Abstract
This study presents the first record of a living dinoflagellate cyst with a hypocystal, antapical archeopyle. It is also the first detailed account of the archeopyle of a living freshwater cyst from the genus Peridinium. The cysts were isolated from sediment traps deployed in Lake Nero di Cornisello, a low-alkalinity high mountain lake of the Adamello mountain range (2233 m above sea level, South Eastern Alps, Italy). The archeopyle is large, clearly hypocystal, polygonal, and slightly peanut-shaped. The species producing this cyst belongs to the Peridinium umbonatum group and is described based on scanning electron microscopy and light microscopy. Partial sequences of SSU rDNA were obtained and compared with previously published sequences from the P. umbonatum group. The taxonomic position of the species is discussed.
Introduction
Resting stages are common in freshwater phytoplankton (Fryxell, Citation1983). Traditionally, the resting stage has been considered a strategy for surviving periods of adverse environmental conditions, such as nutrient depletion, avoiding the death of an entire population (McQuoid & Hobson, Citation1995). Resting stages are, however, also important for the dispersal of certain species in time and space, acting as seed banks and increasing the genetic variation of a population when produced after sexual reproduction (Rengefors et al., Citation1998). Cysts sink relatively fast through the water column and can remain viable over long periods of time when they reach the bottom sediments (Dale, Citation2001). Subsequently cysts can act as inoculum for vegetative growth when favourable conditions are restored (Dale, Citation1983; Anderson et al., Citation1985; Heiskanen, Citation1993). Most studies on dinoflagellate cysts are from marine environments (Head, Citation1996; Dale, Citation2001) and little is known about cysts of freshwater dinoflagellates.
The excystment of a dinoflagellate cyst takes place through an opening in the cyst wall called the archeopyle (Evitt, Citation1963). The archeopyle usually mirrors the position of one or more thecal plates, or can, as in some gymnodinioid dinoflagellates (Fensome et al., Citation1993), simply be a slightly curved rupture (chasmic archeopyle) or a more or less circular or equidimensional opening (tremic archeopyle). Almost all known archeopyles are on the epicyst (upper part of the cyst), frequently on the dorsal side (Fensome et al., Citation1993). Archeopyle types can be subdivided into apical, intercalary, precingular, a combination of one or more plate series, or epicystal (involving the entire epicyst).
No extant dinoflagellate has been unequivocally shown to produce resting cysts with a hypocystal archeopyle. Among the ∼600 fossil dinoflagellate cyst genera (Fensome & Williams, Citation2004), the only ones with a hypocystal archeopyle are Capisocysta Warny & Wrenn, Caligodinium Drugg (Head, Citation1998), Eocladopyxis P. Morgenroth and Geonettia de Verteuil & G. Norris (Head, Citation2000).
This paper will focus on the description of the first ever reported cyst of a freshwater dinoflagellate with a hypocystal (antapical) archeopyle. The cyst belongs to the Peridinium umbonatum F. Stein group and occurs in Lake Nero di Cornisello (Italy). The phylogenetic position of the species is discussed based on morphological and rDNA sequence data.
Materials and methods
Site description
Lake Nero di Cornisello is located 2233 m above sea level in the South Eastern Alps (Adamello mountain range, Trentino, Italy; 46°13′00″N; 10°43′53″E; Tomasi, Citation1962). The lake has a maximum depth of 33 m, is dimictic and oligotrophic. Mean pH is around 6.0–6.5 and the alkalinity values are always <80 µeq l−1 (Cantonati et al., Citation2002).
Field sampling
Lake Nero di Cornisello was monitored once every 3 months from summer 2004 until summer 2005. Sediment traps were placed at 5, 15 and 30 m water depth at the beginning of the monitoring period and subsequently replaced after each sampling. Material trapped in sample containers was left to settle for 48 h after collection in a refrigerator. Excess water was then drained off, ¾ of the trapped sediment was used for sediment and geochemical analyses and the remaining ¼ was kept moist in the dark at 4°C until laboratory studies on dinoflagellate cysts were performed.
Laboratory study
Part of the wet sample was sieved through a 25 µm mesh and sonicated. Sodium polytungstate (3Na2WO4 · 9WO3 · H2O) at a specific gravity of 1.3 (see Bolch, Citation1997) was used to concentrate the dinoflagellate cysts. Individual cysts were isolated into Nunclon 0.5-ml microwells and incubated at different pH (2–9), temperature (4°C and 15°C), in four different freshwater media and at a light level of ∼35 µmol m−2 s−1 under a 16-h : 8-h light–dark cycle. The first incubation was carried out for 21 days, from 12 December 2005 to 2 January 2006, and the second for 24 days, from 17 May to 10 June 2006.
