Abstract
A comparative PCR analysis of the nodularin synthetase gene cluster (nda) of two closely related Nodularia strains from the Baltic Sea was performed. Unlike the nodularin-producing Nodularia spumigena Huebel 1988/306, all 11 tested nda sequences were absent in the non-toxic Nodularia harveyana Huebel 1983/300. Our results suggest that the benthic N. harveyana strain lacks the entire 48-kb nodularin synthetase gene cluster. We describe new primers and the requisite experimental conditions for screening different nda sequences.
Introduction
In the Baltic Sea, mass occurrences of the filamentous heterocystous cyanobacterial genus Nodularia are an annual phenomenon in late summer and may cover areas of 60 000 km2 (Laamanen et al., Citation2001). The cyanobacterial bloom formation is favoured by increasing phoshorus and nitrogen concentrations in the brackish water, particularly due to anthropogenic activity. Nodularia blooms can cause hazardous health risks for humans and animals via the production of the cyclic pentapeptide hepatotoxin nodularin, a potent inhibitor of eukaryotic protein phosphatases 1 and 2A (Yoshizawa et al., Citation1990; Lethimäki, Citation2000). The acute LD50 toxicity of nodularin in mice (i.p.) is 60 µg/kg (Carmichael et al., Citation1988). With the exception of the benthic strain PCC 7804, originating from a thermal spring at Dax, France (Beattie et al., Citation2000; Saito et al., Citation2001), planktonic Nodularia spumigena is the only confirmed nodularin-producing species.
Based on morphological data and ecological properties, Komarek et al. (Citation1993) proposed that Nodularia comprised four planktonic (N. baltica, N. litorea, N. spumigena, N. crassa) and three benthic species (N. harveyana, N. sphaerocarpa, N. willei). Barker et al. (Citation1999) tested the validity of this phenotypic classification by determining nucleotide sequences for the phycocyanin locus, including the non-coding intergenic spacer (PC-IGS), for the IGS between two adjacent copies of the gas vesicle protein gene gvpA, and for the rDNA internal transcribed spacer (rDNA-ITS) in planktonic isolates of Nodularia. They found no consistent correlation between genotype and any phenotypic features and questioned the usefulness of morphological characters for placing Nodularia isolates into meaningful taxonomic groups.
In general, hepatotoxin synthetase genes are the candidate genes used for the discrimination of toxic and non-toxic genotypes via inexpensive and simple molecular PCR techniques (Dittmann & Börner, Citation2005; Dittmann & Wiegand, Citation2006). Neilan et al. (Citation1999) amplified a 758 bp PCR product of a mcyB microcystin synthetase gene analogue with primers originally designed for Microcystis. DNA amplification of strains PCC 73104 (Canada), NSOR10 (Australia), HEM and BY1 (Baltic Sea) of nodularin-producing N. spumigena resulted in PCR products similar to mcyB. Non-toxic N. sphaerocarpa did not possess a mcyB gene analogue. Surprisingly sequence analysis of amplified PCR products (mcyB) of toxic Nodularia strains showed clear differences. Nodularia spumigena BY1 clustered more closely with PCR products of toxic Microcystis strains whereas N. spumigena strains HEM and PCC 73104 were more closely related to amplification products of peptide synthetase genes of non-toxic Microcystis aeruginosa strains (Neilan et al., Citation1999).
Moffit & Neilan (Citation2001) investigated a variety of strains of the non-toxic N. harveyana Huebel 1983/300 with respect to the mcyB gene analogue, but could not identify it within these benthic strains (Moffit & Neilan, Citation2001). Since Moffitt & Neilan (Citation2004) characterized the complete nodularin synthetase gene cluster in N. spumigena NSOR10, the mcyB gene analogue, discovered only in toxic Nodularia strains, was identified as a sequence of the nodularin synthetase gene ndaF. The 48-kb nda gene cluster responsible for the biosynthesis of the hepatotoxic and tumour promoting cyclic pentapeptide consists of nine open reading frames (ORFs), ndaA to ndaI, which are transcribed from a bidirectional regulatory promoter region and encode non-ribosomal peptide synthetase modules (NRPS), polyketide synthase modules (PKS) and tailoring enzymes.
