Abstract
Members of the Galaxauraceae are all calcified and have a wide distribution from tropical to temperate regions. Of the four genera of Galaxauraceae, only Dichotomaria contains two distinct thallus forms, which are (1) a flattened form with either narrow (e.g. D. marginata) or wide (e.g. D. diesingiana) branches, and (2) a terete form with either slender (e.g. D. papillata) or robust (e.g. D. obtusata) branches. In this study, we present a molecular phylogeny of new Dichotomaria samples from the Indo-Pacific region and describe a new species, D. hommersandii S.L. Liu & S.M. Lin, from South Africa, based on rbcL sequence analysis and morphological evidence. Dichotomaria hommersandii is characterized by a thallus consisting of terete smooth branches (0.5–1.5 mm in diameter), a moniliform branching pattern with constrictions at the nodes, ramification of young branches derived from the damaged terminal regions of the old branches, and cystocarps containing a conspicuous fusion cell. Our phylogenetic analyses indicate that the terete thallus morphology could be the ancestral character state in Dichotomaria.
Introduction
The family Galaxauraceae (Nemaliales) consists of a group of calcareous red algae that are widely distributed from tropical to temperate shallow waters (Kjellman, Citation1900; Huisman, Citation2006; Wang et al., Citation2005). Until recently, seven genera were included in the family, including three calcified genera (Actinotrichia, Galaxaura and Tricleocarpa) and four noncalcified genera (Scinaia, Gloiophloea, Nothogenia and Whidbeyella). Huisman et al. (Citation2004a ) established the family Scinaiaceae for the four noncalcified genera, based on an LSU sequence analysis, and resurrected the genus Dichotomaria, which was previously synonymized under Galaxaura. Later, Wang et al. (Citation2005) characterized the genera Dichotomaria and Galaxaura based on cystocarp development and inferred the generic relationships between these calcified algae based on rbcL sequence analyses.
The four genera of the Galaxauraceae can now be separated morphologically by their life history and post-fertilization development of cystocarps. The life history comprises isomorphic thalli in Actinotrichia (Wang & Chiang, Citation2001; Liu & Wang, Citation2009), isomorphic thalli with a dimorphic cortex in Dichotomaria (Kurihara et al., Citation2005; Wang et al., Citation2005), dimorphic thalli in Galaxaura (Huisman et al., Citation2004b ; Wang et al., Citation2005), and heteromorphic thalli in Tricleocarpa (Magruder, Citation1984, as Galaxaura oblongata). Although some might argue that the life history could be described as dimorphic (i.e. with dimorphism of either the thalli or the cortex) in both Galaxaura and Dichotomaria (e.g. Howe, Citation1917, Citation1918; Huisman & Borowitzka, Citation1990), Wang et al. (Citation2005) suggested that the term should be restricted to the dimorphism of the thalli – the non-hairy gametophyte and hairy tetrasporophyte – in the life cycle of Galaxaura, in contrast to the isomorphic thalli seen in Dichotomaria (smooth gametophyte and tetrasporophyte). The reason is that it is preferable to use ‘dimorphism’ to refer to macroscopic differences and the dimorphism in Dichotomaria can only be seen microscopically; hence it is more appropriate to apply the term only to Galaxaura, not to Dichotomaria. With respect to cystocarp development, pericarp filaments in Dichotomaria never extend into the cystocarp cavity and never intermix with the gonimoblast filaments (Wang et al., Citation2005). In contrast, pericarp filaments in both Actinotrichia and Tricleocarpa can extend from the inner cystocarp wall and produce numerous paraphyses that may or may not intermix with the gonimoblast filaments (Huisman & Borowitzka, Citation1990; Liu & Wang, Citation2009). The pericarp of Galaxaura only surrounds the carpogonial branch and never the gonimoblast filaments (Wang et al., Citation2005; Liu et al., Citation2012).
Of the four genera of the Galaxauraceae, only Dichotomaria displays gross variation in thallus morphology, ranging from flattened forms (e.g. D. diesingiana and D. marginata), to terete forms (e.g. D. obtusata) (Huisman et al., Citation2004a ; Wang et al., Citation2005), whereas the other three genera contain only terete species. As yet, it is unknown whether the terete or the flattened thallus represents the most recent common ancestral thallus morphology in Dichotomaria.