Cysts were regularly checked for germination and observations of the different life cycle stages were performed under an Olympus CK30 inverted light microscope (Olympus, Tokyo, Japan). Full and empty cysts and different life cycle stages were photographed using an Olympus BX51 light microscope (LM) equipped with a DP12 camera using a DP-soft software. Full cysts, and empty cysts from those wells where germination took place, were dehydrated with increasing ethanol concentrations and dried in a Bal-Tec CPD 030 Critical Point Dryer. The stubs with the dried cysts were coated with platinum-palladium to a thickness of c. 15 nm and studied using a Jeol JSM-6335F (JEOL, Tokyo, Japan) field emission scanning electron microscope (SEM). By tilting the stubs in the microscope, each cyst was studied from many different angles to be certain of the orientation and placement of the observed features.
Molecular methods
Single cells and cysts were isolated, washed four times with distilled water and stored in 200 µl Eppendorf tubes with a few µl of distilled water at −20°C. The cells were thawed and then frozen in liquid nitrogen. This was repeated three times. The cells were then heated for 5 min at 94°C, and immediately afterwards a PCR was performed as follows: one initial denaturation at 94°C for 2 min followed by 30–32 cycles of 94°C for 30 s, 58°C for 30 s and 72°C for 25 s; and finally 72°C for 6 min using the primers periNLF (TGGAGGTCGTTATCHATAYG) (present study) and ND6R (GATCCTTCTGCAGGTTCACC) (Ekelund et al., Citation2004). A reamplification using 1 µl of the PCR product was performed. The PCR product was sequenced by Macrogen, Korea (www.macrogen.com). Sequences of two single cells were identical (Genbank EF 581380).
The sequence was aligned by eye (using Bioedit 7.0.5.3; Hall, Citation1999) with other SSU rDNA sequences acquired from Genbank. Sequences that appeared close to our strain in a blast search (including e.g. ‘dinophyceae sp.’ and ‘uncultured eukaryote’) were also included in the analyses. The final data set comprised 38 taxa and 622 positions (only positions for which the present species was sequenced were used in the analyses). Modeltest 3.7 was used to determine the evolutionary model that best fitted the data (Posada & Crandall, Citation1998). The likelihood settings were used for maximum likelihood analysis (100 random-addition replicates). Neighbor-joining (set to Log Det, 1000 random-addition replicates) and unweighted maximum parsimony analyses (1000 random-addition replicates) were performed using PAUP* 4.0b10 (Swofford, Citation2002). One thousand (neighbor-joining and maximum parsimony) or 100 bootstrap replicates (maximum likelihood) were performed.
Results
Cyst morphology (Figs )
The dinoflagellate cysts studied showed a size range of 25–35 µm in length and 21–31 µm in width (average 31 × 26.5 µm, length/width ratio = 1.17; n = 30).
Figs 1–9. SEM micrographs of the cyst of Peridinium umbonatum sensu lato. . Cyst with no thecal remains seen in apical view in which the slightly flattened ventral side is visible and the paratabulation by parasutures is discernable. . Cyst partially covered in thecal vestiges seen in dorsal view. . Cyst partially covered in thecal vestiges seen in ventral view. . Detail of showing the apical pore (ap) region with the x-plate (*). . Cysts with no thecal remains in which the paratabulation is discernable by parasutures. . The ventral-dorsal side of the cyst with the first antapical, polygonal, lightly peanut-shaped archeopyle. Key: X′: apical plates; Xa: intercalary plates; X″: precingular plates. Scale bars: 10 µm.
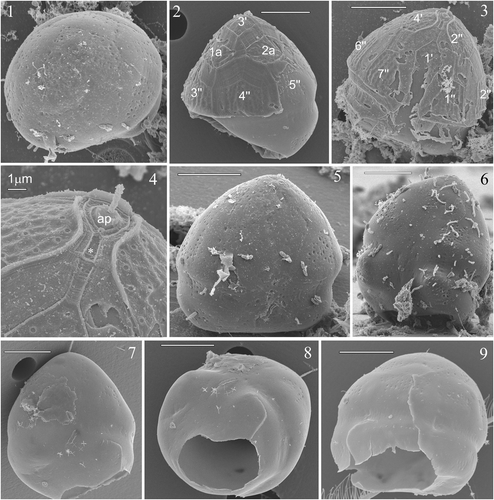
The cyst is slightly flattened on the ventral side (), the epicyst is longer than the hypocyst and bell-shaped (), while the hypocyst is rounded and slightly dorsally slanted on the ventral side in the parasulcal region (). Vestigial remains of the theca were often found on the cyst surface. This allowed recognition of tabulation patterns on the dorsal () and ventral () sides of the epicyst. When thecal remains were not present on the cysts, partial paratabulation could be discerned by patterns of indentations on the cyst wall indicating the parasutures (). The paracingulum is indicated by a narrowing of the cyst and is displaced slightly towards the hypocyst. The alignment of the paracingulum varied from equatorial to very slightly () spiralling to the left (i.e. descending). The parasulcal region tapered off in the epicystal region along the 1′ plate and is enlarged on the hypocyst ().
