Abstract
Total lipids and fatty acid composition were monitored seasonally in exposed and sheltered Caulerpa racemosa settlements along the coast of St Juraj island (Vrsar, Croatia). The underlying sediment in the exposed area was characterized by a lower content of total lipids (0.63 ± 0.18 mg/g d.w.) and coarser grain sizes (median particle diameter [Md] = 1319.5 µm) in comparison to sediment of the sheltered area (0.89 ± 0.06 mg/g d.w.; Md 378.9 µm). Despite the proximities of both populations studied, and, hence, both experiencing similar exposure to seasonal fluctuations, the algal responses were different. Total lipid changes and fatty acid composition of the related settlements indicated the differences in thallus condition during summer of 2004 and to some extent different strategies in cold adaptation during winter 2005. In August 2004, the bad condition of thalli at the sheltered site, expressed by low unsaturation (UNS/SAT 0.45), resulted from its sexual propagation state, in contrast to the advanced condition of vegetative propagation state at the exposed site (UNS/SAT 1.1). The poor thallus condition was related to its slower development at the sheltered habitat, having correspondingly lower biomass. Furthermore, at this site little or no difference in total lipids was observed with season. During the winter period (min. temp. 7.7°C) both settlements altered their fatty acid composition by increasing unsaturation (UNS/SAT up to 1.8), but a pronounced winter peak in total lipids occurred at the exposed site. Under similar environmental conditions, the C. racemosa in this study developed different strategies in cold adaptation and propagation state as a consequence of apparently varying impact of the hydrodynamism at these two proximate sites. This study suggests the mode in which exposure impacts the growth cycle of C. racemosa in temperate regions.
Introduction
Biological invasions in marine habitats are widely reported and represent a recognized threat to the integrity of a native community (Piazzi et al., Citation1997; Balata et al., Citation2004). Caulerpa racemosa (Forsskål) J. Agardh var. cylindracea (Sonder) Verlaque, Huisman et Boudouresque has spread rapidly throughout the Mediterranean Sea (Verlaque et al., Citation2003). Over the last century new documented occurrences of C. racemosa in different regions of the Mediterranean tend to coincide with periods of especially warm waters (Chisholm et al., Citation2000). Since 2000 when the alga was reported for the first time in the Adriatic Sea (Žuljević et al., Citation2003) it has rapidly spread along the Croatian coast. In July 2004 it was recorded in Vrsar (Iveša & Devescovi, Citation2006). Caulerpa species are strong competitors in temperate areas. They tend to eliminate native species and often constitute monospecific beds (Piazzi et al., Citation2001). In comparison to C. taxifolia, C. racemosa showed a higher invasive capacity, differently and more seriously affecting invaded areas. Such extensive spreading of C. racemosa might be additionally supported by its successful sexual reproduction (Panayotidis & Žuljević, Citation2001). The colour changes of the thalli enable visual recognition of sexual reproduction in the field (Clifton & Clifton, Citation1999). Among beneficial factors promoting the growth of Caulerpa species, substrata enriched with organic matter have also been suggested (Chisholm et al., Citation1997).
One of the prerequisites for meeting changes in environmental factors is the maintenance of a certain fluidity of the cell membrane (Chintalapati et al., Citation2004; McElhaney, Citation1982). Changes in membrane lipids, particularly polyunsaturated fatty acids (PUFA), as the most responsive constituents, play a major role in the adaptation process. Physiological response of various Caulerpa species to the seasonality of environmental factors (primarily temperature) in temperate regions is well studied (Terrados & Lopezjimenez, Citation1996; Iveša et al., Citation2004) and their successful adaptation confirmed. Moreover, in the Adriatic Sea Caulerpa species achieve their maximum development during winter-fall periods when seawater temperatures decrease to 8°C (Iveša et al., Citation2004; Iveša & Devescovi, Citation2006). In contrast, in the northwestern Mediterranean, in spite of the higher seawater temperatures during the same period, drastic regression of Caulerpa species takes place (Piazzi et al., Citation2001; Balata et al., Citation2004; Ruitton et al., Citation2005).
