Abstract
Herdmania litoralis is a heterotrophic, sand-dwelling dinoflagellate with morphological characters that do not provide clear evidence for its systematic position in any existing family of dinoflagellates. Protoperidinium minutum is a heterotrophic, planktonic species that has a typical tabulation for the genus Protoperidinium. In order to infer the phylogenetic positions of these two species more confidently, we characterized the thecal plate patterns and determined small-subunit and large-subunit ribosomal DNA sequences (SSU rDNA and LSU rDNA, respectively) from both species. Intraindividual and intraspecific diversity of SSU and LSU rDNA data were characterized in H. litoralis using a combination of single-cell PCR approaches and analyses of PCR clones derived from multi-cell DNA extractions. The results of the molecular phylogenetic analyses demonstrated a novel, well-supported clade comprising both sand-dwelling species (H. litoralis and Thecadinium dragescoi) and planktonic species (P. minutum). Because the establishment of this clade also demonstrated that P. minutum is not a member of Protoperidinium, we reinstated and emended the genus Archaeperidinium Jörgensen Citation1912.
Introduction
Dinoflagellates comprise approximately 300 genera encompassing 2500 extant species of microscopic eukaryotes. They inhabit a wide variety of environments, including marine and freshwater plankton, marine sand, and the tissues of other eukaryotes (Hoppenrath et al., Citation2009a ). Many species of dinoflagellates form cellulosic thecal plates and are consequently called ‘thecate’ or ‘armoured’ (Fensome et al., Citation1993). Diversity in the arrangement of thecal plates, called tabulation patterns, has provided the basis for classification schemes based on comparative morphological data accumulated mainly from planktonic species (Fensome et al., Citation1993). The diversity and detailed morphology of benthic (sand-dwelling) dinoflagellates has only been investigated relatively recently, and the unusual thecal patterns found in these species have been difficult to categorize based on existing taxonomic criteria.
One such example is the genus Herdmania, which was erected by Dodge (Citation1981) with only a single species, Herdmania litoralis Dodge emend. Hoppenrath (Hoppenrath, Citation2000b ). This sand-dwelling species has a dorsoventrally flattened cell with a distinct, hook-like apical notch. Hoppenrath (Citation2000b ) examined this species by light and scanning electron microscopy (SEM) and established the plate formula: P 3′ 2a 6′′ ‘x’ 7c 3s 6′′′ 1p 1′′′′. Its cingulum is incomplete because of a large plate, the ‘x’ plate, between the right cingular end and the sulcus. Hoppenrath (Citation2000b ) suggested that this species should be placed in the order Peridiniales (rather than the Gonyaulacales) based on (1) a symmetrical apical pore complex, (2) the shape of the first apical plate, (3) possession of two anterior intercalary plates and (4) possession of only three sulcal plates. Nonetheless, its morphological characters and plate formula gave no clear evidence for its systematic position in any existing family of dinoflagellates.
By contrast, Protoperidinium minutum (Kofoid) Loeblich III is a marine planktonic species that has a typical tabulation for the genus Protoperidinium, with a low apical horn and no antapical extension. Ribeiro et al. (Citation2010) undertook the first molecular phylogenetic study of this species, collected from Portugal, using small-subunit and large-subunit ribosomal DNA sequences (SSU rDNA and LSU rDNA, respectively) and showed that P. minutum did not belong to either of the two well-supported clades of Protoperidinium (one of these comprises the majority of Protoperidinium species, while the other comprises Protoperidinium steidingerae Balech and species in the section Oceanica). The phylogenetic position of P. minutum was also different in the trees inferred from SSU rDNA and the trees inferred from LSU rDNA. Therefore, the phylogenetic position of P. minutum within dinoflagellates has hitherto been indeterminable.
In order to infer the phylogenetic position of H. litoralis, we determined its SSU rDNA and LSU rDNA sequences. We also determined SSU rDNA and LSU rDNA sequences from a Pacific morphotype of P. minutum and compared the morphological features (e.g. the plate tabulation patterns) of P. minutum with several members of the ‘P. minutum species-complex’, which are morphologically similar to P. minutum but possess characters that differ from the original description (Kofoid, Citation1907; Zonneveld & Dale, Citation1994; Hoppenrath et al., Citation2009a ; Ribeiro et al., Citation2010). All of these analyses enabled us to establish a new clade consisting of both benthic and planktonic species and propose revisions to the current classification system for thecate dinoflagellates.
Materials and methods
Sampling
Sand samples for Herdmania litoralis were collected during low tide at Centennial Beach, Boundary Bay, BC, Canada on 31 July 2007, 17 March 2009 and 2 and 17 September 2009. A melting seawater-ice method (Uhlig, Citation1964) was used with a 100 µm mesh-size filter to concentrate organisms from the sand. Samples containing Protoperidinium minutum were collected using a 40 µm pore-size plankton net (NXXX25, RIGOSHA, Saitama, Japan) in the inner part of Victoria Harbour, BC, Canada on 14, 16 and 21 March 2006.
Scanning electron microscopy (SEM)
Samples extracted from sand were fixed with Lugol's solution (final concentration 2%). Individual cells of H. litoralis were isolated and placed in a small container covered on one side with a 10 µm polycarbonate membrane filter (Corning Separations, Acton, MA, USA). They were then washed three times in filtered seawater to remove the fixative, dehydrated using graded concentrations of ethanol, and critical point dried with CO2 using a Tousimis Samdri 795 CPD (Rockville, MD, USA). The dried filters were subsequently mounted on aluminium stubs and sputter-coated with gold (5 nm thickness) using a Cressington high-resolution sputter coater (Cressington Scientific Instruments, Watford, UK). They were viewed with a Hitachi S4700 scanning electron microscope at an accelerating voltage of 3 kV. SEM images were manipulated using Adobe Photoshop Elements 8 (Adobe Systems, San Jose, CA), to present cells against a black background.
DNA extraction for H. litoralis
Extractions were performed twice, from 25 (31 July 2007) and 21 (17 September 2009) individual cells, respectively, which were isolated and washed three times in sterilized and membrane-filtered (0.2 µm pore-size) seawater. Genomic DNA was extracted according to the protocol in a Total Nucleic Acid Purification kit (Epicentre, Madison, WI, USA).
Light microscopical observations and isolation of cells for single-cell polymerase chain reaction (SC-PCR)
Single living cells of H. litoralis and P. minutum were isolated by micropipette under an inverted microscope, transferred to a glass slide with a vinyl tape frame (Horiguchi et al., Citation2000), and sealed with a cover glass. Cells of H. litoralis were examined with a Zeiss Axioplan 2 microscope equipped with differential interference contrast (DIC) and fluorescence optics, and connected to a Leica DC 500 colour digital camera. In order to assess thecal plate boundaries, the cells were stained with 0.1% Fluorescent Brightener 28 (Sigma, UK) and photographed using an excitation wavelength of 350 nm. After a photographic record had been made, each cell was rinsed three times by transferring it through a series of drops of filtered seawater. Finally, the cell was transferred to a 200 µL PCR tube containing 10 µL of Quick Extract™ FFPE DNA Extraction Solution (Epicentre, Madison, WI, USA) and incubated for 1 h at 56°C, then for 2 min at 98°C. The resulting extract was used as a DNA template for PCR amplification. Cells of P. minutum were observed and photographed using a Nikon Eclipse 80i microscope connected to a Nikon DS-5M colour digital camera. Immediately after photography, each cell was washed and broken with a sharp glass needle and transferred to a 200 µL PCR tube containing 10 µL of distilled water. Each PCR tube was incubated for 2 min in boiling water and subsequently stored at 4°C.
