Abstract
The effect of temperature on the growth of Skeletonema ardens, S. costatum sensu stricto, the S. marinoi–dohrnii complex, S. japonicum, S. menzelii, S. pseudocostatum and S. tropicum isolated from Dokai Bay in southern Japan were examined under five to seven different temperatures and an irradiance of 150 µmol m−2 s−1. The effect of irradiance on the growth of the seven Skeletonema species was also examined under a wide range of irradiances ranging from 7–700 µmol m−2 s−1 at 20°C. All Skeletonema species were able to grow at temperatures ranging from 15 to 25°C. Intra-species differences in specific growth rates of four strains for S. menzelii, and five strains for S. ardens, the S. marinoi–dohrnii complex, S. japonicum and S. tropicum were not significant (Kruskal–Wallis test, P > 0.05). Significant inter-species differences in specific growth rates were observed at 10, 15, 25 and 30°C (Kruskal–Wallis test, P < 0.01; Steel–Dwass test, P < 0.01). The S. marinoi–dohrnii complex and S. japonicum grew faster than other species at the lower temperatures of 10 and 15°C, and S. ardens and S. menzelii grew at the highest temperature of 35°C. The maximum specific growth rates (μ max) from growth–irradiance curves ranged from 1.50 to 3.44 d−1. Threshold values of irradiance (I 0) and saturation irradiance (S) for growth ranged from 3.9 to 7.6 µmol m−2 s−1, and from 250 to 740 µmol m−2 s−1, respectively. In Dokai Bay, our results suggested that the occurrence of Skeletonema species should be affected mainly by temperature and less by irradiance. In particular, only two species, the S. marinoi–dohrnii complex and S. japonicum could maintain their populations in the surface water during the cold season, whereas during other periods, all seven species could maintain their population under the strong influence of estuarine circulation, which rapidly flushed the surface water out of Dokai Bay. Temperature and irradiance dependent growth values were in good agreement with their geographical distributions. The S. marinoi–dohrnii complex and S. japonicum were capable of growing in cold regions, but our results suggested that S. ardens and S. menzelii will tend to prefer tropical regions.
Introduction
The centric diatom genus Skeletonema occurs worldwide and often forms dense blooms in coastal regions (Karentz & Smayda, Citation1984; Aké-Castillo et al., Citation1995; Huang et al., Citation2007). Traditionally, only a few species were recognized, based on morphological features recognizable in light microscopy. These were Skeletonema costatum, S. menzelii, S. subsalsum and S. tropicum in marine and brackish environments, and S. potamos in fresh water. More recent studies utilizing electron microscopy and large subunit rDNA sequences gathered from marine strains revealed that S. costatum sensu lato (s.l.) consists of a series of genetically and morphologically distinct species (Medlin et al., Citation1991; Zingone et al., Citation2005; Sarno et al., Citation2005, Citation2007). As of now the following species have been described or amended: S. ardens, S. costatum sensu stricto (s.s.), S. dohrnii, S. grethae, S. grevillei, S. japonicum, S. marinoi, S. menzelii, S. pseudocostatum, S. subsalsum and S. tropicum. Kooistra et al. (Citation2008) analysed many strains of Skeletonema collected from sites worldwide and concluded that, although these 11 species were genetically and morphologically distinct, multiple genetically distinct taxa, which may represent cryptic diversity, exist within S. menzelii and S. tropicum. Skeletonema dohrnii and S. marinoi, which form part of a genetically diverse species complex (Godhe et al., Citation2006; Kooistra et al., Citation2008), were initially thought to be morphologically distinct, but later studies have indicated that they are cryptic (Ellegaard et al., Citation2008). Kooistra et al. (Citation2008) also showed differences in the distribution patterns of the various species and several species can occur at the same site, although they generally bloom in different seasons. These differences in distribution patterns and the seasonality strongly suggest the existence of differences in growth responses to physiological parameters such as temperature and irradiance.
In the Northwest Pacific, nine species of Skeletonema (S. ardens, S. costatum s.s., S. dohrnii, S. grevillei, S. japonicum, S. marinoi, S. pseudocostatum, S. subsalsum and S. tropicum) have been reported (Cheng et al., Citation2008; Kooistra et al., Citation2008). It is well known that Skeletonema species are some of the dominant diatoms in the coastal areas of Japan, including our study area, Dokai Bay (Yamada et al., Citation1988; Suzuki & Takahashi, Citation1995; Tada et al., Citation2001).
Dokai Bay, which is located in the northern part of Kyushu Island, is well known as one of the most eutrophic embayments in Japan, owing to serious water pollution by untreated effluent from industry along the coast near this bay in the 1960s (Yamada, Citation2000). Following various attempts to treat the effluent entering this bay since the 1970s, the water quality has improved (Yamada et al., Citation1988, 1991). Two Skeletonema species (S. costatum s.l. and S. tropicum) occur abundantly when the temperature is >20°C in Dokai Bay (Kitakyushu Municipal Institute of Environmental Health Science, Citation1994; Yamada & Kaziwara, Citation2004; Yamada et al., Citation2009). Yamada et al. (Citation2010) analysed the species diversity of Skeletonema in Dokai Bay throughout the year, using the identification criteria of Sarno et al. (Citation2005, Citation2007). Seven species of Skeletonema (S. ardens, S. costatum s.s., the S. marinoi–dohrnii complex, S. japonicum, S. menzelii, S. pseudocostatum and S. tropicum) were found in water samples, and the occurrences of these Skeletonema species revealed seasonal fluctuations. Water temperature and irradiance were strongly suggested as major factors controlling the seasonal succession of Skeletonema species in this area (cf. Tada et al., Citation2001). More generally, growth of phytoplankters under natural conditions is predominantly determined by the interaction of physicochemical factors (Yamaguchi & Honjo, Citation1989; Yamaguchi et al., Citation1991, Citation1997; Sarthou et al., Citation2005).