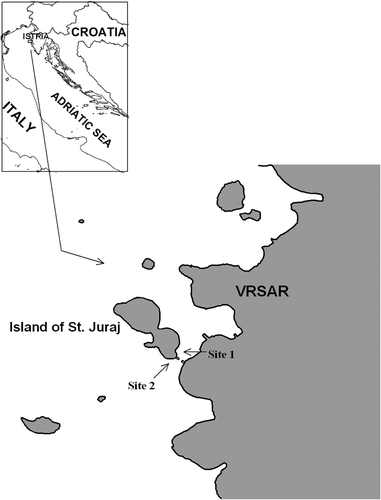
This research was taken within the framework of monitoring the vegetative development of the invasive C. racemosa in two habitats in the region of Vrsar in the northern Adriatic Sea, the northernmost (45°8′52″N; 13°35′59″E) and coldest point of its distribution in the Mediterranean. Previous study demonstrated significant and continuous differences in biomass dry weight, number of erect axes and stolon length between the exposed and the sheltered habitat (Iveša & Devescovi, Citation2006). In order to clarify the factors that contribute to differences in its biomass and growth we investigated and compared qualitative responses in fatty acid profile of C. racemosa in sheltered and exposed habitats to seasonal conditions. The major changes in lipid metabolism correlate with specific developmental events and are controlled by environmental factors.
Materials and methods
Along the coast of St Juraj island in the northern Adriatic Sea near Vrsar, Croatia (45°8′52″N; 13°35′59″E) C. racemosa colonized two distinct sites (). Site 1 was located in the southern part of the Vrsar harbour. Due to its isolated position and less intense mixing with the sea body we consider Site 1 as sheltered. In contrast, Site 2 situated on the external side of the island was considered as more exposed to the open sea with a more unstable environment, particularly during winter water cooling and extended periods of strong NE wind (Bora). Seawater temperature was measured hourly at both sites from November 2004 to August 2005 using Data Loggers.
Sampling of the green alga was performed seasonally in August and November 2004, as well as February, May and August 2005. Caulerpa racemosa was collected together with its stolons, on both sides of the island, by SCUBA divers using the quadrat sampling method. Three 20 × 20 cm quadrats were randomly scattered in positions of maximum coverage (e.g. 100%) for a given date of sampling except for May 2005 when the coverage was 5–10%. Sediment samples were also collected by SCUBA divers with plastic core samplers of 5.5 cm i.d.
Sediment dry weight (d.w.) was recorded as weight loss after drying at 100°C for 24 h to constant weight. Ignition loss (IL) of the surface sediment samples was recorded after heating dried sediment samples at 450°C for 4 h in the muffle furnace.
For grain-size analysis the samples were wet sieved, whilst silt and clay fractions were analysed areometrically. The type of sediment was determined by the percentage of gravel, sand, silt and clay according to Folk's classification (Folk, Citation1954).
Two sub-samples of C. racemosa thalli were extracted in a Soxhlet apparatus for 12 h in a chloroform-methanol mixture 1 : 1. The chloroform phases were purified by adding a salt solution and then evaporated to dryness using rotary evaporation at 30°C (De Rosa et al., Citation1988). After weighing, total extracts were saponified by addition of 1.2 M NaOH in 50% aqueous methanol solution. The tubes were placed in a boiling bath for 30 min. After cooling, the saponificate was acidified with 6 M HCL (pH < 2), 12% BF3 in methanol was added and the mixture was heated for 10 min in a near-boiling water bath. After cooling the fatty acid methyl esters (FAME) were extracted in dichloromethane.
FAME were analysed by gas-liquid chromatography (GLC) on a 6890N Network GC System equipped with 5973Network Mass Selective Detector with a capillary column (30 m × 0.25 mm × 0.25 µm; cross linked 5% phenylmethylsiloxan) and ultra high purity helium as the carrier gas. The GLC settings were as follows: programmed column temperature 145°C by 4°C/min up to 270°C and constant column pressure 15 psi. Retention time and peak areas were recorded on ChemStation Software. FAME in seaweed samples were identified by family plot of equivalent chain length data (ECL) for GC standards for GC column used. Fatty acid methyl esters mix C18-C20 and polyunsaturated fatty acids standards (PUFA1 and PUFA3, Supelco, USA), cod liver oil and various individual pure standards of fatty acid methyl esters (Sigma, Germany) were used.