Figs 10–12. Light micrographs of the cyst of Peridinium umbonatum sensu lato. . Antapical view of an empty cyst with the operculum (arrow) attached at the ventral side. . Full cyst. . Empty cyst with the archeopyle (arrow) detectable in the antapical position.
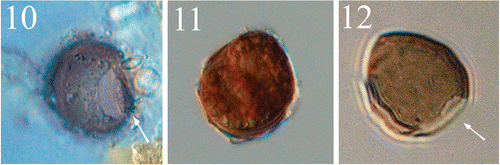
The archeopyle is antapical, large, polygonal and slightly peanut-shaped (). The position of the archeopyle most closely corresponded to the position of the first antapical plate of the thecate stage (1″″; ). The operculum is usually detached, but when attached is connected to the ventral side (). Full cysts were brown in colour () and empty cysts were generally lighter ().
Vegetative stages (Figs )
The first germination of the isolated cysts occurred after 5 days. The first cell was athecate () and brown, with chloroplasts distributed around the periphery of the cell. This was still a non-motile stage, which, in many cases, imploded near the empty cyst. In a few instances, the life-cycle continued with a moving naked dinoflagellate, which could divide into two moving cells after a few days. The first thecate non-motile cell () was seen 13 days after the beginning of the experiment, and started swimming on day 14. A maximum of five swimming cells were obtained from one cyst, but we never obtained a stable culture, although many different growth conditions were tested (unpublished data). The thecate vegetative cells had a mean size of 17 × 15 µm (n = 5). As we hoped that the living cells would continue to divide, observations on the tabulation were primarily done on empty thecae found in the wells (presumably shed in the course of cell division).
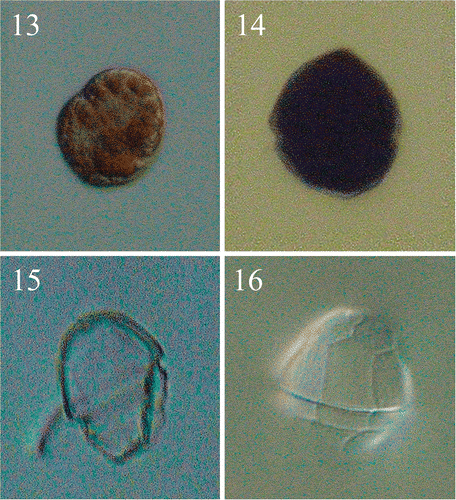
Figs 13–16. Light micrographs of the vegetative cells of Peridinium umbonatum sensu lato obtained from germinated cysts. . Non-thecated stage cell in which cingular portion is visible and radially arranged chloroplasts detectable. . Thecated stage cell. . Empty theca in lateral view, showing the slanting down profile of the hypocone. . Empty theca in dorsal view, showing the longer 4″ plate and the ‘remotum’ tabulation pattern.
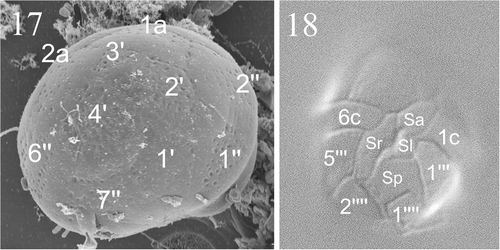
Figs 17, 18. Micrographs showing tabulation of cyst and vegetative cell. . SEM of a cyst of Peridinium umbonatum sensu lato showing details of the epicyst paratabulation. . LM showing the tabulation of the ventral side of the vegetative cell. Key: X′: apical plates; Xa: intercalary plates; X″: precingular plates; Xc: cingular plates; Sa: apical sulcal plate; Sp: posterior sulcal plate; Sr: right sulcal plate; Sl: left sulcal plate; X′″: postcingular plates; X″″: antapical plates).
Figs 19–26. SEMs of the vegetative cells of Peridinium umbonatum sensu lato collected in the water column of Lake Nero di Cornisello. Key: X′: apical plates; Xa: intercalary plates; X″: precingular plates; Xc: cingular plates; X′″: postcingular plates. Scale bars: 1 µm.