Irradiance and temperature have been identified as important environmental factors regulating the intrinsic growth of Skeletonema (Yoder, Citation1979). However, although the taxonomy of Skeletonema species has been refined through observations of ultrastructural characteristics combined with molecular analyses (Sarno et al., 2005, 2007; Cheng et al., Citation2008; Kooistra et al., Citation2008), information on the ecophysiological characteristics of the new or amended species is extremely limited (Saggiomo et al., Citation2006; Suksomjit et al., Citation2009).
The present study was carried out to determine the specific growth rates of strains belonging to seven Skeletonema species collected from Dokai Bay, under nutrient-replete laboratory conditions at various temperatures and irradiances that Skeletonema species experience in the field. The temperature experiments were conducted on four or five strains belonging to each species, so that we could study not only differences in growth response among species but also intraspecies differences, as suggested by Wood & Leatham (Citation1992) and Lakeman et al. (Citation2009). The growth characteristics of the different species are discussed in relation to their seasonality in Dokai Bay (Yamada et al., Citation2010) and their geographical distributions (Kooistra et al., Citation2008).
Materials and methods
Organisms and culture conditions
The strains used in this study are summarized in . The strains of S. menzelii and S. pseudocostatum were obtained from bottom sediment using the extinction dilution method originally developed for counting viable bacteria in water and sediments (most probable number method; Imai et al., Citation1984); other strains were isolated from the water column. LSU rDNA sequences were identical among strains belonging to each of the seven species of Skeletonema isolated from Dokai Bay, but all species were genetically distinct (; redrawn from Yamada et al., 2010). Yamada et al. (Citation2010) presented further information on the taxonomy of Skeletonema species from Dokai Bay. The stock culture was maintained in ESM medium (Okaichi et al., Citation1983) at 20°C under 60 µmol m−2 s−1 of cool-white fluorescent illumination with a 14:10 h light:dark cycle. Cultures were grown in ESM medium (Okaichi et al., Citation1983), modified by the addition of silicate (88 µM Na2SiO3 9H2O), and the omission of soil extract and Tris buffer. Stock cultures (26 strains belonging to seven Skeletonema species) were not axenic, but bacterial contamination was minimized by using sterile techniques and serial transfers during exponential growth.
Fig. 1. Neighbour-joining (NJ) phylogram inferred from partial LSU rDNA sequences (795-bp long including alignment gaps) from 48 strains of 11 morphologically distinct taxa within Skeletonema with Cyclotella meneghiniana and Thalassiosira rotula as outgroups. NJ trees (Saitou & Nei, Citation1987) were constructed using the genetic distance estimated by the Kimura's two-parameter method (Kimura, Citation1980). Alignment gaps were excluded in the estimation of genetic distance, but the gaps were included in the pair-wise comparisons. Bootstrap values were calculated with 1000 replicates under the same settings as in the NJ analysis.
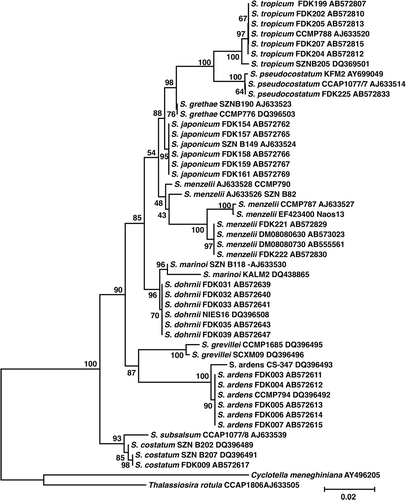
Table 1. Isolated Skeletonema strains, strain ID, GenBank accession number, and date when each strain was isolated from Dokai Bay.
Effect of temperature on growth of Skeletonema species
The cultures were grown at 10, 15, 20, 25 and 30°C and an irradiance of 150 µmol m−2 s−1 on a 14:10 h light:dark cycle. The range of temperature in Dokai Bay, determined from field observations, varied from 8.7 to 29.4°C during the year (Yamada et al., Citation2010). When the maximum specific growth rate was observed at 30°C, additional experiments were conducted at 35 and 40°C to clarify the optimal temperature for each strain. Pre-conditioning to the experimental conditions was conducted through stepwise transfers of stock cultures to each temperature regime. If the transferred cells did not grow under the experimental temperature, the growth experiment was terminated. Acclimated stock cultures (more than five cell generations) were inoculated in triplicate into borosilicate glass test tubes including 30 mL of ESM medium for each temperature. A significant correlation between cell density and in vivo chlorophyll a fluorescence was obtained (r 2 ≥ 0.962, data not shown) for the five Skeletonema species isolated from the water column (S. ardens, S. costatum s.s., the S. marinoi–dohrnii complex, S. japonicum and S. tropicum) under laboratory conditions at 20°C and 150 µmol m−2 s−1. Therefore, growth was determined by measuring in vivo chlorophyll a fluorescence one to three times per day using a Turner Designs Model 10-AU fluorometer (Turner Designs, Sunnyvale, USA). Specific growth rates (d−1) were calculated using data from the exponential portion of the growth curve by least squares regressions of the natural logarithm of in vivo fluorescence against the number of days, using the following formula:
Effect of irradiance on growth of Skeletonema species
A single strain of each species was used (Skeletonema ardens, FDK003; S. costatum s.s., FDK009; the S. marinoi–dohrnii complex, FDK031; S. japonicum, FDK154; S. menzelii, FDK221; S. pseudocostatum, FDK225; S. tropicum, FDK207; ). Seven or eight different irradiance levels (7, 10, 32, 60, 168, 389 and 700 µmol m−2 s−1 or 8, 20, 35, 60, 91, 231, 406 and 700 µmol m−2 s−1) were established by wrapping the test tubes with UV-absorbing vinyl screens. The specific growth rate was determined in triplicate at 20°C at each irradiance level, as described above for the temperature experiment. Modified hyperbolic functions were fit to the irradiance and specific growth rate data, using the equation originally proposed for the photosynthesis–irradiance curve without photoinhibition (Lederman & Tett, Citation1981).
