Abstract
A desktop incubator with temperature control over the range 1–20°C (±0.5°C) was designed to hold two microtitre plates. Illumination of individual wells in the plate was by a matrix of 96 light-emitting diodes, whose intensity, period and pulsation could be controlled individually in each of 12 rows of eight chambers. The incubator was used to test how the length of light period affected cell length of the planktonic diatom Aulacoseira baicalensis, which only grows below 4°C. Short cells (mean length 26 µm) were formed under a 8-h : 16-h light–dark cycle, intermediate cells (mean length 35 µm) under a 4-h : 20-h light–dark cycle and longer cells (mean 38 µm) under a 2-h : 22-h light–dark cycle. These were similar to changes in mean cell length from 28 to 49 µm found in Lake Baikal during a decrease in light period caused by increased convective mixing from 15 m under-ice to 94 m after ice break-up. The laboratory experiments confirmed that decreasing light period was the environmental cue that initiated the production of long cells that then developed into resting stages.
Introduction
The dominant phytoplanktonic diatoms in Lake Baikal grow mainly at temperatures below 4°C (Skabichevsky, Citation1929; Bondarenko et al., Citation2006; Bidoshvili et al., Citation2007; Jewson et al., Citation2008). Studying them in the laboratory using currently available incubators, or constant temperature rooms requires considerable space and expense. So, we have adapted a ‘desktop’ incubator that holds two microtitre plates (Safonova et al., Citation2007) to include a matrix of light-emitting diodes that provides control of the light field in individual ‘wells’ of the plates. An advantage of using microtitre plates is that changes in the number and morphology of cells can be observed directly on an inverted microscope. This then makes it possible to follow changes over time in related cells along a filament without disturbing them. This technique was used to investigate whether the length of light period affected cell length in the phytoplanktonic diatom Aulacoseira baicalensis (K. Meyer) Simonsen. This is one of the largest phytoplankton species in freshwaters and has been observed to undergo large seasonal changes in cell length (Skabichevsky, Citation1929; Bidoshvili et al., Citation2007; Jewson et al., in prep).
Materials and methods
The incubator was based on an earlier design that holds two 96-well microtitire plates (Safonova et al., Citation2007). The internal surface of the incubator was lined with aluminum sheeting overlying Styrofoam thermo-insulation. The aluminum side plates were in contact with 56 W Peltier elements, which were electronic devices that pumped heat from inside the casing and discharged it into the air via a radiator equipped with a fan. The range of temperatures that could be maintained in the incubator was from 1°C to 20°C ± 0.5. The incubator had two methods of illumination. One was a general illumination using six ‘superbright’ light-emitting diodes in the lid of the incubator that provided light exposure in a range from 0 up to 45 µmol m−2 s−1 (). The other was a matrix of 96 light-emitting diodes, which was held in position by placing it in a 96-well, black polystyrene glass-bottomed microplate (Whatman, New Jersey, USA) that was then put on top of a second black polystyrene microtitre plate containing the individual wells to be illuminated. The board containing the diode matrix was slightly raised (5 mm) above the supporting microtitre plate by using four supports at each corner (). This reduced heating from the diodes by allowing air circulation. A connector on the internal surface of the cooled box allowed additional sensors (e.g. temperature) and devices working on Microlan protocol to be connected and controlled through a microprocessor. Light period, intensity (0 to 35 µmol m−2 s−1) and pulse rate (with periods down to 10 µs) could be varied but the latter was not used in the experiment presented here. The new incubator had a control unit, with keyboard and LCD display ().
Fig. 1. The incubator with lid open and two microtitre plates in place but without the diode matrix attached.

