Abstract
A detailed study of the type specimen of Plocamium cartilagineum and other original material of European species of Plocamium revealed that the nomenclatural reorganization proposed by Saunders & Lehmkuhl in 2005 must be revised. The main consequences of the new interpretation are: (1) the use of Plocamium lyngbyanum to name their molecular entity EUR1 (previously considered to be equivalent to P. cartilagineum s.s.); and (2) adopting P. cartilagineum for their molecular entity EUR2, which renders P. subtile Kützing a taxonomic synonym. Using a combination of morphological, anatomical and molecular information (barcoding), we conclude that the colour and consistency of the thallus, the number of ramuli per series, the morphology and arrangement of the tetrasporangial stichidia, the length of the tetrasporangia, and the type of habitat are the most reliable characters of classical taxonomy for discriminating between the species of Plocamium occurring in northern Europe.
Introduction
The genus Plocamium includes approximately 40 species (Wynne, Citation2002) and is widely distributed throughout the world's oceans, but it is most diverse in the southern hemisphere. Traditional taxonomy distinguishes between Plocamium species largely on the basis of the number of ramuli in alternating series, the morphology of the lower ramulus, the length, width, colour and consistency of the thallus, and the morphology and arrangement of tetrasporangial stichidia and cystocarps (Simons, Citation1964; Womersley, Citation1971; South & Adams, Citation1979; Gabrielson & Scagel, 1988). Biogeographical criteria, however, are also important, because many species or groups of species have a restricted distribution. In this context, the reportedly cosmopolitan distribution of the type species, Plocamium cartilagineum (Linnaeus) P.S. Dixon, is rather unexpected. Furthermore, this species, whose original material is almost certainly from the coasts of northern Europe (Dixon, Citation1967), is assumed to have considerable morphological variability.
After a molecular study of numerous specimens from northern Europe and many other parts of the world, Saunders & Lehmkuhl (Citation2005) showed that the name P. cartilagineum sensu lato (s.l.) actually concealed at least eight divergent cryptic species, most still undescribed. In the particular case of northern Europe, P. cartilagineum s.l. was the only species recognized at the time of Saunders & Lehmkuhl's study but their data for specimens collected from Norway to Brittany revealed the presence of at least four distinct molecular entities. Moreover, these four, initially designated as EUR1 to EUR4, were among some of the most divergent of all Plocamium species worldwide, and were separated by genetic distances much greater than those found among Plocamium species from other geographical regions, higher even than between genera in some red algal families. Entities EUR3 and EUR4, seemingly scarce and restricted in their distribution, were described as two new species of European Plocamium (P. nanum Saunders & Lehmkuhl, and P. maggsiae Saunders & Lehmkuhl, respectively), while the widespread and more abundant EUR1 and EUR2 were assigned to the existing names P. cartilagineum sensu stricto (s.s.) and P. subtile Kützing, respectively.
Saunders & Lehmkuhl (Citation2005) noted that specimens of EUR1 and EUR2 can be difficult to discriminate because of their similar habit and considerable morphological variability. Non-tetrasporangial specimens are particularly challenging because the most useful taxonomic attributes for distinguishing the two species are tetrasporangium size and the morphology of tetrasporangial stichidia. Thus, entity EUR1 is characterized by compound stichidia and tetrasporangia 37–60 µm in length while EUR2 has mostly simple stichidia and larger tetrasporangia (60–90 µm in length). Kützing (Citation1866) illustrated compound stichidia similar to those found in EUR1 for P. cartilagineum (as P. coccineum), while he drew simple stichidia very similar to those found in EUR2 for his P. subtile. Viewing only a vegetative portion of the original lectotype collection for P. cartilagineum, Saunders & Lehmkuhl (Citation2005) mostly followed the criterion of Kützing on this attribute and assigned EUR1 to P. cartilagineum s.s. and EUR2 to P. subtile. The comparative analysis of other morphological traits seen in the original lectotype material and regarded by Saunders & Lehmkuhl as putatively diagnostic characters (degree of branching, type of surface cortication, and morphology of growing tips), provided weak support for this decision.
As part of a wider taxonomic revision of the species of Plocamium from the Iberian Peninsula and Balearic Islands (No Couto & Cremades, Citation2001; Cremades et al. Citation2007), one of the co-authors of this study (J.C.) had the opportunity to examine the lectotype for P. cartilagineum in detail. This examination, partly unpublished, revealed that one of the two specimens included in the lectotype sheet designated by Dixon (Citation1967) is a tetrasporophyte bearing simple stichidia and large tetrasporangia similar to those described by Saunders & Lehmkuhl (Citation2005) for EUR2. This finding, together with a critical examination of the original material of other Plocamium species from the European Atlantic (notably Delesseria plocamium var. uncinata C. Agardh, P. binderianum Kützing, P. lyngbyanum Kützing and P. subtile Kützing), and with a morphogenetic study of numerous specimens, leads us to suggest a revised taxonomic and nomenclatural proposal for molecular entities EUR1 and EUR2. In the light of the anatomical data reported here, we generated COI-5P (5′ region of the mitochondrial cytochrome oxidase subunit I gene) data for representative samples from the Saunders & Lehmkuhl (Citation2005) study, as well as from novel collections, to expand on their molecular observations and provide finer resolution to test for the presence of additional overlooked species. Although the variable regions of LSU rDNA (large subunit of the ribosomal cistron) used by Saunders & Lehmkuhl (Citation2005) do separate many species, they are not as variable as COI-5P in the red algae and thus may not discriminate between closely related species pairs (see Sherwood et al., Citation2010). By including samples for other species of Plocamium from additional geographical regions in our COI-5P analyses, we establish that overlooked diversity in this genus is not a problem unique to Europe.
