p = .48; number of trials to reach the learning criterion: p = .88) as well as for the interference memory task (number of learned card locations: p = .70; number of trials to reach the learning criterion: p = .24; ).
Control tasks and sleep data
Subjects showed normal sleep patterns; and time spent in single sleep stages did not differ between the odour and vehicle conditions (all p > .28; odour, in minutes: total sleep time 47.68 ± 2.33, stage 1 sleep 4.58 ± 0.84, stage 2 sleep 17.53 ± 1.82, SWS 24.58 ± 0.70, REM sleep 0.00 ± 0.00; vehicle, in minutes: total sleep time 48.34 ± 3.22, stage 1 sleep 4.58 ± 0.84, stage 2 sleep 17.34 ± 2.36, SWS 23.47 ± 0.93, REM sleep 0.37 ± 0.37 (only one subject showed 7 min of REM sleep)). The number of stimulations during SWS was closely comparable between the odour and vehicle conditions (22.89 ± 0.63 vs. 22.26 ± 0.87; p = .55). There was also no significant difference between the odour and vehicle conditions in reaction times in the vigilance task as well as in subjective sleepiness (all p > .13 for main effects “odour/vehicle” and interactions “odour/vehicle” × “session”), despite generally lower vigilance and higher sleepiness at the final recall test during the night (all p < .01 for main effects “session”). Accuracy in the odour detection test was on average >95% and was comparable between the odour and vehicle conditions (all p > .07).
Discussion
Contrary to our hypothesis, reactivating an interference task during sleep did not impair the ongoing sleep-dependent consolidation of the target memory task. Interestingly, reactivation of the interference task even seemed to stabilise the target memory, evidenced by a reduced incorporation of intrusions from the interference task during recall of the target memory task. These findings are in line with our previous study, in suggesting that reactivation during SWS stabilises memories and protects them against interference (Diekelmann et al., Citation2011). However, these findings are (once more) in contrast to the idea that memory reactivation and consolidation during sleep involves labilisation processes similar to those observed during wakefulness.
We have previously proposed that the transient labilisation of memories upon reactivation during SWS could be a mechanism that allows for the gradual strengthening and integration of newly acquired memories into the networks of pre-existing long-term memory (Rasch & Born, Citation2007). Such a process of labilisation during sleep could be similar to labilisation observed in the wake state upon reactivation, requiring reconsolidation for memories to persist (Nader & Hardt, Citation2009; Sara, Citation2000). In the absence of external input, labilised memories during sleep might be protected against interference and disruption and could be strengthened through repeated processes of labilisation and restabilisation (Forcato et al., Citation2014). Our present results, together with the findings from our previous study (Diekelmann et al., Citation2011), may suggest that during sleep, other than during wakefulness, reactivation does not labilise but immediately stabilise memories. On a behavioural level, we have shown that neither introducing interference shortly after reactivation during sleep (Diekelmann et al., Citation2011), nor introducing interference directly in the reactivation phase during sleep (present study), impairs memory performance at subsequent testing.
However, we believe that our findings do not necessarily rule out the possibility that memory labilisation occurs upon reactivation during sleep. There might be mechanisms in place that protect ongoing sleep-dependent consolidation processes (possibly accompanied by labilisation/restabilisation) from behavioural interference. Memory interference can be considered a kind of updating or new learning; and a recent study suggests that new learning is blocked during sleep-dependent consolidation via a protein synthesis-dependent process (Levy et al., Citation2016). It is conceivable that this protective mechanism makes it very hard or even impossible to interfere behaviourally with memory traces during sleep or shortly after awakening. Thus, similar labilisation-restabilisation processes might take place during sleep and wakefulness, with the difference that interference can disrupt reactivated memories during wakefulness, while interference is blocked during sleep. Alternatively, instead of being completely blocked, interference might have to be much stronger during or after sleep compared to wakefulness in order to produce a detrimental effect on the target memory (Wichert, Wolf, & Schwabe, Citation2013). From an evolutionary perspective, such a divergence would be beneficial in allowing for an updating of labilised memories with new information during wakefulness, while at the same time enabling the strengthening and integration of labilised memories during sleep without potential disruptions. Considering that new learning might be blocked or be much harder during and shortly after sleep, future studies should test the idea of a memory labilisation during sleep using different approaches, such as electrophysiological or pharmacological methods. First evidence from rodents showed that fear-conditioned memories can be impaired if the memories are reactivated during sleep by presenting a learning-associated odour together with prior injections of anisomycin (a protein synthesis inhibitor) into the basolateral amygdala, suggesting that the reactivation of fear-conditioned memories during sleep triggers labilisation of the memory trace, with the restabilisation being dependent on de novo protein synthesis (Rolls, Makam, Kroeger, & Colas, Citation2013).