PCR amplification, cloning and sequencing
The initial polymerase chain reaction (PCR) of H. litoralis was performed using a total volume of 25 µL and a PuRe Taq Ready-To-Go PCR bead (GE Healthcare Bio-Sciences, Québec, Canada). Nearly the entire SSU rRNA gene and part of the LSU rRNA gene were amplified using universal eukaryote primers (NPF1: 5′-TGCGCTACCTGGTTGATCC-3′ and R4: 5′-GATCCTTCTGCAGGTTCACCTAC-3′ for SSU rDNA; LSU D1R: 5′-ACCCGCTGAATTTAAGCATA-3′ and LSU R2: 5′-ATTCGGCAGGTGAGTTGTTAC-3′ for LSU rDNA). The PCR protocol had an initial denaturation stage at 94°C for 1 min; 35 cycles of denaturation at 94°C for 30 s, annealing at 53°C for 30 s, and extension at 72°C for 30 s; and final extension at 72°C for 7 min. Amplified DNA fragments corresponding to the expected size were separated by agarose gel electrophoresis and cleaned using the UltraClean™ 15 DNA Purification Kit (Mo Bio Laboratories, CA, USA). The cleaned DNA was cloned using the TOPO TA Cloning Kit (Invitrogen, CA, USA). Plasmids of the correct insert size were sequenced using BigDye 3.1 and the vector forward and reverse primers, and an internal primer (525F: 5′-AAGTCTGGTGCCAGCAGCC-3′), with an Applied Biosystems 3730S 48-capillary sequencer. We also performed direct sequencing for the single-cells collected on 17 March 2009 and the extracted DNA from the 21 cells collected on 17 September 2009. The first PCR products of SSU rDNA (primers; NPF1 and R4) were used as a DNA template for a second semi-nested round of PCR, where the following combinations of primer pairs were used separately: NPF1 and 1050MRD (5′-GCCTYGCGACCATACTCC-3′), 525F and R4. Amplified DNA fragments were separated and cleaned using the same procedure mentioned above. The results were confirmed by sequencing both forward and reverse strands. The primers used for PCR amplification and direct sequencing of the SSU and LSU rDNA from P. minutum were those listed in Yamaguchi et al. (Citation2006), and the PCR and sequencing protocols were as described by Takano & Horiguchi (Citation2004). New sequences have been deposited in DDBJ/EMBL/GenBank under the accession numbers AB564298–AB564310 in Table S1 (see supplementary material, which is available on the supplementary context tab of the article's online page at http://dx.doi.org/10.1080/09670262.2011.564517).
Sequence alignments and phylogenetic analyses
SSU rDNA sequences were aligned with ClustalW (Higgins et al., Citation1994) using the MEGA (Molecular Evolutionary Genetics Analysis) program version 4 (Tamura et al., Citation2007) and manually refined in MacClade (Maddison & Maddison, Citation2000). The final alignment consisted of 70 taxa and contained 1636 unambiguously aligned characters. Perkinsus marinus was used as the outgroup species. LSU rDNA sequences were aligned manually based on the datasets of Yamaguchi et al. (Citation2006) and the apicomplexan Neospora caninum was used as outgroup. The final alignment consisted of 49 taxa and contained 871 unambiguously aligned characters. The GenBank accession numbers for all species used in the analyses are listed in Table S1 (see supplementary material, available online). The alignments are available from the authors upon request.
Phylogenetic trees were constructed using maximum likelihood (ML) and Bayesian analysis. For ML, the alignments were analysed at a distant server (http://8ball.sdsc.edu:8889/cipres-web/Home.do) running the program RAxML, version 7.0.4 or 7.2.6 (Stamatakis, Citation2006; Stamatakis et al., Citation2008). The search for the optimal model, using hierarchical likelihood-ratio tests as implemented in the software jModeltest 0.1.1 (Posada, Citation2008), indicated that GTR+I+G and GTR+G model were the best-fit substitution models for the SSU rDNA and LSU rDNA datasets, respectively. For the SSU rDNA dataset, the 100 RAxML rapid bootstrap inferences were executed with RAxML 7.2.6 using a GTRCATI substitution model and thereafter a thorough ML search was performed and the final tree was evaluated and optimized under GAMMA+P-Invar Model parameters. For the LSU rDNA dataset, the 500 RAxML rapid bootstrapping and subsequent ML search were performed with RAxML version 7.0.4, using GTRGAMMA substitution model.
MrBayes version 3.1.2 was used to perform Bayesian analyses on both alignments (Huelsenbeck & Ronquist, Citation2001; Ronquist & Huelsenbeck, Citation2003). The program was set to operate with a gamma distribution and four Monte-Carlo–Markov chains (MCMC) starting from a random tree. A total of 2 000 000 generations were calculated with trees sampled every 50 generations and with a prior burn-in of 100 000 generations (2000 sampled trees were discarded). A majority rule consensus tree was constructed from 38 000 post-burn-in trees. Posterior probabilities (PP) correspond to the frequency at which a given node was found in the post-burn-in trees. Independent Bayesian runs on each alignment yielded the same results.
Results
Morphology of Herdmania litoralis
Cells of H. litoralis from Centennial Beach, Canada, were flattened dorsoventrally and rounded in ventral view, with a distinct apical notch pointing to the left lateral side of the cell (). Cells were 14.3–29.5 µm ( x ± SD = 17.7 ± 3.5 µm, N = 16) in width, with an approximately equal length : width ratio. No chloroplast, eyespot or recognizable food particles were observed. The cytoplasm was colourless and numerous globules were present (). A large pusule was located in the hypocone and a second smaller pusule was in the middle of the left cell half (). In SEM, no striations were observed on the boundaries between plates and the thecal plates were smooth with scattered round pores ().
Figs 1–5. Single cell of Herdmania litoralis used for SSU and LSU rDNA sequencing, DIC optics (1, 2) and fluorescence microscopy (3–5). 1, 2. Two focuses showing the incomplete cingulum, apical hook-like notch (arrow), globules (arrowheads) and two pusules (p). 3. Ventral view, showing the hexagonal ‘x’ plate (or S.a. plate). 4. Ventral view, showing the four-sided 6′′′ plate (or S.d. plate). 5. Dorsal view. Labels: 4′′ and 5′′, precingular plates; 3a, third anterior intercalary plate; 5′′′ and 6′′′, postcingular plates. Scale bars: 10 µm.