Results
Intraspecific variation in the effects of temperature on growth rates
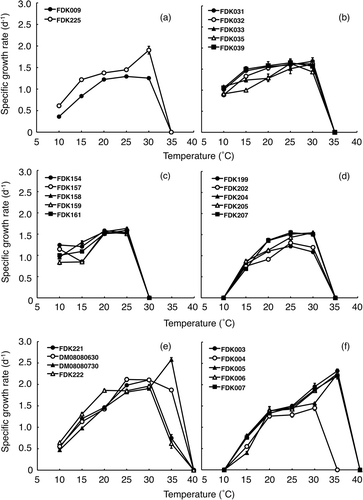
The strains belonging to Skeletonema costatum s.s., S. pseudocostatum and the S. marinoi–dohrnii complex grew over a range of 10–30°C (). Specific growth rates of strains of S. costatum s.s. and S. pseudocostatum ranged from 0.36 to 1.29 d−1, and 0.61 to 1.90 d−1, respectively, and maximum specific growth rates occurred at 25 and 30°C. Minimum specific growth rates for both species were observed at 10°C. For the S. marinoi–dohrnii complex, a maximum specific growth rate (1.69 d−1) was recorded for strain ID FDK031 at 30°C, and a minimum specific growth rate (0.89 d−1) was obtained at 10°C for strain ID FDK032. The strains of S. japonicum grew over a 10–25°C range (), and specific growth rates varied from 0.84 to 1.63 d−1. Specific growth rates of S. japonicum obtained at lower temperatures (10 and 15°C) were more variable among strains than those at higher temperatures (20 and 25°C). Maximum and minimum specific growth rates were recorded at 25°C (1.63 d−1; strain ID FDK158) and 10°C (0.84 d−1; strain ID FDK159). The strains of S. tropicum grew over the range 15–30°C (), and intra-specific variations in specific growth rates at 15°C were smaller than those at higher temperatures (20–30°C). The maximum specific growth rate (1.55 d−1) for S. tropicum was observed at 20 and 25°C (strain ID FDK207 at 20°C and strain ID FDK205 at 25°C), and strain ID FDK207 exhibited a minimum specific growth rate (0.69 d−1) at 15°C. The strains of S. menzelii grew over a 10–35°C range (), and had specific growth rates of 0.47 to 2.58 d−1. Although the variations in specific growth rate within the strains were small between 10 and 30°C, specific growth rates at 35°C were considerably different among the four strains. Maximum and minimum specific growth rates of S. menzelii were obtained from strain ID FDK222 at 10°C (0.47 d−1) and 35°C (2.58 d−1). The strains of S. ardens grew over a 15–35°C range (). The minimum specific growth rate observed at 15°C was 0.40 d−1 (strain ID FDK005), and the maximum specific growth rate obtained at 35°C was 2.32 d−1 (strain ID FDK003). Four out of five strains of S. ardens showed extremely high specific growth rates at 35°C; however, one strain did not grow at 35°C. When the specific growth rate data for all temperatures were compared for each strain, intra-specific differences were not significant for S. ardens, the S. marinoi-dohrnii complex, S. japonicum, S. menzelii and S. tropicum (Kruskal–Wallis test, P > 0.05). Intra-specific comparisons were not tested for S. costatum s.s. and S. pseudocostatum, since only one strain was used.
Fig. 2. Specific growth rates for seven species of Skeletonema at various temperatures for (a) S. costatum s.s. (FDK009) and S. pseudocostatum (FDK225), (b) the S. marinoi–dohrnii complex, (c) S. japonicum, (d) S. tropicum, (e) S. menzelii and (f) S. ardens. Error bars indicate ± standard deviation (n = 3). Strain identities are indicated throughout (see ).
Interspecific variation in the effects of temperature on growth rates
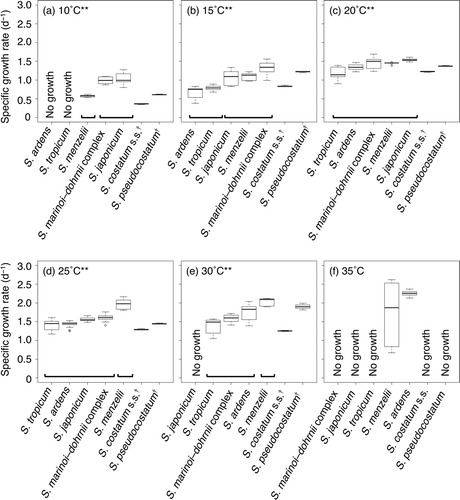
At each temperature (10, 15, 20, 25 and 30°C), interspecific differences were tested with the Kruskal–Wallis and Steel–Dwass tests (). Since the specific growth rates of S. costatum s.s. and S. pseudocostatum were determined for only one strain, these two species were excluded from interspecific comparisons. Kruskal–Wallis test at each temperature revealed a statistically highly significant tendency for at least one species to give higher specific growth rate than at least one of the other species (; Kruskal–Wallis test; P < 0.01). At 10°C, five of the seven species (S. costatum s.s., S. pseudocostatum, the S. marinoi–dohrnii complex, S. japonicum and S. menzelii) grew well, and a specific growth rate was calculated ( and ). The specific growth rate of S. menzelii was significantly lower than the S. marinoi–dohrnii complex and S. japonicum (Steel–Dwass test; P < 0.01, ). The specific growth rates of S. costatum s.s. and S. pseudocostatum at 10°C were similar to those of S. menzelii (). Note that the growth rates for S. costatum s.s. and S. pseudocostatum were excluded from statistical tests, since the specific growth rate was determined for only one strain of each. All species grew at 15°C, and the S. marinoi–dohrnii complex, S. japonicum and S. menzelii exhibited significantly higher specific growth rates than S. ardens and S. tropicum (Steel–Dwass test; P < 0.01, ). Specific growth rates of S. costatum s.s. and S. pseudocostatum were similar to those of S. tropicum and that of S. menzelii, respectively (). Although the population median of specific growth rate of each species at 20°C was significantly different (Kruskal–Wallis test; P < 0.01), multiple comparisons revealed no significant differences within the specific growth rates of tested species (Steel–Dwass test; P > 0.01, ). Specific growth rates of S. costatum s.s. and S. pseudocostatum were almost equal to those of S. tropicum and S. ardens, respectively (). Specific growth rates of S. tropicum, S. ardens, S. japonicum and the S. marinoi–dohrnii complex at 25°C revealed no significant differences, but the growth rate of S. menzelii was significantly higher than other species (Steel–Dwass test; P < 0.01, ). S. pseudocostatum showed similar specific growth rates as S. ardens, and S. costatum s.s. had the lowest growth rate within the tested species when compared as average values (). Although S. japonicum showed no growth at 30°C, other species showed rapid growth and the specific growth rate of S. menzelii was higher than those of other species (Steel–Dwass test; P < 0.01, ). Specific growth rates of S. costatum s.s. and S. pseudocostatum at 30°C were comparable to those of S. tropicum and S. menzelii, respectively ().