Apart from the finding that the reactivation of the interference task did not impair target memory recall, we observed that subjects displayed even fewer intrusion errors following reactivation. This unexpected reduction of intrusions may suggest that participants were better able to discriminate between the target memory and the interference memory if the interference task was reactivated during SWS. It is important to note, however, that intrusions were overall at a very low level and the difference in intrusions between odour and vehicle conditions was no longer significant when five outliers were included in the analysis. Accordingly, the difference in intrusions should be interpreted with caution and we can only speculate about possible explanations for this effect. One possibility is that the reactivation of the interference task during sleep triggered or facilitated processes of pattern separation that supported the consolidation of discrete memory traces for the two tasks and thereby reduced the likelihood of interference errors during subsequent recall. Previous studies have shown that odour reactivation during sleep particularly activates hippocampal regions (Diekelmann et al., Citation2011; Rasch et al., Citation2007) and the hippocampal formation is known to be strongly implicated in pattern separation (Bakker, Kirwan, Miller, & Stark, Citation2008; Lacy, Yassa, Stark, Muftuler, & Stark, Citation2011; Yassa & Stark, Citation2011). Interestingly, this effect was only evident for the target memory task but not for the interference memory task itself. Possibly, reactivation of the interference task prevented the incorporation of parts of the interference memory into the target memory but not the other way around.
Alternatively, the odour serving as a strong context cue for the interference memory task might have generalised to the target memory task, such that the subsequent presentation of the odour during sleep directly facilitated the reactivation and consolidation of the target memory. Although the target memory itself was never paired with the odour, the target memory task was very similar to the interference memory task and was learned in the same context as the interference memory task (same room, same experimenter and same experimental setup). Simply being in the same general context for the learning of the target memory task on Day 2, might have reactivated the memory episode from Day 1, including the odour as a strong context cue (Hupbach, Hardt, Gomez, & Nadel, Citation2008; Kuhl, Shah, Dubrow, & Wagner, Citation2010; Sahakyan, Citation2010). This cue might then have become associated with the learning of the target memory task. Such a process would indicate that a reactivation cue does not necessarily have to be directly associated with the to-be reactivated learning content but could also generalise from different strongly related contents. This possibility should be tested systematically, for example by introducing different environmental contexts for the learning of the interference task and the target memory task.
A third and related explanation might be that the target memory and the interference memory share sufficiently strong overlapping memory features, such that the reactivation of the interference task indirectly also reactivates the strongly related target memory task. Both memory traces might be part of one common memory network and the odour might have reactivated the whole network, consequently stabilising both memories. Speaking against this possibility is the finding that the reduction of intrusion errors was specific for the target memory task but was not evident in the interference memory task. However, it could be speculated that the interference memory was too weak (after the 24-h interval) to benefit from the odour reactivation, evidenced by considerably lower memory recall and higher intrusion rates in the interference task compared to the target memory task. Previous evidence suggests that reactivation cues fail to benefit memories when the memories are too weak or too strong (Creery, Oudiette, Antony, & Paller, Citation2015). This possibility should be tested in future studies by directly manipulating the strength of the different memory traces.
A potential limitation of the present study relates to the observation that the recall of the target memory task was very high. Because of the short retention interval for the target memory task (∼1.5 h), subjects performed very well at testing and showed virtually no forgetting, such that we might have been unable to capture any enhancing effects of the reactivation on target memory recall. On a descriptive level, target memory recall was higher after odour reactivation compared to the vehicle condition, but due to the generally high performance level our task might not have been sensitive enough to detect this effect. However, this limitation does not affect our original hypothesis, considering that we predicted a reduction in target memory performance after odour reactivation of the interference task. Thus, we can safely exclude that the interference reactivation impaired target memory performance. Future studies will have to test whether the reactivation of interference might even enhance target memory recall. Another limitation is that the target memory task was always recalled first, followed by the recall of the interference memory task. The order of task recall was held constant on purpose because we were mainly interested in recall of the target memory task. However, recall of the target memory task might have introduced proactive interference to subsequent recall of the interference memory task (this might also have contributed to lower recall performance in the interference memory task, apart from the longer retention interval). Therefore, the results of the interference memory task should be interpreted with caution, considering that different results may be observed if recall of the interference memory task is tested first or if recall of the target memory task and the interference memory task are counterbalanced. Finally, in the present design the interference memory task was always learned 24 h before the target memory task, such that the interference memory task might have already been consolidated during the first night of sleep. Accordingly, we cannot be absolutely certain that it is possible to reactivate the interference memory task during the second night to interfere with the target memory task. An alternative possibility would be to have subjects learn both tasks in the same session before the experimental night to hold the consolidation level constant across tasks. However, this alternative design has the disadvantage that the two tasks might interfere already during encoding (or that there might be pattern separation or pattern completion or generalisation processes already during encoding), which would be difficult to disentangle from the hypothesised interference effect during sleep. To avoid these limitations, it would be essential to develop new experimental designs to dissociate and control for these effects in future studies.
