Abstract
A parasitic chytrid that attacks the green alga Haematococcus pluvialis was recently isolated in our laboratory and identified as a novel species from the phylum Blastocladiomycota, named herein Paraphysoderma sedebokerensis (nom. prov.). A method for early and precise detection of chytrid infections was developed using the fluorescent dye, Nile red, which stained the chytrids’ sporangia. Using this technique we determined the specificity of Paraphysoderma sedebokerensis for 13 algal species belonging to the Chlorophyta. Algal species tested including: Chlamydomonas nivalis, Chlorella emersonii, Chlorella vulgaris, Chlorococcum sp., Chlorogonium elongatum, Monoraphidium braunii, Muriella zofigiensis, Scenedesmus obliquus, Scenedesmus vacuolatus (two strains), and Scotiellopsis oocystiformis were either resistant to infection, or only experienced slight levels of infection during exponential growth. During exponential growth phase 100% Chlorella zofigiensis Donz cells were infected, but none developed any infection during resting stage. Only in cultures of H. pluvialis did infections develop rapidly (3–4 days) and intensively (100% cells infected) during both the logarithmic and stationary stages of growth. We suggest that the newly isolated chytrid, Paraphysoderma sedebokerensis (nom. prov.), is highly specific for H. pluvialis, but has a limited capacity to infect other green algae.
Introduction
Microalgae are often plagued by harmful contaminants, including viruses, bacteria, protists, fungi, and various grazers, both in their natural habitats and in aquaculture. These parasites include zoosporic true fungi, or chytrids, which belong to the fungal phylum Chytridiomycota (Canter-Lund & Lund, Citation1995), or Blastocladiomycota (James et al., Citation2006). Members of this class of fungi have been found to cause extreme fluctuations in algal populations in natural fresh and marine water bodies (Blinn & Button, Citation1973; Bruning et al., Citation1992). Chytrid infections of algae have also been described in sewage oxidation ponds (Abeliovich & Dikbuck, Citation1977) and in outdoor algal mass-culture systems (Lukavsky, Citation1970). However, many aspects of the ecology of chytrids still remain poorly understood, including the nature of the interactions between parasites and their hosts (Gleason et al., Citation2008).
A novel parasite was recently isolated in our laboratory (Hoffman et al., Citation2008). It was found to contaminate cultures of the green alga Haematococcus pluvialis (Chlorophyta, Volvocales), the best natural source of the commercially exploited ketocarotenoid, astaxanthin (Olaizola, Citation2003). The life cycle of the parasite was determined and its phylogeny characterized, based on its 18S ribosomal DNA sequence data. These indicated that it is a novel chytrid, closely related to the vascular plant pathogen Physoderma (Blastocladiomycota), therefore herein it has been named Paraphysoderma sedebokerensis (nom. prov.). In the initial stages of infection, only the presence of an attached chytrid indicates contamination. As the infection proceeds, the chytrid sporangium grows bigger, as it degrades and consumes the host cytoplasm and the algal remnants turn into a brown, reduced, clumpy mass (Hoffman et al., Citation2008).
Chytrid species exhibit considerable variation in host ranges (Barr & Hickman, Citation1967 a, b; Ibelings et al., Citation2004). Canter & Jaworski (Citation1982) observed attraction of chytrid zoospores to a wide range of hosts. However in contrast, narrow host specificity based on species-specific interactions (Holfeld, Citation1998), or strain-specific interactions (De Bruin et al., Citation2008) have also been demonstrated. In some cases extreme specificity for a particular stage in the hosts life cycle has been reported (Canter-Lund & Lund, Citation1995).
The chytrid that attacks H. pluvialis has been shown to infect both the palmelloids (green cells) and the aplanospores (red cyst). However, the flagellates (young green cells released from ‘mother cells’) were not susceptible to the chytrid infection (Hoffman et al., Citation2008). In this study, the host-range of the chytrid for different species of green algae was tested using a newly developed method based on staining employing Nile red fluorescent dye.