Fig. 3. Box plots of specific growth rates for seven Skeletonema species at six temperatures: (a) 10, (b) 15, (c) 20, (d) 25, (e) 30 and (f) 35°C. Data for all strains of each species (see ) were pooled and plotted at each temperature. Asterisks beside the temperature and lines under plots indicate significant differences of specific growth rate among the tested species (Kruskal–Wallis test, P < 0.01; Steel–Dwass test, P < 0.01). Skeletonema costatum s.s. and S. pseudocostatum were excluded from the statistical tests, since their specific growth rates were obtained from only one strain.
Effect of irradiance on growth of Skeletonema species
Specific growth rates of Skeletonema species as a function of irradiance are shown in . Although S. japonicum did not grow at 8 µmol m−2 s−1, other species grew at all irradiance levels. The specific growth rates increased with irradiance from 7 to 406 µmol m−2 s−1 and photo-inhibition was observed at 700 µmol m−2 s−1 by all species. The relationships between specific growth rate and irradiance between 7 and 389 µmol m−2 s−1, or between 8 and 406 µmol m−2 s−1 were well described by a rectangular hyperbola (Equation 2). The derived parameters (μ max, Ks and I 0) are given in , and Equation (2) for each species is superimposed in .
Fig. 4. Specific growth rates of strains of seven Skeletonema species as a function of irradiance. Curves were fit to a hyperbolic equation (see text for details). (a) S. ardens, (b) S. costatum s.s. (c) the S. marinoi–dohrnii complex, (d) S. japonicum, (e) S. menzelii, (f) S. pseudocostatum and (g) S. tropicum.
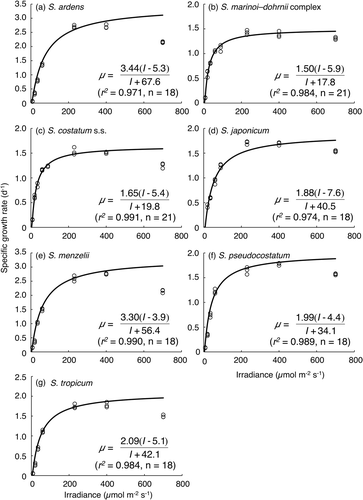
Table 2. Strain ID, maximum specific growth rate (μ max), half saturation constant (K s), threshold value of irradiance (I 0), saturation irradiance (S) and affinity index (α = μ max/K s) of seven Skeletonema species at 20°C.
According to the method of Matsuda et al. (Citation1999), the saturation irradiance for specific growth rate (S; µmol m−2 s−1) was calculated as 740, 237, 257, 491, 607, 389 and 486 µmol m−2 s−1 for S. ardens, S. costatum s.s., the S. marinoi–dohrnii complex, S. japonicum, S. menzelii, S. pseudocostatum and S. tropicum, respectively (). The affinity index (α = μ max/KS ) was also calculated as 0.044, 0.050, 0.053, 0.034, 0.051, 0.046 and 0.039 for S. ardens, S. costatum s.s., the S. marinoi–dohrnii complex, S. japonicum, S. menzelii, S. pseudocostatum and S. tropicum, respectively ().
Discussion
Influence of temperature and light on the growth of Skeletonema species
Wood & Leatham (Citation1992) pointed out that it is important to estimate the variation of physiological parameters within phytoplankton species if it is to be possible to determine the significance of differences observed between species. In addition, Lakeman et al. (Citation2009) commented that a cultured phytoplankton strain is not a static snapshot of a natural algal population, but rather a dynamic, ever-changing laboratory population. In this study, we have shown that specific growth rates at various temperatures were significantly different among the five Skeletonema species (Kruskal–Wallis test; P < 0.01), whereas intra-species differences were not significant (Kruskal–Wallis test; P > 0.05). At low temperatures (10 and 15°C), the S. marinoi–dohrnii complex and S. japonicum grew faster than other species (Steel–Dwass test; P < 0.01). By contrast, S. ardens and S. menzelii tended to grow fast under high temperatures (30 and 35°C) (S. costatum s.s. and S. pseudocostatum were excluded from inter-specific comparisons). Two patterns of irradiance–growth curve were observed. The first occurs in S. ardens and S. menzelii, which have very high maximum growth rates. The second is found in the other five species, which have relatively low maximum growth rates compared with S. ardens and S. menzelii. These results strongly suggest that the Skeletonema species established after recent taxonomic revisions are distinct from each other not only genetically and morphologically (cf. Sarno et al., 2005, 2007; Zingone et al., Citation2005), but also physiologically.
