Abstract
Species composition and abundance of present rocky shore assemblages at Cap Corse (Corsica, North Western Mediterranean) dominated by the fucalean alga Cystoseira crinita are compared with similar data obtained almost 50 years ago. Fifteen sites at five different localities where dense C. crinita assemblages were present in the past were revisited in June 2007. Possible differences between the two sampling times were investigated, applying various multivariate analysis techniques available in the statistical package PRIMER v.6. Dense assemblages dominated by C. crinita were found at 14 out of the 15 surveyed sites, showing a similar structure and composition to the assemblages studied in the past. PERMANOVA indicated slight differences in species composition and abundances between old and new surveys. These differences can be summarized as a higher abundance of encrusting species (up to 3 times greater cover), and more sciaphilic turf-forming species (3 to 60-fold greater, depending on the species) and Cladophora spp. (20 times greater) in the present study than in the old study. Furthermore, the present assemblages showed a lower abundance of photophilic turf-forming species. These differences could be due to different taxonomic competence between observers, seasonal fluctuations or long-term changes. Assemblages dominated by Cystoseira crinita in Cap Corse were confirmed to be common, as they were found and sampled at 22 new sites around the island.
Introduction
Brown algae in the orders Laminariales and Fucales are the main ecosystem engineer species dominating rocky shallow areas in temperate seas all around the world (Ribera et al., Citation1992; Steneck et al., Citation2002). Seaweed beds dominated by these macroalgae are very productive and their three-dimensional structure supplies habitat and shelter for a large number of species (Feldmann, Citation1937; Giaccone, Citation1973; Mann, Citation1973; Dayton, Citation1985a ; Graham, Citation2004). Over the last few decades, assemblages dominated by kelps and fucoids have experienced a general decline related to urbanization (Airoldi & Beck, Citation2007; Connell et al., Citation2008) and overfishing, through the effect of trophic cascades (Estes & Duggins, Citation1995; Steneck, Citation1998) leading to habitat and ecosystem services loss (Dobson et al., Citation2006).
Cystoseira C. Agardh (Fucales) is the main genus of erect macroalgae functioning as ecosystem engineers in the Mediterranean Sea (Giaccone, Citation1973; Ballesteros, Citation1992). Cystoseira spp. assemblages harbour a large number of algal and invertebrate species (e.g. Ballesteros, Citation1990a , Citation1990b ; Ballesteros et al., Citation2009) and these assemblages appear in most of the infralittoral and upper circalittoral rocky bottoms in unimpacted areas (Giaccone, Citation1973). The genus Cystoseira is believed to have speciated in the ancient Tethys Sea and to have recolonized the Mediterranean after the Messinian crisis about 6 million years ago (Roberts, Citation1978; Amico et al., Citation1985). Currently, it is especially abundant and diversified in the Mediterranean Sea and on the adjacent Atlantic coasts (Roberts, Citation1978).
Floristic composition and structure of the assemblages dominated by Cystoseira species are fairly well known (e.g. Boudouresque, Citation1969, Citation1971, Citation1985; Giaccone, Citation1973; Ballesteros, Citation1992; Giaccone et al., Citation1994). Spatial trends of these assemblages have been studied at local (Feldmann, Citation1937; Ercegovic, Citation1952; Giaccone & Bruni, Citation1973) and regional scales (Báez et al., Citation2005). Temporal trends have been reported on a seasonal scale (Ballesteros, Citation1988, Citation1990a , Citation1990b ; Pizzuto, Citation1999). In addition, long-term studies have mainly reported disappearances (Munda, Citation1982, Citation1993; Cormaci & Furnari, Citation1999; Thibaut et al., Citation2005; Serio et al., Citation2006) but also recovery of Cystoseira species (Zavodnik et al., Citation2002) at a local scale. The loss of Cystoseira populations has often been attributed to eutrophication (Munda, Citation1974, Citation1993; Soltan et al., Citation2001; Arévalo et al., Citation2007) but other possible causes include overgrazing, climate change and invasive species (Arenas et al., Citation1995; Rico & Fernández, Citation1997; Thibaut et al., Citation2005; Serio et al., Citation2006). A very slow recovery of Cystoseira assemblages has been observed, probably due to its low dispersal ability (Díez et al., Citation2009). However, Zavodnik et al. (Citation2002) and Hanel (Citation2002) reported recolonization by many fucalean species in the Northern Adriatic, which was attributed to decreased herbivory pressure.