Results
Among saturated, monounsaturated and polyunsaturated fatty acids of C. racemosa, sampled over the seasons at both sites, the dominating components were palmitic (C16:0), palmitoleic (C16:1(n–7)) and linolenic acid (C18:3(n–3)).
The particle composition of the two sites differed in the percentage of sand and gravel. The exposed area (Site 2) was sandy gravel (gravel 32%; sand 63.9%; silt 1.6%; clay 2.5%), while the sheltered area (Site 1) was gravelled sand (gravel 9.2%; sand 83.6%; silt 0.8%; clay 6.4%) according to Folk (Citation1954). Consequently, the median particle size of the sediment from the exposed area (median particle diameter [Md] = 1, 319.5 µm) was much higher than that from the sheltered area (Md = 378.9 µm). ILs in the sediment underlying C. racemosa meadows were 2.4% and 3.2%, while in the sediment outside the meadows they were lower, 2.1% and 2.5%, at Site 2 and 1, respectively. The underlying sediment in the harbour area was more enriched in total lipids (0.89 ± 0.06 mg/g d.w.) than the sediment in exposed areas (0.63 ± 0.18 mg/g d.w.), irrespective of the season.
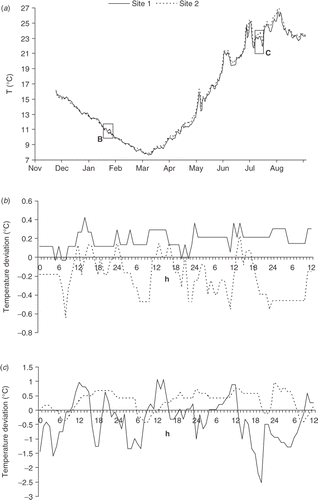
With changing seasons, C. racemosa was faced with significant changes in seawater temperatures, abruptly decreasing below 10°C at the end of January 2005. Minimum values, fluctuating between 7.7°C and 7.8°C, lasted from 1 to 12 March 2005. Daily temperature means, calculated from hourly values, were very similar at the two sites (). However, supporting the argument that these two sites are differently influenced by the open sea, the deviation of hourly temperature from the daily mean temperature for representative three day periods in summer and winter differs at the two sites. During the period of winter water cooling () the temperature conditions at Site 2 (exposed site) are more unstable: hourly values are lower and more variable than at Site 1 (harbour). In contrast during summer, thermal changes at Site 1 are more pronounced: during the day Site 1 is warmer than Site 2 but it is colder during the night ().
Fig. 2. Caulerpa racemosa: (a) daily seawater temperature at 4 m depth in two settlements from 25 November 2004 to 30 August 2005, (b) winter and (c) summer temperature oscillation pattern.
Total lipids extracted from the C. racemosa thalli from Site 2 increased till the coldest month, February, when maximum values were attained. In that period total lipids were significantly higher than those from the harbour area, where generally small variations in total lipids were found ().
Fig. 3. Caulerpa racemosa: (a) total lipids (mean ± SD), (b) α-linolenic acid (18:3n–3) and (c) seasonal variations in total biomass (mean ± SD) at two sites.
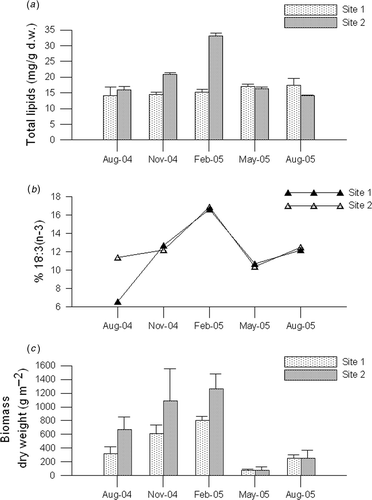
Seasonal changes were evident in the relative distribution of saturated (SFA), mono- (MUFA) and polyunsaturated fatty acids (PUFA) at both sites. SFA varied inversely to PUFA. MUFA hardly varied at both sites. The dominant component of the total PUFA, linolenic acid (18:3n–3), generates a pattern of PUFA behaviour (). PUFA gradually increased from August to February when the highest values were observed. Similarly, unsaturated to saturated fatty acid ratios (UNS/SAT) were highest in February (1.75) at both sites, while lowest in August 2004 at Site 1 (0.47), as well as the proportion of linolenic acid. In contrast at Site 2 in August 2004 the UNS/SAT ratio was significantly higher (1.0). Generally, unsaturated to saturated fatty acids ratio varied inversely with temperature.