Moffitt & Neilan (Citation2001) considered that non-toxic Nodularia strains differ from toxic strains via peptide synthetase and polyketide synthase sequences. In differentiating toxic and non-toxic strains, several authors (Moffitt & Neilan, Citation2001; Lyra et al., Citation2005; Surakka et al., Citation2005, Jungblut & Neilan, Citation2006; Koskenniemi et al., Citation2007) focussed solely on the detection of subunit ndaF because of its essential role in nodularin biosynthesis. The aim of this study was to develop different, high-stringency, PCR methods to discriminate toxic and non-toxic genotypes, in order to investigate which nda sequences (beyond ndaF) are lacking in the non-toxic N. harveyana Huebel 1983/300 compared to the toxic N. spumigena Huebel 1988/306. Despite their different ecological strategies, both strains were selected because of their close morphological and phylogenetical relationship as well as their geographical origin, near Ruegen island in the Baltic Sea (Laamanen et al., Citation2001).
Materials and methods
Nodularia strains were cultivated at 25°C with continuous illumination of 80 µmol of photons m−2 s−1 in BG-11 medium with the addition of 6 g NaCl and adjusted to pH 9.0.
To extract total genomic DNA, cells were broken in lysis buffer (7 M urea, 0.3 M NaCl, 1% lauryl sarcosin, 20 mM Na2EDTA, 50 mM Tris-HCl, pH 8.0) by homogenization with glass beads, extracted with phenol/chloroform/isoamyl alcohol (25:24:1) and then precipitated with isopropanol. The pellet was washed twice with 80% ethanol and resuspended in pure water with the addition of RNase. DNA purity was assessed via photometric detection and calculated by the A260/A280 and A230/A260 ratios.
Total RNA isolation was performed following a modified protocol of Chomczynske & Sacchi (Citation1987) using aliquots of exponentially growing Nodularia cultures which were lysed via homogenization with sterile glass beads. RNA extracts were treated with DNase followed by thermal inactivation of the enzyme. The cDNA synthesis was performed by the cDNA first strand synthesis kit (Fermentas) following the instruction manual.
We investigated the occurrence of all nine nda genes in both Nodularia strains, including different primers and target sequences for the detection of ndaF. Independently, we designed all primers () used in this study via Primer3 Software (http://frodo.wi.mit.edu/cgi-bin/primer3/primer3_www.cgi) used and the complete sequence of the nodularin synthetase gene cluster (Moffitt & Neilan, Citation2004) were deposited in GenBank under accession number AY210783. At least one sequence (mostly including the start codon) of every gene was tested. The 10427 bp ndaF gene encoding the hybrid PKS-NRPS enzyme complex NdaF was tested in two different target sequences, one near the start codon, both of which differed from the sequence of Neilan et al. (Citation1999) and Koskenniemi et al. (Citation2007).
Table 1. Oligonucleotides.
In addition, we investigated the usefulness of the applied primer pairs for cDNA amplification and for expression studies. Based on cpcBA (encoding the 946 and 945 subunits of phycocyanin) sequence alignments of different Nodularia strains, we designed the primer pair cpcBA-LP and cpcBA-RP as a genus-specific positive PCR control to exclude false negative results. Amplification was performed in a 25-µl reaction mixture containing 1 × PCR buffer (KCl, Fermentas), 2 mM MgCl2 (Fermentas), 0.2 mM of each deoxynucleoside triphosphates (Fermentas), 1 U Taq DNA polymerase (Fermentas), 0.5 μM of each primer (MWG Biotech) and template DNA at a concentration of 10–100 ng µl−1. There were no significant performance changes over the range of DNA template concentrations used. Thermal cycling () was performed in MWG Primus 96 plus PCR machines.
Table 2. PCR programmes.
Results and discussion
PCR experiments indicated a lack of nodularin synthetase genes (ndaA-I) in the non-toxic N. harveyana Huebel 1983/300, whereas the nodularin-producing N. spumigena Huebel 1988/306 contained all tested genes as expected (; ). We tested at least three different high-purity DNA isolates per strain and repeated the PCR analysis for each primer pair and target sequence several times. Furthermore, the cpcBA positive amplification control clearly excludes a false negative due to co-extracted polymerase inhibiting agents within DNA isolates of the non-toxic strain ().
Fig. 1. PCR analysis, gel montage. (M) DNA marker; (1, 3, 5, 7, 9, 11, 13, 15, 17, 19, 21, 24) Nodularia spumigena Huebel 1988/306; (2, 4, 6, 8, 10, 12, 14, 16, 18, 22, 25) Nodularia harveyana Huebel 1983/300; (23) water control, (26) Spirulina platensis DNA negative control; (1, 2) ndaA; (3, 4) ndaB; (5, 6) ndaC1; (7, 8) ndaC2; (9, 10) ndaD; (11, 12) ndaE; (13, 14) ndaF1; (15, 16) ndaF2; (17, 18) ndaG; (19, 20) ndaH; (21, 22) ndaI; (23–26) cpcBA.