Cystoseira crinita Bory has been reported from most of the Mediterranean countries (Ribera et al., Citation1992). Assemblages dominated by this species are generally in the upper infralittoral zone of semi-exposed areas (Gómez-Garreta et al., Citation2000). Cystoseira crinita-dominated assemblages were originally described as an algal association (Cystoseiretum crinitae) by Molinier (Citation1960) at Cap Corse (northern Corsica). He considered them to be the most complex photophilic Mediterranean seaweed assemblage that develops in shallow rocky areas. However, most Mediterranean shallow water assemblages dominated by Cystoseira are devoid of C. crinita but support a similar species, Cystoseira brachycarpa var. balearica (e.g. Verlaque, Citation1987; Giaccone et al., Citation1994). Thus, concern about the identification of C. crinita by Molinier (Citation1960) arose (Verlaque, Citation1987).
The present work is aimed at determining possible changes in species composition and relative abundances of C. crinita assemblages at Cap Corse by a comparison of Molinier's data (obtained in 1958) and data obtained by the authors in 2007. The specific objectives are:
-
To check the presence and possible disappearances of C. crinita assemblages at Cap Corse and to obtain information about the distribution and state of these assemblages for the entire island of Corsica.
-
To describe possible alterations in the species composition of the assemblages from Cap Corse.
Materials and methods
Study area
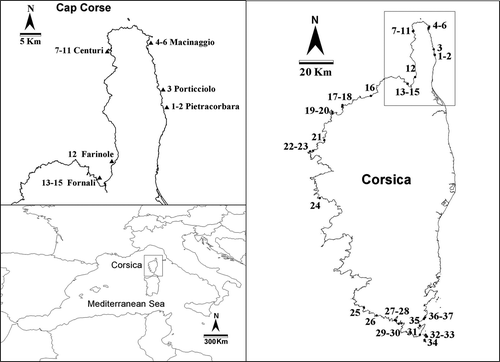
Corsica (France) is the fourth largest island in the Mediterranean Sea, situated in the NW Mediterranean close to the north of Italy and the south-east of France (). The island has 1000 km of coastline. Its northern and western coasts have extensive rocky shores with several coves, while on the eastern coast there are long sandy beaches and estuaries. Cap Corse, a prominent peninsula in the north of the island, has several coves and small bays suitable for the development of shallow water algal assemblages. Human influence is generally low in Cap Corse since only a few small villages and towns exist. Moreover, there is no industrial development and, although livestock is present, the topography of the zone does not allow extensive cultivation of land.
Sampling
Molinier's descriptions of site locations were carefully reviewed before our sampling was carried out. The 15 sites reported by Molinier (Citation1960) as having dense populations of C. crinita (assemblages attributed to the sub-association Cystoseiretum crinitae typicum) were revisited in June 2007. Moreover, 22 new study sites were visited around Corsica. At each site, the presence of such assemblages was checked for and, whenever present, they were sampled using Molinier's (Citation1960) procedure. A frame of 25 × 25 cm – which is greater than the minimal sampling area and is largely representative both of the species composition and the relative species abundances (Coppejans, Citation1980; Ballesteros, Citation1992) – was haphazardly placed within a dense C. crinita stand at each site, and a species list was created estimating algal species cover as follows: + (<1%), 1 (1–5%), 2 (5–25%), 3 (25–50%), 4 (50–75%), 5 (>75%) (Braun-Blanquet, Citation1951). Species that could not be identified in the field were collected, fixed in 4% formalin:seawater and subsequently identified in the laboratory.
Data analysis
A matrix of algal species abundances (data both from Molinier's samples and from 2007 samples) was constructed for each site. Scientific names of species listed by Molinier were updated according to Algaebase (Guiry & Guiry, Citation2008) and, as taxonomic resolution was higher in our lists, some species in some genera were merged (). Species from our lists with a total abundance lower than 1% and not recorded by Molinier were not considered for the analysis to avoid the presence of rare species whose abundance would not be properly quantified by the sampling areas used.