Materials and methods
Algal cultures
Most of the species were obtained from the Culture Collection of Algae of the University of Göttingen (SAG), Göttingen, Germany. Haematococcus pluvialis Flotow 1844em.Wille K-0084 was obtained from the Scandinavian Culture Collection of Algae and Protozoa (SCCAP) at the University of Copenhagen, Denmark. Chlorococcum sp. UTEX 819 and Chlamydomonas nivalis UTEX 2824 were purchased from UTEX, the culture collection of algae at the University of Texas, Austin. Chlorella zofigiensis Donz CCAP 211/14 and Chlorella emersonii CCAP 211/11N were obtained from the Culture Collection of Algae and Protozoa (CCAP), at the Scottish Association for Marine Science, UK.
Most of the species were grown in a modified BG11 medium (Boussiba & Vonshak, Citation1991). Chlorogonium elongatum SAG 12-2b and Chlamydomonas nivalis UTEX 2824 were grown in a basal medium (Kobayashi et al., Citation1991).
Algal cultures at the logarithmic stage were obtained as described in Boussiba & Vonshak (Citation1991) to give a H. pluvialis cell density of 2 × 105 cells ml−1 after inoculation. Other algae were inoculated at similar initial chlorophyll content i.e. ∼5 µg chl ml−1.
To obtain stressed cultures, algal cells harvested from the logarithmic stage were re-suspended in nitrogen depleted medium to give 1 µg ml−1 chlorophyll, a concentration indicative for their optimum exposure to illumination. Cultures (100 ml) in 250 ml Erlenmeyer flasks were then incubated on rotary shaker for 10 days (temperature 25°C, illumination 80 µmol photon m−2 s−1, rotation speed 125 rpm).
Chytrid growth conditions
Chytrids were grown in chytrid growth medium (CGM) (Hoffman et al., Citation2008), to which were added 1.25 g l−1 yeast extract, 2.5 g l−1 peptone and 5 g l−1 glucose (enriched CGM). This inoculum contained thriving fungal cells (reaching OD of ∼3, within 3–5 days, from initial OD of 0.025).
Chytrid inoculation of algal cultures
An axenic, 5-day-old chytrid culture at its logarithmic stage (virulent toward Haematococcus), was used to inoculate samples of either exponentially growing, or stressed algal cultures. Cultures (20 ml) in 50 ml Erlenmeyer flasks, or 5 ml/well in 6-well plates (1 × 106 cells ml−1) were inoculated with the chytrid culture to yield a final concentration of about 0.025 OD600 ml−1. To ensure that all algal species were subjected to identical experimental procedures, algal densities in the Erlenmeyer flasks and micro-well plates were based on the equivalent dry weight of 106 H. pluvialis cells ml−1. The medium used for the experiments was either mBG11, or mBG11 without nitrogen, to investigate the susceptibility to infection of the logarithmic, or the stressed stage, respectively. The inoculated cultures were then incubated at 30°C, an optimal growth temperature for the chytrid (Hoffman et al., Citation2008), on an orbital shaker (125 rpm), under continuous dim white light (15 µmol photon m−2 s−1). Under these experimental conditions the pathogen is fully viable and the algae can survive, but do not grow. The cultures were observed daily over a period of seven days for the development of contamination.
Nile red staining and epidemic determination
Pellets containing 2 × 105 cells were stained with 100–200 µg Nile red l−1 in 2% DMSO, mixed and washed immediately with 1 ml DDW (10 s; 13,400 g). The pellet was re-suspended in 20–40 µl DDW to obtain a dense sample suitable for microscopic observation and mounting. Samples were observed on a Axioskop1 (Zeiss) microscope employing the light filter allowing maximum exposure to the excitation wavelength (450–490 nm) and a 520 nm cut-off filter.