Materials and methods
Samples were collected as indicated in . Specimens collected specifically for this study were preserved in 4% formalin–seawater; after study, vouchers were deposited in the SANT-Algae herbarium at the University of Santiago de Compostela (Spain). Other specimens had been previously collected by G.W. Saunders and are deposited in the phycological collection at Connell Herbarium, University of New Brunswick (Canada). In all cases, a small portion of each specimen was preserved in silica gel for DNA analyses. Herbarium-mounted specimens were scanned using an Epson Perfection V750 Pro scanner. Vegetative and reproductive structures were excised and mounted in 50% Karo in 4% formalin–seawater for microscopical observation. Photomicrographs were taken with an Olympus C-5060 digital camera mounted on an Olympus BX50 light microscope and edited with Adobe Photoshop (http://www.adobe.com/).
Table 1. Collection information for specimens of Plocamium used in this study.
Specimens were assigned to genetic species groups using COI-5P (Saunders, Citation2005; Robba et al., Citation2006). Sequence data were generated following protocols described by Saunders (Citation2005) and the specific primer combination used for each sample is available at both the Barcode of Life Database (BOLD: www.boldsystems.org/) and Genbank (see for accession numbers). COI-5P sequences were aligned by eye in MacClade version 4.06 (Maddison & Maddison, Citation2003) and species groups were visualized with neighbour-joining analysis (general time reversible model) in PAUP (Swofford, Citation2003) (as implemented in Geneious Pro 5.0.3; available at www.geneious.com). The resulting tree provided a visual representation of species diversity and relative divergence, but should not be interpreted as a robust phylogenetic analysis.
Results and discussion
EUR2 = Plocamium cartilagineum (Linnaeus) Dixon sensu stricto
BASIONYM: Fucus cartilagineus Linnaeus Citation1753: 151.
SYNONYMS: Fucus plocamium Gmelin Citation1768: 153, nom. illegit. Fucus coccineus Hudson Citation1778: 586, nom. illegit. Plocamium vulgare J.V. Lamouroux Citation1813: 50, nom. illegit. Delesseria coccinea C. Agardh Citation1817: xiv, nom. illegit. Plocamium coccineum Lyngbye Citation1819: 39, nom. illegit. Delesseria plocamium C. Agardh Citation1822: 181, nom. illegit. Delesseria plocamium var. uncinata C. Agardh Citation1822: 181. Plocamium coccineum var. subtile Kützing Citation1843: 449. nom. illegit. (non Plocamium coccineum var. subtile Lyngbye). Plocamium coccineum var. uncinatum (C. Agardh) Kützing Citation1849: 884. nom. illegit. Plocamium subtile Kützing Citation1866: 15, pl. 42a, b. Plocamium uncinatum (C. Agardh) Kützing Citation1866: 16. Plocamium cartilagineum var. uncinatum (C. Agardh) Guiry ex Benhissoune, Boudouresque, Perret-Boudouresque & Verlaque 2002: 398. Plocamium cartilagineum var. uncinatum (C. Agardh) M.J. Wynne Citation2002: 350, nom. illegit.
LECTOTYPE: Leiden (Herb. Lugdb. Bat. 910.184.14).
TYPE LOCALITY: ‘in Oceano australiore’ (more likely northern Europe fide Dixon, Citation1967).
Observations on the original material
Although it is impossible to be certain, the lectotype collection for Fucus cartilagineus () probably comes from the Netherlands (cf. Dixon, Citation1967). The lectotype sheet includes one tetrasporophyte and one female gametophyte, each about 11 cm in height (). Both plants are dark red, delicate in construction, and slightly cartilaginous. The main axes are subcylindrical in section and up to 0.8 mm in width. The lowermost ramulus in each series is narrow and pointed distally, up to 1 mm in length and 0.25 mm in width, and up to 8 times longer than wide. Although the branching appears somewhat unordered, plants are increasingly branched distally. The ramuli are alternate in series of 2 or 3 (or 4) above the basal (quiescent) ramuli; they are barely incurved or even slightly extrorse at times (). Under the microscope, the cortication appears loose and incomplete () and the apices inconsistently have a chevron-like appearance (). In the female gametophyte, the cystocarps are sessile or have a short peduncle, c. 1 mm in diameter, and are found in the axil of the basal ramulus as well as along the margins of the main axis. The tetrasporangial specimen has stichidia replacing ramuli. Juvenile stichidia are simple, fusiform or bifurcated, up to 125 µm in diameter, and have a short stalk, which is narrower than the fertile portion, c. 70 µm in diameter (). When older, simple stichidia may have a long, sterile stalk while bifurcated stichidia are dichotomously branched from the middle of their length (). Tetrasporangia are up to 80 × 50 µm (). All the fragments examined for this study were host to Cocconeis scutellum (), a common pioneer diatom in Europe (Munda, Citation2005). Unfortunately, this diatom is rather cosmopolitan in distribution and its presence does not clarify the geographical origin of the lectotype.
Figs 1–10. Plocamium cartilagineum. 1. Lectotype of Fucus cartilagineus Linnaeus. 2. Ramuli alternating in series of 2 or 3 above the basal ramuli. 3. Cortication. 4, 5. Apices with and without chevron-like appearance. 6. Cocconeis scutellum as epiphyte. 7. Simple young stichidia. 8. Simple and dichotomous old stichidia. 9. Cross-section of stichidia showing tetrasporangia. 10. Cross-section of a subcylindrical empty sporangial chamber.