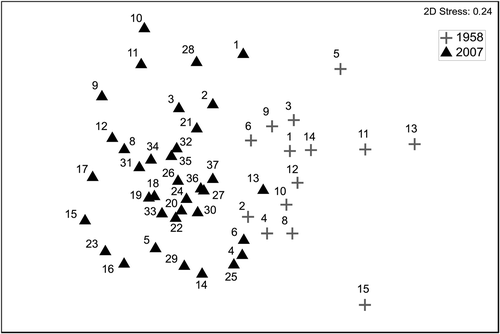
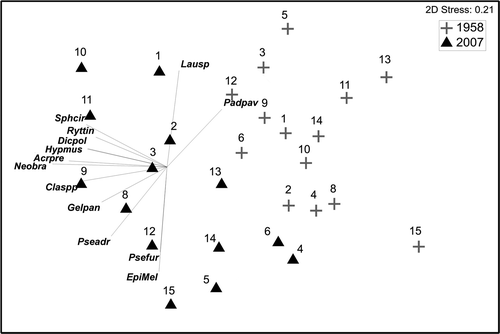
The combined transformation of Van der Maarel (Citation1979) was applied to the dataset in order to convert Braun–Blanquet indices to numerical data. Next, a distance matrix was created using Bray–Curtis distance (Bray & Curtis, Citation1957). Finally, a MDS ordination (Kruskal & Wish, Citation1978) was performed on the whole dataset and in a subset of the data from Cap Corse (using old and new data) to visualize multivariate patterns of distribution of the samples. Species which showed a Spearman correlation >0.55 were represented as overlaid vectors in the second MDS (data only from Cap Corse).
The sites from Cap Corse were grouped into five locations, as some of them were very close to one another (tens to 100 m). Three or four sites were present within each location, except at Farinole, where there was only one sampled site. A PERMANOVA (Anderson, Citation2001) with the factors time (1958, 2007) and location (Pietracorbara plus Porticciolo, Macinaggio, Centuri and Fornali; Farinole was excluded from the analysis) was applied to the data in order to test for differences in species composition and abundances between sampling times and among locations. Pairwise PERMANOVA tests were done for the factor time in each level of the factor location, as a significant interaction between the two tested factors was found. Monte Carlo P-values were calculated as the number of replicates per locality was small (3 or 4). In addition, SIMPER analysis was carried out in order to identify the species which contributed most to the differences between the samples from the two studies. All multivariate analyses were performed with PRIMER v.6 (Clarke & Gorley, Citation2006).
Results
Seaweed assemblages dominated by Cystoseira crinita were found at all the sites previously sampled by Molinier, except at Centuri harbour (Site 7, ‘Centuri: interieur du grand jetée’). Moreover, the presence of assemblages dominated by C. crinita was confirmed at 22 more sites around Corsica Island (see location of the sites in ).
A complete species list with abundances per site generated for the 15 sites from Cap Corse is presented in . A reduced list (the one used for the statistical analyses), with the species merged as mentioned in the previous section and showing the species average abundance per sampling time (1958 and 2007), is presented in . The cover of C. crinita was high in the current samples (mean 78%, ) and this species was sometimes mixed with other Cystoseira species like C. barbata C. Agardh, C. compressa (Esper) Gerloff & Nizamuddin and C. spinosa Sauvageau var. tenuior (Ercegovic) Cormaci et al. (see ). Although the average cover of C. crinita was generally higher in our samples than in Molinier's (78.6% vs. 67.9%, ), a similar structure with different layers (encrusting, turf, erect and epiphytes) appeared in both groups of samples. The encrusting layer was characterized by the coralline Neogoniolithon brassica-florida (Harvey) Setchell & Mason and the brown alga Pseudolithoderma adriaticum (Hauck) Verlaque. The most common species of turf-forming algae in both groups of samples were the green alga Dasycladus vermicularis (Scopoli) Krasse, the red algae Laurencia Lamouroux spp., and the brown algae Stypocaulon scoparium (Linnaeus) Kützing (formerly Halopteris scoparia) and Padina pavonica (Linnaeus) Thivy, as well as other species belonging to the Dictyotaceae. Finally, the epiphyte layer was characterized by the articulated coralline Haliptilon virgatum (Zanardini) Garbary & H.W. Johansen and Jania rubens (Linnaeus) Lamouroux, and the brown alga Sphacelaria cirrosa (Roth) C. Agardh ( and ).
Table 1. Complete species list for the 15 Cap Corse sites. Abundances of the species are on the Braun–Blanquet scale. a
Table 2. Reduced list of species used for the comparison between data from 1958 and 2007. The acronyms shown represent the species which are correlated with the ordination in . Results of SIMPER analysis are shown as cumulative per cent contribution to dissimilarity between 1958 and 2007.