Filaments of A. baicalensis were collected from under the ice at 7 km offshore from Cape Berezovy (51° 81′N, 104° 89′E) in the south basin of Lake Baikal on 25 March 2007 and then returned immediately to the lakeside laboratory at the Baikal Museum where single filaments were washed and placed in individual chambers (300 µl) of 96-well, black polystyrene glass-bottomed microplates (Whatman, New Jersey, USA). Two plates were then placed side by side in the incubator. One plate was kept in the dark to act as a control, while the other plate was illuminated by the 96-diode matrix. The plates were made of black polystyrene but to avoid any risk of light seepage one row of ‘wells’ was left in the dark between each illuminated row. So, out of 12 rows in the plate, two rows of eight wells were given 2 h light per day, two rows were given 4 h per day and a further two rows 8 h per day. Valve lengths of 100 individuals from six wells in each light period were measured on digital images collected with a Pixera Penguin 600CL camera and VideoTest Razmer 5.0 software on an inverted microscope at ×200 magnification (Karl Zeiss, Axiovert 2000). This reduced handling time by allowing measurements to be completed later. Measurements were made of 100 valves from the natural population for each sample date on an inverted microscope (Olympus Ltd, model IM) at ×500 using a digital video and analysis system, model RMC-D4 (Brian Reece Scientific, UK). Measurements were made to the nearest 1 µm. Microscopes were calibrated using 50 × 2 µm calibration slides (Graticules Ltd, Tonbridge, UK).
Results
Both valve and cell lengths of A. baicalensis were measured but only cell lengths are presented here. The mean cell lengths of A. baicalensis under the ice on the 24 March 1997 in the south basin of Lake Baikal were 28 µm (). The measurements excluded any cells lengthening before division to lay down new daughter valves. At that time, filaments were mixed vertically in the water column by convection from 0 to 15 m, so they remained in the light zone throughout the day, which was 8 hours. The estimates of mixing depth were based on the isothermal distribution of temperature and homogeneous depth distribution of A. baicalensis cell concentration (Jewson, unpublished). By 22 April the length of cells had increased to a mean of 40 µm (). By this time, the depth of mixing had increased to 63 m. After ice break-up on 22 May 1997, the mixing depth reached 94 m and the mean cell length increased to 49 µm ().
Fig. 3. Cell length distributions of Aulacoseira baicalensis, expressed as relative frequency, are shown for (a) cultures grown in the same microtitre plate in the incubator but in three different light–dark photoperiods, 8-h : 16-h (8), 4-h : 20-h (4) and 2-h : 22-h (2) and (b) for populations under the ice of Lake Baikal on 24 March 1997 (closed circles) and 22 April (open triangles) and after ice break-up 22 May 1997 (closed triangles).
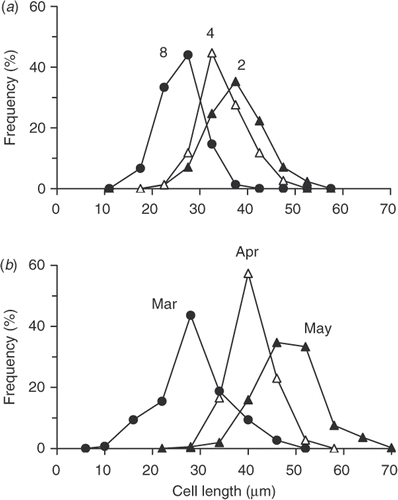
Using the incubator, an experiment was carried out over 3 weeks (25.vi.07 to 18.v.07) to test the effect of different lengths of photoperiod on valve and cell length of A. baicalensis (). Three different light periods were used, i.e. 2, 4 and 8 h per day. In 8-h : 16-h light–dark photoperiod, the mean cell length was 26 µm. In 4-h : 20-h light–dark photoperiod, the mean cell length increased to 35 µm. In the shortest light period, 2-h : 22-h light–dark photoperiod, the mean length was 38 µm. Experiments were carried out under an illumination of 14 µmol m−2 s−1. In an initial experiment at higher light (35 µmol m−2 s−1), cells failed to grow and then died.
Discussion
Ecological understanding of species is greatly improved if field observations are supported by experiments in the laboratory but this has often proved difficult and time consuming using traditional techniques, especially for temperature-sensitive species. In this study, the desktop incubator (Figs , ) provided an opportunity to investigate cell response () in a convenient way in related cells but it could be used in a variety of other situations. It is small enough to be put on a bench, desk or shelf and the diode matrix can be adjusted to provide higher light intensities in microtitre plate wells of different sizes. Also, it is relatively easily transported, so it can be taken to remote locations for experiments during field expeditions.
