Abstract
When the chlorophyte alga Dunaliella tertiolecta is placed in darkness, a form of programmed cell death with many similarities to apoptosis (including the induction of caspase-like proteases) is induced. Many uncertainties about this process remain, two of which are whether it requires protein synthesis and whether there is potential viral involvement. In order to examine the relationship between the induction and/or activation of proteolytic activities and the cell death event, we used inhibitors of cytoplasmic protein synthesis (cycloheximide, CHX), organellar protein synthesis (chloramphenicol, CAP) and DNA synthesis (mitomycin C, MMC). Use of MMC also allowed us to examine whether temperate viruses were present in the D. tertiolecta isolate, since MMC treatment has been shown to induce lytic cycles. Addition of protein synthesis inhibitors (100 μM CHX or 1500 μM CAP applied singly or both inhibitors added together) did not prevent cell death from occurring when cultures were placed in the dark. There were no differences in caseinolytic activities visualized using zymograms, or in caspase 1, 3, 8 and 9 -like activities. Surprisingly, 100 μM MMC prevented the cell death event. Caseinolytic activities that appeared in darkness in controls did not appear in MMC-treated cultures, and caspase-like activities remained the same as in controls maintained in the light. The lack of effect of CHX and CAP suggests that the cell death programme we observe does not depend on protein synthesis, but rather on post-translational modification of pre-existing proteins. Results for MMC discount the involvement of temperate virus, but are difficult to interpret. MMC affects DNA synthesis and presumably transcription, but since inhibitors of translation did not prevent cell death, it is not clear why inhibiting transcription would. MMC affects cell cycle progression and cell division cycles, thus these processes may play as yet unexplained roles in mediating the cell death process observed.
Introduction
Apoptosis (an important process in programmed cell death) is ubiquitous in multicellular systems and is essential for normal growth and development (Leist & Nicotera, Citation1997). Cells undergoing apoptosis suffer a series of characteristic changes, including chromatin condensation and margination, ordered DNA cleavage while the cytoplasm and organelles remain unchanged (Cohen, Citation1997). Such changes are brought about by specific proteases called caspases (cysteinyl aspartate-specific proteases, Thornberry, Citation1999) with an unusual and stringent requirement for cleavage after aspartic acid. Apoptosis-like phenomena occur in vascular plants (Greenberg, Citation1996; Pennell & Lamb, Citation1997; Lam & del Pozo, Citation2000; Lam et al., Citation2001), and they have also been reported in unicellular organisms, including chlorophytes (Berges & Falkowski, Citation1998; Segovia et al., Citation2003), dinoflagellates (Vardi et al., Citation1999; Franklin et al., Citation2004; Franklin & Berges, Citation2004), yeast (Frohlich & Madeo, Citation2000) and bacteria (Lewis, Citation2000), including cyanobacteria (Berman-Frank et al., Citation2004).
In 1998, Berges and Falkowski reported a form of autocatalyzed cell death in the single celled chlorophyte alga Dunaliella tertiolecta. D. tertiolecta is an obligate photoautotroph that cannot use dissolved organic compounds and does not reproduce sexually in culture. Members of the genus Dunaliella are well known for their extraordinarily high tolerance to salt stress, high light and relatively high temperatures. When deprived of light, however, cell cultures undergo catastrophic cell death between the second and sixth day. Cell death is preceded by a reduction in photosynthetic capability and cell numbers (Berges & Falkowski, Citation1998). Upon triggering the cell death process, the cells literally dissolve, and the culture, which on the previous day had been green, becomes transparent (Segovia et al., Citation2003). Zymograms and protein profiles, before and during the culture decline, revealed the induction of novel proteases in the cells (Berges & Falkowski, Citation1998).
Further work by Segovia et al. (Citation2003) provided compelling evidence for the apoptotic nature of this phenomenon, including DNA fragmentation, increases in caspase-like activity (defined as cleavage of the fluorogenic caspase-specific substrates WEHD (caspase 1), DEVD (caspase 3), IETD (caspase 8) and LEHD (caspase 9)) during light deprivation, and changes in nuclear structure such as chromatin margination. Moreover, antibodies raised against mammalian caspases cross-reacted with specific proteins in the alga. The pattern of expression of these immunologically-reactive proteins was correlated with the onset of cell death. Under light deprivation, nuclear disintegration took place, while the cytoplasm and organelles remained intact. It was concluded that this form of cell death is an active process resulting from protein synthesis or caspase activation within cells (Segovia et al., Citation2003).
The occurrence of apoptosis in unicells is puzzling because, unlike multicellular organisms, it results in complete loss of the organism and must therefore be maladaptive. Arguments have been made that this may be an ‘altruistic’ phenomenon in other unicells (e.g. Frohlich & Madeo, Citation2000), but this seems unlikely for Dunaliella (Berges & Falkowski, Citation1998), and is also at odds with current ecological theory. Alternatively, it has been suggested that apoptosis plays roles in cell defence from pathogens such as viruses. Lawrence et al. (Citation2001) noted that Heterosigma akashiwo cells infected by HaRNAV, a single-stranded RNA virus (Tai et al., Citation2003), took on apoptotic morphologies. During a time-course experiment, the onset of cell lysis was indicated by a decrease in the relative fluorescence of the cultures and the heterochromatin of infected cells was found at the margin of the nucleoplasm (a clear hallmark of the apoptotic event). Berges and Falkowski (Citation1998) argued that viral lysis was unlikely given the identical kinetics at different cell concentrations. Moreover, Berges and Brussaard (unpublished) were unable to detect free virus during lysis events using SYBR green staining. Nonetheless, there remains the possibility that our D. tertiolecta culture harbours temperate viruses and that they can be activated by darkness. The presence of important components of cell death pathways in some of the earliest-evolved organisms (Berman-Frank et al., Citation2004) suggest that their origins are truly ancient, and it has been speculated that they may be the result of viral-eukaryote genomic mixing during ancient evolutionary history (Berges & Falkowski, Citation1998; Segovia et al., Citation2003).