Table 3. PERMANOVA results for species composition and abundances of C. crinita assemblages and factors time and location.
Several species recorded in 2007 and not in 1958 included members of the genus Ceramium Roth, Acrothamnion preissii (Sonder) Wollaston, Hypnea musciformis (Wulfen) Lamouroux, Rytiphlaea tinctoria (Clemente) C. Agardh, Chondracanthus acicularis (Roth) Fredericq, Parviphycus tenuissimus (Feldmann & Hamel) Santelices and Pseudochlorodesmis furcellata (Zanardini) Børgesen. A few species, like Erythrocystis montagnei (Derbès & Solier) Silva, Liagora viscida (Forskal) C. Agardh and Wrangelia penicillata C. Agardh, were found only in 1958 ( and ).
PERMANOVA showed a significant interaction between the factors time and location. Pair-wise tests for the factor time in each location showed significant differences between old and current assemblages only in Centuri, although the P-value for Pietracorbara was quite close to 0.05 ().
Patterns observed in both MDS ordinations segregated old and new data according to the species composition and abundances ( and ). According to the species overlain in the MDS on the Cap Corse data () and the SIMPER analysis () there were several species which differed in average abundance between historical and present samples. The encrusting algae Pseudolithoderma adriaticum and Neogoniolithon brassica-florida were much more abundant in 2007 than in 1958 (). Some photophilic algae, like Haliptilon virgatum, Padina pavonica, Laurencia spp. and Dasycladus vermicularis appeared to be more abundant in 1958 than in 2007 (). The opposite pattern was observed for Cladophora spp. and for some sciaphilic species such as Valonia utricularis (Roth) C. Agardh, Flabellia petiolata (Turra) Nizzamuddin, Peyssonnelia spp. and Dictyopteris polypodioides (De Candolle) Lamouroux ().
Discussion
The finding of Cystoseira crinita at the majority of the study sites as the most abundant Cystoseira species suggests that the identification of the species by Molinier (Citation1960) was correct. The distribution of C. crinita in the North Western Mediterranean is restricted to shallow and relatively sheltered areas (Ballesteros, Citation1992; Sales & Ballesteros, Citation2009). This is also true for Corsica, as we always found C. crinita in this kind of environment and we also observed that Cystoseira brachycarpa var. balearica replaced C. crinita in deeper waters. Thus, although we confirm the presence of C. crinita in the localities studied by Molinier, its presence was restricted to shallow (<1 m) sheltered areas and with a reduced surface coverage. In contrast, Molinier (Citation1960) suggested that C. crinita-dominated assemblages were representative of shallow rocky shores in the entire Mediterranean.
C. crinita-dominated assemblages were present in 14 of the 15 places that Molinier sampled in 1958 (with C. crinita cover >60%), as well as at 22 other sites around Corsica. The only study site where C. crinita had disappeared was Centuri harbour and, therefore, the decline of C. crinita in Cap Corse seems to be restricted to places where the natural habitat has been profoundly altered. These findings are in contrast with the decline of the species of the genus Cystoseira observed in other areas where anthropogenic disturbances are considered to be small (Cormaci & Furnari, Citation1999; Thibaut et al., Citation2005; Serio et al., Citation2006).
Slight changes in the species composition and abundances between the assemblages sampled in 1958 and in 2007 were detected by PERMANOVA. The only location where the differences were significant was Centuri, and a near-significant P-value was found for Pietracorbara. Although C. crinita-dominated assemblages had disappeared from the harbour of Centuri, outside the harbour the changes detected in the assemblages do not seem to correspond to human pressure as we did not find any species indicating pollution or stress.
Despite the small differences evidenced by PERMANOVA, several species differed in average abundance between the samples from 1958 and 2007, as shown by SIMPER analysis. Several explanations can be suggested for these differences. The first is the possible difference between observers in sorting accuracy, taxonomic competence or quantification estimates by visual cover. For example, there is no apparent reason for the higher values of cover by encrusting species in 2007 samples. The subjectivity in the estimation of visual per cent cover is high, as different observers can provide different values. However, comparisons between this methodology and other more accurate methodologies, like random-point quadrats, have demonstrated that visual estimation is a legitimate technique for estimating benthic organism cover, even by different observers (Meese & Tomich, Citation1992; Dethier et al., Citation1993). Differences in taxonomic competence between observers, as pointed out by Rindi & Guiry (Citation2004), could have resulted in some records of species only found in one of the studies. The causes could be misidentifications or different ability of observers to detect small species like Ceramium spp. (only found in 2007) or Erythrocystis montagnei (only found in 1958).