The type sheet for Plocamium subtile [Leiden, L 0491621. The type locality is given as ‘In mari atlantico’ (Kützing, Citation1866: 15), but the type clarifies that the source was St. Malo, northwest coast of France; consists of a single tetrasporangial specimen about 5 cm in diameter that is firmly attached to paper (). The morphology of this specimen shows that it served as the model for Kützing's (Citation1866) , b. It is dark red with a pink or yellow tinge in some areas, and the main axis is very narrow (maximum width 0.5 mm), subcylindrical in section, and slightly cartilaginous at the base. The absence of a holdfast, spherical appearance, extrorse branches, and lack of multicellular epiphytes in the type are characteristics of the loose-lying plants often found on sand, gravel or maërl seabeds along European Atlantic coasts. The branching is profuse with ramuli arranged in series of (2 or) 3 (or 4) above the basal ramuli. The basal ramuli are long (up to 1.5 mm) and narrow (up to 0.15 mm) in section, pointed, and gradually attenuated from the base; up to 12 times longer than wide; their margins are entire and the general outline is from falcate to extrorse (). No chevron-like appearance was observed in the apices of ramuli (). As in the type specimen of P. cartilagineum, the surfaces of the axes are host to Cocconeis scutellum (). The type specimen seems to have reached a fertile stage shortly before collection, as only young tetrasporangial stichidia were seen. The tetrasporangial stichidia are simple and fusiform or just terminally bifurcated. They replace ramuli, are up to 125 µm wide in the fertile portion, and have a short, narrow stalk (c. 70 µm in width); the tetrasporangia are up to 70 × 50 µm (). Adventitious stichidia were not noted on the axil of the basal ramulus. Kützing (Citation1866) did not specifically state that P. subtile (type locality ‘In mari atlantico’) was an elevation of his earlier illegitimate name Plocamium coccineum var. subtile Kützing (Citation1843), for which he listed ‘Helgoland’ as the type locality. However, a comparison of the two protologues suggests that it is the same taxon.
Figs 11–17. Plocamium cartilagineum. 11. Lectotype of Plocamium subtile Kützing. 12. Ramuli alternating in series of 2–4 (usually 3) above the basal ramuli. 13. Apex without chevron-like appearance. 14. Cocconeis scutellum as epiphyte. 15. Simple and bifurcated young stichidia showing tetrasporangia. 16. Lectotype for Delesseria plocamium var. uncinata. 17. Detail of the lectotype material showing fruticose habit and ramuli in series of 3 or 4 above the basal ramulus.
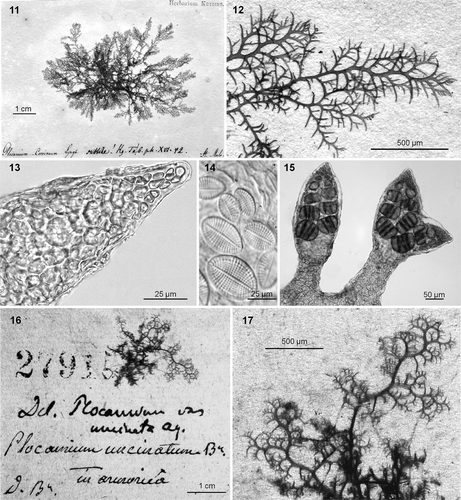
The type sheet for Delesseria plocamium var. uncinata C. Agardh (Lund 27915 in Herbarium Agardh; type locality ‘ad litora Armoricae’, northwestern coast of France; ) contains a single fragment of a sterile specimen. The thallus is cartilaginous, highly branched, dark pink-red, and is loosely attached to the paper (). Its axes are narrow (up to 0.5 mm) and subcylindrical; the fragment has a fruticose morphology. The ramuli are arranged in series of 3 or 4 above the basal ramulus and are always narrow, pointed, and very extrorse (). The basal ramulus reaches up to 1 × 0.15 mm diameter in section and its length:width ratio never exceeds 10. The habit is typical of plants grown under low water motion (Cremades et al., Citation2007).
Observations on contemporary collections ()
Our COI-5P data (discussed below; ) for 13 specimens establish conspecificity with molecular entity EUR2 of Saunders & Lehmkuhl (Citation2005). The 13 specimens yielded four distinct COI-5P haplotypes differing by 1–3 nucleotides (0.2–0.5% sequence divergence). All collections from northern Europe (Ireland, France) had the same haplotype, which was also detected in one collection from Galicia in northwest Spain. The other three haplotypes were detected in collections from locations around Spain.
In these specimens of the EUR2 clade, the thalli are erect, about 10 cm in height (up to 20 cm). The main axes are subcylindrical (0.5–) 1 (–1.5) mm in width (), and are more distinct in the lower two-thirds of the thallus (). Plants are attached to the substratum by small basal discs and haptera linked by prostrate stolons. The colour ranges from dark red with a tinge of brown, to pink-red; dried plants often turn black. The plants, particularly the lower portions of the main axes, are cartilaginous, but young thalli may have a more fleshy consistency. The branches and ramuli are repeatedly pinnate and alternate in series of (2 or) 3 (or 4) above the basal ramulus (), which is always quiescent; some plants have a particularly sparse appearance owing to a predominance of alternate series of only 2 ramuli. The basal ramulus is usually long and slender, acute at the apex, (0.5–) 2.0 (–4.5) × (0.10–) 0.25 (–0.50) mm in section, and (4–) 9 (–14) times longer than wide (). While the typical basal ramulus is often falcate at the tip, it ultimately becomes patent or even extrorse, resulting in a fruticose morphology, particularly in unattached plants or in specimens grown in little water movement (). The cortication is complete and consists of 2 or 3 layers of rather compact cortical cells. In vivo, cortical cells often have a refractive globule, possibly a kind of oil body, as well as abundant small discoid or somewhat elongated plastids ().