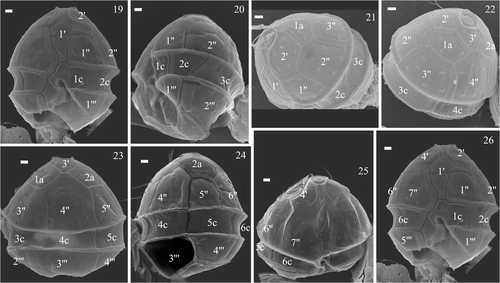
The empty thecae () showed the same slightly dorsally slanting profile in the ventral region of the hypotheca () as in the hypocyst. The tabulation pattern was discerned from the thecal remains on the cysts (), from the parasutures on the cyst surface (), from the empty thecae () and on SEM micrographs of vegetative cells of the species collected in the water column of Lake Nero di Cornisello (). The apical (1′) plate followed the entire length of the epicone, with an x-plate close to the C-type apical pore (Figs , ; see Pfiester et al., Citation1984; Toriumi & Dodge, Citation1993). The 3′ plate was rhomboidal (, ) and the two anterior intercalary plates pentagonal (, ). The pattern relationship between the 3′ and 4″ plates was mainly of the ‘remotum’ (Figs ) type (see Elbrächter & Meyer, Citation2001). The 4′ plate was longer than the other precingular plates (, , ). On a few specimens sutures were visible in the sulcus, indicating that there were at least four sulcal plates: an anterior sulcal plate (Sa) by the left end of the cingulum, a posterior sulcal plate (Sp) bordering both antapical plates, a long right sulcal plate (Sr) bordering the right side with the 7″, 6c and 5′″ plates, and a small and undulate left sulcal plate (Sl) (Figs ). The sutures running more or less through the centre of the sulcus from top to bottom formed a characteristic wavy structure. The plate pattern of the cingular plates is shown in : the first (1c) and the second (2c) cingular plates end at the middle of 1″ and 2″ respectively (), 3c and 4c align with 3″ and 4″ respectively (), and 5c and 6c have the same width of the fourth and the fifth post cingular plates. In some SEM micrographs (see e.g. ) the last cingular suture seemed to be aligned with the suture between 6″ and 7″, which suggests that there should be a seventh cingular plate. Future studies will investigate the possibility that there are seven cingular plates at a particular stage of the life cycle. The precise plate pattern of the hypocone () was difficult to determine as the thecate cells were mostly broken in the antapical area.
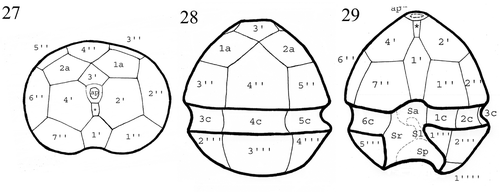
illustrates the plate pattern from the ventral, dorsal and apical sides: the tabulation pattern was 4′ 2a 7″ 6c 4?s 5′″ 0p 2″″.
Figs 27–29. Drawings of the plate pattern of Peridinium umbonatum sensu lato of Lake Nero di Cornisello in the apical (), dorsal (), and ventral () sides. Key: ap: apical pore; *: x-plate; X′: apical plates; Xa: intercalary plates; X″: precingular plates; Xc: cingular plates; Sa: apical sulcal plate; Sp: posterior sulcal plate; Sr: right sulcal plate; Sl: left sulcal plate; X′″: postcingular plates; X″″: antapical plates.
Phylogenetic inferences
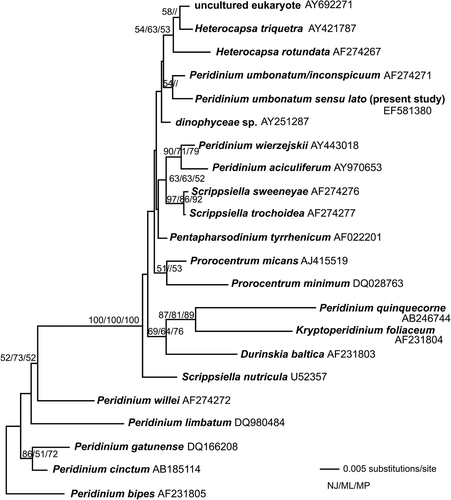
The phylogenetic studies of a region of the SSU nuclear-encoded rDNA revealed that the closest relative to the present strain was P. umbonatum/P. inconspicuum (UTEX strain LB2255) (GenBank accession number AF274271), but bootstrap support was low (), or the relationship was unresolved. Of the data set of 622 base pairs, seven base pair differences were found between our strain and the Genbank sequence, including five transitions, one transversion and one insertion.
Fig. 30. Neighbor joining tree based on parts of the SSU the nuclear encoded rDNA. The tree is unrooted. The bootstrap values indicated on the branches represent bootstrap values from neighbor joining (500 replicates, before slash), (maximum likelihood 1000 replicates, between slashes) and parsimony (1000 replicates, after slash). Only values above 50% are shown.
Cells of the UTEX strain and a P. inconspicuum strain from CCAP (1140/3) were measured for comparison. The UTEX strain had a mean size of 19 × 17 µm (SD: ± 2 µm and ±2.7 µm, respectively) and the CCAP strain 17 × 15 (SD: ± 1.7 µm and ±1.2 µm, respectively). The general morphology of both strains was similar to the strain from Nero di Cornisello.