Secondly, seasonality could also explain divergences between 1958 and 2007, as it is very important in Mediterranean shallow benthic communities (Ballesteros, Citation1991, Citation1992). For example, the photophilic species Haliptilon virgatum, Padina pavonica and Laurencia spp., more abundant in 1958 than in 2007, usually have their highest abundance at the end of the summer. An opposite pattern is seen in several sciaphilic species, more abundant in 2007 than in 1958, which generally show maximum abundance in spring (Ballesteros, Citation1992; Pizzuto, Citation1999). Late spring was chosen for sampling in the present study, because it coincides with the maximum development of littoral communities (Ballesteros, Citation1992). No information on date of collection is provided by Molinier (Citation1960), but he could have carried out his surveys during summer and not in late spring. In that case, some of the observed differences could be due to seasonal changes.
Finally, the observed differences could also be related to long-term changes in community composition caused by natural disturbances, such as increased herbivory or storms. These disturbances can cause cyclic or occasional fluctuations, leading to states in which canopy algae completely dominate the seascape and to other states in which these algae partially or completely disappear. Such natural fluctuations have been described for kelp-dominated assemblages (Dayton, Citation1985a , Citation1985b ; Estes & Duggins, Citation1995) and may explain long-term changes in the presence and abundance of Fucales in western Ireland (Rindi & Guiry, Citation2004).
An unequivocal change between 1958 and 2007 is the appearance of the introduced filamentous red alga Acrothamnion preissii, which was reported for the first time in the Mediterranean by Cinelli & Sartoni (Citation1969). This alga is highly invasive on rhizomes of Posidonia oceanica (L.) Delile associated with a decrease in biodiversity of flora and fauna inhabiting these biogenic structures as epiphytes (Piazzi & Cinelli, Citation2000; Piazzi et al., Citation2002). However, although the species was found at seven out of the 14 locations surveyed, it was always very low in abundance.
In conclusion, C. crinita assemblages still remain in most of the places where they were present 50 years ago. This pattern is probably general for the whole island of Corsica, as we have observed similar habitats and assemblages at several sites around the island. In fact, some areas of Corsica are considered to be reference situations for the implementation of the European Water Framework Directive when using macroalgae as biological elements (Ballesteros et al., Citation2007; Pinedo et al., Citation2007), which is in agreement with the small changes in algal assemblages detected between 1958 and 2007.
Acknowledgements
Marta Sales was supported by a FI fellowship from the regional government of Catalunya (Generalitat de Catalunya), Spain, co-funded by the European Social Fund. Financial support was provided by Institut Menorquí d’Estudis, GRACCIE project C5D2007-0067 (Spanish Ministry of Science and Innovation) and Agència Catalana de l’Aigua. We thank colleagues from Massey University, Auckland, for revising the English text. We are also grateful to Susana Pinedo for her assistance with the statistical analyses. We wish to thank Pierre-Alain Mannoni for providing us with the Corsica coastline shape file for and Agries Canals for her assistance with producing figures.
References
- Airoldi , L and Beck , MW . 2007 . Loss, status and trends for coastal marine habitats of Europe . Oceanogr. Mar. Biol. Ann. Rev. , 45 : 345 – 405 .
- Amico , V , Giaccone , G , Colombo , P , Mannino , AM and Randazzo , R . 1985 . Un nuovo approccio allo studio della sistematica del genere Cystoseira C. Agardh (Phaeophyta, Fucales) . Boll. Acc. Gioenia Sci. Nat. , 18 : 887 – 985 .
- Anderson , MJ . 2001 . A new method for non-parametric multivariate analysis of variance . Aust. Ecol. , 26 : 32 – 46 .
- Arenas , F , Fernández , C , Rico , JM , Fernández , E and Haya , D . 1995 . Growth and reproductive strategies of Sargassum muticum (Yendo) Fensholt and Cystoseira nodicaulis (Whit.) Roberts . Sci. Mar. , 59 : 1 – 8 .
- Arévalo , R , Pinedo , S and Ballesteros , E . 2007 . Changes in the composition and structure of Mediterranean rocky-shore communities following a gradient of nutrient enrichment: descriptive study and test of proposed methods to assess water quality regarding macroalgae . Mar. Pollut. Bull. , 55 : 104 – 113 .