Acknowledgements
We would like to thank Florian Hobrack and Daysi Lorena Prieto Rojas for helping with data collection.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Antony, J. W., Gobel, E. W., O’Hare, J. K., Reber, P. J., & Paller, K. A. (2012). Cued memory reactivation during sleep influences skill learning. Nature Neuroscience, 15, 1114–1116. doi: 10.1038/nn.3152
- Bakker, A., Kirwan, C. B., Miller, M., & Stark, C. E. (2008). Pattern separation in the human hippocampal CA3 and dentate gyrus. Science, 319, 1640–1642. doi: 10.1126/science.1152882
- Cairney, S. A., Durrant, S. J., Hulleman, J., & Lewis, P. A. (2014). Targeted memory reactivation during slow wave sleep facilitates emotional memory consolidation. Sleep, 37, 701–707, 707A. doi: 10.5665/sleep.3572
- Creery, J. D., Oudiette, D., Antony, J. W., & Paller, K. A. (2015). Targeted memory reactivation during sleep depends on prior learning. Sleep, 38, 755–763. doi: 10.5665/sleep.4670
- Diekelmann, S., & Born, J. (2010). Slow-wave sleep takes the leading role in memory reorganization. Nature Reviews Neuroscience, 11, 218. doi: 10.1038/nrn2762-c2
- Diekelmann, S., Buchel, C., Born, J., & Rasch, B. (2011). Labile or stable: Opposing consequences for memory when reactivated during waking and sleep. Nature Neuroscience, 14, 381–386. doi: 10.1038/nn.2744
- Dudai, Y., Karni, A., & Born, J. (2015). The consolidation and transformation of memory. Neuron, 88, 20–32. doi: 10.1016/j.neuron.2015.09.004
- Exton-McGuinness, M. T., Lee, J. L., & Reichelt, A. C. (2015). Updating memories – the role of prediction errors in memory reconsolidation. Behavioural Brain Research, 278, 375–384. doi: 10.1016/j.bbr.2014.10.011
- Forcato, C., Fernandez, R. S., & Pedreira, M. E. (2014). Strengthening a consolidated memory: The key role of the reconsolidation process. Journal of Physiology-Paris, 108, 323–333. doi: 10.1016/j.jphysparis.2014.09.001
- Forcato, C., Fernandez, R. S., & Pedreira, M. E. (2013). The role and dynamic of strengthening in the reconsolidation process in a human declarative memory: what decides the fate of recent and older memories? PloS one, 8(4), e61688–NaN. doi: 10.1371/journal.pone.0061688
- Forcato, C., Rodríguez, M. L., Pedreira, M. E., & Maldonado, H. (2010). Reconsolidation in humans opens up declarative memory to the entrance of new information. Neurobiology of learning and memory, 93(1), 77–161. doi: 10.1016/j.nlm.2009.08.006
- Forcato, Cecilia, Rodríguez, María L. C., Pedreira, María E., & Robertson, Edwin. (2011). Repeated labilization-reconsolidation processes strengthen declarative memory in humans. PLoS ONE, 6(8), e23305. http://dx.doi.org/10.1371/journal.pone.0023305
- Hupbach, A., Gomez, R., Hardt, O., & Nadel, L. (2007). Reconsolidation of episodic memories: A subtle reminder triggers integration of new information. Learning & Memory, 14, 47–53. doi: 10.1101/lm.365707
- Hupbach, Almut, Gomez, Rebecca, & Nadel, Lynn. (2009). Episodic memory reconsolidation: Updating or source confusion? Memory, 17(5), 502–510. http://dx.doi.org/10.1080/09658210902882399
- Hupbach, A., Hardt, O., Gomez, R., & Nadel, L. (2008). The dynamics of memory: Context-dependent updating. Learning & Memory, 15, 574–579. doi: 10.1101/lm.1022308
- Ji, D., & Wilson, M. A. (2007). Coordinated memory replay in the visual cortex and hippocampus during sleep. Nature Neuroscience, 10, 100–107. doi: 10.1038/nn1825
- Kuhl, B. A., Shah, A. T., Dubrow, S., & Wagner, A. D. (2010). Resistance to forgetting associated with hippocampus-mediated reactivation during new learning. Nature Neuroscience, 13, 501–506. doi: 10.1038/nn.2498
- Lacy, J. W., Yassa, M. A., Stark, S. M., Muftuler, L. T., & Stark, C. E. (2011). Distinct pattern separation related transfer functions in human CA3/dentate and CA1 revealed using high-resolution fMRI and variable mnemonic similarity. Learning & Memory, 18, 15–18. doi: 10.1101/lm.1971111
- Lee, J. L. (2009). Reconsolidation: Maintaining memory relevance. Trends in Neurosciences, 32, 413–420. doi: 10.1016/j.tins.2009.05.002
- Lee, A. K., & Wilson, M. A. (2002). Memory of sequential experience in the hippocampus during slow wave sleep. Neuron, 36, 1183–1194. doi: 10.1016/S0896-6273(02)01096-6
- Levy, R., Levitan, D., & Susswein, A. J. (2016). New learning while consolidating memory during sleep is actively blocked by a protein synthesis dependent process. Elife., 5, e17769. doi: 10.7554/eLife.17769
- Lewis, P. A., & Durrant, S. J. (2011). Overlapping memory replay during sleep builds cognitive schemata. Trends in Cognitive Sciences, 15, 343–351. doi: 10.1016/j.tics.2011.06.004
- Nadasdy, Z., Hirase, H., Czurko, A., Csicsvari, J., & Buzsaki, G. (1999). Replay and time compression of recurring spike sequences in the hippocampus. Journal of Neuroscience, 19, 9497–9507.