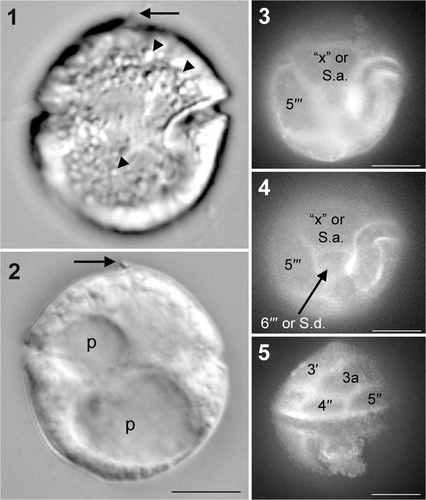
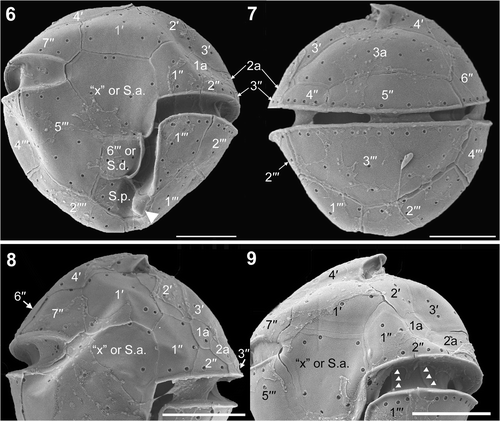
The first apical plate (1′) was pentagonal (). The anterior part of fourth apical plate (4′) formed an apical notch (). There are three anterior intercalary plates (). The third anterior intercalary (3a) plate is large and six-sided (, ). The fifth precingular plate (5′′) was longer than the fourth (4′′) (). The sixth precingular plate (6′′) borders 4′, 3a, 5′′ and the seventh precingular plate (7′′) (). The ventral view showed a slightly ascending cingulum, incompletely encircling the cell (, ). Only the left end of the cingulum is in contact with the sulcus (). The large plate between the right cingular end and the sulcus is called the ‘x’ plate (, ) and formed a narrow list at its sulcal border (). SEM indicated that the cingulum comprised five plates (, 9–11). However, the cingulum plate line positioned under plate 7′′ was noticeable in only one SEM micrograph and we could not completely confirm the tabulation of the sulcus. Therefore, we represent this plate line with a dotted line in . The third postcingular plate (3′′′) was large and covered the main part of the dorsal hypotheca (). The sixth postcingular plate (6′′′) was four-sided and its left side had a smooth list bordering the sulcus (). A spine-like posterior ventral list was part of the posterior sulcal plate (S.p.) (). Different plate interpretations are possible (). The ‘x’ plate could also be interpreted as an anterior sulcal plate (S.a.) and 6′′′ as the right sulcal plate (S.d.) (, , ); if so, the cingulum would make contact with the sulcus at both sides of the cell (, , ).
Figs 6–9. Herdmania litoralis, SEM. 6. Ventral view showing a spine-like posterior ventral list (arrowhead). 7. Dorsal view. 8. Ventral view of epitheca. 9. Ventral view of epitheca, showing the plate boundaries of the cingulum (arrowheads). Labels: 1′ to 4′, apical plates; 1′′ to 7′′, precingular plates; 1a to 3a, anterior intercalary plates; 1′′′ to 6′′′, postcingular plates; ‘x’, the ‘x’ plate; S.a., the anterior sulcal plate; S.d., the right sulcal plate; S.p., the posterior sulcal plate. Scale bars: 5 µm.
Figs 10, 11. Herdmania litoralis: plate pattern. 10. Ventral view; note the spine-like posterior ventral list (arrowhead). 11. Dorsal view. Labels: 1′ to 4′, apical plates; 1′′ to 7′′, precingular plates; 1a to 3a, anterior intercalary plates; 1′′′ to 6′′′, postcingular plates; 1′′′′ and 2′′′′, antapical plates; ‘x’, ‘x’ plate; S.a., anterior sulcal plate; S.d., right sulcal plate; S.p., posterior sulcal plate; S.m., median sulcal plate.
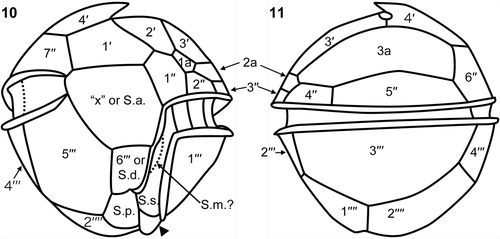
Table 1. Possible plate interpretations (plate formulas) of selected dinoflagellates.
Morphology of Protoperidinium minutum
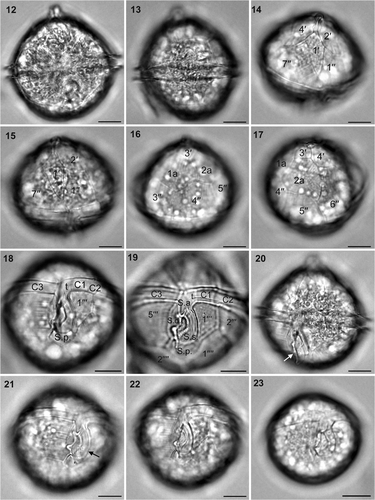
Most cells from Victoria, Canada, measured 40.0–46.7 µm in diameter, but P. minutum #5 was smaller and measured 32.2 µm in diameter. The cells were spherical in ventral view with a conspicuous apical horn and no antapical extension (). Plate 1′ was rhombic (ortho-type) and asymmetrical and did not connect with the cingulum (). The anterior part of the sulcal anterior plate (S.a.) was narrow, extended between plates 1′′ and 7′′, and was pointed (). The two anterior intercalary plates were hexagonal and subequal (). The cingulum was equatorial with no displacement (). The right sulcal plate (S.d.) connected with the cingulum plate () and had a well-developed fin that covered the sulcal area (). The left sulcal plate (S.s.) was long and formed a J-shaped curve. The anterior part of this plate connected with S.a. and the transitional plate (t) (). The posterior sulcal plate (S.p.) was pentagonal and almost symmetrical (). Protoperidinium minutum cells #1–#4 had broad intercalary bands (), but P. minutum #5 did not (). The thecal surface contained large pores that were sparsely distributed ().
Figs 12–23. Archaeperidinium (Protoperidinium) minutum, bright field optics. Specimens #1 and #5 were used for SSU and LSU rDNA sequencing. 12. Ventral view of specimen #3. 13. Left view of specimen #4. 14. Ventral epitheca of specimen #1. 15. Ventral epitheca of specimen #3. 16. Dorsal epitheca of specimen #1. 17. Right dorsal epitheca of specimen #1. 18. Ventral hypotheca of specimen #1. 19. Ventral (empty) hypotheca of specimen #2. 20. Left ventral view of specimen #3, showing a flagellar fin (arrow). 21. Right ventral view of the hypotheca of specimen #3, showing flagellar fin (arrow). 22. Ventral view of the hypotheca of specimen #3, showing sparsely distributed large pores on the thecal surface. 23. Ventral view of specimen #5. Labels: 1′ to 4′, apical plates; 1′′ to 7′′, precingular plates; 1a and 2a, anterior intercalary plates; C1 to C3, cingular plates; S.a., anterior sulcal plate; S.d., right sulcal plate; S.s., left sulcal plate; S.p., posterior sulcal plate; 1′′′ to 5′′′, postcingular plates; 1′′′′ and 2′′′′, antapical plates. Scale bars: 10 µm.