Many studies have reported specific growth rates of Skeletonema costatum s.l. (i.e. corresponding to some or all of S. ardens, S. costatum s.s., S. dohrnii, S. grethae, S. grevillei, S. japonicum, S. marinoi and S. pseudocostatum) and S. tropicum obtained under various temperatures and irradiances for strains collected from various regions. Although these studies were conducted on only one strain of a species, the growth characteristics observed can be used to some extent for comparison. Generally speaking, specific growth rates obtained in previous studies on S. costatum s.l. were similar to those found in our study. For example, the value of 1.10 d−1 at 15°C reported from Trondheim fjord (Sakshaug et al., Citation1989) is comparable to the 1.06 ± 0.20 d−1 for S. japonicum and 1.16 ± 0.13 d−1 for S. menzelii at 15°C in this study. Again, the 0.60–1.70 d−1 between 10 and 22°C obtained for a strain from Narragansett Bay (Yoder, Citation1979) is comparable to 0.55 ± 0.06 d−1 for S. menzelii and 0.61 ± 0.01 d−1 for S. pseudocostatum at 10°C, and to 1.60 ± 0.09 d−1 for the S. marinoi–dohrnii complex and 1.94 ± 0.12 d−1 for S. menzelii at 25°C in this study. Finally, the range recorded for S. costatum s.l. of 0.70–1.50 d−1 for 10–25°C, and 0.90 d−1 at 30°C obtained for a strain from Tokyo Bay (Suzuki & Takahashi, Citation1995) was reasonably similar to the range from 0.99 ± 0.09 to 1.60 ± 0.09 d−1 at 10–30°C in the S. marinoi–dohrnii complex in this study. For S. tropicum, the lowest temperature to limit growth in our study (15°C) is consistent with the limit of 13–15°C observed by Hulburt & Guillard (Citation1968) and the 12.5°C in Dokai Bay (Yamada et al., Citation2009). Although the specific growth rates of S. tropicum at the various temperatures were also similar to those reported by Tada et al. (Citation2004), the maximum specific growth rate in this study (1.40 d−1 at 25°C) was significantly lower than the 2.08 d−1 at 25°C recorded by Hulburt & Guillard (Citation1968). The specific growth rates of the S. marinoi–dohrnii complex were similar to those of S. dohrnii obtained from the Gulf of Naples and San Francisco Bay (<0.35 d−1 at 5–10°C and 1.0–1.7 d−1 at 15–24°C: Saggiomo et al., Citation2006).
It has been well documented that the dependence of growth rate on photosynthetically active radiation in terms of irradiance differs among diatoms (Chan, Citation1978; Falkowski & Owens, Citation1980; Popovich & Gayoso, Citation1999; Nishikawa & Yamaguchi, Citation2006, 2008). Suksomjit et al. (Citation2009) reported specific growth rates of a strain of S. japonicum isolated from Dokai Bay to be 1.47–1.95 d−1 under several irradiances (58–450 µmol m−2 s−1). Our results for S. japonicum were similar (0.93–1.71 d−1 at 60–406 µmol m−2 s−1, ). shows the effect of irradiance on the growth rate of some marine diatoms. Since Chan (Citation1978) conducted his experiments under continuous light, direct comparisons are not possible. Although the data presented in were obtained from a single strain for each species, the growth characteristics of each species could be compared to some extent via an affinity index (α = µ max /KS ). For S. costatum s.l., Chan (Citation1978) found that the lowest saturation irradiance and lower maximum specific growth rates resulted in a moderate affinity index, when compared to Chaetoceros sp., Cylindrotheca fusiformis, Thalassiosira floridana and T. eccentrica. The affinity indices of Skeletonema species obtained in this study (0.03–0.05) are similar to those of S. costatum s.l. at 15°C (0.06: Falkowski & Owens, Citation1980) and Eucampia zodiacus (0.05: Nishikawa & Yamaguchi, Citation2006). Although the half-saturation constants of Coscinodiscus wailesii and C. granii (84 and 47 µmol m−2 s−1: Nishikawa & Yamaguchi, Citation2008) are similar to those of Skeletonema species in this study (30–78 µmol m−2 s−1), Skeletonema species showed higher maximum growth rates and affinity indices than the two Coscinodiscus species (Nishikawa & Yamaguchi, Citation2008). The high maximum specific growth rates of Skeletonema species suggest that they are more competitive at high irradiances than C. wailesii and C. granii (Nishikawa & Yamaguchi, Citation2008). In addition, the high affinity indices of Skeletonema species suggest that Skeletonema species are also more tolerant of low irradiance than the Coscinodiscus species (Nishikawa & Yamaguchi, Citation2008). For S. ardens and S. menzelii, there were some discrepancies between the specific growth rates at 20°C and 150 µmol m−2 s−1 shown in and the growth rates estimated from . The specific growth rates of these two species in (1.35 d−1 for S. ardens and 1.55 d−1 for S. menzelii) were considerably lower than those estimated from (2.29 d−1 for S. ardens and 2.34 d−1 for S. menzelii). It is clear that their optimal conditions are higher than 20°C and 150 µmol m−2 s−1, and this might be one of the reasons for the discrepancies observed in specific growth rates at 20°C and 150 µmol m−2 s−1 between temperature experiments and irradiance experiments. To clarify the discrepancies, irradiance experiments should be performed at their optimal temperatures, 30 or 35°C. In the future, interspecies differences on growth characteristics against the irradiance (represented as µ max, KS , I 0 and α) should be clarified by gathering data on not only many species, but also many strains belonging to one species.