Regardless of its origins or evolutionary meanings, the existence of cell death phenomena in phytoplankton has important implications for species successions and biogeochemical cycling in aquatic ecosystems (Walsh, Citation1983; Brussaard et al., Citation1995; Van Boeckel et al., Citation1992; Heiskanen, Citation1993; Brussaard et al., Citation1996; Kirchman, Citation1999). If cell death is truly the result of a programme activated by environmental stresses, then it is important to understand how activation happens and what occurs within the cell in response. One obvious starting point is the proteolytic activities that mediate cell death.
The aim of this work was to gain insight into the regulation of proteolytic activities during the apoptotic event in D. tertiolecta and, specifically, whether proteases are synthesized de novo or activated through post-translational modifications. We studied the effect of cycloheximide (CHX) and chloramphenicol (CAP) on the induction of proteolytic activities during dark-induced cell death in D. tertiolecta. CHX inhibits both peptide chain initiation and elongation by blocking the peptidyl transferase of 80S eukaryotic ribosomes (Smith et al., Citation1997) resulting in inhibition of cytoplasmic protein synthesis. CAP inhibits elongation by inhibiting translation on the 50S prokaryotic ribosomal subunit at the peptidyltransferase step and thus prevents protein synthesis in organelles. The effectiveness of either of these inhibitors would indicate a role for de novo protein synthesis in cell death. In addition, we examined the effects of mitomycin C (MMC), which has the ability to alkylate one strand of DNA at the C-1 position forming cross links between the complementary strands of DNA preventing its replication (Ueda & Komano, Citation1984). If MMC enhanced the decline there would be evidence of a viral-mediated decline and / or for the activation of caspases (Park et al., Citation2000). Here we report that proteolytic activities detected in D. tertiolecta are constitutive, and that de novo protein synthesis is not required during the cell death event. Thus, it appears that they are activated through a post-translational mechanism.
Materials and methods
Culture conditions
Dunaliella tertiolecta (CCAP strain 19/6) was grown in 1-litre semi-continuous batch cultures in artificial seawater medium (Goldman & McCarthy, Citation1978) enriched with f/2 nutrients (Guillard & Ryther, Citation1962) at 16°C under continuous white light at 200 μmol quanta m− 2 s− 1, while maintaining gentle stirring and bubbling with filtered air. When cultures reached mid log-phase they were placed in complete darkness under the same conditions of temperature, stirring and bubbling. Cultures were sampled daily. For cell counts, blanks were done on gravity-filtered (GF/F) medium. For Fv/Fm the fluorometer was set to read zero on the culture medium. At key points in the experiment, samples of culture were filtered through 25 mm GF/F filters in Swinnex holders and measured in the fluorometer. Blanks were never more than 0.1% of the fluorescence measured as F0.
Chlorophyll a fluorescence and cell counts
The optimal quantum yield for Photosystem II (PS 2) fluorescence (Fv/Fm) was measured during light deprivation by using a Turner-Designs TD700 fluorometer according to Berges & Falkowski (Citation1998). The initial fluorescence emitted when all the reaction centres are open (F0) was measured in 15 min dark-adapted cells and the maximal fluorescence corresponding to all the reaction centres closed (Fm) was measured after addition of 10 μM 3’-(3, 4-dichlorophenyl)-1′-1′-di-methylurea (DCMU) (final concentration) to the samples (Berges & Falkowski, Citation1998). Fv was calculated as Fm – F0. Cells were preserved in Lugols iodine and counted in a Model Z2 Coulter-counter equipped with a 100 μm aperture (Beckman-Coulter, Fullerton, California). Samples were measured in duplicate for Fv/Fm and triplicate for cell counts.
Inhibition of protein synthesis and DNA replication
The roles of protein synthesis and DNA replication in the cell death process were examined using specific antibiotic inhibitors. Final concentrations of 100 μM cycloheximide (CHX), 100 μM mitomycin C (MMC), 1500 μM chloramphenicol (CAP), and 100 μM CHX plus 1500 μM CAP were each prepared from stocks dissolved in dimethylsulphoxide (DMSO; all chemicals from Sigma), and were added to the cultures just before they were placed in darkness. An equivalent amount of DMSO without antibiotics was added to the control culture to account for non-specific effects. Two replicate cultures for each of the treatments were used.
Zymograms
Zymograms of caseinolytic activities were prepared as described by Berges & Falkowski (Citation1996). Samples were collected by centrifugation, homogenized and sonicated in 50 mM Tris-HCl buffer, pH 7.5, under non-denaturing conditions at 4°C. Samples were centrifuged at 7000 × g to remove cell fragments and then loaded onto a 10% native gel. After electrophoresis, caseinolytic activities were detected by incubating the gels in 2% (w/v) casein dissolved in 50 mM Tris-HCl buffer, pH 7.5, for 1 h and staining with Coomassie blue R 250. Clear bands were apparent after destaining, indicating the position of the caseinolytic activities.
SDS – PAGE
Samples for SDS – PAGE were prepared as described by Berges & Falkowski (Citation1996), loaded on an equal protein basis, separated on 15% gradient polyacrylamide gels and stained with Coomassie blue R250. The intensity of protein bands was analysed using Kodak 1D Image Analysis software (Version 3.6.1, Eastman Kodak, New Haven, Connecticut). Net intensity (relative units) was calculated by measuring intensity in the centre of the bands and subtracting averaged background intensity in blank lanes.