Intraspecific and intraindividual variability in small and large subunit ribosomal DNA sequences of Herdmania litoralis
We obtained eight different SSU rDNA sequences and two different LSU rDNA sequences from H. litoralis. Three of the SSU rDNA sequences were cloned PCR products generated from a single cell (, H. litoralis SC clone-1 to clone-3), and one was directly sequenced from a PCR product (, H. litoralis SC direct) acquired from a single cell. Out of a total of 1724 base pairs (bp), these four sequences differed by 0.1–0.9%. The remaining four SSU rDNA sequences were derived from two different multi-cell extractions of genomic DNA: the 21-cell extraction in September 2009 and the 25-cell extraction in July 2007. The three sequences derived from the 2009 extraction (, H. litoralis 2009 clone-1 and clone-2 and H. litoralis 2009 direct) differed by 0.1–0.9% from each other. The sequence derived from the July 2007 extraction showed the largest divergence from other H. litoralis sequences, differing from them by 2.1–2.7%.
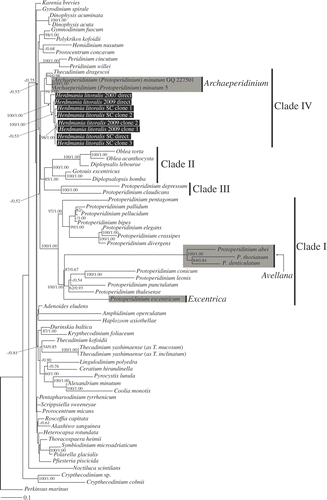
Fig. 24. Maximum-likelihood tree inferred from SSU rDNA sequences. RAxML bootstrap values (ML) over 50 and Bayesian posterior probabilities (PP) over 0.50 are shown at the nodes (ML/PP). Clades I–IV are labelled and marked with vertical lines on the right. The species highlighted by grey boxes are those hitherto assigned to Protoperidinium subgenus Archaeperidinium. The scale bar represents inferred evolutionary distance in changes site–1. DNA sequences generated in this study are indicated in black boxes.
We also obtained two LSU rDNA sequences, one cloned sequence from a single-cell of H. litoralis collected in March 2009 (, H. litoralis SC clone, 1204 bp) and one cloned sequence derived from genomic DNA extracted from 21 cells of H. litoralis collected in 17 September 2009 (, H. litoralis clone, 1149 bp). Out of the total of 1149 matched bp, these two sequences differed by 4.3%.
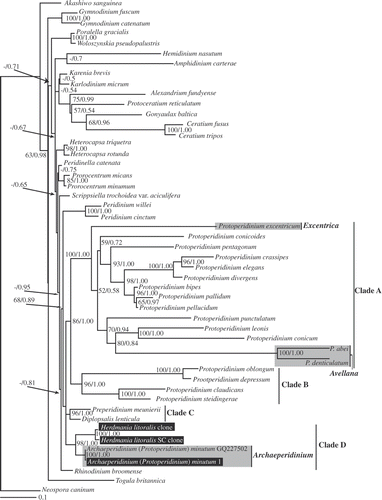
Fig. 25. Maximum-likelihood tree inferred from LSU rDNA sequences. RAxML bootstrap values (ML) over 50 and Bayesian posterior probabilities (PP) over 0.50 are shown at the nodes (ML/PP). Clades A–D are labelled and marked with vertical lines on the right. The species highlighted by grey boxes are those hithero assigned to Protoperidiniu subgenus Archaeperidinium. The scale bar represents inferred evolutionary distance in changes site–1. DNA sequences generated in this study are indicated in black boxes.
SSU and LSU rDNA sequences of Protoperidinium minutum
Five individual cells of P. minutum from Victoria, Canada were observed, photographed and isolated for DNA extraction and SC-PCR (P. minutum #1–#5, ). We generated two SSU rDNA sequences, each from a different cell (P. minutum #1, 1606 bp; P. minutum #5, 1685 bp), and one LSU rDNA sequence (P. minutum #1, 1002 bp). Out of a total of 1563 bp, the SSU rDNA sequences of P. minutum #1 and #5 differed by only 1 bp.
Phylogenetic analysis
SSU rDNA. Herdmania litoralis, Thecadinium dragescoi and Protoperidinium minutum formed a robust clade with 99% ML bootstrap and 1.00 Bayesian PP. Within this clade, P. minutum and T. dragescoi clustered with 94% ML bootstrap and 0.98 Bayesian PP. The species within the Protoperidiniaceae fell into four well-supported clades (). Three out of the four clades were concordant with Clades I–III named by Ribeiro et al. (Citation2010). Clade I included only Protoperidinium species, namely the sect. Avellana (P. abei, P. denticulatum and P. thorianum), sect. Conica (P. conicum, P. leonis, P. pentagonum, P. punctulatum and P. thulesense), sect. Divergentia (P. crassipes, P. divergens and P. elegans), sect. Excentrica (P. excentricum) and sect. Protoperidinium (P. bipes, P. pallidum and P. pellucidum). Clade II included five species of the Diplopsaloideae (Diplopsalis lebourae, Diplopsalopsis bomba, Gotoius excentricus, Oblea acanthocysta and O. torta). Clade III comprised species within Protoperidinium sect. Oceanica (P. claudicans and P. depressum). In this study, we refer to the clade comprising H. litoralis, T. dragescoi and P. minutum as ‘Clade IV’.
Clades II and III were sister groups with 85% ML bootstrap and 1.00 Bayesian PP. Thecadinium kofoidii, T. yashimaense (as T. inclinatum) and T. yashimaense (as T. mucosum) formed a clade with 54% ML bootstrap and 0.85 Bayesian PP and were separated from T. dragescoi. The deep branches in the tree were short and without statistical support from either ML bootstrapping (<50%) or Bayesian PP (<0.5).
LSU rDNA. The sequences from H. litoralis and P. minutum clustered together and formed a strongly supported clade (98% ML bootstrap and 1.00 Bayesian PP). As in trees inferred from SSU rDNA, species within the Protoperidiniaceae formed four well-supported clades (). Clade A comprised the same sections as clade I in the SSU analyses (), and Clade B comprised the section Oceanica plus P. steidingerae (the clade naming scheme conforms to Ribeiro et al., Citation2010). Two diplopsalid species (Diplopsalis lenticula and Preperidinium meunieri) formed a separate clade, referred to here as Clade C, while H. litoralis and Protoperidinium minutum formed a clade referred to here as Clade D. Clades A, B, C and D together comprised a more inclusive clade, albeit with low statistical support. A clade comprising Peridinium willei and P. cinctum was positioned sister to the more inclusive clade consisting of Protoperidinium Clades A–D, but with only 68% ML bootstrap support and 0.89 Bayesian posterior probability.