Figs 18–28. Plocamium cartilagineum. Barcoded specimens. 18. Habit of a large robust morph from the NE Atlantic (SANT 24199). 19. Ramuli alternate in series of 3 or 4 above the basal ramuli. 20. Subcylindrical cross-section in a main axis. 21. Compact cortication; in vivo, cortical cells with discoid plastids and a refractive globule. 22. Female gametophyte with cystocarps. 23. Upper portion of a branch, showing whitish appearance, profuse branching and abundant spermatangia. 24. Juvenile simple and apically bifurcated stichidia. 25. Simple and di-trichotomous old stichidia. 26. Tetrasporangia. 27. Small delicate individuals from the Mediterranean (SANT 24202). 28. Typical habit for plants occurring loose-lying on maërl beds (SANT 24200).

The cystocarps are globular, sessile, smooth, and lack a carpostoma; they are up to 1 mm in diameter and are distributed along the margins of the main axes as well as on ramuli (). In male gametophytes, spermatangia are plentiful on the surface of the upper portions of some branches (), which, in contrast to sterile ones, are whitish and more profusely branched. The tetrasporangial stichidia replace ramuli or are rarely adventitious in the axils of basal ramuli, and are circular in section. Juvenile stichidia are simple and fusiform (), and sometimes terminally bi- (less commonly tri-) furcate, (90–) 120 (–150) µm in diameter, and have a short stalk that is much narrower than the fertile portion (c. 70 µm in diameter). Over time, simple stichidia develop a long, sterile stalk, while bifurcated stichidia ultimately become dichotomously branched (). The zonate tetrasporangia are (70–) 80 (–90) µm in length () and are arranged in two parallel rows.
Plocamium cartilagineum s.s. has considerable morphological variability, apparently linked to water motion and nutrient availability. It can range from large strong morphs in nutrient-rich, exposed Atlantic sites () to small delicate individuals in oligotrophic, sheltered western Mediterranean locations (). It is predominantly epilithic, less commonly epiphytic, and occurs in the lower intertidal to higher subtidal of semi-exposed sites; it is likewise common as loose-lying plants () on sand, gravel or maërl seabeds. Plocamium cartilagineum s.s. is usually found at low densities, so that individual plants are readily distinguishable; individuals may also occur in clumps, but this species does not form turfs. It is common to find thalli with a mostly naked main axis covered by Melobesia membranacea and bryozoans.
European distribution: Atlantic Ocean from North Ireland to South Iberian Peninsula, Mediterranean Sea.
Comments
As shown in , important taxonomic characters such as colour, texture, thallus width, number of ramuli in a series, morphology and dimensions of the basal ramulus, tetrasporangial stichidia, and spores are all similar in the types of P. cartilagineum and P. subtile, as well as in specimens of molecular entity EUR2. Some uncertainty may remain in the case of the type of Delesseria plocamium var. uncinata, because it contains sterile material only. Still, the number of branches per series, the morphology of the basal ramulus, and the consistency of the thallus suggest that it probably belongs to the same species.
Table 2. Attributes of type material examined in this study, compared to contemporary collections assigned via molecular data to either EUR1 (P. lyngbyanum) or EUR2 (P. cartilagineum).
EUR1 = Plocamium lyngbyanum Kützing Citation 1843 : 450.
SYNONYM: ?Plocamium coccineum var. subtile [subtilis] Lyngbye Citation1819: 39.
LECTOTYPE: Leiden (L 0491620).
TYPE LOCALITY: ‘Faröer’ Faeroe Islands, Norwegian Sea, North Atlantic.
Observations on the original material
The type sheet for P. lyngbyanum () comprises three tetrasporangial fragments (two attached to the paper, the other loose). The two fragments attached to the paper seemingly belong to the same specimen; the largest is about 12 cm in height, with original annotations by Kützing (), and can be unequivocally identified as the model for Kützing's (Citation1866) . This specimen is dark pink-red, mostly membranous except for a cartilaginous base, and has relatively wide (up to 1.5 mm) and compressed main axes. The lower thallus is sparsely branched owing typically to substantial development of only the subapical ramulus in a series, while the upper portions of the thallus are more richly branched because two or three ramuli in each series reach a similar degree of development. When branching is profuse, branches are slightly attenuated at the base. Ramuli alternate in series of (3 or) 4 (or 5) above the basal ramulus; the latter is comparatively short and broad (up to 2.5 × 0.5 mm, length:width ratio up to 5.5), somewhat attenuated at the base, has entire margins, and has blunt or truncated tips (). Under the microscope, the apices of the ramuli are obtuse and do not have a chevron-like appearance (). In surface view, the cortication can be seen to be incomplete and fenestrated (), and appears partially covered by a thin layer of encrusting coralline algae (bryozoans were not noted).
Figs 29–38. Plocamium lyngbyanum. 29. Lectotype of Plocamium lyngbyanum Kützing. 30. Ramuli alternate in series of 4 or 5 above the basal ramuli. 31. Apex without chevron-like appearance. 32. Incomplete cortication. 33. Juvenile stichidia palmately divided. 34. Old compound stichidium with third-order ramification. 35. Slightly compressed cross-section of a stichidium showing tetrasporangia. 36. Tetrasporangia in detail. 37. Clusters of stichidia alternating along the bare section of a main axis. 38. Stichidia growing at the tip of basal ramuli can mask the sympodial look typical of the genus.