Recently, we obtained specimens from South Africa that were identified by Max H. Hommersand as an unknown Galaxaura species. The external morphology of these specimens was similar to Galaxaura in having a narrow terete thallus. However, an rbcL sequence generated from one of these samples suggested that it was phylogenetically allied to Dichotomaria. In this study, the goals were twofold. First, we made a wider study of Galaxauraceae, using rbcL sequence and parsimonious trait evolution analyses, to examine phylogenetic relationships and the evolution of thallus development in Dichotomaria. Second, we aimed to describe the unknown ‘Galaxaura’ species formally (as a new Dichotomaria), based on molecular and morphological evidence, and to provide a comparative table to aid identification.
Materials and methods
Sample collection and morphological examinations
Samples were collected from exposed rocks at low tide. After collecting, each new specimen was immediately subdivided, with part of the specimen preserved in silica gel for molecular analysis and the remainder placed in 5–10% formalin for morphological observations. The 1983 collection was only preserved in 5–10% formalin. Voucher specimens were deposited at the University of California Herbarium at Berkeley in USA (UC), the Department of Biology, University of North Carolina in USA (UNC), the Department of Life Science, Tunghai University in Taiwan (TUNG), and the Herbarium, Biodiversity Center, Academia Sinica, Taipei in Taiwan (HAST). For morphological observations, terminal portions of branches were decalcified by immersing in 1–3% HCl solution and then either squashed or sectioned by hand using a razor blade. Squashed or transverse sections were stained either in 1% aniline blue (AB) overnight or with aceto-haematoxylin-chloral hydrate (AHC) (Wittmann, Citation1965). The AB-treated materials were mounted in 50% Karo corn syrup solution, whereas the AHC-treated materials were mounted in 50% Hoyer's mounting medium. Photographs were taken using a Canon EOS600D digital camera (Tokyo, Japan) mounted on a Leica DM750 light microscope (Frankfurt, Germany). Drawings of female reproductive structures were made with the aid of a Leica camera lucida (Frankfurt, Germany).
DNA sequencing and phylogenetic analyses
Genomic DNA was extracted using the DNeasy Plant Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer's instruction manual. Gene amplification and DNA sequencing procedures followed Lin et al. (Citation2001) and Wang et al. (Citation2005). Specific primers used for gene amplification and sequencing are listed in Lin et al. (Citation2001) and Saunders (Citation2005). The sequencing was conducted using an ABI 3730 DNA Sequencer (Applied Biosystems, Foster, California) at the Mission Biotechnology Company (Taipei, Taiwan). The rbcL and COI-5P sequences for D. hommersandii were deposited in GenBank (accessions JX072968 and KC253901, respectively). Information concerning the taxa included in the phylogenetic analysis is given in the Supplementary Table.
For phylogenetic analyses, additional rbcL sequences from 26 representative ingroup taxa belonging to the Galaxauraceae were downloaded from GenBank (Supplementary Table). Two members of the family Scinaiaceae were also obtained from GenBank and served as the outgroup (Supplementary Table). Prior to the phylogenetic analyses, the sequences were aligned using MUSCLE with the default settings (Edgar, Citation2004), then manually refined using BioEdit (Hall, Citation1999). Phylogenetic analysis was conducted using a Maximum Likelihood (ML) method and a Bayesian Inference method. Before ML analysis, we used MEGA5 (Tamura et al., Citation2011) to find the best nucleotide substitution model for our dataset, out of the 24 models included. The Tamura–Nei model (Tamura & Nei, Citation1993) + gamma distribution + invariable sites was selected as the best fit because of the lowest BIC (Bayesian information criterion) score. The parameters were as follows: assumed nucleotide frequencies A = 0.3191, T = 0.3077, C = 0.1609, G = 0.2123; nucleotide substitution matrix with A–T = 0.04, A–C = 0.02, A–G = 0.09, T–A = 0.04, T–C = 0.18, T–G = 0.03, C–A = 0.04, C–T = 0.35, C–G = 0.03, G–A = 0.13, G–T = 0.04, G–C = 0.02; proportion of sites assumed to be invariable = 0.5837; and rates for variable sites assumed to follow a gamma distribution with the shape parameter = 1.5025. Phylogenetic inference by ML was also computed using MEGA5. Support for nodes was determined by using the bootstrapping method (Felsenstein, Citation1985) with 1000 bootstrap replicates of ML analyses. The pairwise divergence of rbcL sequences among different specimens or between any two different lineages was estimated using a Maximum Likelihood method. When calculating the pairwise divergence, the function “pairwise deletion” was used in MEGA5, because the rbcL sequences of some taxa were incomplete. In the Bayesian Inference method, four chains of the Markov Chain Monte Carlo (one hot and three cold) were implemented starting with a random tree using the software MrBayes v3.1.2 (Huelsenbeck & Ronquist, Citation2001). One tree from every 1000 generations was sampled over the course of 1 000 000 generations. Seventy-five per cent of trees at the stationary phase were saved when the average standard deviation of split frequencies was below 0.008. The phylogenetic inference was based on one of those saved trees. The posterior probabilities for nodes were estimated by computing the 50% consensus tree [majority rule as implemented in PAUP* v.4.0 beta 10 (Swofford, Citation2003)] from the saved trees.