Quantification of fungal infection was estimated by determining the prevalence of infection, Pr (Bruning, Citation1991; Holfeld, Citation2000):
Results
Nile-red staining of chytrid sporangia
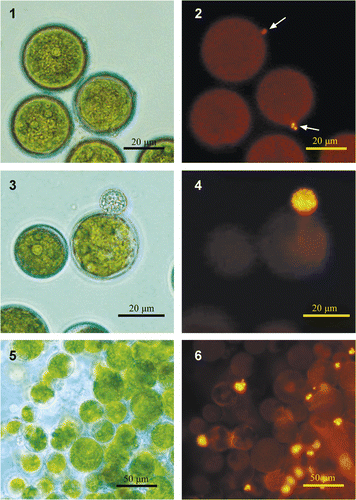
To visualize chytrid sporangia mycologists use Calcofluor white to stain chitin, the major constituent in the chytrids' cell walls (Kagami et al., Citation2004). However, this dye also stains the cell wall of H. pluvialis (Montsant et al., Citation2001), thus limiting the ability to differentiate between chytrid sporangia and the algal cells. We found that staining with the lipophilic dye Nile red (Greenspan et al., Citation1985) facilitates the efficient detection of both, very young and mature, chytrid sporangia of Paraphysoderma sedebokerensis (nom. prov.) (). Staining was evident as yellow/gold fluorescence (emission at >520 nm), under blue excitation (450–490 nm). This new, simple staining method does not require fixation and there is no need for prolonged incubation with the dye. In fact, the threshold of detection of the colourless sporangia, at up to 1% of infected cells without staining, is remarkably reduced upon Nile red staining, (). Furthermore the sensitivity of the technique allows detection of young sporangia even in dense algal cultures.
Figs 1–6. Infected Haematococcus pluvialis culture stained with Nile red. Images are either in phase contrast (1, 3 and 5), or under fluorescent light (2, 4 and 6). : Nile-red staining enables very young sporangium to be detected. : lipid globules inside the chytrid's sporangium. : Staining of infected culture allows for the algal cells carrying fungal sporangia to be counted. Arrows indicate the location of a chytrid sporangium, which is undetectable under phase contrast in dense culture.
Chytrid specificity towards different microalgae
Thirteen algal species, including representatives of the major/most common Chlorophyte genera, were tested for their susceptibility to infection by Paraphysoderma sedebokerensis (nom. prov.). The susceptibility test was conducted at 30°C, a temperature optimal for the growth of the chytrid, to facilitate epidemic development (Hoffman et al., Citation2008). During the 7 days of experiment the algae did not grow, but remained viable, unless they were infected by the chytrid. The kinetics of infection development in the algal species that were infected at their logarithmic stage is depicted in . Epidemics developed rapidly and intensively only in H. pluvialis and C. zofigiensis Donz, i.e. after 3–4 days almost all the cells were infected and their contents depleted. Three additional species–S. vacuolatus (SAG 211-8b); M. zofigiensis and S. oocystiformis–served as hosts for the pathogen during their logarithmic growth stages, although the infection developed at a slower rate and did not affect more than 20% of the algal cells, even after 7 days of exposure. The remaining eight species, including other species of Chlorella and Scenedesmus, were not infected at all (data not shown). After 7 days incubation, cultures of H. pluvialis and C. zofigiensis Donz collapsed and turned brown in colour. Since a 7-day incubation period was sufficiently to result in complete ‘collapse’ of H. pluvialis cultures the prevalence of infection in the other algal species after 7 days was used as measure for characterization of strain susceptibility ().
Fig. 7. Development of ‘epidemics’ in different algal species at their logarithmic stage, during the period of 7 days. Each point on the graph represents the percentage of algal cells observed carrying Paraphysoderma sedebokerensis (nom. prov.) sporangium. Haematococcus pluvialis Flotow 1844em.Wille SCCAP K-0084 (•), Chlorella zofigiensis Donz CCAP 211/14 (▪), Scenedesmus vacuolatus SAG 211-8b(○), Muriella zofigiensis SAG 4.80 (▴), Scotiellopsis oocystiformis SAG 277-1(♦).
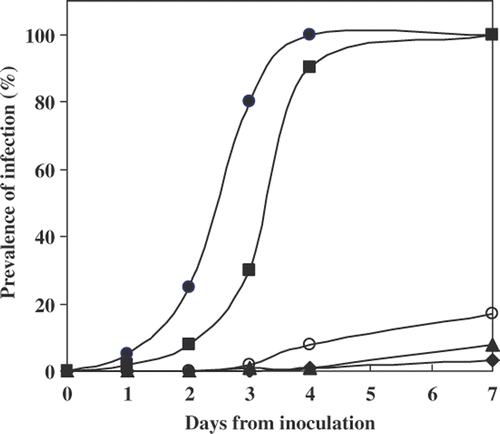
Table 1. A survey of the susceptibility of algal species to infection by P. sedebokerensis in vegetative and nitrogen-starved stages.