Table 3. Literature values for maximum specific growth rate (μ max), half saturation constant for irradiance (Ks), threshold value of irradiance (I 0), saturation irradiance (S) and affinity index (α = μmax /Ks) of some marine diatoms.
Occurrence of Skeletonema species in Dokai Bay
The seven Skeletonema species were isolated from Dokai Bay, which is characterized by high phytoplankton biomass during summer but low biomass during other seasons, although nutrients are saturating for phytoplankton growth throughout the year (Tada et al., Citation2001). In this bay, strong estuarine circulation is dominant, and the offshore water intrudes into the bottom layer while riverine water flows out in the surface layer. The phytoplankton in the surface water mass at the head of the bay are transported out of the bay within 2–2.5 days throughout the year by the strong estuarine circulation without vertical mixing (Yanagi et al., Citation1996; Yanagi & Yamada, Citation2000; Tada et al., Citation2007). Thus, phytoplankters cannot maintain their populations in the surface waters of the bay and will be flushed out within two days, unless the doubling time is higher than about 1.0 division d−1 (equating to a specific growth rate of more than 0.7 d−1) [note that the doubling time is calculated by base-two logarithms and specific growth rate is calculated by natural logarithm: see Materials and methods, Equation (1)]. Assuming that irradiance is saturating for growth (>150 µmol m−2 s−1), the temperature range where each species could maintain its population in Dokai Bay can be estimated from , which shows the range where specific growth rates exceed 0.7 d−1. Between 15 and 30°C, most species could maintain their populations (). However, only the S. marinoi–dohrnii complex and S. japonicum could maintain their populations at 10°C, while S. japonicum will not grow at 30°C; the occurrence of these species in the field is in good agreement with this estimation (Yamada et al., Citation2010). The strains of S. ardens were isolated from Dokai Bay in October 2007 and September 2008 when the water temperature was 27°C (Yamada et al., Citation2010). From the data obtained from monthly observations between January 2008 and February 2009, the temperature range for each species that was isolated, varied from 22 to 27°C for S. costatum s.s., from 9 to 28°C for S. marinoi–dohrnii complex, from 9 to 26°C for S. japonicum and from 15 to 27°C for S. tropicum, respectively (Yamada et al., Citation2010). Comparable data cannot be provided for S. menzelii and S. pseudocostatum, because the strains used in this study were obtained from the bottom sediment. The threshold values of irradiance to maintain their population in this bay can be estimated from Equation (2) and were 20, 20, 24, 24, 25, 27 and 36 µmol m−2 s−1 for S. menzelii, S. tropicum, S. ardens, the S. marinoi–dohrnii complex, S. pseudocostatum, S. costatum s.s. and S. japonicum, respectively. These values are much lower than the average irradiances in Dokai Bay during mid-summer which are ∼580 µmol m−2 s−1 in the surface water and usually >200 µmol m−2 s−1 at 3 m, according to Suksomjit et al. (Citation2009). Although the irradiance data are limited to mid-summer, considering that the maximum water depth in the bay is 8–10 m and that there is a strong estuarine circulation, it seems most likely that the occurrence and distribution of Skeletonema species will be influenced mainly by water temperature and little by irradiance.
Table 4. Temperature and specific growth rate ranges where Skeletonema species can maintain their population against flush-out of surface water of Dokai Bay (see text).
Comparison between growth physiology and geographic distribution of Skeletonema species
Kooistra et al. (Citation2008) analysed 184 strains of Skeletonema from marine and estuarine sites worldwide, identifying them according to the criteria established by Sarno et al. (2005, 2007). Although there was good agreement in most species between our experimental results and the geographical distributions given by Kooistra et al. (Citation2008) – for example, S. japonicum and the S. marinoi–dohrnii complex exhibit high specific growth rates at low temperatures, agreeing with their distribution in cold waters – there were some differences. According to Kooistra et al. (Citation2008), S. ardens and S. menzelii have a wide geographical distribution, S. ardens having been collected from the coasts of Singapore, China and Australia and S. menzelii from the coasts of Italy, the Netherlands, Uruguay, Panama and the USA, and also from the Sargasso Sea. Judging from the results of our temperature experiments, however, S. ardens and S. menzelii might be expected to prefer tropical regions where they would experience water temperatures over 30°C. These two species also grew faster than other species, especially under high irradiances (>91 µmol m−2 s−1), which would also agree with adaptation to tropical waters. Further growth studies would be valuable, for example into aspects of nutritional physiology and better sampling of tropical seas.
Acknowledgements
We thank Mayumi Kawaguchi of Fukuoka Women's University for help with isolation and identification of Skeletonema species and Akira Tanaka of Kagawa University for providing the data for cell counts and fluorescence relationships. We wish to especially thank the anonymous reviewers who kindly provided helpful insights on the manuscript.
References
- Aké-Castillo , J.A. , Meave-del Castillo , M.E. and Hernandez-Becerril , D.U. 1995 . Morphology and distribution of species of the diatom genus Skeletonema in a tropical coastal lagoon . Eur. J. Phycol. , 30 : 107 – 115 .
- Chan , A.T. 1978 . Comparative physiological study of marine diatoms and dinoflagellates in relation to irradiance and cell size. I. Growth under continuous light . J. Phycol. , 14 : 396 – 402 .
- Cheng , J. , Li , Y. , Liang , J. , Gao , Y. , Wang , P. , Kin-Chung , H. and Lin , X. 2008 . Morphological variability and genetic diversity in five species of Skeletonema (Bacillariophyta) . Prog. Nat. Sci. , 18 : 1345 – 1355 .
- Ellegaard , M. , Godhe , A. , Härnström , K. and McQuoid , M. 2008 . The species concept in a marine diatom: LSU rDNA-based phylogenetic differentiation in Skeletonema marinoi/dohrnii (Bacillariophyceae) is not reflected in morphology . Phycologia , 47 : 156 – 157 .