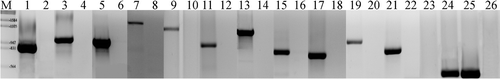
Table 3. PCR analysis.
We decided to try at least one additional method to verify our results and to demonstrate the utility of the oligonucleotides for mRNA expression studies. For this reason we isolated the total RNA and synthesized the corresponding cDNA via reverse transcriptase. RT-PCR analysis approved the absence of all tested nodularin synthetase gene sequences () as anticipated. In practice, cDNA amplification seems to be more vulnerable to false negative results then amplification of genomic DNA. Nevertheless, the developed primers are useful to detect nda gene transcripts in potentially nodularin-producing cyanobacterial strains to semi-quantitatively evaluate the nda gene expression, for instance, in different Nodularia strains or under variable growth conditions in further studies.
Previous work has suggested that the ability to synthesize the hepatotoxins microcystin and nodularin is an ancestral feature that was possibly lost by non-toxic strains as they adopted the benthic habitat (Rantala et al., Citation2004), along with the loss of gas vacuoles (Lyra et al., Citation2005). The selective pressures acting on these cyanobacterial features are different and evolutionary coherence is questionable, but the presence or absence of nodularin might be strongly influenced by the habitat, benthic or planktonic, as is the occurrence of gas vacuoles.
Interestingly, we detected no conserved nda sequences in the non-toxic Nodularia strain. This study provides the first report of the absence of the complete nda gene cluster in N. harveyana Huebel 1983/300. Whether this is typical for all non-toxic N. harveyana strains, or only for some, must be investigated in other studies using many more strains, particularly since N. harveyana is the phylogentically most diverse species within Nodularia (Lyra et al., Citation2005). Therefore the PCR methods reported here are powerful tools for determining the possible occurrence of nda subunits or conserved nda sequences, and whether or not they are transcribed.
The stringency of all these PCR methods is good to acceptable. In particular, amplification of the ndaC2 target sequence only infrequently produced false negatives in the toxigenic strain. The stringency could be optimized with higher amounts of Taq polymerase (1–2.5 U). Amplification of ndaF2 also sometimes showed short, non-specific bands in the non-toxic N. harveyana strain. Higher annealing temperatures, lower amounts of Taq DNA polymerase (0.5 U rather than 1.0 U) and less MgCl2 (1.5 mM rather than 2 mM) solved the problem and increased the stringency of the method. Amplification of longer target sequences, especially ndaC2, ndaD and ndaF1, was more susceptible to interference due to variation in the annealing temperature (±0.5 C) than other target sequences with fewer than 1000 bp (±1.0 C). On the one hand, lower temperatures could possibly result in non-specific PCR products, but on the other hand, higher temperatures will prevent primer-template binding. For longer amplification products it was necessary to reduce the maximum cycle number from 40 to 35. It emerged that the performance of the primers for ndaE and ndaF1 increased with higher concentrations in the reaction mixture (1.0 instead of 0.5 µM).
Unlike the cpcBA amplification, the oligonucleotides for ndaA-I detection were based on single genes because of the lack of sequence and thus conservation data. Specificity may therefore be increased with lower detection stringency of analogue genes in related species. Further comparative studies of our primers and PCR methods could expand our knowledge of the evolution of sporadically distributed nodularin synthetase genes within Nodularia. The evolutionary process of nda loss in benthic strains is of interest with respect to the biological role of toxin production.
Acknowledgements
We are grateful to Dr Norbert Wasmund (IOW, Rostock, Germany) for donating the strains. We also thank Dr Bationa Shahollari, Dr Paul Hein and Yvonne Venus (University of Jena, Germany) for helpful advice. Special thanks go to Dr Eileen J. Cox for improving the English.
References
- Barker , GLA , Hayes , PK , O'Mahony , SL , Vacharapiyasophon , P and Walsby , AE . 1999 . A molecular and phenotypic analysis of Nodularia (Cyanobacteria) from the BalticSea . J. Phycol , 35 : 931 – 937 .
- Beattie , KA , Kaya , K and Codd , GA . 2000 . The cyanobacterium Nodularia PCC 7804, of freshwater origin, produces [L-Har2]nodularin . Phytochemistry , 54 : 57 – 61 .