Discussion
The archeopyle
Evitt & Wall (Citation1968) published descriptions of the plates involved in the cyst archeopyles of Peridinium wisconsinense S. Eddy and P. limbatum (A. Stokes) Lemmermann. Empty cysts of P. wisconsinense have an apical archeopyle with a ventrally attached operculum, equivalent to plates 1′–4′; in P. limbatum the cyst wall ruptures along the wall sutures (transapical archeopyle suture). The first detailed studies of life cycles and cysts of Peridinium species were published in the 1970s for P. cinctum cf. ovoplanum Er. Lindemann (Pfiester, Citation1975), P. willei Huitfeldt-Kaas (Pfiester, Citation1976) and P. gatunense Nygaard (Pfiester, Citation1977). All three species produced cysts with three wall layers, but the descriptions of these cysts did not include features of the archeopyle. Later studies (see e.g. Sako et al., Citation1984, Citation1985, Citation1987) reported the presence of Peridinium cysts, but only brief descriptions of the archeopyle were given for some of these. In P. willei (Boltovsky, Citation1984), the archeopyle consists of a partial separation of the epicyst and in P. gatunense (Boltovsky, Citation1973a) the archeopyle was reported as separation of the whole epicyst. In a recent overview of the freshwater dinoflagellates of the British Isles, 10 species of Peridinium are reported to form cysts, but the related description was not detailed, and the archeopyle was not reported at all (Lewis & Dodge, Citation2002). A cyst of P. inconspicuum was described (Pfiester et al., Citation1984) as having an unarmoured wall that was continuously shed and formed during the enlargement and elongation of the cyst, finally attaining a peanut shape 35–40 µm long. Again, no reference was made to the archeopyle. Our observations were performed on cysts several months after collection and storage in dark conditions at 4°C, indicating that the cysts were mature. In contrast to the observations of Pfiester et al. (Citation1984), our cysts were not peanut-shaped and had a mean size of 31 × 26.5 µm. Detailed SEM cysts observations often showed thecal remains on the external part of the cyst wall, probably from the planozygote phase.
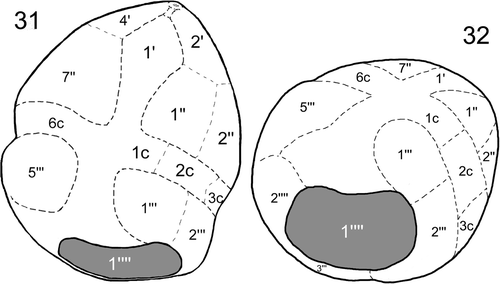
Our study represents the first detailed description of an archeopyle in the cyst of a freshwater dinoflagellate belonging to the genus Peridinium, as well as the first report of a hypocystal archeopyle in an extant dinoflagellate. The position of the large, polygonal, and slightly peanut-shaped archeopyle corresponds to the position of the first antapical plate of the thecate stage (1″″), as illustrated in . Two living dinoflagellates, Pyrophacus steinii (J. Schiller) D. Wall et B. Dale (producing the cyst Tuberculodinium vancampoae M. Rossignol) and Pyrophacus horologium F. Stein have been associated with a hypocystal archeopyle (Head, Citation1998). However, Matsuoka et al. (Citation1998) have demonstrated that the archeopyle of T. vancampoae (P. steinii) is epicystal. Similarly, detailed studies of P. horologium have been performed (see e.g. Montresor & Marino, Citation1994), but the presumed hypocystal location of the archeopyle of this species (Wall & Dale, Citation1971) has never been demonstrated.
Figs 31, 32. Drawings of the cyst of Peridinium umbonatum sensu lato of Lake Nero di Cornisello illustrating the first antapical plate position (1″″) of the archeopyle in relation with the plate pattern of the thecate stage in the semi-ventral () and semi-antapical () side. Key: dashed lines: visible lines of paratabulation; dotted lines: interpreted lines of paratabulation; X′: apical plates; X″: precingular plates; Xc: cingular plates; X′″: postcingular plates; X″″: antapical plates).
Two fossil dinoflagellate cysts of the genus Capisocysta have been described as having archeopyles uniquely formed by the extensive and exclusive dissociation of hypocystal plates (Head, Citation1998). This dissociation included plates of the postcingular series, so that, upon excystment, only the epicyst, some sulcal plates, and the first precingular plate usually remained intact. Two other fossil genera have archeopyles involving both hypocystal and epicystal plates: the genus Geonettia loses epicystal and hypocystal plates during excystment (see also Head, Citation2000), and Eocladopyxis has archeopyles that involve epicystal plates, although some dissociation of hypocystal plates may occur. Hence, Eocladopyxis and Geonettia cannot strictly be considered to have hypocystal archeopyles because epicystal plates are also involved in the archeopyle (Head, Citation1998).