In the experiment described here, we wanted to test if we could use the incubator to investigate whether the length of light period influenced the length of cells through changing the length of daughter valves of A. baicalensis. Filamentous diatoms are useful organisms to study for this because related cells stay attached. Also, they have a rigid silica cell wall made of two valves (Round et al., Citation1990), which separate when they divide to lay down two new valves. So, each daughter cell has one valve from its parent and one new one. It is then relatively easy to measure the lengths of related valves. The lengths of light periods chosen were based on the probable conditions that cells might experience in the lake. This is a function of both day-length and the depth to which cells are mixed. So, under the ice in February and March, cells experienced their longest light periods, because the depth of convective mixing was then similar to the depth of the photic zone and, as a result, they spent most of the day (8 h) in the light. The short cells produced at that time () helped the filaments stay in suspension (Granin et al., Citation1999). However, although day-length then increased, cells gradually spent less time in the light because the photic zone stayed relatively constant while convective mixing went on increasing until after ice break-up in June, when it reached over 100 m. The increase in cell length () is related to the formation of a resting stage. A more detailed description of this will be published elsewhere (Jewson et al., currently unpublished data) but it was important to establish experimentally that photoperiod could act as an environmental cue. The large changes in cell length of A. baicalensis were first noted by Skabichevsky (Citation1929) and have been confirmed by later studies (Bidoshvili et al., Citation2007; Jewson et al., unpublished data). From other similar combined field and laboratory studies in Lake Baikal, we now know that the closely related species Aulacoseira skvortzowii uses low phosphate rather than light as its environmental cue for initiating resting stage formation (Jewson et al., Citation2008). So, it is an enormous benefit to understanding if field observations can be confirmed by laboratory experimentation. The size and convenience of this incubator system makes it much easier to do this, especially when access to well-established laboratory facilities are limited.
Acknowledgements
We thank M. Grachev, N. Granin, Ye. Likhoshway, L. Obolkina, R. Gnatovsky, A. Zhdanov, N. Bondarenko and staff of the Limnology Institute for their help. This study was partially supported by the program of the Presidium of the Russian Academy of Sciences RAS, No. 10.4. We also thank the Royal Society, who has supported parts of this work.
Notes
*Laboratory closed in 2000.
References
- Bidoshvili , YE.D. , Bondarenko , NA , Sakirko , MV , Khanayev , IV and Likhoshway , YE.V. 2007 . The change in the length of colonies of the planktonic diatom Aulacoseira baicalensis in various stages of the annual cycle in Lake Baikal . Hydrobiol. J. , 43 : 79 – 86 .
- Bondarenko , NA , Timoshkin , OA , Ropstorf , P and Melnik , NG . 2006 . The under-ice and bottom periods in the life cycle of Aulacoseira baicalensis (K. Meyer) Simonsen, a principal Lake Baikal alga . Hydrobiol. , 568 : 107 – 109 .
- Granin , NG , Jewson , D , Gnatovsky , R.YU. , Levin , LA , Zhdanov , AA , Averin , AI , Gorbunova , LA , Tsekhanovskii , VV , Doroschenko , LF , Minko , NP and Grachev , MA . 1999 . Turbulent mixing in the water layer just below the ice and its role in development of diatomic algae in Lake Baikal . Dokl. Akad. Nauk , 366 : 835 – 839 .
- Jewson , DH , Granin , NG , Zhdarnov , AA , Gorbunova , LA , Bondarenko , NA and Gnatovsky , R.YU. 2008 . Resting stages and ecology of the planktonic diatom Aulacoseira skvortzowii in Lake Baikal . Limnol. Oceanogr. , 53 : 1125 – 1136 .
- Round , FE , Crawford , RM and Mann , DG . 1990 . The Diatoms , Cambridge, , UK : Cambridge University Press .
- Safonova , TA , Aslamov , IA , Basharina , TN , Chenski , AG , Vereschagin , AL , Glyzina , OY and Grachev , MA . 2007 . Cultivation and automatic counting of diatom algae cells in multi-well plastic plates . Diatom Res. , 22 : 189 – 195 .
- Skabichevsky , AP . 1929 . On the biology of Melosira baicalensis (K. Meyer) Wisl . Russische Hydrobiologische Zeitschrift , 8 : 93 – 114 . (in Russian)