- Báez , JC , Olivero , J , Real , R , Vargas , JM and Flores-Moya , A . 2005 . Analysis of geographical variation in species richness within the genera Audouinella (Rhodophyta), Cystoseira (Phaeophyceae) and Cladophora (Chlorophyta) in the western Mediterranean . Bot. Mar. , 48 : 30 – 37 .
- Ballesteros , E . 1988 . Estructura y dinámica de la comunidad de Cystoseira mediterranea Sauvageau en el Mediterráneo Noroccidental . Invest. Pesq. , 52 : 313 – 334 .
- Ballesteros , E . 1990a . Structure and dynamics of the community of Cystoseira zosteroides (Turner) C. Agardh (Fucales, Phaeophyceae) in the Northwestern Mediterranean . Sci. Mar. , 54 : 217 – 229 .
- Ballesteros , E . 1990b . Structure and dynamics of the Cystoseira caespitosa Sauvageau (Fucales, Phaeophyceae) community in the North-Western Mediterranean . Sci. Mar. , 54 : 155 – 168 .
- Ballesteros , E . 1991 . Structure and dynamics of North-western Mediterranean marine communities: a conceptual model . Oecol. Aquat. , 10 : 223 – 242 .
- BALLESTEROS, E. (1992). Els vegetals i la zonació litoral: espècies, comunitats i factors que influeixen en la seva distribució. Arxius de la Secció de Ciències, 101. Institut d'Estudis Catalans, Barcelona
- Ballesteros , E , Garrabou , J , Hereu , B , Zabala , M , Cebrian , E and Sala , E . 2009 . Deep-water stands of Cystoseira zosteroides (Fucales, Phaeophyta) in the Northwestern Mediterranean: insights into assemblage structure and population dynamics . Estuar. Coast. Shelf Sci. , 82 : 477 – 484 .
- Ballesteros , E , Torras , X , Pinedo , S , Garcia , M , Mangialajo , L and De Torres , M . 2007 . A new methodology based on littoral community cartography for the implementation of the European Water Framework Directive . Mar. Pollut. Bull. , 55 : 172 – 180 .
- Boudouresque , CF . 1969 . Étude qualitative et quantitative d'un peuplement algal à Cystoseira mediterranea dans la région de Banyuls sur Mer . Vie Milieu , 20 : 437 – 452 .
- Boudouresque , CF . 1971 . Contribution à l'étude phytosociologique des peuplements algaux des côtes varoises . Vegetatio , 22 : 83 – 184 .
- Boudouresque , CF . 1985 . Groupes écologiques d'algues marines et phytocoenoses benthiques en Méditerranée Occidentale: une revue . Giorn. Bot. Ital. , 118 ( suppl. 2 ) : 7 – 42 .
- Braun-Blanquet , J . 1951 . Pflanzensociologie , Wien : Springer .
- Bray , JR and Curtis , JT . 1957 . An ordination of the upland forest communities of southern Wisconsin . Ecol. Monogr. , 27 : 325 – 349 .
- Cinelli , F and Sartoni , G . 1969 . Acrothamnion J. Ag. (Rhodophyta, Ceramiaceae): genere algale nuovo per il mare Mediterraneo . Pubbl. Staz. Zool. Napoli , 37 : 567 – 574 .
- CLARKE, K.R. & GORLEY, R.N. (2006). Primer v6: User Manual/Tutorial. Primer-E Ltd, UK
- Connell , SD , Russell , BD , Turner , DJ , Shepherd , SA , Kildea , T , Miller , D , Airoldi , L and Cheshire , A . 2008 . Recovering a lost baseline: missing kelp forests from a metropolitan coast . Mar. Ecol. Prog. Ser. , 360 : 63 – 72 .
- Coppejans , E . 1980 . “ Phytosociological studies on Mediterranean algal vegetation: rocky surfaces of the photophilic infralittoral zone ” . In The Shore Environment, Vol. 2: Ecosystems , Edited by: Price , JH , Irvine , DEG and Farnham , WF . 371 – 393 . London : Academic Press .
- Cormaci , M and Furnari , G . 1999 . Changes of the benthic algal flora of the Tremiti Islands (southern Adriatic), Italy . Hydrobiologia , 398–399 : 75 – 79 .