- Carmichael , WW , Eschedor , JT , Patterson , GM and Moore , RE . 1988 . Toxicity and partial structure of a hepatotoxic peptide produced by the cyanobacterium Nodularia spumigena Mertens emend. L575 from New Zealand . Appl. Environ. Microbiol. , 54 : 2257 – 2263 .
- Chomczynski , P and Sacchi , N . 1987 . Single-step method of RNA isolation by acid guanidinium thiocyanate phenol-chloroform extraction . Anal. Biochem. , 162 : 156 – 159 .
- Dittmann , E and Börner , T . 2005 . Genetic contributions to the risk assessment of microcystin in the environment . Toxicol. Appl. Pharmacol. , 203 : 192 – 200 .
- Dittmann , E and Wiegand , C . 2006 . Cyanobacterial toxins–occurrence, biosynthesis and impact on human affairs . Mol. Nutrit. Food Res. , 50 : 7 – 17 .
- Jungblut , A-D and Neilan , BA . 2006 . Molecular identification and evolution of the cyclic peptide hepatotoxins, microcystin and nodularin, synthetase genes in three orders of cyanobacteria . Arch. Microbiol. , 185 : 107 – 114 .
- Komarék , J , Hübel , M , Hübel , H and Šmarda , J . 1993 . The Nodularia studies 2. Taxonomy . Algol. Stud. , 68 : 1 – 25 .
- Koskenniemi , K , Lyra , C , Rajaniemi-Wacklin , P , Jokela , J and Sivonen , K . 2007 . Quantitative real-time PCR detection of toxic Nodularia Cyanobacteria in the Baltic Sea . Appl. Environ. Microbiol. , 73 : 2173 – 2179 .
- Laamanen , MJ , Gugger , MF , Lehtimäki , JM , Haukka , K and Sivonen , K . 2001 . Diversity of toxic and nontoxic Nodularia isolates (Cyanobacteria) and filaments from the Baltic Sea . Appl. Environ. Microbiol. , 67 : 4638 – 4647 .
- Lehtimäki , J . 2000 . “ Characterisation of cyanobacterial strains originating from Baltic Sea with emphasis on Nodularia and its toxin, nodularin ” . In Academic dissertation in microbiology Helsinki, , Finland
- Lyra , C , Laamanen , M , Lehtimäki , JM , Surakka , A and Sivonen , K . 2005 . Benthic cyanobacteria of the genus Nodularia are non-toxic, without gas vacuoles, able to glide and genetically more diverse than planktonic Nodularia . Int. J. System. Evol. Microbiol. , 55 : 555 – 568 .
- Moffitt , MC and Neilan , BA . 2001 . On the presence of peptide synthetase and polyketide synthase genes in the cyanobacterial genus Nodularia . FEMS Microbiol. Lett. , 196 : 207 – 214 .
- Moffitt , MC and Neilan , BA . 2004 . Characterization of the nodularin synthetase gene cluster and proposed theory of the evolution of cyanobacterial hepatotoxins . Appl. Environ. Microbiol. , 70 : 6353 – 6362 .
- Neilan , BA , Dittmann , E , Rouhiainen , L , Bass , RA , Schaub , V , Sivonen , V and Börner , T . 1999 . Nonribosomal peptide synthesis and toxigenicity of cyanobacteria . J. Bacteriol. , 181 : 4089 – 4097 .
- Rantala , A , Fewer , DP , Hisbergues , M , Rouhiainen , L , Vaitomaa , J , Börner , T and Sivonen , K . 2004 . Phylogenetic evidence for the early evolution of microcystin synthesis . Proc. Natl Acad. Sci. USA , 101 : 568 – 573 .
- Saito , K , Konno , A , Ishii , H , Saito , H , Nishida , F , Toshihiko , A and Chen , C . 2001 . Nodularin-Har: A new nodularin from Nodularia . J. Nat. Products , 64 : 139 – 141 .
- Surakka , A , Sihvonen , LM , Lehtimäki , JM , Wahlsten , M , Vuorela , P and Sivonen , K . 2005 . Benthic cyanobacteria from the Baltic Sea contain cytotoxic Anabaena, Nodularia, and Nostoc strains and an apoptosis-inducing Phormidium strain . Environ. Toxicol. , 20 : 285 – 292 .
- Yoshizawa , S , Matsushima , R , Watanabe , MF , Harada , K , Ichihara , A , Carmichael , WW and Fujiki , H . 1990 . Inhibition of protein phosphatases by microcystins and nodularin associated with hepatotoxicity . J. Cancer Res. Clin. Oncol. , 116 : 609 – 614 .