- Falkowski , P.G. and Owens , T.G. 1980 . Light–shade adaptation, two strategies in marine phytoplankton . Plant Physiol. , 66 : 592 – 595 .
- Godhe , A. , McQuoid , M.R. , Karunasagarb , I. , Karunasagarb , I. and Rehnstam-Holm , A.-S. 2006 . Comparison of three common molecular tools for distinguishing among geographically separated clones of the diatom Skeletonema marinoi Sarno & Zingone (Bacillariophyceae) . J. Phycol. , 42 : 280 – 291 .
- Huang , C.-J. , Wang , C. , Dong , Q.-X. and Lin , X.-P. 2007 . Population dynamics of Skeletonema costatum in Zhelin Bay of Eastern Guangdong . Acta Ecol. Sin. , 27 : 142 – 151 .
- Hulburt , E.M. and Guillard , R.R.L. 1968 . The relationship of the distribution of the diatom Skeletonema tropicum to temperature . Ecology , 49 : 337 – 339 .
- Imai , I. , Itoh , K. and Anraku , M. 1984 . Extinction dilution method for enumeration of dormant cells of red tide organisms in marine sediments . Bull. Plankt. Soc. Japan , 31 : 123 – 124 .
- Karentz , D. and Smayda , T.J. 1984 . Temperature and seasonal occurrence patterns of 30 dominant phytoplankton species in Narragansett Bay over a 22-year period (1959–1980) . Mar. Ecol. Prog. Ser. , 18 : 277 – 293 .
- Kimura , M. 1980 . A simple method for estimating evolutionary rate of base substitutions through comparative studies of nucleotide sequences . J. Mol. Evol. , 16 : 111 – 120 .
- Kitakyushu Municipal Institute of Environmental Health Science . 1994 . Comprehensive Investigation Report of Dokai Bay III , 263 Bunshindou : Fukuoka . (in Japanese)
- Kooistra , W.H.C.F. , Sarno , D. , Balzano , S. , Gu , H. , Andersen , R.A. and Zingone , A. 2008 . Global diversity and biogeography of Skeletonema species (Bacillariophyta) . Protist , 159 : 177 – 193 .
- Lakeman , M.B. , von , Dassow , P. and Cattolico , R.A. 2009 . The strain concept in phytoplankton ecology . Harmful Algae , 8 : 746 – 758 .
- Lederman , T.C. and Tett , P. 1981 . Problems in modeling the photosynthesis – light relationship for phytoplankton . Bot. Mar. , 24 : 125 – 134 .
- Matsuda , A. , Nishijima , T. and Fukami , K. 1999 . Effects of nitrogenous and phosphorus nutrients on the growth of toxic dinoflagellate Alexandrium catenella . Nippon Suisan Gakk. , 65 : 847 – 855 .
- Medlin , L.K. , Elwood , H.J. , Stickel , S. and Sogin , M.L. 1991 . Morphological and genetic variation within the diatom Skeletonema costatum (Bacillariophyta): evidence for a new species, Skeletonema pseudocostatum . J. Phycol. , 27 : 514 – 524 .
- Nishikawa , T. and Yamaguchi , M. 2006 . Effect of temperature on light-limited growth of the harmful diatom Eucampia zodiacus Ehrenberg, a causative organism in the discoloration of Porphyra thalli . Harmful Algae , 5 : 141 – 147 .
- Nishikawa , T. and Yamaguchi , M. 2008 . Effect of temperature on light-limited growth of the harmful diatom Coscinodiscus wailesii, a causative organism in the bleaching of aquacultured Porphyra thalli . Harmful Algae , 7 : 561 – 566 .
- Okaichi , T. , Nishio , S. and Imatomi , Y. 1983 . “ Mass culture of marine phytoflagellates: an approach to new sources of biologically active compounds ” . In IUPAC Pesticide Chemistry , Edited by: Miyamoto , J. and Kearney , P.C. Vol. 2 , 141 – 144 . New York : Pergamon Press .
- Popovich , C.A. and Gayoso , A.M. 1999 . Effect of irradiance and temperature on the growth rate of Thalassiosira curviseriata Takano (Bacillariophyceae), a bloom diatom in Bahîa Blanca estuary (Argentina) . J. Plankt. Res. , 21 : 1101 – 1110 .
- Saggiomo, M., Kooistra, W.H.C.F., Sarno, D., Montresor, M., Lopez, Burn, C., Cloern, J., Hargraves, P. Zingone, A. (2006). Differences in Skeletonema distribution among three distant temperate coastal sites. J. Phycol., 42 (suppl. s1): 31–32. [http://onlinelibrary.wiley.com/doi/10.1111/jpy.2006.42.issue-s1/issuetoc]
- Saitou , N. and Nei , M. 1987 . The neighbor-joining method: a new method for reconstructing phylogenetic trees . Mol. Biol. Evol. , 4 : 406 – 425 .
- Sakshaug , E. , Andersen , K. and Kiefer , D.A. 1989 . A steady state description of growth and light absorption in the marine planktonic diatom Skeletonema costatum . Limnol. Oceanogr. , 34 : 198 – 205 .
- Sarno , D. , Kooistra , W.H.C.F. , Medlin , L.K. , Percopo , I. and Zingone , A. 2005 . Diversity in the genus Skeletonema (Bacillariophyceae). II. An assessment of the taxonomy of S. costatum-like species with the description of four new species . J. Phycol. , 41 : 151 – 176 .
- Sarno , D. , Kooistra , W.H.C.F. , Hargraves , P.E. and Zingone , A. 2007 . Diversity in the genus Skeletonema (Bacillariophyceae). III. Phylogenetic position and morphology of Skeletonema costatum and Skeletonema grevillei, with the description of Skeletonema ardens sp. nov . J. Phycol. , 43 : 156 – 170 .