Results
Molecular analyses
The COI-5P sequence (KC253901) was not used for phylogenetic analysis but was obtained to enable future barcoding comparisons. The rbcL sequence alignment initially included 1467 characters, but the first 87 sites were excluded from further phylogenetic analysis because information was missing at the 5′ end of the rbcL sequences in many species. In total, 1380 nucleotide positions were used to infer the phylogenetic relationships of Galaxauraceae.
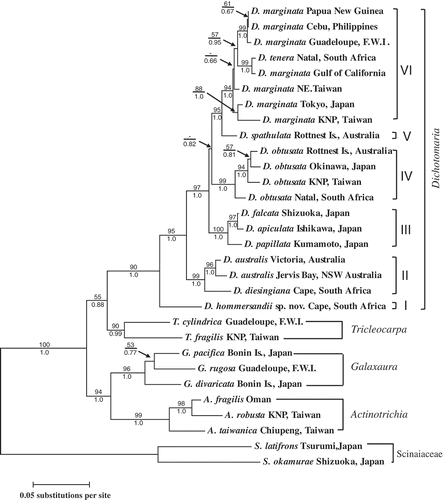
The tree topology derived from the ML method was largely congruent with that of the Bayesian analysis, and only the ML tree is presented (). The molecular analyses showed that the species from the four genera (Dichotomaria, Tricleocarpa, Galaxaura and Actinotrichia) grouped into four monophyletic assemblages with moderate to strong support. Within the Dichotomaria assemblage, the species grouped into six well-supported clades (I–VI). The basal clade (clade I) consisted of our newly generated sequence from the South African entity (described here as D. hommersandii sp. nov.: see below), which has a terete thallus. The rbcL pairwise genetic distances between D. hommersandii and the other Dichotomaria species were 6.8–8.3% (Tamura–Nei model). The second clade (clade II) consisted of three different flattened specimens: D. diesingiana from South Africa and two specimens identified as D. australis from southern Australia. The third lineage (clade III) consisted of one terete species from Japan, D. papillata, and two different flattened species from Japan, D. apiculata and D. falcata. The fourth lineage (clade IV) was the D. obtusata assemblage, which is as of yet relatively uncharacterized with regard to morphological features. This assemblage showed more genetic variation (with percentage divergences of 1.3–2.7%) than the D. marginata assemblage and included four different terete specimens from Natal (South Africa), Kenting National Park (Taiwan), Okinawa (Japan) and Rottnest Island (Australia). The fifth lineage (clade V) contained a flattened species, D. spathulata, from Rottnest Island. The final lineage (clade VI), referred to here as the D. marginata assemblage, comprised several flattened taxa, including D. tenera from South Africa and several D. marginata specimens from different locations worldwide. The D. marginata assemblage has not yet been completely characterized, due to a lack of detailed morphological information. Although the D. obtusata assemblage was sister to the monophyletic group composed of D. spathulata and the D. marginata assemblage, there was no support for this relationship from bootstrapping in the ML analyses and only weak support in the Bayesian analysis (< 0.85; posterior probabilities from Bayesian analyses usually inflate statistical support: Yang & Rannala, Citation2012). Therefore, it is possible that the D. obtusata assemblage might also be a sister clade of the third lineage (i.e. D. papillata, D. apiculata and D. falcata) and the exact phylogenetic placement of the D. obtusata assemblage among other flattened species requires further investigation. In contrast to the other three genera that only possess terete thalli, Dichotomaria possesses a wider variability of the thallus morphology from an evolutionary standpoint, switching back and forth between the terete and flattened thallus morphologies (see for details).