Samples of C. racemosa with the most similar fatty acid compositions were grouped by cluster analysis-complete linkage method ( and ). Two groups of samples with similar behaviour were identified while the sample from August 2004 (Site 1) remained ungrouped. Group 2 included late fall-winter samples (November–February) with the highest mean values and ranges for PUFA (45.4–52.4; 48.5) and the lowest for SFA (36.4–43; 39.6) and consequently the highest UNS/SAT ratio (1.3–1.75; 1.54). Group 1 included spring and summer samples (May–August) characterised by lower PUFA and higher SFA, while proportions of MUFA were essentially the same. The ungrouped sample, C. racemosa thalli from the sheltered area in August 2004, differed substantially from the thalli sampled at the exposed site in August 2004, as well as thalli sampled in August 2005 at both sites. It is worth noting that algae also differed in colour. In contrast to the healthy green coloured thalli usually sampled, in August 2004 at Site 1 the alga was white to very pale green. PUFA were at a minimum (18.2%), while SFA were maximum (67.9%). Both C16 (4.1%) and C18 PUFA (11%) were half as much as that found in other August samples.
Fig. 4. Caulerpa racemosa: cluster analysis dendrogram of fatty acid composition during seasons 2004/2005 at two sites.
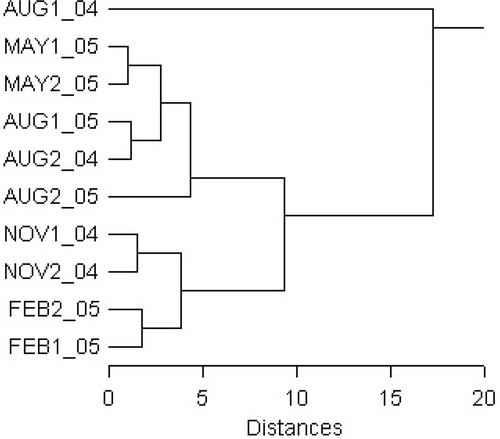
Table 1. Caulerpa racemosa: fatty acid composition in two groups of samples identified using K-means.
The general seasonal pattern of C. racemosa biomass was similar at both sites. Total biomass (g dry wt/m2) increased from August 2004 through November 2004, reaching maximum values in February 2005 (803 ± 62 g/m2; 1260 ± 221 g/m2 in the sheltered and exposed habitats, respectively). The biomass significantly decreased in May 2005 while recovering by the following August ().
Discussion
The fatty acid composition of C. racemosa collected in the northern Adriatic Sea showed a pattern of dominating components, C16:0, C16:1(n–7) and C18:3(n–3), among saturated, monounsaturated and polyunsaturated fatty acids, respectively. Additionally, specific taxonomic features for Caulerpa species, i.e. C18:3(n–3) and C16:3(n–3), dominating among C18 PUFA and C16 PUFA, were also recognized in this alga. Generally, the fatty acid composition found in C. racemosa is in good agreement with other results reported for Caulerpa species (Aknin et al., Citation1992; Khotimchenko, Citation1995).
The sediment underlying C. racemosa meadows with higher biomass (exposed settlement) was less enriched in organic matter as well as in total lipids. Our findings are in contrast to some others (Chisholm et al., Citation1997; Jaubert et al., Citation2003) suggesting that, due to a high content of organic matter associated with urban pollution, sediment conditions promote the growth of invasive Caulerpa species. In accordance with recent laboratory experiments (Terrados & Marbà, Citation2006) our results do not support more efficient growth or facilitate survival of C. racemosa due to higher organic matter content of the sediment. This difference in organic matter enrichment between the two sites (exposed and sheltered) underlying C. racemosa meadows in Vrsar most probably derives from a different granulometric composition of the sediments. The absorptive capacity for organic molecules and the degree of organic matter preservation is lower in the exposed area due to coarser grain sizes.