Discussion
Morphological variability of Herdmania litoralis
The tabulation of the Canadian specimens differed in some aspects from that of German cells described by Hoppenrath (Citation2000b ). The general morphology of both isolates was very similar, however, and assignment to the genus Herdmania was unambiguous. The Canadian specimens had an additional apical plate, an additional anterior intercalary plate (1a), and an additional precingular plate (2′′). When considering the apical plate series, it is possible that the somewhat cryptic suture in the Canadian specimens was unnoticed in the German specimens. The plates labelled 1′′′′ and S.p. by Hoppenrath (Citation2000b ) are interpreted in the present study as S.p. and S.s., respectively. Then the plate 1′′′′ interpreted by Hoppenrath (Citation2000b ) would actually consist of two plates, which would automatically change the plate interpretation to the one given in the present study, consisting of a new S.p. and two antapical plates instead of one posterior intercalary and one antapical plate. Because of these uncertainties about the completeness of the plate observations of the German material and our limited knowledge about the plate variability of the epitheca of H. litoralis, we are hesitant to describe the Canadian isolates as a new species based on two additional plates (1a and 2′′) alone. Moreover, plate variability in benthic dinoflagellates has been described previously in other taxa (e.g. Hoppenrath, Citation2000c ). A re-investigation of the tabulation pattern and molecular phylogenetic data from the German specimens of H. litoralis is needed.
Identification of Protoperidinium minutum from Canada
We identified cells collected from Victoria, Canada as P. minutum. However, the shape of the anterior sulcal plate (S.a.) in our isolates differed from the original description (Kofoid, Citation1907: his illustrations are redrawn as our ). In our material, the anterior part of the S.a. is extended narrowly between plates 1′′ and 7′′ (, ); by contrast, Kofoid (Citation1907) described cells in which S.a. lacks an extension and the 1′ plate touches the cingulum ().
Figs 26–41. Illustrations of the thecal plate patterns in the Archaeperidinium (Protoperidinium) minutum complex. 26–29. Peridinium minutum, redrawn from Kofoid (Citation1907). 30. Archaeperidinium (Protoperidinium) minutum, this study. 31, 32. Peridinium constricta, redrawn from Abé (Citation1936). 33. Peridinium monospinum, redrawn from Paulsen (Citation1907). 34, 35. Protoperidinium monospinum, redrawn from Zonneveld & Dale (Citation1994). 36. Protoperidinium aspidiotum, redrawn from Balech (Citation1964a ). 37. Peridinium aspinum, redrawn from Meunier (Citation1919). 38. Peridinium monospinum, redrawn from Lebour (Citation1925). 39. Protoperidinium minutum, redrawn from Fukuyo et al. (Citation1977). 40, 41. Protoperidinium minutum, redrawn from Balech (Citation1964b ). Labels: 1′ to 4′, apical plates; 1′′ to 7′′, precingular plates; 1a and 2a, anterior intercalary plates; S.a., anterior sulcal plate; S.d., right sulcal plate.
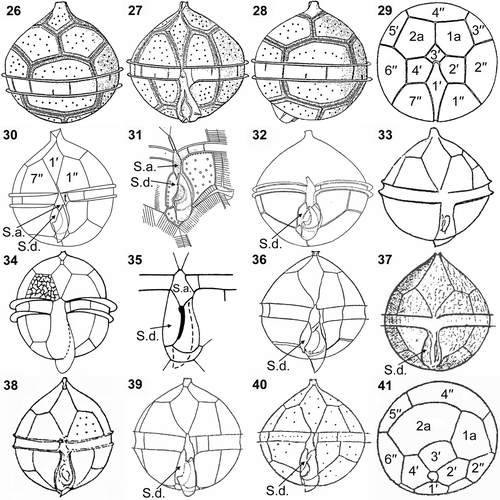
The shape of S.a. in our specimens was similar to that in an illustration of Peridinium constricta by Abé (Citation1936; our ). However, Abé (Citation1936) also illustrated the specimen as P. constricta, with an S.a. that only barely extended between 1′′ and 7′′ (). The description indicated the size of P. constricta to be considerably larger (transverse diameter 60–67 µm) than our cells.
Peridinium constricta was later interpreted to be a junior synonym of P. monospinum Paulsen by Abé (Citation1981). Peridinium monospinum (Paulsen, Citation1907; our ) has a very similar morphology to Protoperidinium minutum () but, although Peridinium monospinum is larger (48–56 µm in diameter), subsequent researchers have treated it as a synonym of P. minutum (Schiller, Citation1937; Balech, Citation1974; Dodge, Citation1982); a new combination, Protoperidinium monospinum, was proposed by Zonneveld & Dale (Citation1994; our ), with an emended description based on a distinctive cyst and theca morphology. Protoperidinium monospinum differs from P. minutum in having a finely reticulate cell wall ().
The right sulcal plates (S.d.) of our cells are in contact with the cingulum (, ), which is similar to P. minutum as illustrated by Ribeiro et al. (Citation2010) and P. monospinum as illustrated by Lebour (Citation1925; our ) and Zonneveld & Dale (Citation1994; our ), and also to P. aspidiotum (Balech, Citation1964a ; our ) and P. aspinum (Meunier, Citation1919; our ). The S.d. of the P. minutum material described by Fukuyo et al. (Citation1977; our ) is also in contact with the cingulum but it exceeds the anterior line of the cingulum in ventral view. By contrast, the S.d. of P. minutum shown by Abé (Citation1936; our ), Balech (Citation1964b ; our ) and Okolodkov (Citation2005), and also the S.d. of P. constricta, tapers towards a point before reaching the posterior margin of the sulcus. As in Kofoid's illustration (), the two anterior intercalary plates observed in our specimens were almost equal in size (). Balech (Citation1964b ) described P. minutum with a second anterior intercalary plate (2a) that was larger than the first anterior intercalary plate (1a) ().
The SSU and LSU rDNA sequences of our Canadian isolates were closely related to the sequences of P. minutum published by Ribeiro et al. (Citation2010). Their specimens were smaller (27–37 µm in diameter) than ours (40.0–46.7 µm in diameter, but one cell measured 32.2 µm in diameter). The S.a. of the cells described by Ribeiro et al. (Citation2010) extended shortly between plates 1′′ and 7′′, and the transitional plate was hourglass-shaped. The transitional plates of our specimens were broader and four-sided (, ). Otherwise, with respect to cell shape, and to the character of the S.d., the cingulum and the two symmetrical anterior intercalary plates, our isolates were similar to those described by Ribeiro et al. (Citation2010).