Caspase-like activities
Caspase-like activities were measured according to Segovia et al. (Citation2003) using commercial kits (R&D Systems, MN, USA). Extracts were mixed with 50 μM of 7-amino-4-fluoromethyl coumarin (AFC)-labelled substrates for caspases 1 (WEHD), 3 (DEVD), 8 (IETD) and 9 (LEHD) and lysis buffer provided by the kit. Fluorescence was measured (excitation 400 nm, emission 505 nm) in an FL × 800 Bio-Tek microplate fluorescence reader.
Statistics
Differences in Fv/Fm, cell counts, and caspase activities under the different inhibitor treatments were tested by one-way ANOVA followed by Tukey test comparisons (p < 0.05), using the SigmaSTAT statistical package (SPSS Inc. Chicago, IL, USA).
Results
Variable fluorescence and cell numbers
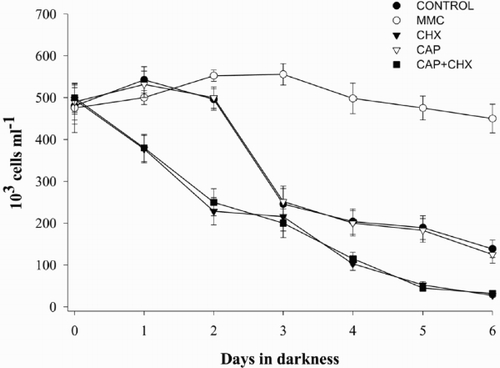
Fv/Fm values decreased in all treatments after placing the cultures in darkness (). However, in cultures containing CHX and MMC, the pattern was different to that observed for the Control. In the Control experiment, Fv/Fm remained high during the first 2 days and suddenly dropped 4-fold between days 2 and 3 in darkness as previously described (Berges & Falkowski, Citation1998; Segovia et al., Citation2003). A more gradual decrease in variable fluorescence was observed with MMC, while Fv/Fm decreased sharply from the first day under light deprivation onwards in the presence of CHX (). Cell numbers paralleled the decrease in photochemical efficiency in Control and CHX treatments (); cell numbers were reduced by half in the Control experiment at the point of the decline in Fv/Fm, and dropped steadily from day 0 in presence of CHX. When cells were grown in presence of CHX but in light, neither Fv/Fm nor cell numbers dropped (data not shown). CAP also provoked a decrease in Fv/Fm although the drop was not as sharp as with CHX. When cells were grown in the presence of both CHX and CAP, the effect was the same as with only CHX. Cells numbers with CAP followed the same pattern as the Control in darkness, while with CHX + CAP the behaviour was similar to that observed for only CHX. However, MMC had a very different effect. The number of cells remained constant throughout the whole light deprivation period and the culture did not show the characteristic decline (). Results for both Fv/Fm and cell numbers were significantly different between the Control and CHX from day 2 onwards, and between the Control and MMC, as well as for MMC and CHX from days 2 to 6 under light deprivation (ANOVA and Tukey tests, p < 0.05). Treatments with CAP and CHX + CAP did not show significant differences during the dark period.
Fig. 1. Variation of PS II optimum quantum yield (Fv/Fm) under light deprivation in Dunaliella tertiolecta. Cultures were placed in darkness immediately after the day 0 measurement (culture in light). Control culture (•); culture containing 100 μM MMC (○); culture containing 100 μM CHX (▾); culture containing 1500 μM CAP (▿); culture containing 100 μM CHX + 1500 μM CAP (▪). Symbols are means of duplicate measurements and error bars indicate standard deviations. * indicates significant F-value in 1-way ANOVA for each sample time.
Zymograms and SDS – PAGE
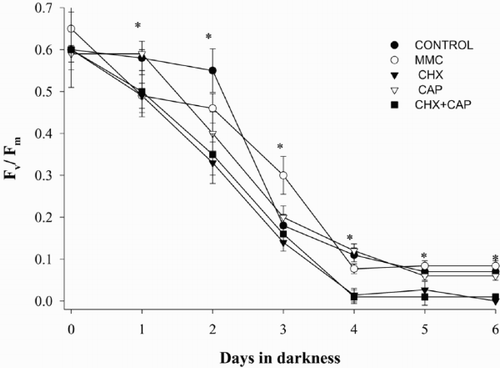
Casein zymograms revealed changes in the pattern of caseinolytic activities depending on the antibiotic used; samples taken after 6 days in darkness were chosen as examples (, ). Because gels were run under native conditions, the molecular weight (MW) of the bands cannot be accurately estimated, however we used denatured pre-stained protein standards in order to be able to compare the relative position of the bands between gels and assign apparent MW. We observed a band of 20 kDa apparent MW in all cultures, (, lanes 1 – 5) and the appearance of novel bands of 60 kDa apparent MW in cultures without and with inhibitors after 6 days in darkness (, lanes 2 – 5). There was slight variation in the banding patterns between experiments, for example, we observed either single or multiple bands at around 60 kDa in cultures showing declines, and the constitutive low MW protease varied between approximately 15 and 20 kDa in apparent MW (compare control and dark exposed cultures in , ). However, overall results were consistent between gels and between experiments. CHX had no apparent effect on the appearance of the proteases (, lane 4), indicating that caseinolytic activities did not result from synthesis of new proteins in the cytoplasm. In contrast, MMC-treated cultures showed no evidence of the higher MW proteases; samples resembled pre-darkness Controls (). Thus MMC effectively prevented the appearance of caseinolytic activities related to the cell death process in D. tertiolecta. We also observed a new band at about 48 kDa apparent MW in those treatments including CAP (, lanes 3, 5).