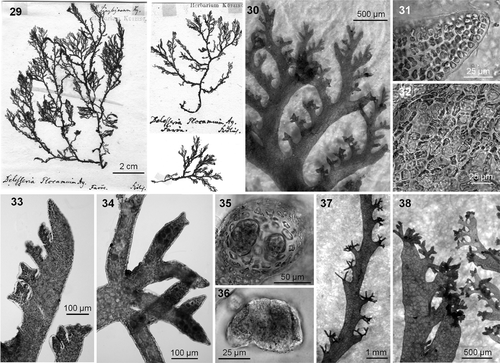
Figs 39–51. Plocamium lyngbyanum. Barcoded specimens. 39. Habit of a large robust morph from NW Spain (SANT 24196). 40. Ramuli alternate in series of 3–5 above the basal ramuli. 41. Main axis with slightly compressed cross section. 42. Incomplete cortication with fenestrated conformation. 43. Cystocarps on a female gametophyte. 44. Branch of a male gametophyte showing abundant spermatangia. 45. Palmately divided young stichidia. 46. Old compound stichidia with third-order branching. 47. Tetrasporangia. 48. Dense axillary clusters of compound stichidia in older main axes. 49. Compound stichidia growing on the tip of basal ramuli (GW001801, North Ireland). 50. Basal ramulus with serrate margin (SANT 24195). 51. Main axes infested by the bryozoan Electra pilosa.

The tetrasporangial stichidia are compound and replace ramuli in the series, as well as forming in axils and/or at the tips of basal ramuli. Juvenile stichidia are produced in series of (1 or) 2–4 (or 5), are palmately divided, and possess a short and poorly differentiated stalk (). As stichidia develop, they continue to grow in length and divide so that mature structures (up to 100 µm wide in section) may have up to third-order branching (). Tetrasporangia are up to 70 × 50 µm in dimensions (). The production of stichidia in axils, as well as at the tips of basal ramuli, results in clusters of stichidia alternating along the bare sections of the main axes (). As a result fertile branches can lose the typical sympodial look () of this genus. Lyngbye (Citation1819, table 9, fig. b2) illustrated a fertile tip with ramuli in series of four, compound stichidia, and stichidia also forming at the tips of basal ramuli for his P. coccineum from the Faeroe Islands.
Observations on contemporary collections ()
We sequenced 11 specimens, resolving three COI-5P haplotypes differing by 1 or 2 nucletotides (0.2 or 0.3%). One haplotype dominated throughout the sampled range (nine specimens from Northern Ireland to Galicia in Northwest Spain); the other two were found in single collections from Galicia and Brittany, respectively. In contrast to the low intraspecific variability, sequence divergence relative to EUR2 was considerable and ranged from 51–55 nucleotide changes (8.6–9.1%). Our COI-5P data establish conspecificity with the molecular entity EUR1 of Saunders & Lehmkuhl (Citation2005).
Our EUR1 specimens have erect thalli, (9−) 12 (−20) cm in length. They are pink-red and some plants are pale with the central axial filament visible through the cortex in the younger parts; the fronds can have a gentle blue iridescence when submerged. Individual thalli are often readily distinguishable because they never grow in dense clumps; however, several axes may still arise from a single point. The thalli are membranous, somewhat fleshy and quite flexible; only the lower portions of the thallus are cartilaginous. They are attached by short basal stolons from which several broad, (1–) 1.7 (−2.5) mm wide and slightly compressed main axes arise (); the latter, by sympodial growth, produce fronds divided (up to 5 or 6 orders) in a single plane (). Ramuli alternate in series usually of 4 or 5, more rarely 3 or 6 above the basal ramulus (). The basal ramulus is obtuse, wide, (0.8–) 2 (−3) × (0.15−) 0.4 (−0.7) mm and (3−) 5 (−8) times longer than wide, slightly recurved, somewhat attenuated at the base, with an often wavy or even irregularly serrate outer margin (). The terminal and subterminal ramuli in a series are equally developed; this produces a flabellate appearance, accentuated by the presence of pseudodichotomies, which distort the main axes distally ().
The cortication is incomplete and consists of 1 or 2 layers of cortical cells (); some areas resemble the fenestrated conformation typical of P. raphelisianum (cf. Cremades et al., Citation2007, ). In vivo, cortical cells have abundant, somewhat elongated, small discoid plastids, and often a refractive globule (possibly a kind of oil body).
The cystocarps are globular, sessile, up to 1 mm in diameter, and occur singly or in groups of 2 or 3, and are formed along the margins of the main axes and on the ramuli (). Spermatangia are distally plentiful on the surface of some branches, which are lighter-coloured and more delicate than sterile ones (). Tetrasporangial stichidia are cylindrical or slightly compressed in section, and replace ramuli or arise in axillary locations (); they are rarely located at the tip of the basal ramulus (). Juvenile stichidia are (1 or) 2–4 (or 5) times palmately divided, with a short and poorly differentiated stalk () and branches (75–) 100 (–125) µm wide. Over time, they increase in length and continue to divide so that mature stichidia display up to third-order branching () and eventually form dense clusters in older specimens (). Tetrasporangia are (50–) 60 (–70) µm long ().
At least in the northwest Iberian Peninsula, where it has been extensively studied, P. lyngbyanum is almost exclusively epiphytic on Gelidium corneum and less frequently on Chondrus crispus. Host plants are always heavily infested by Electra pilosa, suggesting that this bryozoan may facilitate the epiphytism of Plocamium. The same bryozoan is likewise common on the lower portions of the Plocamium itself (). Fertile adult plants occur throughout the year. Typical of exposed sites, P. lyngbyanum occurs from lower intertidal pools down to 15 m depth; in the Iberian Peninsula, its optimum is at 2–3 m.