Levandowski & Kaneta (Citation1987) linked the almost exclusive epicystal location of archeopyles in dinoflagellates to their swimming direction. Head (Citation1998) did not consider the connection to be obviously functional because the protoplast exits the cyst by amoeboid movement, not by flagellar propulsion, although flagella may be formed on the protoplast prior to excystment (Dale, Citation1983; and references therein). We observed that most of the cysts (and vegetative cells) observed by SEM rested on their hypocystal regions. The empty cysts appeared ‘sitting’ on their archeopyle. However, evolution seems to have favoured the epicystal location of archeopyles (e.g. Fensome et al., Citation1993). The functional significance of the antapical archeopyle position remains unresolved. However, we observed that vegetative stages collected from the plankton indicate a tendency to open in the antapical region. Before undergoing division the cell will exit from the theca through an opening on the hypotheca (see also Lefèvre, Citation1932). The same tendency for the theca to break in an archeopyle-equivalent location was reported by Boltovsky (Citation1973 a, Citation b , Citation1984) for P. willei and P. gatunense.
Identity of the species
The Kofoid system of classification (used in this paper) was also applied by Popovský & Pfiester (Citation1990) in the most recent overview of freshwater dinoflagellate species. The species described in the present paper belongs to the P. umbonatum group, which was defined as having a non-spherical shape, a symmetrical plate pattern (4′, 2a, 7″, 5′″, 2″″), an hypotheca smaller than the epitheca and an apical pore. The cingular plate pattern proposed by Bourrelly (Citation1985) for this group has been confirmed in our observation on motile cells from plankton samples. The dorsal thecal tabulation may vary, with 3 tabulation variants, depending on whether the plates 3′ and 4″ are in contact along a suture (tabulation ‘conjunctum’), at a single point (tabulation ‘contactum’) or not at all (tabulation ‘remotum’). We observed mainly the ‘remotum’ tabulation patterns. Elbrächter & Meyer (Citation2001) showed that clonal cultures, which they assigned to the P. umbonatum species complex as P. umbonatum sensu lato, had all three tabulation patterns.
At present, many authors prefer to distinguish the species included in P. umbonatum by Popovský & Pfiester (Citation1990), such as P. inconspicuum and P. umbonatum sensu stricto. The only difference between the two species when comparing the original descriptions is that the cells are more ovoid in P. umbonatum sensu stricto and have a cingulum that divides the cell in a ⅔ epitheca and ⅓ hypotheca, whereas in P. inconspicuum the cells are approximately pentagonal and the cingulum is more equatorial. Additional morphological differences between the two species, reported by different authors, are listed in . The specimens we studied displayed features characteristic of both species. Hence, from a morphological point of view, we consider it more appropriate to designate our species as P. umbonatum sensu lato, confirming the need for a taxonomical revision of this group, as suggested by Elbrächter & Meyer (Citation2001).
Table 1. Morphological characteristics of P. umbonatum and P. inconspicuum as described by other authors.
The phylogenetic analyses showed that the cells from Lake Nero di Cornisello were most similar to sequences in Genbank corresponding to P. umbonatum (UTEX LB2255). However, in the UTEX collection (http://www.utex.org/) this strain is called P. inconspicuum. Apparently there is confusion over the identity of the strain. Another putative P. inconspicuum from CCAP (1140/3) was sequenced by Ramiro Logares and had the same SSU rDNA as the Genbank sequence (Ramiro Logares pers. comm.). Thus, either P. inconspicuum and P. umbonatum share the same SSU rDNA sequence or only one species has been sequenced. This apparent confusion illustrates the current taxonomical problems in the P. umbonatum group. Further detailed studies on several strains are needed to reveal the identity of P. umbonatum sensu stricto. Moreover, the position of P. umbonatum/P. inconspicuum in the phylogeny (), although not well supported, indicates that this group of species may not belong to Peridinium sensu stricto. In the previously published phylogeny that included P. umbonatum this group was also separated from other Peridinium species (Saldarriaga et al., Citation2001).
Future studies will focus on the archeopyle location of other peridinioid dinoflagellates currently included in the P. umbonatum group by Popovský & Pfiester (Citation1990) and observed in other Trentino lakes. These studies will aim to determine whether the hypocystal archeopyle is a peculiar characteristic of the Lake Nero di Cornisello population, possibly related to its peculiar environment (e.g. pH), or if it is a common characteristic of the cysts in the P. umbonatum group.
Acknowledgements
The work for this paper was carried out towards the PhD thesis of MT at the University of Pisa (Italy; Doctoral Study Programme in Evolutionary Biology–Protistology). We are grateful to the Autonomous Province of Trento for funding the OLOAMBIENT project; we thank the MTSN research fellows, who assisted us during field work, the Department of Phycology of the Biological Institute of the University of Copenhagen research fellows who assisted us in the laboratory studies, Henk Brinkhuis and André Lotter of the Laboratory of Palaeobotany and Palynology of the Institute of Environmental Biology of the University of Utrecht and SYNTHESYS (DK-TAF) project, financed by the European Commission's framework programmes through a grant from the European-funded (FP 6) Integrated Infrastructure Initiative SYNTHESYS(DK-TAF). NL was funded by grant number 2111-04-0011 from the Danish Agency for Science Technology and Innovation.
References
- Anderson , DM , Coats , DW and Tyler , MA . 1985 . Encystment of the dinoflagellate Gyrodinium uncatenatum: temperature and nutrient effects . J. Phycol. , 2 : 200 – 206 .