Fig. 3. Casein zymograms of protease activities from Dunaliella tertiolecta detected after separation of proteins by using 10% native PAGE under non-denaturing conditions. (A) in presence and absence of CHX and/or CAP. CBD: control culture (-CHX-CAP) before darkness; CAD: control culture (-CHX-CAP) after 6 days in darkness; CHX 6d: culture + CHX after 6 days in darkness; CAP 6d: culture + CAP after 6 days in darkness; CAP + CHX 6d: culture + CAP + CHX after 6 days in darkness. (B) in presence and absence of MMC. CBD: control culture (-MMC) before darkness; CAD: control culture (-MMC) after 6 days in darkness; MMC 6d: culture + MMC after 6 days in darkness.
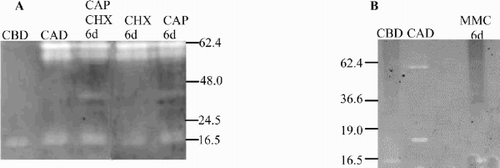
There were few consistent differences in the pattern of protein bands between control cultures and treatments, but there were large differences in apparent densities of bands. Despite the fact that samples were loaded on an equal-protein basis, lanes loaded with either Control cultures (before light deprivation) (, , lane 1) or cultures treated with MMC and exposed to darkness (, lane 3) stained more densely with Coomassie blue than did cultures that had been placed in darkness either without treatment (, lane 2) or with CHX treatment (, lane 4) and CAP and CAP + CHX treatment (, lane 2, 3). Cell death events were manifested by large decreases in certain proteins, such as the prominent band at approximately 55 kDa, representing the large subunit (LSU) of Rubisco (see arrow, , ). Differing backgrounds in different gels made statistical comparisons more difficult. If relative densities of the light Control (CBD) were scaled to 1.00, then 95% confidence limits on replicates averaged about 0.15 units. MMC-treated samples were no different from CBD (averaging 1.00), while the LSU band of Rubisco measured in light-deprived control cultures (CAD) averaged 0.74 vs 0.68 for the CHX-treated sample, 0.58 for CAP and 0.49 for CHX + CAP.
Fig. 4. Total protein composition from Dunaliella tertiolecta detected after separation in a 15% gradient SDS – PAGE and stained with Coomasie blue R 250. (A) in presence and absence of CHX or MMC. (B) in presence and absence of CAP and CAP + CHX. Abbreviations as in .
Based on replication across different gels, the 95% confidence limits on our estimates of molecular mass were ± 12 kDa for a band of approximately 60 kDa, and ± 3 kDa for a band of approximately 20 kDa.
Caspase-like activities
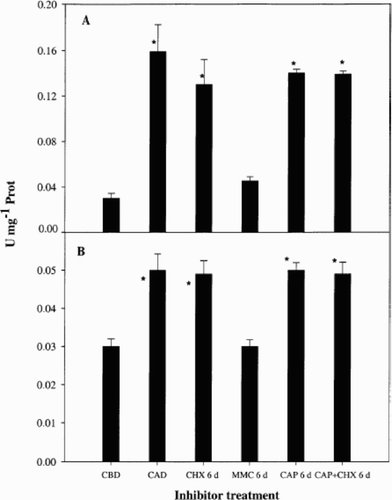
Caspase 3 and 9-like activities were similar in the Control before light deprivation and in MMC treated cultures after 6 days in darkness, whilst darkness increased caspase activity, both in the presence and absence of CHX (). Therefore, MMC prevented any increases in caspase-like activities. Enzymatic activity of caspase 9 in both Control and CHX treatments was about 3-fold higher than activity of caspase 3. Caspase 1 activity was in the same range as caspase 3 (0.02 to 0.06 U mg− 1 Prot) and showed the same pattern. Caspase 8 activity was in the same range as caspase 9 (0.04 to 0.16 U mg− 1 Prot) and the behaviour was similar (data not shown). No differences were found between activities in dark controls and CHX, CAP or CHX + CAP -treated cultures (p > 0.05). In contrast, there were no significant differences between the Control in light and MMC treated cultures (p > 0.05), but these two treatments were significantly lower in caspase activities than any of the other treatments (p < 0.05).
Fig. 5. Caspase-like activity in Dunaliella tertiolecta. Caspase-like activity was measured as hydrolysis of 7-amino-4-fluoromethyl coumarin-labelled substrates specific for caspases 9 (LEHD, panel A) and 3 (DEVD, panel B) and before and after 6 d dark in the presence and absence of CHX, MMC, CAP and CAP + CHX. Abbreviations as in . Bars are means of triplicate measurements and error bars represent standard deviations. U is the activity of one unit of enzyme defined as 1 μmol of AFC-labelled substrate hydrolysed per minute. * indicate significant differences between each treatment and the control culture in darkness (Tukey test).