Morphological observations
Based on the rbcL sequence analyses, as well as the morphological features described below, it was evident that the narrow terete species from South Africa is distinct from any currently recognized species in Dichotomaria or Galaxaura, although it must be noted that rbcL sequences have yet to be obtained for many putative Dichotomaria species. Therefore, we describe the South African species here as a new species. A tabular key to the terete species of Dichotomaria and Galaxaura that have been described is given in .
Table 1. Comparison of the external and vegetative characteristics of terete Dichotomaria and some Galaxaura species that are morphologically similar to the genus Dichotomaria. + = present, – = absent
Dichotomaria hommersandii S.-L. Liu & S.-M. Lin, sp. nov.
(Figs 2–30)
Description: Thalli up to 20 cm tall, dark red when dried, heavily calcified, articulated, dichotomously branched every 5–20 mm, arising from a small hirsute discoid holdfast up to 0.5 cm in diameter. Branches terete, up to 1.5 mm in diameter, composed of pseudoparenchymatous cortex with 3 (or 4) cell layers and a filamentous medulla. Dioecious. Carpogonial branches three-celled, consisting of a carpogonium, a hypogynous cell, and a basal cell. Post-fertilization carpogonium cutting off a gonimoblast initial laterally, this subsequently producing numerous gonimoblast filaments directly. Hypogynous cell cutting off several sterile branches with enlarged nuclei during cystocarp development, and the basal cell producing involucral filaments that form a pericarp surrounding the sterile branches from the hypogynous cell and the gonimoblast filaments. The pit connections between several inner gonimoblast cells, the basal gonimoblast initial, the carpogonium, the hypogynous cell, and the basal cell break down and a distinct long fusion cell is formed. Cystocarp conceptacles hemispherical, 550–700 μm in diameter, with moderately diffuse gonimoblasts. Carposporangia obovoid to ovoid, 8–20 μm wide by 20–50 μm long.
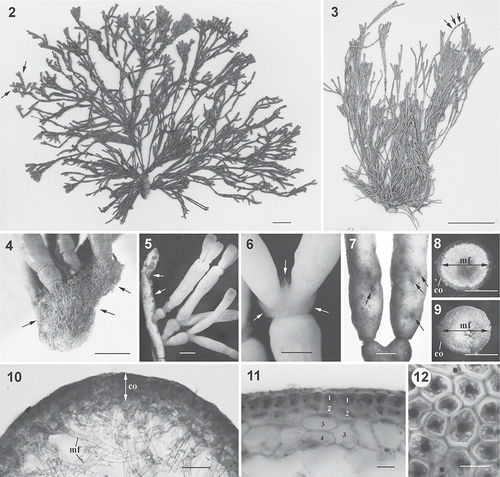
Figs 2. Dichotomaria hommersandii. Habit and vegetative structure (Natal, South Africa). 2. Holotype: a female plant showing ramified branchlets (arrows) at upper part and narrower terete branches at lower part of thallus. 3. Isotype: a female plant showing constrictions at internodes (arrows) that form the moniliform thallus. 4. A silica gel-preserved, female plant showing a holdfast consisting of numerous rhizoidal filaments (arrows), and well-developed proliferations (upper). 5. Upper part of thallus showing numerous young branches arising from lesions of a senescent branch (arrows). 6. Nodes showing production of numerous long assimilatory filaments (arrows). 7. Close-up of ostioles of cystocarps (arrows). 8. Cross-section through a branch showing cortical layer (co) and central medullary filaments (mf) with heavily calcification (white in colour). 9. Cross-section through a branch showing cortical layer (co) and central medullary filaments (mf) after decalcification. 10. Detail of cross-section through a branch, showing outer layer of cortical region (co) and inner layer of medullary filaments (mf). 11. Detail of cortex showing 3- or 4-celled cortical structure. Number indicates the different layer of cortical cells. 12. Surface view of upper part of smooth branch showing 5- or 6-sided polygonal cortical cells. Scale bars = 2 cm (Fig. 2), 5 cm (Fig. 3), 1 mm (Figs 4, 5), 0.5 mm (Figs 6–9), 100 µm (Fig. 10), 25 µm (Fig. 11) and 15 µm (Fig. 12).