- Dayton , PK . 1985a . Ecology of kelp communities . Annu. Rev. Ecol. Syst. , 16 : 215 – 245 .
- Dayton , PK . 1985b . The structure and regulation of some South American kelp communities . Ecol. Monogr. , 55 : 447 – 468 .
- Dethier , MN , Graham , ES , Cohen , S and Tear , LM . 1993 . Visual versus random-point percent cover estimations: 'objective' is not always better . Mar. Ecol. Prog. Ser. , 96 : 93 – 100 .
- Díez , I , Santolaria , A , Secilla , A and Gorostiaga , JM . 2009 . Recovery stages over long-term monitoring of the intertidal vegetation in the ‘Abra de Bilbao’ area and on the adjacent coast (N. Spain) . Eur. J. Phycol. , 44 : 1 – 14 .
- Dobson , A , Lodge , D , Alder , J , Cumming , GS , Keymer , J , Mcglade , J , Mooney , H , Rusak , JA , Sala , O , Wolters , V , Wall , D , Winfree , R and Xenopoulos , MA . 2006 . Habitat loss, trophic collapse, and the decline of ecosystem services . Ecology , 87 : 1915 – 1924 .
- ERCEGOVIC, A. (1952). Jadranske Cistozire. Fauna i Flora Jadrana, Vol 2. Institut d'Océanographie et de Pêche, Split
- Estes , JA and Duggins , DO . 1995 . Sea otters and kelp forests in Alaska: generality and variation in a community ecological paradigm . Ecol. Monogr. , 65 : 75 – 100 .
- Feldmann , J . 1937 . Recherches sur la végétation marine de la Méditerranée: la côte des Albères . Rev. Algol. , 10 : 1 – 340 .
- Giaccone , G . 1973 . Écologie et chorologie des Cystoseira de Méditerranée . Rapp. Com. Int. mer Médit. , 22 : 49 – 50 .
- Giaccone , G , Alongi , G , Pizzuto , F and Cossu , A . 1994 . La vegetazione marina bentonica fotofila del Mediterraneo: II. Infralitorale e Circalitorale. Proposte di aggiornamento . Boll. Accad. Gioenia Sci. Nat. Catania , 27 : 1 – 47 .
- Giaccone , G and Bruni , A . 1973 . Le Cistoseire e la vegetazione sommersa del Mediterraneo . Atti. Ist. Veneto Sci., Lett. ed Arti , 131 : 59 – 103 .
- Gómez-Garreta , A , Barceló , MC , Gallardo , T , Pérez-Ruzafa , IM , Ribera , MA and Rull , J . 2000 . Flora Phycologica Iberica. Vol. 1: Fucales , Murcia : Universidad de Murcia, Servicio de Publicaciones, Murcia .
- Graham , MH . 2004 . Effects of local deforestation on the diversity and structure of southern California giant kelp forest food webs . Ecosystems , 7 : 341 – 357 .
- GUIRY, M.D. & GUIRY, G.M. (2008). AlgaeBase. World-wide electronic publication. National University of Ireland, Galway. www.algaebase.org (accessed 8 October 2009)
- Hanel , R . 2002 . Recovery of Fucacean associations and associated fish assemblages in the vicinity of Rovinj, Istrian Coast, northern Adriatic . Period. Biol. , 104 : 159 – 163 .
- Kruskal , JB and Wish , M . 1978 . Multidimensional Scaling , Sage Publications : Beverly Hills, CA .
- Mann , KH . 1973 . Seaweeds: their productivity and strategy for growth . Science , 182 : 975 – 981 .
- Meese , RJ and Tomich , PA . 1992 . Dots on the rocks: a comparison of percent cover estimation methods . J. Exp. Mar. Biol. Ecol. , 146 : 193 – 203 .
- Molinier , R . 1960 . Étude des biocenoses marines du Cap Corse . Vegetatio , 9 : 217 – 331 .
- Munda , I . 1974 . Changes and succession in the benthic algal associations of slightly polluted habitats . Rev. Intern. Oceanogr. Med. , 34 : 37 – 52 .
- Munda , I . 1982 . The effects of organic pollution on the distribution of fucoid algae from the Istrian coast (vicinity of Rovinj) . Acta Adriat. , 23 : 329 – 337 .
- Munda , I . 1993 . Changes and degradation of seaweed stands in the Northern Adriatic . Hydrobiologia , 260–261 : 239 – 253 .