In summary, P. minutum, P. constricta, P. monospinum of Paulsen (Citation1907) and P. aspinum are all very similar, with only slight morphological differences between them. In agreement with previous interpretations treating these species as synonyms of P. minutum (Schiller, Citation1937; Abé, Citation1981), we interpret the differences as intraspecific variability within P. minutum rather than indications of cryptic species. However, molecular phylogenetic investigations of many more different variants of P. minutum are required to confirm species boundaries in this part of the dinoflagellate tree.
Intraspecific and intraindividual variability in small and large subunit ribosomal DNA sequences of Herdmania litoralis
Intraspecific variation of SSU and LSU rDNA sequences and intraindividual variability of SSU rDNA were found in H. litoralis, but monophyly of all H. litoralis sequences was strongly supported (, ). Intraspecific variability in nuclear ribosomal genes has been found in a wide range of eukaryotes, including several other species of dinoflagellates (e.g. Gribble & Anderson, Citation2007; Handy et al., Citation2009). Gribble & Anderson (Citation2007) detected not only intraspecific variability but also intraindividual variability in the D1–D6 region of LSU rDNA sequences in eight different species within the Protoperidiniaceae from cultures and environmental samples. Differences among sequences included single base-pair substitutions, single base-pair indels and also larger indels. The differences we observed in the SSU and LSU rDNA sequences were single base-pair substitutions; indels were not observed. The SSU rDNA sequence obtained from the 2007 extraction was the most divergent among all of the sequences obtained for H. litoralis, but current knowledge is insufficient to determine whether this degree of variation constitutes evidence for cryptic species.
Molecular phylogenetic inferences
Our data provide evidence for a new, well-supported clade comprising both sand-dwelling and planktonic dinoflagellates: SSU rDNA analyses strongly supported a close relationship between T. dragescoi, P. minutum and H. litoralis (), and the LSU rDNA phylogeny demonstrated monophyly of a clade comprising H. litoralis and P. minutum (). Thecadinium dragescoi, P. minutum and H. litoralis do not have chloroplasts and have thecal tabulation patterns that are consistent with the Peridiniales (Fensome et al., Citation1993; Hoppenrath, Citation2000b ; Hoppenrath et al., Citation2004). If we adopt the interpretation of the thecal plate pattern of T. dragescoi by Hoppenrath et al. (Citation2004, ), and if the sixth postcingular plate of H. litoralis is interpreted as an S.d. plate (), then all three species possess four apical plates, two or three anterior intercalary plates, seven precingular plates, five postcingular plates and two antapical plates (). It should be noted here that Crypthecodinium sp., C. cohnii and Hemidinium nasutum are considered to have an ‘x’ plate; however, these species have a very different overall tabulation pattern to the species considered here (Fensome et al., Citation1993) and are distantly related to Herdmania litoralis and T. dragescoi (, ).
Species classified in the Protoperidiniaceae form four well-supported clades in molecular phylogenetic analyses (Clades I–IV in and A–D in ). All of the species within three of these clades (Clades I–III and A–C) have a cingulum comprised of three plates and a transitional plate, congruent with the diagnosis of the Protoperidiniaceae (Fensome et al., Citation1993). By contrast, the fourth clade (Clade IV in and Clade D in ) contains (1) P. minutum, which has a typical tabulation pattern for the Protoperidiniaceae; (2) H. litoralis, which is supposed to have five cingular plates in Canadian isolates and seven cingular plates in German isolates (Hoppenrath, Citation2000b ); and (3) T. dragescoi, which has six or seven cingular plates (Hoppenrath, Citation2000a ). Ribeiro et al. (Citation2010) showed that P. minutum is not closely related to other Protoperidinium species. In the present study, P. minutum branched with H. litoralis and T. dragescoi and was only very distantly related to the clade (SSU rDNA clade I, LSU rDNA clade A) that contains the type species of Protoperidinium, P. pellucidum (, ). These data demonstrate that P. minutum should not be classified within the genus Protoperidinium.
In the current taxonomic system, P. minutum is assigned to the subgenus Archaeperidinium (e.g. Balech, Citation1974; Taylor, Citation1976; Dodge, Citation1982). Jörgensen (Citation1912) originally established the genus Archaeperidinium for species with only two anterior intercalary plates, whereas Peridinium was retained for species with three anterior intercalary plates. He designated Peridinium minutum (described by Kofoid, Citation1907) as the type species for Archaeperidinium, as Archaeperidinium minutum (Kofoid) Jörgensen. At the time, he assigned only A. minutum and A. monospinum (Paulsen) Jörgensen to this genus and suggested that the two species were probably synonyms. Archaeperidinium, defined by the possession of two anterior intercalary plates and regarded as subgenus of Protoperidinium, is now recognized to contain about 20 species (Hoppenrath et al., Citation2009a ). By contrast, Abé (Citation1936) established a classification system that placed considerable emphasis on the sulcal plates and classified P. minutum within the Monovela group within Peridinium, which was characterized by the possession of a flat sulcal area, a flagellar fin, a circular girdle and a marked asymmetry of the posterior three plates of the sulcal area (Abé, Citation1936, 1981). Taylor (Citation1976) proposed three sections within the subgenus Archaeperidinium: (1) Avellana in which the girdle is left-handed and the anterior intercalaries are symmetrical; (2) Archaeperidinium (= Monovela of Abé), in which the girdle has no displacement; and (3) Excentrica, in which the anterior intercalary plates are very unequal in size.
Our phylogenetic analyses included five species from the subgenus Archaeperidinium, namely P. abei, P. denticulatum, P. excentricum, P. minutum and P. thorianum (, ). According to Taylor's (Citation1976) system, P. abei, P. denticulatum and P. thorianum are members of sect. Avellana, P. excentricum belongs to sect. Excentrica and P. minutum belongs to sect. Archaeperidinium. The species representing sections Avellana and Excentrica lay within Clade I (SSU rDNA) and Clade A (LSU rDNA), which also include the type species of Protoperidinium. Molecular phylogenetic analyses show that subgenus Archaeperidinium, as circumscribed by Taylor (Citation1976), is not monophyletic, and P. minutum can be distinguished morphologically from species within Protoperidinium sects Avellana and Excentrica by the structure of the sulcal area and girdle displacement (Abé, Citation1936; Taylor, Citation1976). Accordingly, we propose here to reintroduce and emend the genus Archaeperidinium Jörgensen.