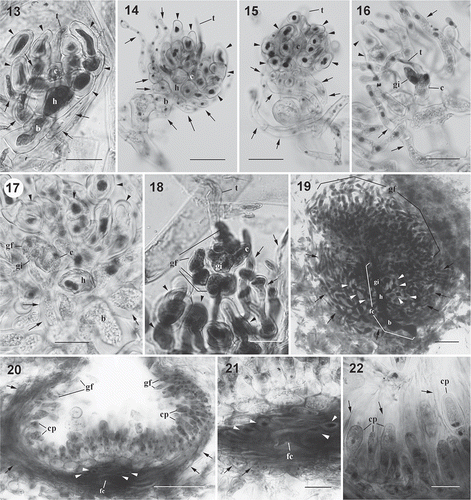
Figs 3. Dichotomaria hommersandii, cystocarp development. (Natal, South Africa). 13. Young carpogonial branch showing carpogonium (c) with trichogyne (t), hypogynous cell (h) producing several enlarged sterile branches (arrowheads), and basal cell (b) bearing few involucral filaments (arrows). 14. Developing carpogonial branch showing carpogonium (c) with trichogyne (t), hypogynous cell (h) producing some enlarged sterile branches (arrowheads) with enlarged stained nuclei, and basal cell (b) bearing several involucral filaments (arrows). 15. Developed carpogonial branch showing carpogonium (c) with trichogyne (t), hypogynous cell (h) producing some enlarged sterile branches (arrowheads) with enlarged stained nuclei, and basal cell (b) bearing more involucral filaments (arrows) that surround the basal cell and the hypogynous cell. 16. Post-fertilization carpogonial branch showing fertilized carpogonium (c) with trichogyne (t), gonimoblast initials (gi), enlarged sterile cells (arrowheads) with dark stained nuclei derived from hypogynous cell, and involucral filaments (arrows) originated from basal cell. Note that the disconnection between the gonimoblast initial and the hypogynous cell is caused by the squashing preparation. 17. Post-fertilization carpogonial branch showing fertilized carpogonium (c), gonimoblast initials (gi) cut off the first cell of the gonimoblast filament (gf), hypogynous cell (h) producing enlarged sterile cells (arrowheads) with dark stained nuclei, and basal cell (b) bearing some involucral filaments (arrows). 18. Post-fertilization carpogonial branch showing fertilized carpogonium (c) with trichogyne (t), gonimoblast initials (gi) bearing few gonimoblast filaments (gf), enlarged sterile cells (arrowheads) derived from hypogynous cell, and involucral filaments (arrows) originated from the basal cell. 19. Cross-section through a young cystocarp showing developing gonimoblast filaments (gf) borne on a large fusion cell (fc) that incorporates gonimoblast initial (gi), hypogynous cell (h) and basal cell (b). Enlarged sterile cells (arrowheads) produced from the hypogynous cell and numerous loosely arranged involucral filaments (arrows) issued from basal cell are not involved in the formation of the fusion cell. 20. Cross-section of mature cystocarp showing gonimoblast filaments (arrowheads) terminally bearing carposporangia (cp), a distinct fusion cell (fc) at basal position of cystocarp with enlarged sterile cells (arrowheads) that originated from hypogynous cell, and modified involucral filaments (arrows) forming a pericarp that surrounds the cystocarp. 21. Close-up of fusion cell (fc) showing sterile cells (arrowheads) that originated from hypogynous cell and involucral filaments (arrows) cut off from the basal cell. 22. Closer view of mature cystocarp showing inner gonimoblast filaments (arrows) and carposporangia (cp) within the remnant of old cell walls (arrows). Scale bars = 25 µm (Figs 13, 16–18, 21, 22), 50 µm (Figs 14, 15, 19) and 100 µm (Fig. 20).