Discussion
Effects of treatments on Fv/Fm and cell numbers
In the Control cultures Fv/Fm and cell numbers declined in a similar manner, probably as a direct consequence of the apoptotic phenomenon taking place (Berges & Falkowski, Citation1998). In contrast, the patterns were quite different when inhibitors were used. CHX had immediate effects on both Fv/Fm and cell numbers. Although CHX is well known for inhibiting cytoplasmic protein synthesis, it also has been reported to have direct effects on chloroplastic protein synthesis and also indirect effects upon the thylakoid electronic transport chain and enzymes related to photosynthetic and cell metabolism. For example, CHX caused a rapid 70 – 80% reduction in levels of mRNA for the chloroplast elongation factor Tu (tufA) in asynchronously growing Chlamydomonas sp. (Kawazoe et al., Citation2000), and also inhibited chloroplast protein synthesis in desiccation-tolerant mosses (Proctor & Smirnoff, Citation2000). The pattern in Fv/Fm observed in our experiments was probably due to failure to renew reaction centre proteins such as D1 (a phenomenon well known during nitrogen deprivation; cf. Berges et al., Citation1996). The effect observed in D. tertiolecta might be due to cessation of cytoplasmic protein synthesis related to photosynthesis, therefore affecting mostly reaction centre proteins. CHX may have a severe effect on energy metabolism and this could lead to an accidental death (i.e. not genetically driven or programmed), with the programmed death due to darkness starting later in the survivors. However, when cultures were grown in the presence of CHX in light, both Fv/Fm and cell numbers remained constant (data not shown) and did not drop, as would be predicted by this hypothesis. The effects of CHX on photosynthesis are likely to be mediated through a number of cytoplasmic proteins, e.g. a nuclear encoded protease from Arabidopsis thaliana that performs GTP-dependent primary cleavage of the photodamaged D1 protein and hence catalysing the key step in the repair cycle in plants (Haussuhl et al., Citation2001); a FtsH protease from thylakoid membranes, involved in the turnover of photosynthetic protein complexes. FtsH comprises a protein family that is encoded by 12 different nuclear genes in A. thaliana. The more rapid losses of cells observed in presence of CHX and decreased Fv/Fm when compared to dark-exposed controls may be due to CHX accelerating the execution of a cell death programme by means of inhibition of key proteins synthesized in the cytoplasm, but targeted to the chloroplast such as the small subunit of ribulose-1,5-bisphosphate carboxylase/oxygenase, several light harvesting chlorophyll protein complex apoproteins and molecular chaperones involved in the mechanism of protein import into the chloroplast (Reinbothe et al., Citation1993).
The decrease in Fv/Fm and cell numbers when cells were treated with CAP was not as drastic as with CHX. However, we suggest that CAP and/or CAP + CHX induced the decline in both fluorescence and cell numbers due to the degradation of proteins such as D1. CAP has been reported to exert several effects upon PSII and cell survival. The use of CAP and CHX in Chlamydomonas reinhardtii suggested that photosystem stoichiometry adjustments (lowering of the PSI/PSII ratio) occurs by suppression of de novo biosynthesis of PSI components and, therefore, by dilution of the PSI complex in the thylakoid membrane, rather than by active degradation of assembled PSI in chloroplasts (Murakami et al., Citation1997). Green et al. (Citation1992) demonstrated that recovery from iron limitation was completely inhibited by either CHX or CAP in D. tertiolecta and in the marine diatom Phaeodactylum tricornutum. Uptake of nitrate from the external medium and the recovery of Fv/Fm, chlorophyll content, and protein accumulation were inhibited when either cytosolic or chloroplastic protein synthesis was prevented by CHX or lincomycin in D. tertiolecta (Young & Beardall, Citation2003). As for reduction in cell numbers, the morphological change from vegetative to cyst cells of the unicellular green alga Haematococcus pluvialis was prevented by CAP, resulting in algal death (Kobayashi et al., Citation1997). CAP also inhibited tobacco cell growth as shown by a reduction (34%) of cell mass 4 days after treatment (Zhang et al., Citation1999).
The drop in Fv/Fm when cells were treated with MMC was more gradual than the patterns observed for CHX and for the control. Although the cultures containing MMC did not undergo apoptosis, the decrease in Fv/Fm reflected the effect that light deprivation had on the electron transport chain. However, the loss of photochemical efficiency was not paralleled by the loss of cells. These results suggest that MMC does not have a direct effect upon the electron transport chain per se but it does so upon components of the cell cycle.
Effects of treatments on protease activities and cell death programmes
Neither the caseinolytic activities that appeared in D. tertiolecta during cell death, nor the increases in caspase-like activities were affected by CHX and/or CAP, but they were effectively suppressed by MMC treatment. CHX effectively prevents translation in the cytoplasm, and CAP does so in the organelles. Based on other organisms so far examined, the caspases and the rest of the conventional apoptotic machinery appear to be nuclear-encoded. CAP has been reported to have apoptotic effects in mammals (Guimaraes & Linden, Citation2000; Ramachandran et al., Citation2002), but there is no direct link so far between CAP and inhibition of the synthesis of caspase-like proteins. This suggests that the caspase activation we observed does not depend on transcription/translation, but rather on some form of post-translational regulation of pre-existing proteins.
Mitochondrial DNA in mammals can clearly be damaged by MMC (Pritsos et al., Citation1997), and thus transcription could be prevented; indeed MMC has been reported to inhibit transcription of superoxide dismutase (sod1) in hepatocytes (Cho et al., Citation1997). However, effects on transcription would ultimately have manifested themselves in proteins and, since inhibiting translation had no effects on cell death, this does not offer an explanation. Other possible explanations for the effects of MMC may be related to its effects in other areas of cell metabolism. For example, if MMC prevented cells from synthesizing DNA, then their cell cycles may have arrested at a point where they simply could not initiate the cell death programme; the same argument might apply to organelle DNA synthesis as well. In mammalian cells, apoptotic cell death can be activated by aggregation of the cell surface death receptor, CD95, after viral infection. MMC treatment prevented up-regulation of CD95 and inhibited both caspase-8 cleavage and apoptotic cell death (Sheard & Vojtesek, Citation2002). However, it seems more likely that damage to DNA by MMC would cause rather than prevent cell death; indeed, MMC has been shown to trigger apoptosis in mammalian cells (Park et al., Citation2000; Pirnia et al., Citation2002). It is important to recognize that MMC-induced cell death in mammalian cells may proceed through different pathways. Vit et al. (Citation2001) showed that, when apoptosis was induced in human B-lymphoblasts by ionizing radiation or MMC, apoptosis proceeded without the normally-required activation of caspase 8. Replication of the gene encoding the catalytic subunit of DNA polymerase zeta (AtREV3) in A. thaliana was sensitive to MMC (Sakamoto et al., Citation2003). Curiously, in the higher plant Pisum sativum, it has been reported that MMC treatment mimics some aspects of fungal pathogenesis (triggering transcription of pathogenesis-related genes), but does not result in death through the hypersensitive response (HR) or the programmed cell death pathway (Choi et al., Citation2001). Although many mechanisms of MMC action appear possible in D. tertiolecta, it is unclear as yet how we might distinguish them experimentally.