- Bolch , CJS . 1997 . The use of sodium polytungstate for the separation and concentration of living dinoflagellate cysts from marine sediments . Phycologia , 36 : 472 – 478 .
- Boltovsky , A . 1973a . Peridinium gatunense Nygaard. Estructura y estereoultraestructurab tecal (Dinoflagellida) . Physis Sección B Buenos Aires , 32 : 331 – 344 .
- Boltovsky , A . 1973b . Formacion del arqueopilo en tecas de dinoflagellados . Revista Español de Micropalaeontología , 5 : 81 – 98 .
- Boltovsky , A . 1984 . Relacio huesped-parasito entre el quiste de Peridinium willei y oomicete Aphanomycopsis peridiniella n. sp . Limnobios , 2 : 635 – 645 .
- Bourrelly , P . 1985 . Les Algues d’Eau Douce. Initiation à la Systèmatique. Tome II. Les Algues Bleues et Rouges. Les Euglénins, Peridiniens et Cryptomonadines , Paris, , France : Société Nouvelle des Editions Boubée .
- Cantonati, M., Tolotti, M., & Lazzara, M., editors. (2002). I laghi del Parco Naturale Adamello-Brenta, Documenti del Parco, 14. Parco Naturale Adamello-Brenta, Strembo, Italy
- Dale , B . 1983 . “ Dinoflagellate resting cysts: ‘benthic plankton’ ” . In Survival Strategies of the Algae , Edited by: Fryxell , GA . 69 – 136 . Cambridge, , UK : Cambridge University Press .
- Dale , B . 2001 . The sedimentary record of dinoflagellate cysts: looking back into the future of phytoplankton blooms . Sci. Mar. , 65 : 257 – 272 .
- Ekelund , F , Daugbjerg , N and Fredslund , L . 2004 . Phylogeny of Heteromita, Cercomonas and Thaumatomonas based on SSU rDNA sequences, including the description of Neocercomonas jutlandica sp. nov., gen nov . Eur. J. Protistol. , 40 : 119 – 135 .
- Elbrächter , M and Meyer , B . 2001 . Plate pattern variability and plate overlap in a clonal culture of the freshwater dinoflagellate Peridinium umbonatum Stein species complex (Dinophyceae) . N. Jb. Geol. Paläont. Abh. , 219 : 221 – 227 .
- Evitt , WR . 1963 . A discussion of proposal concerning fossil dinoflagellates, hystricospheres, and acritarchs, I . Proc. Nat. Acad. Sci. , 49 : 158 – 164 .
- Evitt , WR and Wall , D . 1968 . Dinoflagellate studies. IV. Theca and cyst of recent freshwater Peridinium limbatum (Stokes) Lemmermann. Stanford Univ. Publ . Geol. Sci. , 12 : 1 – 15 .
- Fensome , RA , Taylor , FJR , Norris , G , Sarjeant , WAS , Wharton , DI and Williams , GL . 1993 . A Classification of Living and Fossil Dinoflagellates , Micropaleontology, Special Publication 7 New York, , USA : American Museum of Natural History .
- Fensome , RA and Williams , GL . 2004 . The Lentin and Williams Index of Fossil Dinoflagellates , Dallas, , USA : American Association of Stratigraphic Palynologists Contributions Series Number 42 .
- Fryxell , GA . 1983 . “ Introduction ” . In Survival Strategies of the Algae , Edited by: Fryxell , GA . 1 – 22 . New York, , USA : Cambridge University Press .
- Hall , TA . 1999 . BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT . Nucl. Acids. Symp. Ser. , 41 : 95 – 98 .
- Head , MJ . 1996 . “ Modern Dinoflagellates cysts and their biological affinities ” . In Palynology: Principles and Applications , Edited by: Jansonius , J and McGregor , DC . Vol. 3 , 1197 – 1248 . Dallas, , USA : American Associations of Stratigraphic Palynologists Foundation .
- Head , MJ . 1998 . New goniodomacean dinoflagellates with a compound hypotractal archeopyle from the late Cenozoic: Capisocysta Warny and Wrenn, emend . J. Paleont. , 72 : 797 – 809 .
- Head , MJ . 2000 . Geonettia waltonensis, a new goniodomacean dinoflagellate from the Pliocene of the North-Atlantic region, and its evolutionary implications . J. Paleont. , 74 : 812 – 827 .
- Heiskanen , A-S . 1993 . Mass encystment and sinking of dinoflagellates during a spring bloom . Mar. Biol. , 116 : 161 – 167 .
- Lefèvre , M . 1932 . Monographie des espèces d'eau douce du genre . Peridinium. Archives de Botanique , 2 : 1 – 208 .
- Lemmermann , E . 1899 . Ergebnisse einer Reise nach dem Pacific: Planktonalgen . Abhandlungen Herausgegeben vom Naturwissenschaftlichen Vereine zu Bremen , 16 : 313 – 398 .