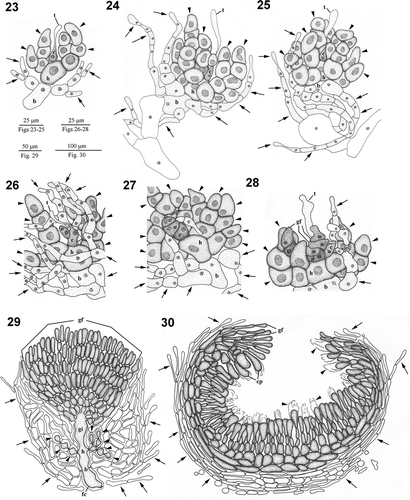
Figs 4. Dichotomaria hommersandii. Hand drawings of cystocarp development (Natal, South Africa). 23. Young carpogonial branch showing carpogonium (c) with trichogyne (t), hypogynous cell (h) producing several enlarged sterile branches (arrowheads), and basal cell (b) bearing few involucral filaments (arrows). 24. Young carpogonial branch showing carpogonium (c) with trichogyne (t), hypogynous cell (h) producing some enlarged sterile branches (arrowheads) with enlarged stained nuclei, and basal cell (b) bearing several involucral filaments (arrows). 25. Developed carpogonial branch showing carpogonium (c) with trichogyne (t), hypogynous cell (h) producing some enlarged sterile branches (arrowheads) with enlarged stained nuclei, and basal cell (b) cut off involucral filaments (arrows) surrounding both basal and hypogynous cell. 26. Post-fertilization carpogonial branch showing fertilized carpogonium (c) with trichogyne (t), gonimoblast initials (gi), enlarged sterile cells (arrowheads) with dark stained nuclei derived from hypogynous cell, and involucral filaments (arrows) originated from the basal cell. 27. Post-fertilization carpogonial branch showing fertilized carpogonium (c), gonimoblast initials (gi) cut off the first cell of the gonimoblast filament (gf), hypogynous cell (h) producing enlarged sterile cells (arrowheads) with dark stained nuclei, and basal cell (b) bearing some involucral filaments (arrows). 28. Post-fertilization carpogonial branch showing fertilized carpogonium (c) with trichogyne (t), gonimoblast initials (gi) bearing few gonimoblast filaments (gf), enlarged sterile cells (arrowheads) derived from hypogynous cell, and involucral filaments (arrows) originated from basal cell. 29.Young cystocarp showing developing gonimoblast filaments (gf) borne on a large fusion cell (fc) that incorporates gonimoblast initial (gi), hypogynous cell (h), and basal cell (b). Enlarged sterile cells (arrowheads) cut off from hypogynous cell and numerous loosely arranged involucral filaments (arrows) issued from basal cell are not involved in the fusion cellformation. 30. Cross-section through mature cystocarp showing gonimoblast filaments (gf) bearing terminal carposporangia (cp) with remaining cell walls (arrowheads), and modified involucral filaments (arrows) forming a pericarp.
Holotype: University Herbarium, University of California at Berkeley, USA (UC1966724).
Isotypes: Department of Biology, University of North Carolina, USA (NCU598867), Herbarium of Academia Sinica, Taiwan (HAST-133253), and Department of Life Science, Tunghai University, Taiwan (TUNG_DiHo_19830811).
Paratype: Department of Life Science, Tunghai University, Taiwan (TUNG_DiHo_19930720.1-2).
Topotype: University Herbarium, University of California at Berkeley, USA (PR-XLVIII-159).
Type locality: Reef at Three Sisters, Riet River Rocks, Port Alfred, Cape Province, South Africa.
Etymology: The species epithet ‘hommersandii’ honours Professor Max Hommersand in recognition of his long-lasting contributions to the study of the reproductive biology and systematics of red algae.
Misapplied name (presumably in part): Galaxaura obtusata sensu Papenfuss et al. (Citation1982, p. 437, ).