In any case, our results using MMC argue strongly against the idea that the phenomenon we have observed is virus-related. MMC is commonly used for inducing the lysogenic cycle in temperate viruses (Weinbauer & Suttle, Citation1996). We expected that, if temperate viruses were present, the culture treated with MMC would have declined as a consequence of the inhibition of DNA replication and cell cycle arrest in G1, as has been described in mammals (Ueda & Komano, Citation1984). However, the results obtained here were the opposite. Viral involvement in the process can be effectively discounted due to the lack of lysis in the presence of MMC. In addition, no viral particles were found at all during the apoptotic phenomenon, as shown by epifluorescence microscopy (Segovia & Berges, unpublished data) and TEM (Segovia et al., Citation2003).
The cell death process in D. tertiolecta confers no obvious ecological advantage or evolutionary fitness to this organism and it is strange that a seemingly maladaptative strategy should have evolved in a unicellular organism. Segovia et al. (Citation2003) hypothesized that key elements of cell death pathways may have been viral in origin. They suggested that viral genes were transferred to the nuclear genome of early eukaryotes through ancient viral infections in the Precambrian Ocean and that these genes were subsequently appropriated for cell death programmes in both metazoan and higher plant lineages. Alternatively, there is the possibility that the transfer took place before the origin of eukaryotic life itself; a cell death programme has been identified in the cyanobacterium Anabaena (Ning et al., Citation2002), caspase activity occurs in the marine cyanobacterium Trichodesmium sp (Berman-Frank et al., Citation2004), and a reverse transcriptase gene of retroviral origin is contained and maintained within a nitrogen assimilation operon in Trichodesmium as well (Anton Post, pers. comm.; see Segovia et al., Citation2003).
If this is true, then endosymbiosis and cell death may have developed in tandem. The endosymbiotic theory (Margulis, Citation1981) postulates that eukaryotic life arose when a prokaryotic host cell engulfed an aerobic bacterium which ultimately became the mitochondrion. Photosynthetic eukaryotes evolved later, as the result of at least two distinct endosymbiotic events incorporating photosynthetic bacteria, which became chloroplasts, and there is ample evidence of lateral gene transfer among host and endosymbiont genomes (see Falkowski et al., Citation2004). Kroemer (Citation1997) first pointed out that apoptosis itself evolved together with the endosymbiotic incorporation of aerobic bacteria (the precursors of mitochondria) into ancestral unicellular eukaryotes and, quite recently, Bidle and Falkowski (Citation2004) argued that the presence of eukaryotic cell-death domains (e.g. genes that encode metacaspases, a family of proteases found in higher plants, unicellular protists, fungi and specialized bacteria) in the genomes of Proteobacteria, indicates that these genes have a bacterial origin.
In conclusion, we have demonstrated that increases in caspase activities were not affected by CHX and/or CAP, but that MMC essentially prevented increases in caspase activity and subsequent cell death. Taken together, these results suggest that caspase increases are most likely due to post-translational modifications, as first proposed by Segovia et al. (Citation2003), and that caspase-like activities seem to be nuclear encoded in D. tertiolecta. Further, the different effects of CHX, CAP and MMC suggest that at least some features of the cell death pathway in D. tertiolecta depend on chloroplast or mitochondrial transcription/translation and that particular events may be dependent on the stage of the cell cycle.
However, this needs further research to study chloroplasts and mitochondria as intermediates of the signalling pathway after reception of the apoptotic stimuli (as shown by the banding pattern in the casein zymograms). Some questions will be answered by ongoing work: are there cell death proteins in D. tertiolecta encoded by chloroplastic and mitochondrial DNAs? Is all of the cell death machinery nuclear encoded, and part of it imported into the organelles? How well preserved during evolution are the organellar proteins which are involved in the cell death event?
We have shown in this work that at least part of the cell death programme we observe does not depend on protein synthesis, but rather on post-translational modification of pre-existing proteins. This finding means that understanding the potential importance of cell death processes in phytoplankton populations in nature will probably require biochemical measurements of specific features of cell death, rather than measurement of gene expression. Further, our results discount the involvement of temperate virus during the cell death process and raise the possibility that non-viral cell death in phytoplankton is a significant process in nature.
Acknowledgements
This research was supported by an Individual Marie Curie Postdoctoral Fellowship (EU) to Maria Segovia, and grants from the Natural Environment Research Council (UK) and the Nuffield Foundation to John A. Berges. We thank Willie Wilson (Marine Biological Association, UK) for suggestions regarding the use of MMC.