- Levandowsky , M and Kaneta , PJ . 1987 . “ Behaviour in dinoflagellates ” . In The Biology of Dinoflagellates. Botanical Monographs , Edited by: Taylor , EJR . Vol. 21 , 360 – 397 . Oxford, , UK : Blackwell Scientific .
- Lewis , JM and Dodge , JD . 2002 . “ Phylum Pyrrophyta (Dinoflagellates) ” . In The Freshwater Algal Flora of the British Isles , Edited by: John , DM , Whitton , BA and Brook , AJ . 186 – 210 . Cambridge, , UK : Cambridge University press .
- Matsuoka , K , Pholpunthin , P and Fukuyo , Y . 1998 . Is the archeopyle of Tuberculodinium vancampoae (Rossignol) (Gonyaulacales, Dinophyceae) on the hypocyst? . Paleont. Res. , 2 : 183 – 192 .
- Mcquoid , MR and Hobson , LA . 1995 . Importance of resting stages in diatom seasonal succession . J. Phycol. , 31 : 44 – 50 .
- Montresor , M and Marino , D . 1994 . New observations on the life cycle of Pyrophacus horologium Stein (Dinophyceae) . Bollettino della Società Adriatica di Scienze , 75 : 261 – 268 .
- Pfiester , LA . 1975 . Sexual reproduction of Peridinium cinctum f. ovoplanum (Dinophyceae) . J. Phycol. , 11 : 259 – 265 .
- Pfiester , LA . 1976 . Sexual reproduction of Peridinium willei (Dinophyceae) . J. Phycol. , 12 : 234 – 238 .
- Pfiester , LA . 1977 . Sexual reproduction of Peridinium gatunense (Dinophyceae) . J. Phycol. , 13 : 92 – 95 .
- Pfiester , LA , Timpano , P , Skvarla , JJ and Holt , R . 1984 . Sexual reproduction and meiosis in Peridinium inconspicuum Lemmermann (Dinophyceae) . Amer. J. Bot. , 71 : 1121 – 1127 .
- Popovský , J and Pfiester , LA . 1990 . “ Dinophyceae (Dinoflagellida) ” . In Sußwasserflora von Mitteleuropa 6 , Edited by: Ettl , H , Gerloff , J , Heyning , H and Mollenhauer , D . Germany : Jena .
- Posada , D and Crandall , KA . 1998 . Modeltest: testing the model of DNA substitution . Bioinformatics , 14 : 817 – 818 .
- Rengefors , K , Karlsson , I and Hansson , L-A . 1998 . Algal cyst dormancy: a temporal escape from herbivory . Proc. Royal Soc. Ser. B: Biol Sci. , 265 : 1353 – 1358 .
- Sako , Y , Ishida , Y , Kadota , H and Hata , Y . 1984 . Sexual reproduction and cyst formation in the freshwater dinoflagellate . Peridinium cunningtonii. Bull. Japan. Soc. Sci. Fish. , 50 : 743 – 750 .
- Sako , Y , Ishida , Y , Kadota , H and Hata , Y . 1985 . Excystment in the freshwater dinoflagellate . Peridinium cunningtonii. Bull. Japan. Soc. Sci. Fish. , 51 : 267 – 272 .
- Sako , Y , Ishida , Y , Nishijima , T and Hata , Y . 1987 . Sexual reproduction and cyst formation in the freshwater dinoflagellate . Peridinium penardii. Nippon Suisan Gakkaishi , 53 : 473 – 478 .
- Saldarriaga , JF , Taylor , FJR , Kelling , PJ and Cavalier-Smith , T . 2001 . Dinoflagellate nuclear SSU rRNA phylogeny suggests multiple plastid losses and replacements . J. Mol. Evol. , 53 : 204 – 213 .
- Starmach , K . 1974 . Flora Slodkowodna Polski (Volume 4. Cryptophyceae, Dinophyceae, Raphidophyceae) , Warsaw, , Poland : Polska Akademia Nauk .
- Stein , F . 1883 . Der Organismus der Infusionsthiere. III. Flagellaten II , Leipzig, , Germany : Engelmann .
- Swofford , DL . 2002 . PAUP*. Phylogenetic Analysis Using Parsimony (*and other methods). 4.0 , Sunderland, , USA : Sinauer Associates .
- Tomasi , G . 1962 . Origine, distribuzione, catasto e bibliografia dei laghi del Trentino . St. Trent. Sci. Nat. , 1–2 : 1 – 355 .
- Toriumi , S and Dodge , J . 1993 . Thecal apex structure in the Peridiniaceae (Dinophyceae) . Eur. J. Phycol. , 28 : 39 – 45 .
- Wall , D and Dale , B . 1971 . A reconsideration of living and fossil Pyrophacus Stein, 1883 (Dinophyceae) . J. Phycol. , 7 : 221 – 235 .