Plocamium lyngbyanum is known from the Faroe Islands down to the northwest of Spain. Its morphological variability is moderate and seems linked to local conditions, as well as to geographical origin. Thus, changes in water motion seemingly have a considerable effect on branching and thallus size. In northwest Spain, where it is commonly found living with Gelidium corneum in sites of strong water motion, this species typically has a broad robust habit (). Likewise, our observations suggest that the production of stichidia at the tips of basal ramuli may be linked to high latitude specimens, because this particular arrangement is common in the type material, was clearly illustrated by Lyngbye (Citation1819) and Kützing (Citation1866), and was found in one of our collections from the north of Ireland (GW001801, ).
European distribution: Atlantic Ocean: Norway, Denmark (Faeroe Islands), Scotland, Ireland & North Ireland, Brittany and NW Spain.
Comments
shows that the type of P. lyngbyanum and our collections of EUR1 sensu Saunders & Lehmkuhl (Citation2005) match one another well in all the main taxonomic features, such as colour, texture, thallus width, number of ramuli per series, morphology and dimensions of basal ramulus, tetrasporangial stichidia, and tetrasporangial dimensions.
According to Børgesen (Citation1902: 367) there is no type material for P. coccineum var. subtile in the herbarium of Lyngbye and so the taxonomic identity of this taxon remains uncertain. In the original publication, Lyngbye (Citation1819: 39) noted that this variety differed from the typical morph of P. lyngbyanum (as P. coccineum) by its ‘fronde tenuiori’, noting that the variety subtile was as abundant as the typical morph. Interestingly, both Børgesen (Citation1902) and Irvine (Citation1982) reported a single species of Plocamium in the Faeroe Islands, noting that it was extremely abundant and often epiphytic on other algae. It, therefore, seems likely that P. coccineum var. subtile may be only a delicate morph of P. lyngbyanum.
Plocamium raphelisianum has a habit quite similar to that of P. lyngbyanum and, in fact, the two species have often been misidentified in the Iberian Peninsula. However, they have highly divergent COI-5P sequences and P. raphelisianum is readily distinguished by being more sparsely branched – ramuli occur in series of 2 or 3 above the basal ramuli, which are wide and sturdy – and the plants are mostly saxicolous, have a membranous (not fleshy) thallus, are purple-red, and exhibit a strong blue iridescence when submerged (Cremades et al., Citation2007).
Plocamium binderianum Kützing Citation 1843 : 450.
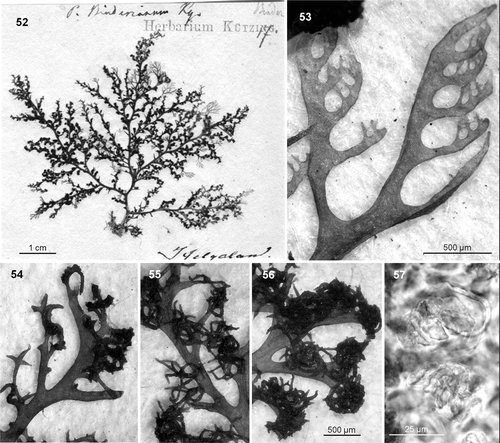
The type material for Plocamium binderianum Kützing (Leiden, L 0491619; type locality: ‘Helgoland’ Germany, North Sea; ) consists of a tetrasporangial plant 6 cm in height, richly branched throughout, and with no clear main axis (). The specimen is firmly attached to the paper and it is quite evident that it served as the model for Kützing's (Citation1866) . The thallus is slightly cartilaginous and dark red, not pinkish or brownish or purple; its lower portions are host to a colony of the bryozoan Electra pilosa. The ramuli are in series of 3 or 4 above the basal ramuli (), which are somewhat long and narrow, gradually attenuated from the base, up to 1.25 × 0.15 mm in section, and up to 10 times longer than wide, sometimes slightly patent or recurved with entire margins. The tetrasporangial stichidia replace ramuli and are long, strongly recurved, proliferous, and have stalks 75 µm wide (). They are likewise common in the axil of the basal ramulus. Despite the size and abundance of stichidia, tetrasporangia are scarce, seemingly immature (maximum size 40 × 32 µm, ) and they occur loose and disordered within the stichidia (as in Kützing's drawings).
Figs 52–57. Plocamium binderianum. 52. Lectotype of Plocamium binderianum Kützing. 53. Ramuli alternate in series of 4 above the basal ramuli. 54–56. Burgeoning long and strongly recurved stichidia (the three figures are at the same scale). 57. Surface view of the scarce and anomalous tetrasporangia.
Comments
Given its peculiar morphology, it is difficult to tell whether P. binderianum represents a distinct species or just an aberrant form of one of the previous two species. Several pieces of evidence suggest that the type material may be a particularly long-lived specimen, viz. the stichidia are tremendously developed, new adventitious shoots occur scattered throughout the thallus, and there is an abundance of epiphytic diatoms (Cocconeis scutellum) and bryozoans in basal areas. Longevity, or even some infectious process, may thus explain the unusual morphology of the stichidia, as well as the paucity of tetrasporangia. Undoubtedly, it was this bizarre morphology that led Kützing to separate P. binderianum as a new species. Fortunately, even if P. binderianum was an aberrant specimen of either EUR1 or EUR2, it would not be the nomenclatural type of either. In the case of EUR2, the name P. cartilagineum has priority over P. binderianum, while in the case of EUR1, although the names P. binderianum and P. lyngbyanum have equal priority, the type of P. lyngbyanum undoubtedly is far more representative of the species and should thus be preferred.