The seasonal cycle of the C. racemosa thalli fatty acid composition showed a unimodal pattern, similar in both settlements. This pattern was quite similar to that found in native algal species of temperate regions (Nelson et al., Citation2002). Lower and similar PUFA levels were recorded in spring and summer periods, respectively in spite of opposite phases of its growth cycle. In the overwintering remnants of C. racemosa meadows, sampled in both areas during the period of minimum coverage (May 2005), the condition of the thalli was almost restored and the fatty acid composition was not different from that of the healthy growing thalli in the summer period (August 2005).
The increasing proportions of PUFAs in C. racemosa thalli coincided with a sharp and continuous decrease in water temperature (November–February) reaching its maximum in the coldest period. By enhancing unsaturation C. racemosa decreases its lower temperature threshold and successfully survives the winter without visible necrotic parts or chilling injuries. The low temperatures elicit gelling effects in the membrane lipids (Chintalapati et al., Citation2004). Under such conditions strong induction of adaptive membrane restructuring occurs in order to avoid the harmful consequences of altered membrane function (McElhaney, Citation1982).
In comparison to C. taxifolia (Iveša et al., Citation2004) a much higher proportion of PUFA, and consequently, a higher degree of unsaturation was measured in C. racemosa throughout the entire seasonal cycle. Because of its greater degree of unsaturation, which provides more efficient regulation of viscosity by maintaining lipids in a liquid state, C. racemosa expresses more pronounced chilling resistance than C. taxifolia under low temperatures. Such characteristics are usually shared by many low-temperature-adapted species and/or species associated with cold (or permanently cold) environments (Hazel & Williams, Citation1990). However, low water temperatures during winter, fluctuating between 7.7°C and 7.8°C for almost 2 weeks, could be the reason for the rather slower recovery of C. racemosa during summer 2005, compared to the summer of 2004 in both habitats. One of the important factors that formed the basis of a spontaneous regression of C. taxifolia in Malinska was the persistent winter low temperatures (Iveša et al., Citation2006).
However, the seasonal changes in total lipids in C. racemosa thalli were different at the two sites. The sheltered site showed little or no difference in total lipids with season, while a pronounced winter peak occurred at the exposed site. These results indicate that in spite of the short distance between the sites, the two Caulerpa settlements integrated the same environmental change in different ways. Lipid accumulation and unsaturation imply additional need for freezing protection in the exposed settlement, suggesting it is adapted to colder environment than the sheltered one. The effect of low temperature might be also intensified by the winter swell. The more unstable environment, probably because of the more intense turbulent mixing at the exposed station, had lower and more variable temperatures at this site. However, no harmful effects on the algal abundance occurred unlike in other Mediterranean areas where significant reduction of settlements exposed to violent winter swell has been reported (Balata et al., Citation2004; Ruitton et al., Citation2005).
Caulerpa racemosa, like many other green algae, has varied and complex modes of both sexual and asexual propagation (Clifton, Citation1997). Although sexual as well as vegetative reproduction plays an important role in the algal dynamics in its native tropical environment (Clifton & Clifton, Citation1999), in temperate regions it is believed that it additionally supports its spreading capability (Panayotidis & Žuljević, Citation2001). Thalli that underwent sexual reproduction were sampled in August 2004 in the sheltered settlement of the Vrsar harbour. Emission of gametes, which has a high energetic cost, resulted in very poor thallus condition. The lowest degree of unsaturation resulted from the sharp depletion of polyunsaturated fatty acids, most probably as a consequence of their incorporation in the gametes. During the fertile stage protoplasmic contents, together with fatty acids as the principal energy source, are converted into gametes enabling their persistence through unfavourable periods (Clifton, Citation1997). Thus, in addition to a fundamental role in the membrane fluidity regulation and maintenance, PUFAs account for another very important physiological feature, long-term viability of gametes. In its native environment C. racemosa dies after spawning over a few months when over 95% of its meadow decays (Clifton & Clifton, Citation1999). This clearly negative impact of sexual reproduction on local algal abundance was not observed in a temperate region, however it is reflected in diminished abundance and biomass, as well as reduced growth throughout the year. However, only a short distance away the exposed settlement was not found in a sexual phase, but only in vegetative propagation state. These differences in propagation state of the two C. racemosa settlements during summer 2004 reflect important variations in environmental conditions at these two sites. Presumably the temperature conditions at Site 1 were coupled more closely to short term changes in heat transfer than those at Site 2 because of its isolated position and less intense mixing with the open sea. Large day-night temperature oscillation followed by weak hydrodynamism apparently created favourable conditions for sexual propagation (Pearson & Serrao, Citation2006).