- Piazzi , L , Balata , D and Cinelli , F . 2002 . Epiphytic macroalgal assemblages of Posidonia oceanica rhizomes in the western Mediterranean . Eur. J. Phycol. , 37 : 69 – 76 .
- Piazzi , L and Cinelli , F . 2000 . Effects of the spread of the introduced Rhodophyceae Acrothamnion preissii and Womersleyella setacea on the macroalgal community of Posidonia oceanica rhizomes in the western Mediterranean sea . Cryptogamie Algol. , 21 : 291 – 300 .
- Pinedo , S , Garcia , M , Satta , MP , De Torres , M and Ballesteros , E . 2007 . Rocky-shore communities as indicators of water quality: a case study in the Northwestern Mediterranean . Mar. Pollut. Bull. , 55 : 126 – 135 .
- Pizzuto , F . 1999 . On the structure, typology and periodism of a Cystoseira brachycarpa J. Agardh emend. Giaccone community and of a Cystoseira crinita Duby community from the eastern coast of Sicily (Mediterranean Sea) . Plant Biosyst. , 133 : 15 – 35 .
- Ribera , MA , Garreta , AG , Gallardo , T , Cormaci , M , Furnari , G and Giaccone , G . 1992 . Check-list of Mediterranean Seaweeds. I. Fucophyceae (Warming, 1884) . Bot. Mar. , 35 : 109 – 130 .
- Rico , JM and Fernández , C . 1997 . Ecology of Sargassum muticum on the North Coast of Spain II. Physiological differences between Sargassum muticum and Cystoseira nodicaulis . Bot. Mar. , 40 : 405 – 410 .
- Rindi , F and Guiry , MD . 2004 . A long-term comparison of the benthic algal flora of Clare Island, County Mayo, western Ireland . Biodiver. Conserv. , 13 : 471 – 492 .
- Roberts , M . 1978 . “ Active speciation in the taxonomy of the genus Cystoseira C. Agardh ” . In Modern Approaches to the Taxonomy of the Red and Brown Algae , Edited by: Irvine , DEG and Price , JH . 399 – 422 . London : Academic Press .
- Sales , M and Ballesteros , E . 2009 . Shallow Cystoseira (Fucales: Ochrophyta) assemblages thriving in sheltered areas from Menorca (NW Mediterranean): Relationships with environmental factors and anthropogenic pressures . Estuar. Coast. Shelf Sci. , 84 : 476 – 482 .
- Serio , D , Alongi , G , Catra , M , Cormaci , M and Furnari , G . 2006 . Changes in the benthic algal flora of Linosa Island (Straits of Sicily, Mediterranean Sea) . Bot. Mar. , 49 : 135 – 144 .
- Soltan , D , Verlaque , M , Boudouresque , CF and Francour , P . 2001 . Changes in macroalgal communities in the vicinity of a Mediterranean seawage outfall after the setting up of a treatment plant . Mar. Pollut. Bull. , 42 : 59 – 70 .
- Steneck , RS . 1998 . Human influences on coastal ecosystems: does overfishing create trophic cascades? . Trends Ecol. Evol. , 13 : 429 – 430 .
- Steneck , RS , Graham , MH , Bourque , BJ , Corbett , D , Erlandson , JM , Estes , JA and Tegner , MJ . 2002 . Kelp forest ecosystems: biodiversity, stability, resilience and future . Environ. Conserv. , 29 : 436 – 459 .
- Thibaut , T , Pinedo , S , Torras , X and Ballesteros , E . 2005 . Long-term decline of the populations of Fucales (Cystoseira spp. and Sargassum spp.) in the Albères coast (France, north-western Mediterranean) . Mar. Pollut. Bull. , 50 : 1472 – 1489 .
- Van der maarel , E . 1979 . Transformation of cover abundance values in phytosociology and its effects on community similarity . Vegetatio , 39 : 97 – 114 .
- VERLAQUE, M. (1987). Contribution à l'étude du phytobenthos d'un ecosystème photophile thermophile marin en Méditerranée Occidentale. Thése de Doctorat. Université d'Aix-Marseille, Marseille
- Zavodnik , N , Ivesa , L and Travizi , A . 2002 . Note on recolonisation by fucoid algae Cystoseira spp. and Fucus virsoides in the North Adriatic Sea . Acta Adriat. , 43 : 25 – 32 .