Molecular analyses and taxonomic characters in Plocamium
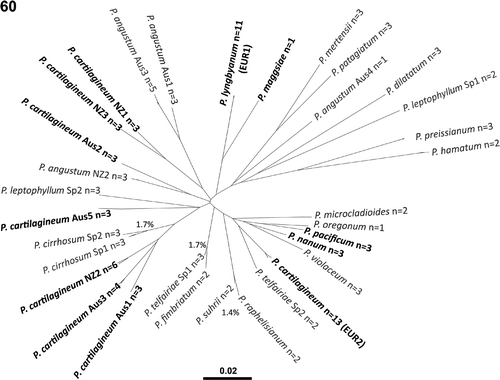
Our molecular analyses are consistent with published data (Saunders & Lehmkuhl, Citation2005) in revealing multiple divergent lineages for specimens typically assigned to Plocamium cartilagineum s.l. (, bold type). Of particular importance here, four European lineages (viz. P. cartilagineum s.s., P. lyngbyanum, P. maggsiae and P. nanum) were uncovered that are highly divergent in COI-5P sequence. While the improved resolution of COI-5P over LSU rDNA (used by Saunders & Lehmkuhl, Citation2005) did not uncover additional cryptic species in northern Europe, broadening our taxonomic sample to assess the relative divergence of the European specimens has established that overlooked species represent a chronic problem in the genus Plocamium (e.g. P. angustum, P. cirrhosum, P. leptophyllum and P. telfairiae; ), indicating that considerable taxonomic work remains to be done.
While molecular data (LSU and COI-5P: Saunders & Lehmkuhl, Citation2005, and this paper) show that P. cartilagineum and P. lyngbyanum are clearly distinct species, discriminating them exclusively on the basis of morphology remains difficult (). Non-tetrasporangial specimens present a particular challenge, because the most reliable attributes for species discrimination are the morphology and size of stichidia and tetrasporangia. Unfortunately, even when these reproductive features are present, they must be used with caution because it is not uncommon to find specimens with intermediate or even variable stichidial morphology, and tetrasporangium size has been found to vary with position within the stichidia, maturation or even latitude (noted here for P. lyngbyanum). Therefore, the other morphological attributes conventionally employed for distinguishing species of Plocamium in other areas of the world (colour, consistency, thallus width and section shape, number of ramuli per series, morphology and size of basal ramulus, habitat: see Simons, Citation1964; Womersley, Citation1971; South & Adams, Citation1979; Gabrielson & Scagel, Citation1989; Wynne, Citation2002) are a useful, although imperfect, complement for species discrimination in northern Europe.
We have observed that a number of other characters (a chevron pattern in growing tips, ramuli with gall-like tips and/or marginal serrations, the curvature of ramuli, the degree of cortication) possibly depend more on age, growth rate, and/or habitat than on species identity. Therefore, and instead of the diagnostic value attributed to these features by Saunders & Lehmkuhl (Citation2005), we recommend that these traits should not be used for species discrimination. For example, the chevron pattern often shows differences among growing tips even within a single specimen (evident in compressed active tips, blurred in cylindrical less active ones; ); moreover, it often becomes more evident when growing tips are fixed with formalin than when they are examined fresh. Similarly, the presence of truncated branches with gall-like tips possibly results from abrasion against a hard substratum (e.g. in saxicolous and loose-lying specimens). Also, Saunders & Lehmkuhl (Citation2005) considered the presence of marginal serrations a distinctive attribute of P. cartilagineum (as P. subtile), but we have found that it is relatively common in P. lyngbyanum (identity confirmed by molecular data) (), as well as in other European species of Plocamium (P. maggsiae, P. raphelisianum). Cortication seems dependent on light intensity and/or desiccation resistance. Thus, subtidal plants living under low light intensity usually have a clearly fenestrated cortication (i.e. the cortical cell layer is discontinuous and mostly clumped above the junctions of the larger inner cells), while in intertidal specimens the outer cortical layer is often complete and highly compact. Finally, we have found that the presence of extrorse branches resulting in a fruticose habit is relatively common in several species and seems linked to poor growth polarity due to a lack of appropriate directional stimuli. Thus, the fruticose habit and gall-like tips are easily found in unattached plants or in specimens living where the water is relatively still (). Indeed, some species are known to have a fruticose morphology only in portions of the thallus subject to these factors, e.g. in extensive basal systems and secondary proliferations grown on some plants after detaching from the substratum (). This phenomenon was already reported for P. raphelisianum (Cremades et al., Citation2007, ).
Figs 58, 59. Plocamium spp. 58. Extensive basal system with fruticose morphology of P. maggsiae. 59. Similar morphology with some gall-like tips in secondary proliferations of a detached specimen of P. lyngbyanum.

Fig. 60. Neighbour-joining tree for the COI-5P sequence data used in this study (as a visual representation of species diversity and relative divergence, not to be interpreted as a robust phylogenetic analysis). Lineages traditionally considered as Plocamium cartilagineum s.l. are indicated in bold type. ‘n =’ indicates the number of samples that resolved within each genetic species group. For closely related genetic groups the per cent COI-5P divergence is indicated.
Conclusions
The taxonomy of Plocamium in northern Europe is experiencing a true revolution. In the last few years we have moved from one supposedly cosmopolitan species to five distinct taxa of more or less restricted distribution. Plocamium maggsiae and P. nanum are two small intertidal species recently described by Saunders & Lehmkuhl (Citation2005). Plocamium nanum seems rather restricted in its distribution, while P. maggsiae may be more widely distributed in Europe (manuscript in preparation). The other three are larger subtidal species with P. cartilagineum and P. lyngbyanum widely distributed in northern Europe while P. raphelisianum, originally described from North Africa, is thus far known in Europe only from the Iberian Peninsula (Cremades et al., Citation2007).