In conclusion, fatty acid composition and its seasonal variations in C. racemosa thalli in both settlements were typical for the algal species in temperate regions and correspond to its growth state and survival requirements. Although habitats of the two Caulerpa settlements are in close proximity with negligible differences in substratum organic enrichment, differences in propagation state of the thalli (summer 2004) and to some extent different cold adaptation strategies (winter 2005) occurred. These results can partly be explained by exposure of the two sites to different hydrodynamism, influencing the growth cycle of C. racemosa in temperate regions by weakening or intensifying temperature oscillations.
Acknowledgements
The authors thank Mrs R. Sanković for laboratory assistance, Dr D. Bogner for granulometric analysis and Dr A. Jaklin for help in the field with a temperature Data Logger. We thank Prof M. Orlić for advice on temperature data analysis. The helpful comments from anonymous referees significantly improved the manuscript. The support of the Ministry of Science, Education and Sport of the Republic of Croatia (Projects 0982705-2729, 0982705-2732 and ‘Jadran’) is greatly appreciated.
References
- Aknin , M , Moellet-Nzaou , R , Cisse , E , Kornprobst , JM , Gaydou , EM , Samb , A and Miralles , J . 1992 . Fatty acid composition of twelve species of Chlorophyceae from the Senegalese coast . Phytochemistry , 31 : 2739 – 2741 .
- Balata , D , Piazzi , L and Cinelli , F . 2004 . A comparison among assemblages in areas invaded by Caulerpa taxifolia and C. racemosa on a subtidal Mediterranean rocky bottom . P.S.Z.N.I: Mar. Ecol. , 25 : 1 – 13 .
- Chintalapati , S , Kiran , MD and Shivaji , S . 2004 . Role of membrane lipid fatty acids in cold adaptation . Cell. Mol. Biol. , 50 : 631 – 642 .
- Chisholm , JRM , Fernex , FE , Mathieu , D and Jaubert , JM . 1997 . Wastewater discharge, seagrass decline and algal proliferation on the Côte d’Azur . Mar. Pollut. Bull. , 34 : 78 – 84 .
- Chisholm , JRM , Marchioretti , M and Jaubert , JM . 2000 . Effect of low water temperature on metabolism and growth of a subtropical strain of Caulerpa taxifolia (Chlorophyta) . Mar. Ecol. Prog. Ser. , 201 : 189 – 198 .
- Clifton , KE . 1997 . Mass spawning by green algae on coral reefs . Science , 275 : 1116 – 1118 .
- Clifton , KE and Clifton , LM . 1999 . The phenology of sexual reproduction by green algae (Bryopsidales) on Caribbean coral reefs . J. Phycol. , 35 : 24 – 34 .
- De Rosa , S , De Stefano , C , Scarpelli , P and Zavodnik , N . 1988 . Terpenes from the red alga Sphaerococcus coronopifolius of the north Adriatic Sea . Phytochemistry , 27 : 1875 – 1878 .
- Folk , RL . 1954 . The distinction between grain size and mineral composition in sedimentary rock nomenclature . J. Geol. , 62 : 344 – 356 .
- Hazel , JR and Williams , EE . 1990 . The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment . Prog. Lipid Res. , 29 : 167 – 227 .