- Sarthou , G. , Timmermans , K.R. , Blain , S. and Treguer , P. 2005 . Growth physiology and fate of diatoms in the ocean: a review . J. Sea Res. , 53 : 25 – 42 .
- Suksomjit , M. , Ichimi , K. , Hamada , K. , Yamada , M. , Tada , K. and Harrison , P.J. 2009 . Ammonium accelerates the growth rate of Skeletonema spp. in the phytoplankton assemblage in a heavily eutrophic embayment, Dokai Bay, Japan . La mer , 47 : 89 – 101 .
- Suzuki , Y. and Takahashi , M. 1995 . Growth responses of several diatom species isolated from various environments to temperature . J. Phycol. , 31 : 880 – 888 .
- Tada , K. , Morishita , M. , Hamada , K. , Montani , S. and Yamada , M. 2001 . Standing stock and production rate of phytoplankton and red tide outbreak in a heavily eutrophic embayment, Dokai Bay, Japan . Mar. Poll. Bull. , 42 : 1177 – 1186 .
- Tada , K. , Ichimi , K. , Yokota , H.T. , Yamada , M. and Montani , S. 2004 . Why flagellates do not produce bloom in Dokai Bay, Japan . Umi no Kenkyu , 13 : 271 – 279 . (in Japanese with English abstract)
- Tada , K. , Ichimi , K. , Hamada , K. , Ueda , N. , Yamada , M. and Montani , S. 2007 . Estuarine circulation and red tide outbreaks in the Dokai Bay, Japan . Bull. Coast. Oceanogr. , 44 : 147 – 155 . (in Japanese with English abstract)
- Tokai , T. 1997 . Maximum likelihood parameter estimates of a mesh selectivity logistic model through SOLVER on MS-Excel . Bull. Jap. Soc. Fish. Oceanogr. , 61 : 288 – 298 . (in Japanese with English abstract)
- Wood , A. and Leatham , M.T. 1992 . The species concept in phytoplankton ecology . J. Phycol. , 28 : 723 – 729 .
- Yamada, M. (2000). Dokai Bay. In Water Pollution Control Policy and Management: The Japanese Experience (Okada, M. & Peterson, S.A.), 161–173. Gyosei, Tokyo
- Yamada , M. , Higashi , T. , Hamada , K. , Ueda , N. , Eguchi , N. and Suzuki , M. 1988 . Improvement and management of environmental conditions in a eutrophicated coastal bay with ecoremediation . Environ. Sci. , 11 : 329 – 340 . (in Japanese with English abstract)
- Yamada , M. , Katsuki , M. , Otsubo , M. , Hamada , K. , Ueda , N. and Montani , S. 2009 . Survival strategy of tropical and subtropical marine diatom Skeletonema tropicum in temperate coastal small bay (Dokai Bay), Japan . Umi no Kenkyu , 18 : 157 – 167 . (in Japanese with English abstract)
- Yamada , M. , Katsuki , E. , Otsubo , M. , Kawaguchi , M. , Ichimi , K. , Kaeriyama , H. , Tada , K. and Harrison , P.J. 2010 . Species diversity of genus Skeletonema (Bacillariophyceae) in the industrial harbor Dokai Bay, Japan . J. Oceanogr. , 66 : 755 – 771 .
- Yamada , M. and Kaziwara , Y. 2004 . Characteristics of phytoplankton occurrence in the hyper-eutrophic environment, Dokai Bay, Japan . Umi no Kenkyu , 13 : 281 – 293 . (in Japanese with English abstract)
- Yamada , M. , Takeuchi , R. , Sueta , S. , Kido , K. , Yabumoto , Y. and Yoshida , Y. 1991 . Recovery of aquatic animals in Dokai Bay, northern Kyushu, Japan . Mar. Poll. Bull. , 23 : 201 – 207 .
- Yamaguchi , M. and Honjo , T. 1989 . Effects of temperature, salinity and irradiance on the growth rate of the noxious red tide flagellate Gymnodinium nagasakiense (Dinophyceae) . Nippon Siuisan Gakk. , 55 : 2029 – 2036 .
- Yamaguchi , M. , Imai , I. and Honjo , T. 1991 . Effects of temperature, salinity and irradiance on the growth rate of the noxious red tide flagellate Chattonella antiquea and C. marina (Raphidophyceae) . Nippon Suisan Gakk. , 57 : 1277 – 1284 .
- Yamaguchi , M. , Itakura , S. , Nagasaki , K. , Matsuyama , Y. , Uchida , T. and Imai , I. 1997 . Effects of temperature and salinity on the growth of the red tide flagellate Heterocapsa circularisquama (Dinophyceae) and Chattonella verruculosa (Raphidophyceae) . J. Plankton Res. , 19 : 1167 – 1174 .
- Yanagi , T. , Inoue , K. , Montani , S. and Yamada , M. 1996 . Tidal current and residual flow in Dokai Bay . Memories Faculty Eng. Ehime Univ. , 15 : 423 – 430 . (in Japanese with English abstract)
- Yanagi , T. and Yamada , M. 2000 . Reason why red tides do not occur during winter in Dokai Bay, Japan . Umi no Kenkyu , 9 : 125 – 132 . (in Japanese with English abstract)
- Yoder , J.A. 1979 . Effect of temperature on light-limited growth and chemical composition of Skeletonema costatum (Bacillariophyceae) . J. Phycol. , 15 : 352 – 370 .
- Zingone , A. , Percopo , I. , Sims , P.A. and Sarno , D. 2005 . Diversity in the genus Skeletonema (Bacillariophyceae). I. A reexamination of the type material of S. costatum with the description of S. grevillei sp. nov . J. Phycol. , 41 : 140 – 150 .