Acknowledgements
We thank P. Lassen (Lund Herbarium) and W. Prud’homme van Reine (Leiden Herbarium) for herbarium loans and consultation. This research was funded by Spain's Dirección General de Enseñanza Superior, Ministerio de Educación y Ciencia (grant PB95-0385-C06-02) and Secretaría de Estado de Investigación, Ministerio de Ciencia e Innovación (grant CTM2007-61011). All of the collectors listed in are acknowledged for their critical involvement in this project. This research was supported through funding to the Canadian Barcode of Life Network from Genome Canada through the Ontario Genomics Institute, NSERC and other sponsors listed at www.BOLNET.ca. Additional support was provided by the Canada Research Chair Program, the Canada Foundation for Innovation and the New Brunswick Innovation Foundation.
References
- Agardh , CA . 1817 . Synopsis algarum Scandinaviae , Lund .
- AGARDH, C.A. (1822). Species algarum. Vol. 1, Part 2. Lund.
- Benhissoune , S , Boudouresque , CF , Perret-Boudouresque , M and Verlaque , M . 2002 . A checklist of the seaweeds of the Mediterranean and Atlantic coasts of Morocco . Bot. Mar. , 45 : 391 – 412 .
- BØRGESEN, F. (1902). The marine algae of the Faeroes. In Botany of the Faeroes. Part 2(H.H. Thiele, editor); Copenhagen.
- Cremades Ugarte , J , Bárbara Criado , I and No Couto , E . 2007 . Sobre la presencia del rodófito Plocamium raphelisianum Dangeard (Plocamiales, Florideophyceae) en las costas meridionales europeas . Anales Jard. Bot. Madrid , 64 : 35 – 42 .
- Dixon , PS . 1967 . The typification of Fucus cartilagineus L. and F. corneus Huds . Blumea , 15 : 55 – 62 .
- Gabrielson , PW and Scagel , RF . 1989 . The marine algae of British Columbia, northern Washington and Southeast Alaska: division Rhodophyta (red algae), class Rhodophyceae, order Gigartinales, families Caulacanthaceae and Plocamiaceae . Can. J. Bot. , 67 : 1221 – 1234 .
- Gmelin , SG . 1768 . Historia fucorum , St. Petersburg .
- Hudson , W . 1778 . Flora anglica, , 2nd , London .
- Irvine , DEG . 1982 . Seaweeds of the Faroes: 1. The Flora . Bull. Brit. Mus. (Nat. Hist.), Bot. , 10 : 109 – 131 .
- Kützing , FT . 1843 . Phycologia generalis , Leipzig .
- Kützing , FT . 1849 . Species algarum , Leipzig .
- Kützing , FT . 1866 . Tabulae phycologicae , Vol. 16 , Nordhausen .
- Lamouroux , JVF . 1813 . Essai sur les genres de la famille des thalassiophytes non articulées . Ann. Mus. Natl. Hist. Nat. , 20 : 21 – 47 . 115–139, 267–293, pls. 7–13
- LINNAEUS, C. (1753). Species plantarum, 2. Stockholm.
- Lyngbye , HC . 1819 . Tentamen hydrophytologiae danicae , Hafniae .
- MADDISON, W. & MADDISON, D. (2003). MacClade, v. 4.06. Sinauer Associates, Massachusetts.
- Munda , IM . 2005 . Seasonal fouling by diatoms on artificial substrata at different depths near Piran (Gulf of Trieste, Northern Adriatic) . Acta Adriat. , 46 : 137 – 157 .
- No Couto , E and Cremades , J . 2001 . Contribución al conocimiento de Plocamium raphelisianum Dangeard (Plocamiales, Rhodophyta) en las costas de la Península Ibérica . Bol. Soc. Esp. Ficol. , 26 : 21
- Robba , L , Russell , SJ , Barker , GL and Brodie , J . 2006 . Assessing the use of the mitochondrial cox1 marker for use in DNA barcoding of red algae (Rhodophyta) . Am. J. Bot. , 93 : 1101 – 1108 .
- Saunders , GW . 2005 . Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications . Phil. Trans. R. Soc. B , 360 : 1879 – 1888 .
- Saunders , GW and Lehmkuhl , K . 2005 . Molecular divergence and morphological diversity among four cryptic species of Plocamium (Plocamiales, Florideophyceae) in northern Europe . Eur. J. Phycol , 40 : 293 – 312 .
- Sherwood , AR , Sauvage , T , Kurihara , A , Conklin , KY and Presting , GG . 2010 . A comparative analysis of COI, LSU and UPA marker data for the Hawaiian florideophyte Rhodophyta: implications for DNA barcoding of red algae . Cryptogamie Algol. , 31 : 451 – 465 .
- Simons , RH . 1964 . Species of Plocamium on the South African Coast . Bothalia , 8 : 183 – 193 .
- South , GR and Adams , NM . 1979 . A revision of the genus Plocamium Lamouroux (Rhodophyta, Gigartinales) in New Zealand . Phycologia , 18 : 120 – 132 .
- SWOFFORD, D.L. (2003). PAUP*. Phylogenetic Analyses Using Parsimony (*and Other Methods). Sinauer Associates, Massachusetts.
- Womersley , HBS . 1971 . The genus Plocamium (Rhodophyta) in southern Australia . Trans. R. Soc. S. Aust. , 95 : 9 – 27 .
- Wynne , MJ . 2002 . A description of Plocamium fimbriatum sp. nov. (Plocamiales, Rhodophyta) from the Sultanate of Oman, with a census of currently recognized species in the genus . Nova Hedwigia , 75 : 333 – 356 .