- Iveša , Lj and Devescovi , M . 2006 . Seasonal vegetation patterns of the introduced Caulerpa racemosa (Caulerpales, Chlorophyta) in the northern Adriatic Sea (Vrsar, Croatia) . Period. Biol. , 108 : 111 – 116 .
- Iveša , Lj , Blažina , M and Najdek , M . 2004 . Seasonal variation in fatty acid composition of Caulerpa taxifolia (M. Vahl.) C. Ag. in the northern Adriatic Sea (Malinska, Croatia) . Bot. Mar. , 47 : 209 – 214 .
- Iveša , Lj , Jaklin , A and Devescovi , M . 2006 . Vegetation patterns and spontaneous regression of Caulerpa taxifolia (Vahl) C. Agardh in Malinska (Northern Adriatic, Croatia) . Aquat. Bot. , 85 : 324 – 330 .
- Jaubert , JM , Chisholm , JRM , Minghelli-Roman , A , Marchioretti , M , Morrow , JH and Ripley , HT . 2003 . Re-evaluation of the extent of Caulerpa taxifolia development in the northern Mediterranean using airborne spectrographic sensing . Mar. Ecol. Prog. Ser. , 263 : 75 – 82 .
- Khotimchenko , SV . 1995 . Fatty acid composition of green algae of the genus Caulerpa . Bot. Mar. , 38 : 509 – 512 .
- McElhaney , RN . 1982 . “ Effects of membrane lipids on transport and enzymic activities ” . In Current Topics in Membranes and Transport , Edited by: Razin , S and Rotem , S . 317 – 380 . New York, , USA : Academic Press .
- Nelson , MM , Phleger , CF and Nichols , PD . 2002 . Seasonal lipid composition in macroalgae of the northeastern Pacific Ocean . Bot. Mar. , 45 : 58 – 65 .
- Panayotidis , P and Žuljević , A . 2001 . Sexual reproduction of the invasive green alga Caulerpa racemosa var. occidentalis in the Mediterranean Sea . Oceanol. Acta , 24 : 199 – 203 .
- Pearson , GA and Serrao , EA . 2006 . Revisiting synchronous gamete release by fucoid algae in the intertidal zone: fertilization success and beyond? . Int. Comp. Biol. , 46 : 587 – 597 .
- Piazzi , L , Balestri , E , Magri , M and Cinelli , F . 1997 . The spread of the tropical alga Caulerpa racemosa (Forsskål) J. Agardh (Bryopsidophyceae, Chlorophyta) along the Tuscan coast (Italy) . Cryptogamie Algol. , 18 : 343 – 350 .
- Piazzi , L , Ceccherelli , G and Cinelli , F . 2001 . Threat to macroalgal diversity: effects of the introduced green alga Caulerpa racemosa in the Mediterranean . Mar. Ecol. Prog. Ser. , 210 : 140 – 159 .
- Ruitton , S , Verlaque , M and Boudouresque , SM . 2005 . Seasonal changes of the introduced Caulerpa racemosa var. cylindracea (Caulerpales, Chlorophyta) at the northwest limit of its Mediterranean range . Aquat. Bot. , 82 : 55 – 70 .
- Terrados , J and Lopezjimenez , JA . 1996 . Fatty acid composition and chilling resistance in the green alga Caulerpa prolifera (Forsskal) Lamouroux (Chlorophyta, Caulerpales) . Biochem. Molecul. Biol. Internatl. , 39 : 863 – 869 .
- Terrados , J and Marbà , N . 2006 . Is the vegetative development of the invasive chlorophycean, Caulerpa taxifolia, favored in sediments with a high content of organic matter? . Bot. Mar. , 49 : 331 – 338 .
- Verlaque , M , Durand , C , Huisman , JM , Boudouresque , CF and Le Parco , Y . 2003 . On the identity and origin of the Mediterranean invasive Caulerpa racemosa (Caulerpales, Chlorophyta) . Eur. J. Phycol. , 38 : 325 – 339 .
- Žuljević , A , Antolić , B and Onofri , V . 2003 . First record of Caulerpa racemosa (Caulerpales: Chlorophyta) in the Adriatic Sea . J. Mar. Biol. Assoc. UK , 83 : 711 – 712 .