Specimens examined: South Africa, Cape Province: (1) Riet River Rocks, coll. G.F. Papenfuss & R.F. Scagel, 9 December 1962 (PR-XLVIII-159, female, pressed specimen); (2) Three Sisters, Riet River, Port Alfred, coll. M.H. Hommersand & F.C. Hommersand, 11 August 1983 (UC1966724, NCU598867, HAST-133253, TUNG_DiHo_19830811, female, pressed and formalin specimens); (3) Three Sisters, Riet River, Port Alfred, coll. M.H. Hommersand, 20 July 1993 (TUNG_DiHo_19930720.1-2, female, silica gel-preserved specimens).
Distribution: Currently known only from Riet River Rocks, Port Alfred, Cape Province, South Africa, but probably more widespread.
Habitat and seasonality: Collections were made in July, August and December. The seasonal occurrence of D. hommersandii is unclear due to insufficient sampling. Plants grew on the exposed rocks that can be reached at low tide (Prof. Max Hommersand, personal communication).
Habit. The thalli are erect, branched, up to 20 cm in height (, ), and dark red to pink in colour when dried. The thalli possess prostrate fibrous holdfasts approximately 0.5–1 cm in diameter (). The main axes are regularly dichotomously branched with internodes 5–20 mm long and 0.5–1.5 mm wide (, ). Branches are heavily calcified and are very stiff in the lower old parts, whereas young branch tips are less calcified and become compressed when dried. Throughout the thallus, the branches appear to be highly articulated due to constrictions forming both at and between nodes (, ), giving a moniliform appearance (). The outer surface of branches, except for the holdfast, is very smooth without any assimilatory filaments in the examined female plants. The thallus grows primarily via apical meristems at the tips of branches or from the lesion regions of old branches () that have regenerated meristems. Occasionally, numerous long assimilatory filaments arise from the joints of branches (). During cystocarp maturation, obvious ostioles can be found scattered over the surface of the upper parts of the thallus, except the nodes ().
Vegetative structure. As only female gametophytes were collected, it is not known whether the tetrasporophyte is externally similar to the gametophyte. Cross sections show that calcification occurs primarily in the medullary region (white deposits in ). After decalcification, the medullary region can be seen to be loosely arranged and surrounded by a firm outer cortical layer (). Except for the fertile region, the cortex consists of three to four layers of cells and is up to 100 μm thick (, ). The cortical cells of the innermost layer are 25–40 μm wide by 40–60 μm long and are rectangular or lobed. Those of the middle layer are ovoid or obovoid and 25–35 μm wide by 25–40 μm long; those in the outermost layer are smaller still and slightly obpyramidal, 15–25 μm wide by 15–25 μm long (). In surface view, the outermost cortical cells are polygonal and four- to six-sided (). The inner part of the internodes is composed of medullary filaments that are approximately 15 μm in diameter ().
Reproductive morphology. Male gametophytes and tetrasporophytes were not found, and only female specimens were examined. Cystocarps are distributed throughout the thallus except for the basal part and the nodes. Carpogonial branch initials are cut off from cortical cells near branch apices. Young carpogonial branches consist of a flask-shaped carpogonium with a long trichogyne, a cylindrical hypogynous cell, and an obovoid basal cell (, ). Before fertilization, three to four sterile branches are produced from the hypogynous cell (, , , ) and several involucral filaments arise from the basal cell (, ). During maturation of the carpogonial branch, the carpogonium remains one-celled, but the involucral filaments develop further to surround the basal cell and the sterile cells derived from the hypogynous cell (, ). Following presumed fertilization, the carpogonium (zygote) divides laterally to produce a gonimoblast initial (, ). The remaining part of the carpogonium is undifferentiated with the long trichogyne, and then the gonimoblast initial produces a primary gonimoblast cell (, ). The primary gonimoblast first divides transversely and then produces a cluster of gonimoblast filaments laterally (, ). The nuclei of the hypogynous cell and its derived sterile branches enlarge (, , , –). At an early stage in post-fertilization development, the pit plugs between the basal gonimoblast cells, the hypogynous cell, and the basal cell broaden, and then the involucral filaments derived from the basal cell divide further to form a pericarp surrounding the hypogynous cell, the sterile branches, and the gonimoblast filaments (, ). During maturation of the cystocarp, the sterile branches derived from the hypogynous cell stop dividing and remain distinct (–, 29). During the further development of the cystocarp, the secondary gonimoblast filaments are cut off from the primary gonimoblast filaments, and the pit plugs between the gonimoblast initial, the innermost cells of the primary gonimoblast filaments, the hypogynous cell, and the basal cell break down and form a distinct fusion cell (, ). Mature cystocarps are hemispherical and 550–700 μm in diameter (, ). Ellipsoid or obovoid carposporangia, 8–20 μm in diameter by 20–50 μm long, are cut off terminally from the secondary gonimoblast filaments (, , ). New carposporangia are often produced within the sheaths of old carposporangial walls, from the terminal cell of secondary gonimoblast filaments (arrows in , ).