- Nader, K., & Hardt, O. (2009). A single standard for memory: The case for reconsolidation. Nature Reviews Neuroscience, 10, 224–234. doi: 10.1038/nrn2590
- Oudiette, D., & Paller, K. A. (2013). Upgrading the sleeping brain with targeted memory reactivation. Trends in Cognitive Sciences, 17, 142–149. doi: 10.1016/j.tics.2013.01.006
- Rasch, B., & Born, J. (2007). Maintaining memories by reactivation. Current Opinion in Neurobiology, 17, 698–703. doi: 10.1016/j.conb.2007.11.007
- Rasch, B., & Born, J. (2013). About sleep’s role in memory. Physiological Reviews, 93, 681–766. doi: 10.1152/physrev.00032.2012
- Rasch, B., Buchel, C., Gais, S., & Born, J. (2007). Odor cues during slow-wave sleep prompt declarative memory consolidation. Science, 315, 1426–1429. doi: 10.1126/science.1138581
- Rechtschaffen, A., & Kales, A. (1968). A manual of standardized terminology, techniques, and scoring system for sleep stages of human subjects. Bethesda, MD: US Department of Health, Education, and Welfare - NIH.
- Rihm, J. S., Diekelmann, S., Born, J., & Rasch, B. (2014). Reactivating memories during sleep by odors: Odor specificity and associated changes in sleep oscillations. Journal of Cognitive Neuroscience, 26, 1806–1818. doi: 10.1162/jocn_a_00579
- Rolls, A., Makam, M., Kroeger, D., Colas, D., de Lecea, L., & Heller, H. C. (2013). Sleep to forget: Interference of fear memories during sleep. Molecular Psychiatry, 18, 1166–1170. doi: 10.1038/mp.2013.121
- Rudoy, J. D., Voss, J. L., Westerberg, C. E., & Paller, K. A. (2009). Strengthening individual memories by reactivating them during sleep. Science, 326, 1079. doi: 10.1126/science.1179013
- Sahakyan, L. (2010). Environmental context change affects memory for performed actions. The Quarterly Journal of Experimental Psychology, 63, 425–433. doi: 10.1080/17470210903414365
- Sara, S. J. (2000). Retrieval and reconsolidation: Toward a neurobiology of remembering. Learning & Memory, 7, 73–84. doi: 10.1101/lm.7.2.73
- Schonauer, M., Geisler, T., & Gais, S. (2014). Strengthening procedural memories by reactivation in sleep. Journal of Cognitive Neuroscience, 26, 143–153. doi: 10.1162/jocn_a_00471
- Spiers, H. J., & Bendor, D. (2014). Enhance, delete, incept: Manipulating hippocampus-dependent memories. Brain Research Bulletin, 105, 2–7. doi: 10.1016/j.brainresbull.2013.12.011
- Stickgold, R., & Walker, M. P. (2013). Sleep-dependent memory triage: Evolving generalization through selective processing. Nature Neuroscience, 16, 139–145. doi: 10.1038/nn.3303
- Walker, M. P. (2009). The role of sleep in cognition and emotion. Annals of the New York Academy of Sciences, 1156, 168–197. doi: 10.1111/j.1749-6632.2009.04416.x
- Wichert, S., Wolf, O. T., & Schwabe, L. (2013). Updating of episodic memories depends on the strength of new learning after memory reactivation. Behavioral Neuroscience, 127, 331–338. doi: 10.1037/a0032028
- Yassa, M. A., & Stark, C. E. (2011). Pattern separation in the hippocampus. Trends in Neurosciences, 34, 515–525. doi: 10.1016/j.tins.2011.06.006