The specific attachment of zoospores onto a particular host, or group of algal species, indicates that specific signals are involved in the process (Kagami et al., Citation2007). To test whether these signals were preserved in algae after they were exposed to stress conditions, we performed an additional susceptibility test on the different species after they were exposed to stress conditions (N starvation). To maintain the different algae in the same physiological state, this susceptibility test was performed in the absence of nitrogen. However, nitrogen by itself has no effect on chytrid virulence, at least when tested in cultures of H. pluvialis (Hoffman et al., Citation2008). Excluding C. vulgaris, all species exposed to N depletion accumulated secondary carotenoids, including Chlorogonium elongatum, S. obliquus and M. braunii, all three of which have not previously been reported as secondary carotenoid producers (data not shown). However, of the N-starved species, only H. pluvialis was susceptible to chytrid infection (). The kinetics of the infection and epidemic development in cultures of this alga after nitrogen starvation were comparable to the effects observed in the green vegetative culture (3–4 days). In all the other species examined, even 7 days after inoculation with the chytrid, they were not infected by the chytrid and no change in their cell density was observed. These results indicate that H. pluvialis is the ‘best’ host for the chytrid as infection and development of the epidemic occurred fastest in the logarithmic and the N-starved H. pluvialis cultures (). The speed with which the epidemic developed in the cultures–within 3 days of inoculation–indicated that H. pluvialis was the most susceptible algal species tested.
Discussion
This study defines host specificity on the basis of epidemic development and for an alga to be defined as susceptible, a zoospore must be able not only to attach to, but also to penetrate, the algal cell wall. Furthermore, the zoospore must utilize the cellular constituents of the alga to grow and proliferate. This definition coincides with the earlier suggestion of Holfeld (Citation1998) that host specificity should be expressed during encystment of the zoospore rather than at the earlier stages of possible recognition.
The Nile-red staining protocol, as described in the present work, allows efficient and sensitive detection of the chytrid sporangia of H. pluvialis–Paraphysoderma sedebokerensis (nom. prov.). It might be also a useful tool for the detection of chytrids in algal culture, or in field, since these pathogens are often undetected by non-specialists, or confused with other organisms (Kagami et al., Citation2007). This is of particular interest in biotechnological applications, since in some cases early detection of the contaminant is crucial to the survival of the culture, especially when the contaminant develops epidemics rapidly. Although Nile red has previously been used to visualize chytrid zoospores (Kagami et al., Citation2004), the protocol described in this study, for staining chytrid sporangia on algae, has a particular advantage for phycologists that most frequently detect a contaminant on algae and need to identify it. Under the blue excitation, as reported in this work the neutral lipids, rather than polar lipid are stained (Greenspan, 1985), emitting a characteristic yellow–gold fluorescence clearly distinguishable from the red chlorophyll fluorescence of the algae (see ). Moreover, the staining does not require sample preparation or incubation time with the dye.
Of all the algae tested H. pluvialis provided the best conditions for chytrid adhesion and proliferation, as indicated by sporangia development. Haematococcus pluvialis is highly susceptible to the chytrid both at the logarithmic and cyst (nitrogen-starved) stages of the alga. In contrary, other susceptible species–C. zofigiensis Donz, S. vacuolatus SAG 211-8b, M. zofigiensis, and S. oocystiformis–were attacked by the chytrid only during logarithmic growth. Furthermore, the latter three species did not develop epidemics. On the basis of this observation we suggest that P. sedebokerensis (nom. prov.) is highly specific for H. pluvialis.
The narrow host range of the chytrid is highlighted when comparing the susceptibilities of different species of the same algal genus. For example, of the different Chlorella species, C. zofigiensis Donz was ‘recognised’ by the chytrid during the alga's logarithmic stage, while the other two species, C. emersonii and C. vulgaris, were not part of the chytrid host range. Among the species of Scenedesmus studied, only S. vacuolatus SAG 211-8b exhibited ongoing recognition, as the other two, S. vacuolatus SAG 211-15 and S. obliquus SAG 276-3a, were apparently not susceptible to infection by the pathogen. These observations are in agreement with those of Gromov (1999), who found that the sensitivity of Chlorococcalean algae to a given strain of chytrid was strain-specific, but not genus or even species specific. However, P. sedebokerensis (nom. prov.) appears to be genus-specific to the Haematococcus genus. Cultures of various strains were readily infected as described in a previous study: H. lacustris NIES-144, H. lacustris UTEX 16, H. pluvialis SAG 192.8, and H. lacustris CCAP 34/19 (Hoffman et al., Citation2008) and an additional 15 strains were susceptible to the pathogen in their palmelloid stage.