We have kept Archaeperidinium minutum, H. litoralis and T. dragescoi in different genera in this study because we could not identify morphological characters that unify these species and distinguish them from all other dinoflagellates: for example, H. litoralis and T. dragescoi do not have cingula like those in the Protoperidiniaceae. The recently described species, Protoperidinium vorax, P. bolmonense and P. tricingulatum, possess only three cingular plates without a transitional plate (Siano & Montresor, Citation2005; Chomérat & Couté, Citation2008; Kawami et al., Citation2009). These species, like Archaeperidinium minutum, have rounded cells without an antapical extension. Despite the peculiarities of the cingulum in these species, these species were assigned into the genus Protoperidinium because their epithecal and hypothecal plate patterns are consistent with the genus. SSU and LSU sequence data for these taxa are desirable to improve our understanding of phylogenetic relationships. Molecular phylogenetic analyses have demonstrated that T. dragescoi is distinct from and only distantly related to the genus Thecadinium (; Hoppenrath et al., Citation2004). It has been suggested that this species is a misnamed member of the peridinialean genus Amphidiniopsis, which is a heterotrophic sand-dwelling genus with diverse cell morphologies and tabulation (; Hoppenrath et al., Citation2004; Saldarriaga et al., Citation2004). The type species of Amphidiniopsis, A. kofoidii, shares features with Archaeperidinium minutum, H. litoralis and T. dragescoi, viz. four apical plates, two or three anterior intercalary plates, seven precingular plates, five postcingular plates and two antapical plates (). Hoppenrath (Citation2000a ) noticed that the square intercalary plate 2a of T. dragescoi was very similar in shape to the 2a plate of Amphidiniopsis kofoidii (Dodge & Lewis, Citation1986) and also resembles the 1a plate of Diplopelta and Preperidinium (both in the Protoperidiniaceae). The morphological variability within Amphidiniopsis suggests that the genus may need to be split (; Hoppenrath et al., Citation2009b ) and, in our opinion, the phylogenetic relationships of Amphidiniopsis species need to be better known before any change is made to the classification of T. dragescoi.
Taxonomic and nomenclatural changes
Class Dinophyceae
Order Peridiniales
Archaeperidinium Jörgensen emend. A. Yamaguchi, Hoppenrath, Pospelova, T. Horiguchi & B.S. Leander
DIAGNOSIS: Dinoflagellatae armatae. Formula laminarum: 4′, 2a, 7′′, 4c(3 + t), 5(6?)s, 5′′′, 2′′′′. Chloroplasti absentes. Differt a speciebus sectionis Avellana et Excentrica generis Protoperidinium sulco plano, pinna flagellate sulcum tegente, cingulo centrale non decendenti non ascendenti.
Armoured dinoflagellate. Plate formula Po, X, 4′, 2a, 7′′, 4c(3+t), 5(6?)s, 5′′′, 2′′′′. Chloroplasts absent. Differing from species of sections Avellana and Excentrica of Protoperidinium by the flat sulcus, the flagellar fin covering the sulcal area, and the equatorial cingulum lacking displacement.
TYPE: Archaeperidinium minutum (Kofoid) Jörgensen [E. Jörgensen (Citation1912): Svenska Hydrografisk-Biologiska Kommissionens Skrifter 4: 6].
BASIONYM: Peridinium minutum Kofoid [C.H. Kofoid (Citation1907). University of California Publications. Zoology. 3(13): 310, pl. 31, figs 42–45.
tejp_a_564517_sup_18338266.pdf
Download PDF (169 KB)Acknowledgements
We are grateful to Dr Shelley Reid and Dr Charmaine Gorrie, University of British Columbia, for helping us with the Latin diagnosis. This work was supported by a postdoctoral research salary to A.Y. and M.H. from the Assembling the Tree of Life grant (NSF #EF-0629624) and operating funds to B.S.L. from the National Science and Engineering Research Council of Canada (NSERC 283091-09); B.S.L. is a fellow of the Canadian Institute for Advanced Research, Program in Integrated Microbial Biodiversity. This work was also partly supported by the Grant-in-Aid (16370039) from the MEXT and by the 21st Century Center of Excellence (COE) Program on ‘Neo-Science of Natural History’ (Program Leader: Hisatake Okada) at Hokkaido University financed by the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan.
References
- Abé , TH . 1936 . Report of the biological survey of Mutsu Bay. 29. Notes on the protozoan fauna of Mutsu Bay. II. Genus Peridinium; subgenus Archaeperidinium . Sci. Rep. Tohoku Univ., Ser. 4, Biology , 10 : 639 – 686 .
- Abé , TH . 1981 . Studies on the family Peridinidae. An unfinished monograph of armoured Dinoflagellata . Publ. Seto Mar. Biol. Lab., Special Publ. Ser. , 6 : 1 – 413 .
- Balech , E . 1964a . El plancton de Mar del Plata durante el periodo 1961–62 (Buenos Aires, Argentina) . Bol. Inst. Biología Marina, Mar del Plata, Argentina , 4 : 1 – 49 .
- Balech , E . 1964b . Tercera contribucion al conocimiento del genero Peridinium . Revista Inst. Nac. Invest. Ci., Hidrobiol. , 1 : 179 – 201 .
- Balech , E . 1974 . El genero Protoperidinium Bergh, 1881 (Peridinium Ehrenberg, 1831, Partim) . Revista Mus. Argent. Ci. Nat., Bernardino Rivadavia Inst. Nac. Invest. Ci. Nat., Hidrobiol. , 4 : 1 – 79 .
- Chomérat , N and Couté , A . 2008 . Protoperidinium bolmonense sp. nov. (Peridiniales, Dinophyceae), a small dinoflagellate from a brackish hypereutrophic lagoon (south of France) . Phycologia , 47 : 392 – 403 .
- Dodge , JD . 1981 . Three new generic names in the Dinophyceae; Herdmania, Sclerodinium and Triadinium to replace Heteraulacus and Goniodoma . Br. Phycol. J. , 16 : 273 – 280 .
- DODGE, J.D. (1982). Marine Dinoflagellates of the British Isles. Her Majesty's Stationery Office, London. 303 pp.
- FENSOME, R.A., TAYLOR, F.J.R., NORRIS, G., SARJEANT, W.A.S., WHARTON, D.I. & WILLIAMS, G.L. (1993). A Classification of Living and Fossil Dinoflagellates. Micropaleontology Special Publication 7. Sheridan Press, Hanover, Pennsylvania.
- Fukuyo , Y , Kittaka , J and Hirano , R . 1977 . Studies on the cysts of marine dinoflagellates. I. Protoperidinium minutum (Kofoid) Loeblich . Bull. Plankt. Soc. Jap. , 24 : 11 – 18 .
- Gribble , KE and Anderson , DM . 2007 . High intraindividual, intraspecific, and interspecific variability in large subunit ribosomal DNA in the heterotrophic dinoflagellates Protoperidinium, Diplopsalis, and Preperidinium (Dinophyceae) . Phycologia , 46 : 315 – 324 .
- Handy , SM , Bachvaroff , TR , Timme , RE , Coats , DW , Kim , S and Delwiche , CF . 2009 . Phylogeny of four dinophysiacean genera (Dinophyceae, Dinophysiales) Based on rDNA sequences from single cells and environmental samples . J. Phycol. , 45 : 1163 – 1174 .
- Higgins , D , Thompson , J , Gibson , T , Thompson , JD , Higgins , DG and Gibson , TJ . 1994 . CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice . Nucleic Acids Res. , 22 : 4673 – 4680 .
- Hoppenrath , M . 2000a . Morphology and taxonomy of the marine sand-dwelling genus Thecadinium (Dinophyceae), with the description of two new species from the North German Wadden Sea . Phycologia , 39 : 96 – 108 .