Additional information
Notes on contributors
John A. Berges
Present address: Departamento de Ecología, Facultad de Ciencias, Universidad de Málaga, Campus Universitario de Teatinos s/n. 29071-Málaga, Spain. e-mail: [email protected]Notes
Present address: Departamento de Ecología, Facultad de Ciencias, Universidad de Málaga, Campus Universitario de Teatinos s/n. 29071-Málaga, Spain. e-mail: [email protected]
References
References
- Berges , JA , Charlebois , DO , Mauzerall , DC and Falkowski , PG . 1996 . Differential effects of nitrogen limitation on photosynthetic efficiency of Photosystems I and II in microalgae . Plant Physiol. , 110 : 689 – 696
- Berges , JA and Falkowski , PG . 1996 . Cell-associated proteolytic enzymes from marine phytoplankton . J. Phycol. , 32 : 566 – 574
- Berges , JA and Falkowski , PG . 1998 . Physiological stress and cell death in marine phytoplankton: Induction of proteases in response to nitrogen or light limitation . Limnol. Oceanogr. , 43 : 129 – 135
- Berman-Frank , I , Bidle , KD , Haramaty , L and Falkowski , PG . 2004 . The demise of the marine cyanobacterium, Trichodesmium spp., via an autocatalyzed cell death pathway . Limnol. Oceanogr. , 49 : 997 – 1005
- Bidle , KD and Falkowski , PG . 2004 . Cell death in planktonic, photosynthetic microorganisms . Nature Rev. Microbiol. , 2 : 643 – 655
- Brussaard , CPD , Gast , GJ , Van Duyl , C and Riegman , R . 1996 . Impact of phytoplankton bloom magnitude on a pelagic microbial food web . Mar. Ecol. Prog. Ser. , 144 : 211 – 221
- Brussaard , CPD , Riegman , R , Noordeloos , AAM , Cadee , GC , Witte , H , Kop , AJ , Nieuwland , G , Van Duyl , FC and Bak , RPM . 1995 . Effects of grazing, sedimentation and phytoplankton cell lysis on the structure of a coastal pelagic food web . Mar. Ecol. Prog. Ser. , 123 : 259 – 271
- Cho , G , Kang , S , Seo , SJ , Kim , Y and Jung , GH . 1997 . The transcriptional repression of the human Cu/Zn superoxide dismutase (sod1) gene by the anticancer drug, mitomycin C (MMC) . Biochem. Mol. Biol. , 42 : 949 – 956
- Choi , JJ , Klosterman , SJ and Hadwiger , LA . 2001 . A comparison of the effects of DNA- damaging agents and biotic elicitors on the induction of plant defence genes, nuclear distortion, and cell death . Plant Physiol. , 125 : 752 – 762
- Cohen , GM . 1997 . Caspases: the executioners of apoptosis . Biochemical J. , 326 : 1 – 16
- Falkowski , PG , Katz , ME , Knoll , AH , Quigg , A , Raven , JA , Schofield , O and Taylor , FJR . 2004 . The evolution of modern eukaryotic phytoplankton . Science , 305 : 354 – 360
- Franklin , DJ , Hoegh-Guldberg , O , Jones , RJ and Berges , JA . 2004 . Cell death and degeneration in the symbiotic dinoflagellates of the coral Stylophora pistillata during bleaching . Mar. Ecol. Prog. Ser. , 272 : 117 – 130
- Franklin , DJ and Berges , JA . 2004 . Mortality in cultures of the dinoflagellate Amphidinium carterae during culture senescence and darkness . Proc. R. Soc. Lond. B. , 271 : 2099 – 2107
- Frohlich , KU and Madeo , F . 2000 . Apoptosis in yeast - a monocellular organism exhibits altruistic behaviour . FEBS Lett. , 473 : 6 – 9
- Goldman , JC and McCarthy , JJ . 1978 . Steady state growth and ammonium uptake of a fast growing marine diatom . Limnol. Oceanogr. , 23 : 695 – 703
- Greenberg , JT . 1996 . Programmed cell death: A way of life for plants . Proc. Nat. Acad. Sci. USA. , 93 : 12094 – 12097
- Greene , RM , Geider , RJ , Kolber , Z and Falkowski , PG . 1992 . Iron-induced changes in light harvesting and photochemical energy-conversion processes in eukaryotic marine-algae . Plant Physiol. , 100 : 565 – 575
- Guillard , RRL and Ryther , JH . 1962 . Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea (Cleve) Gran . Can. J. Microbiol. , 8 : 229 – 239
- Guimaraes , CA and Linden , R . 2000 . Chloramphenicol induces apoptosis in the developing brain . Neuropharmacol , 39 : 1673 – 1679
- Haussuhl , K , Andersson , B and Adamska , I . 2001 . A chloroplast DegP2 protease performs the primary cleavage of the photodamaged D1 protein in plant photosystem II . EMBO J. , 20 : 713 – 722
- Heiskanen , AS . 1993 . Mass encystment and sinking of dinoflagellates during a spring bloom . Mar. Biol. , 116 : 161 – 167
- Kawazoe , R , Hwang , SB and Herrin , DL . 2000 . Requirement for cytoplasmic protein synthesis during circadian peaks of transcription of chloroplast-encoded genes in Chlamydomonas . Plant Mol. Biol. , 44 : 699 – 709
- Kirchman , DL . 1999 . Phytoplankton death in the sea . Nature , 398 : 293 – 294
- Kobayashi , M , Hirai , N , Kurimura , Y , Ohigashi , H and Tsuji , Y . 1997 . Abscisic acid-dependent algal morphogenesis in the unicellular green alga Haematococcus pluvialis . Plant Growth Reg. , 22 : 79 – 85
- Kroemer , G . 1997 . Mitochondrial implication in apoptosis. Towards an endosymbiont hypothesis of apoptosis evolution . Cell Death Diff. , 4 : 443 – 456
- Lam , E and Del Pozo , O . 2000 . Caspase-like protease involvement in the control of plant cell death . Plant Mol Biol. , 44 : 417 – 428