The chytrid parasite studied here is specific to the Haematococcus genus, both at the palmelloid and the cyst stages and rarely recognizes, or infects, other algal species. One possible explanation for the chytrid susceptibility of H. pluvialis, also during its cyst stage, is that the changes in the cell wall that H. pluvialis undergoes upon encystment are quantitative and not qualitative, such that the cell wall components exploited by the parasite–for recognition and adsorption–are not changed by the entrance of H. pluvialis into its cyst stage (Montsant et al., Citation2001).
As suggested by Coder & Goff (Citation1986), narrow host specificity may be related to cell surface properties. One of these properties was suggested to be the sporopollenin fraction found in the cell wall, but not as a single mean. The restriction Coder and Goff put on this component as a single means of protection correlates with our results. Likewise, species that do not contain algaenan (sporopollenin from an algal source), such as Chlorococcum sp. UTEX 819 (Gelin et al., Citation1997) and C. vulgaris SAG 211-8L (Burczyk et al., Citation1999), are apparently resistant to chytrid attack, unlike species that are reported to contain that biomacromolecule, i.e., H. pluvialis (Montsant et al., Citation2001) and S. vacuolatus SAG 211-8b (Derenne et al., Citation1992). The fact that H. pluvialis cell wall contains algaenan and still is penetrated/digested by the chytrid fungus is remarkable. Work is underway to clarify this unusual host range specificity of P. sedebokerensis (nom. prov.) to its prey the green alga H. pluvialis.
References
- Abeliovich , A and Dikbuck , S . 1977 . Factors affecting infection of Scenedesmus obliquus by a Chytridium sp in sewage oxidation ponds . Appl. Environ. Microbiol. , 34 : 832 – 836 .
- Barr , DJS and Hickman , CJ . 1967a . Chytrids and algae I. Host-substrate range and morphological variation of species of Rhizophydium . Can. J. Bot. , 45 : 423 – 430 .
- Barr , DJS and Hickman , CJ . 1967b . Chytrids and algae II. Factors influencing parasitism of Rhizophydium sphaerocarpum on Spirogyra . Can. J. Bot. , 45 : 431 – 440 .
- Blinn , DW and Button , KS . 1973 . Effect of temperature on parasitism of Pandorina-sp by Dangeardia mammillata Schröd in an Arizona mountain lake . J. Phycol. , 9 : 323 – 326 .
- Boussiba , S and Vonshak , A . 1991 . Astaxanthin accumulation in the green-alga Haematococcus pluvialis . Plant Cell Physiol. , 32 : 1077 – 1082 .
- Bruning , K . 1991 . Effects of phosphorus limitation on the epidemiology of a chytrid phytoplankton parasite . Freshwater Biol. , 25 : 409 – 17 .
- Bruning , K , Lingeman , R and Ringelberg , J . 1992 . Estimating the impact of fungal parasites on phytoplankton populations . Limnol. Oceanogr. , 37 : 252 – 260 .
- Burczyk , J , Smietana , B , Terminska-Pabis , K , Zych , M and Kowalowski , P . 1999 . Comparison of nitrogen content amino acid composition and glucosamine content of cell walls of various chlorococcalean algae . Phytochemistry , 51 : 491 – 497 .
- Canter , HM and Jaworski , GHM . 1982 . Some observations on the alga Fragilaria crotonensis Kitton and its parasitism by two chytridiaceous fungi . Am. Bot. , 49 : 429 – 446 .
- Canter-Lund , H and Lund , JWG . 1995 . Freshwater Algae: Their Microscopic World Explored , Bristol, , UK : Biopress .
- Coder , DM and Goff , LJ . 1986 . The host range of the chlorellavorous bacterium (Vampirovibrio chlorellavorus) . J. Phycol. , 22 : 543 – 546 .