- Hoppenrath , M . 2000b . An emended description of Herdmania litoralis Dodge (Dinophyceae) including the plate formula . Nova Hedwigia , 71 : 481 – 489 .
- Hoppenrath , M . 2000c . Morphology and taxonomy of six marine sand-dwelling Amphidiniopsis species (Dinophyceae, Peridiniales), four of them new, from the German Bight, North Sea . Phycologia , 39 : 482 – 497 .
- Hoppenrath , M , Saldarriaga , JF , Schweikert , M , Elbrächter , M and Taylor , FJR . 2004 . Description of Thecadinium mucosum sp. nov. (Dinophyceae), a new sand-dwelling marine dinoflagellate, and an emended description of Thecadinium inclinatum Balech . J. Phycol. , 40 : 946 – 961 .
- HOPPENRATH, M., ELBRÄCHTER, M. & DREBES, G. (2009a). Marine Phytoplankton. Selected Microphytoplankton Species from the North Sea around Helgoland and Sylt. Kleine Senckenberg-Reihe 49. 264 pp.
- Hoppenrath , M , Koeman , RPT and Leander , BS . 2009b . Morphology and taxonomy of a new marine sand-dwelling Amphidiniopsis species (Dinophyceae, Peridiniales), A. aculeata sp. nov., from Cap Feret, France . Mar. Biodiv. , 39 : 1 – 7 .
- Horiguchi , T , Yoshizawa-Ebata , J and Nakayama , T . 2000 . Halostylodinium arenarium, gen. et sp. nov. (Dinophyceae), a coccoid sand-dwelling dinoflagellate from subtropical Japan . J. Phycol. , 36 : 960 – 971 .
- Huelsenbeck , JP and Ronquist , F . 2001 . MRBAYES: Bayesian inference of phylogenetic trees . Bioinformatics , 17 : 754 – 755 .
- Jörgensen , E . 1912 . Bericht über die von der schwedischen hydrographisch-biologischen Kommission in den schwedischen Gewässern in den Jahren 1909–10 eingesammelten Planktonproben . Svenska Hydrograph.-Biol. Komm. Skr. , 4 : 1 – 20 .
- Kawami , H , Wezel. , R , Koeman , RPT and Matsuoka , K . 2009 . Protoperidinium tricingulatum sp. nov. (Dinophyceae), a new motile form of a round, brown, and spiny dinoflagellate cyst . Phycol. Res. , 57 : 259 – 267 .
- Kofoid , CH . 1907 . Dinoflagellata of the San Diego Region, III. Descriptions of new species. Contributions from the Laboratory of the Marine Biological Association of the United Kingdom XVII . University of California Publications. Zoology , 3 : 299 – 340 .
- LEBOUR, M.V. (1925). The Dinoflagellates of the Northern Seas. Marine Biological Association of the United Kingdom, Plymouth. 250 pp.
- Maddison , DR and Maddison , WP . 2000 . MacClade 4: Analysis of Phylogeny and Character Evolution Version 4.0 , Sunderland, MA : Sinauer Associates .
- Meunier , A . 1919 . Microplankton de la mer Flamande, Part III. Les Péridiniens . Mém. Mus. R. Hist. Natur. Belgique , 8 : 1 – 116 .
- Okolodkov , YB . 2005 . Protoperidinium Bergh (Dinoflagellata) in the southeastern Mexican Pacific Ocean: part I . Bot. Mar. , 48 : 284 – 296 .
- Paulsen , O . 1907 . The Peridiniales of the Danish waters . Medd. Komm. Havundersøg. Kbh., ser. Plankt. , 1 : 1 – 26 .
- Posada , D . 2008 . jModelTest: phylogenetic model averaging . Mol. Biol. Evol. , 25 : 1253 – 1256 .
- Ribeiro , S , Lundholm , N , Amorim , A and Ellegaard , M . 2010 . Protoperidinium minutum (Dinophyceae) from Portugal: cyst-theca relationship and phylogenetic position on the basis of single-cell SSU and LSU rDNA sequencing . Phycologia , 49 : 48 – 63 .
- Ronquist , F and Huelsenbeck , JP . 2003 . MRBAYES 3: Bayesian phylogenetic inference under mixed models . Bioinformatics , 19 : 1572 – 1574 .
- Saldarriaga , JF , Taylor , FJR , Cavalier-Smith , T , Menden-Deuer , S and Keeling , PJ . 2004 . Molecular data and the evolutionary history of the dinoflagellates . Eur. J. Protistol. , 40 : 85 – 111 .
- SCHILLER, J. (1937). Dinoflagellatae (Peridineae) in monographischer Behandlung. 2. Teil, Lieferung 4. In Kryptogamen-Flora von Deutschland, Österreich und der Schweiz (Rabenhorst, L., editor). Akademische Verlagsgesellschaft, Leipzig. 589 pp.
- Siano , R and Montresor , M . 2005 . Morphology, ultrastructure and feeding behaviour of Protoperidinium vorax sp. nov. (Dinophyceae, Peridiniales) . Eur. J. Phycol. , 40 : 221 – 232 .
- Stamatakis , A . 2006 . RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models . Bioinformatics , 22 : 2688 – 2690 .
- Stamatakis , A , Hoover , P and Rougemont , J . 2008 . A rapid bootstrap algorithm for the RAxML web-servers . Syst. Biol. , 57 : 758 – 771 .
- Takano , Y and Horiguchi , T . 2004 . Surface ultrastructure and molecular phylogenetics of four unarmored heterotrophic dinoflagellates, including the type species of the genus Gyrodinium . Phycol. Res. , 52 : 107 – 116 .
- Tamura , K , Dudley , J , Nei , M and Kumar , S . 2007 . MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0 . Mol. Biol. Evol. , 24 : 1596 – 1599 .
- TAYLOR, F.J.R. (1976). Dinoflagellates from the International Indian Ocean Expedition: a Report on Material Collected by the R.V. ‘‘Anton Bruun’’ 1963–1964. E. Schweizerbart'sche Verlagsbuchhandlung, Stuttgart. 246 pp.
- Uhlig , G . 1964 . Eine einfach methode zur extraktion der vagilen, mesopsammalen mikrofauna . Helgol. wiss. Meeresunters. , 11 : 178 – 185 .
- Yamaguchi , A , Kawamura , H and Horiguchi , T . 2006 . A further phylogenetic study of the heterotrophic dinoflagellate genus Protoperidinium (Dinophyceae) based on small and large subunit ribosomal RNA gene sequences . Phycol. Res. , 54 : 317 – 329 .
- Zonneveld , KAF and Dale , B . 1994 . The cyst-motile stage relationships of Protoperidinium monospinum (Paulsen) Zonneveld et Dale comb. nov. and Gonyaulax verior (Dinophyta, Dinophyceae) from the Oslo Fjord (Norway) . Phycologia , 33 : 359 – 368 .
Supplementary material
The following supplementary material is available for this article, accessible via the Supplementary Content tab on the article's online page at http://dx.doi.org/10.1080/09670262.2011.564517:
Supplementary material 1. List of species used in molecular study and GenBank accession numbers.