- Lam , E , Kato , N and Lawton , M . 2001 . Programmed cell death, mitochondria and the plant hypersensitive response . Nature , 411 : 848 – 853
- Lawrence , JE , Chan , AM and Suttle , CA . 2001 . A novel virus (HaNIV) causes lysis of the toxic bloom-forming alga Heterosigma akashiwo (Raphidophyceae) . J. Phycol. , 37 : 216 – 222
- Leist , M and Nicotera , P . 1997 . The shape of cell death . Biochem. Biophys. Res. Com. , 236 : 1 – 9
- Lewis , K . 2000 . Programmed cell death in bacteria . Microbiol. Mol. Biol. Rev. , 64 : 503 – 514
- Margulis L 1981 Symbiosis in Cell Evolution: Life and Its Environment on the Early Earth, W.H. Freeman San Francisco
- Murakami , A , Fujita , Y , Nemson , JA and Melis , A . 1997 . Chromatic regulation in Chlamydomonas reinhardtii: Time course of photosystem stoichiometry adjustment following a shift in growth light quality . Plant Cell. Physiol. , 38 : 188 – 193
- Ning , SB , Guo , HL , Wang , L and Song , YC . 2002 . Salt stress induces programmed cell death in prokaryotic organism Anabaena . J. Appl. Microbiol. , 93 : 15 – 28
- Park , IC , Park , MJ , Hwang , CS , Rhee , CH , Whang , DY , Jang , JJ , Choe , TB , Hong , SI and Lee , SH . 2000 . Mitomycin C induces apoptosis in a caspases-dependent and Fas/CD95-independent manner in human gastric adenocarcinoma cells . Cancer Lett. , 158 : 125 – 132
- Pennell , RI and Lamb , C . 1997 . Programmed plant cell in plants . Plant Cell. , 9 : 1157 – 1168
- Pirnia , F , Schneider , E , Betticher , DC and Borner , MM . 2002 . Mitomycin C induces apoptosis and caspase-8 and-9 processing through a caspase-3 and Fas-independent pathway . Cell Death Diff. , 9 : 905 – 914
- Pritsos , CA , Briggs , LA and Gustafson , DL . 1997 . A new cellular target for mitomycin C: A case for mitochondrial DNA . Onc. Res. , 9 : 333 – 337
- Proctor , MCF and Smirnoff , N . 2000 . Rapid recovery of photosystems on rewetting desiccation-tolerant mosses: chlorophyll fluorescence and inhibitor experiments . J. Exp. Bot. , 51 : 1695 – 1704
- Ramachandran , A , Moellering , DR , Ceaser , E , Shiva , S , Xu , J and Darley-Usmar , V . 2002 . Inhibition of mitochondrial protein synthesis results in increased endothelial cell susceptibility to nitric oxide-induced apoptosis . Proc. Nat. Acad. Sci. USA. , 99 : 6643 – 6648
- Reinbothe , S , Reinbothe , C and Parthier , B . 1993 . Methyl jasmonate-regulated translation of nuclear-encoded chloroplast proteins in barley (Hordeum-vulgare l cv salome) . J. Biol. Chem. , 268 : 10606 – 10611
- Sakamoto , A , Lan , VTT , Hase , Y , Shikazono , N , Matsunaga , T and Tanaka , A . 2003 . Disruption of the AtREV3 gene causes hypersensitivity to ultraviolet B light and gamma-rays in Arabidopsis: Implication of the presence of a translesion synthesis mechanism in plants . Plant Cell. , 15 : 2042 – 2057
- Segovia , M , Haramaty , L , Berges , JA and Falkowski , PG . 2003 . Cell death in the unicellular chlorophyte Dunaliella tertiolecta: An hypothesis on the evolution of apoptosis in higher plants and metazoans . Plant Physiol. , 132 : 99 – 105
- Sheard , MA and Vojtesek , B . 2002 . Simian virus-40 infection inhibits DNA damage-induced enhancement of CD95 expression and function . Oncogene , 21 : 190 – 197
- Smith AD Datta SP Smith HG Campbell PN Bentley R McKenzie HA 1997 Oxford Dictionary of Biochemistry and Molecular Biology, Oxford University Press
- Tai , V , Lawrence , JE , Lang , AS , Chan , AM , Culley , AI and Suttle , CA . 2003 . Characterization of HaRNAV, a single-stranded RNA virus causing lysis of Heterosigma akashiwo (Raphidophyceae) . J. Phycol. , 39 : 343 – 352
- Thornberry , NA . 1999 . Caspases: A decade of death research . Cell Death Diff. , 6 : 1023 – 1027
- Ueda , K and Komano , T . 1984 . Sequence-specific DNA damage induced by reduced Mitomycin C . Nucleic Acids Res. , 12 : 6673 – 6683
- Van Boeckel , WHM , Hansen , FC , Riegman , R and Bak , RPM . 1992 . Lysis-induced decline of a Phaeocystis spring bloom and coupling with the microbial food web . Mar. Ecol. Prog. Ser. , 81 : 269 – 276
- Vardi , A , Berman-Frank , I , Rozenberg , T , Hadas , O , Kaplan , A and Levine , A . 1999 . Programmed cell death of the dinoflagellate Peridinium gatunense is mediated by CO2 limitation and oxidative stress . Curr. Biol. , 9 : 1061 – 1064
- Vit , JP , Guillouf , C and Rosselli , F . 2001 . Futile caspase-8 activation during the apoptotic cell death induced by DNA damaging agents in human B-lymphoblasts . Exp. Cell Res. , 269 : 2 – 12
- Walsh , JJ . 1983 . Death in the sea: enigmatic phytoplankton losses . Prog. Oceanogr. , 12 : 1 – 86
- Weinbauer , MG and Suttle , CA . 1996 . Potential significance of lysogeny to bacteriophage production and bacterial mortality in coastal waters of the Gulf of Mexico . App. Env. Microbiol. , 62 : 4374 – 4380
- Young , EB and Beardall , J . 2003 . Photosynthetic function in Dunaliella tertiolecta (Chlorophyta) during a nitrogen starvation and recovery cycle . J. Phycol. , 39 : 897 – 905
- Zhang , Q , Wiskich , JT and Soole , KL . 1999 . Respiratory activities in chloramphenicol-treated tobacco cells . Physiol. Plantarum. , 105 : 224 – 232