- De Bruin , AR , Ibelings , BW , Kagami , M , Mooij , WM and Van Donk , E . 2008 . Adaptation of the fungal parasite Zygorhizidium planktonicum during 200 generations of growth on homogeneous and heterogeneous populations of its host, the diatom Asterionella formosa . Euk. Microbiol. , 55 : 69 – 74 .
- Derenne , S , Largeau , C , Berkaloff , C , Rousseau , B , Wilhelm , C and Hatcher , PG . 1992 . Nonhydrolyzable macromolecular constituents from outer walls of Chlorella fusca and Nanochlorum-eucaryotum . Phytochem. , 31 : 1923 – 1929 .
- Gelin , F , Volkman , JK , De Leeuw , JW and Damste , JSS . 1997 . Mid-chain hydroxy long-chain fatty acids in microalgae from the genus Nannochloropsis . Phytochem. , 45 : 641 – 646 .
- Gleason , F , Kagami , M , Lefevre , E and Sime-Ngandos , T . 2008 . The ecology of chytrids in aquatic ecosystems: Roles in food web dynamics . Fungal Biol. Rev. , 22 : 17 – 25 .
- Greenspan , P , Mayer , EP and Fowler , SD . 1985 . Nile red: a selective stain for intracellular lipid droplets . J. Cell Biol. , 100 : 965 – 73 .
- Gromov , B , Pljusch , A and Mamkayeva , K . 1999 . Cultures of Rhizophydium spp. (Chytridiales) parasites of chlorococalean algae . Algol. Stud. , 95 : 115 – 123 .
- Hoffman , Y , Aflalo , C , Zarka , A , Gutman , J , James , TY and Boussiba , S . 2008 . Isolation and characterization of a novel pathogenic chytrid species from the phylum Blastocladiomycota, parasitic on the green alga Haematococcus . Mycol. Res. , 112 : 70 – 81 .
- Holfeld , H . 1998 . Fungal infections of the phytoplankton: Seasonality, minimal host density, and specificity in a mesotrophic lake . New Phytol. , 138 : 507 – 517 .
- Holfeld , H . 2000 . Infection of the single-celled diatom Stephanodiscus alpinus by the chytrid Zygorhizidium: parasite distribution within host population, changes in host cell size, and host-parasite size relationship . Limnol. Oceangr. , 45 : 1440 – 1444 .
- Ibelings , BW , De Bruin , A , Kagami , M , Rijkeboer , M , Brehm , M and Van Donk , E . 2004 . Host parasite interactions between freshwater phytoplankton and chytrid fungi (Chytridiomycota) . J. Phycol. , 40 : 437 – 453 .
- James , TY , Letcher , PM , Longcore , JE , Mozley-Standridge , SE , Porter , D , Powell , MJ , Griffith , GW and Vilgalys , R . 2006 . A molecular phylogeny of the flagellated fungi (Chytridiomycota) and description of a new phylum (Blastocladiomycota) . Mycologia , 98 : 860 – 871 .
- Kagami , M , De Bruin , A , Ibelings , BW and Van Donk , E . 2007 . Parasitic chytrids: their effects on phytoplancton communities and food-web dynamics . Hydrobiologia. , 578 : 113 – 129 .
- Kagami , M , Van Donk , E , De Bruin , A , Rijkeboer , M and Ibelings , BW . 2004 . Daphnia can protect diatoms from fungal parasitism . Limnol. Oceanogr. , 49 : 680 – 685 .
- Kobayashi , M , Kakizono , T and Nagai , S . 1991 . Astaxanthin production by a green-alga, Haematococcus pluvialis accompanied with morphological-changes in acetate media . J. Ferment. Bioeng. , 71 : 335 – 339 .
- Lukavsky , J . 1970 . Phlyctidium scenedesmi, a chytrid destroying an outdoor mass culture of Scenedesmus obliqus . Nova Hedwig. , 19 : 775 – 777 .
- Montsant , A , Zarka , A and Boussiba , S . 2001 . Presence of a nonhydrolyzable biopolymer in the cell wall of vegetative cells and astaxanthin-rich cysts of Haematococcus pluvialis (chlorophyceae) . Mar. Biotechnol. , 3 : 515 – 521 .
- Olaizola , M . 2003 . Commercial development of microalgal biotechnology: From the test tube to the marketplace . Biomol. Eng. , 20 : 459 – 466 .