Discussion
The rbcL sequence analyses show that Dichotomaria comprises at least six distinct clades with two thallus forms (, clades I–VI). The genus is distributed in various geographical localities in the Caribbean Sea, and the western Pacific and Indian Oceans. The basal clade (I) is a terete species (our newly recognized D. hommersandii) that has been found only at the Riet River Rocks, South Africa, but may be more widely distributed in colder regions of the Indian Ocean when more collections are made or herbarium specimens from the region are examined. Our analyses indicate that not all flattened species of Dichotomaria are closely related. Dichotomaria diesingiana and D. australis (clade II, flattened form) are only found in the cold-temperate areas of the Cape region (Papenfuss et al., Citation1982; Huisman, Citation2006), South Africa, and southeast Australia, and are sister to a large assemblage containing the majority of Dichotomaria species with both flattened and terete thalli (clades III–VI). Interestingly, clade III comprises a terete species, D. papillata from southern Japan and two flattened species, D. falcata and D. apiculata, which are distributed in Japan and Korea (Lee & Lee, Citation1989; Kurihara et al., Citation2005), indicating that this evolutionary clade may be restricted to the cold-temperate region of the northwestern Pacific Ocean. Dichotomaria obtusata (clade IV, terete form) and species of the D. marginata–tenera complex (clade VI, flattened form) are widely distributed from the tropical to warm temperate areas within each of the Caribbean Sea and the Indo-Pacific region (Papenfuss et al., Citation1982; Huisman & Borowitzka, Citation1990), whereas D. spathulata (clade V, flattened form) is primarily distributed in southwestern Australia (Huisman, Citation2006), indicative of an endemic species in the cold-temperate area in the southeastern Indian Ocean. A restricted biogeographical distribution of D. spathulata has been recently revisited by Huisman (Citation2006) and its broad distribution record in AlgaeBase (Guiry & Guiry, Citation2012) may be due to a liberal species concept (references cited in Huisman, Citation2006). Alternatively, the sampling effort for this species has still not reached saturation.
Overall, two trends can be observed in our phylogenetic analyses. First, except for the species complexes in D. marginata and D. obtusata, different clades of Dichotomaria seem to be restricted to different oceanic regions, implying that the speciation of Dichotomaria may have been largely allopatric (i.e. associated with isolation by geographical barriers). Second, the three evolutionary lineages of terete species (clade I, D. papillata in III, and clade IV) seem to contain fewer species than the other clades (II, D. falcata–apiculata in III, V and VI), suggesting that the gain of a flattened thallus morphology might facilitate species diversification. However, these suggestions are only tentative and need to be reassessed following better sampling of the genus on a global scale.
However, in contrast to the prevailing evidence of allopatric speciation in Dichotomaria, we found two unexpected cases in which geographically distant specimens of flattened species differed very little in rbcL sequence. The first case is D. marginata, in which specimens from southeast Asia (Papua New Guinea and the Philippines) differ from those from the Caribbean Sea (Guadeloupe) by only 0.3% (); the second case is the 0.6% divergence between South African and Gulf of California D. marginata–tenera (). In general, an rbcL sequence divergence of > 2% in red algae represents different species (e.g. Freshwater & Rueness, Citation1994). If we apply this to the two cases mentioned above, it would require that some Dichotomaria species have exhibited long-range dispersal across separated oceanic basins. How could this occur? One possible explanation is that D. marginata has been introduced recently via human activities, either intentionally (by aquaculture) or unintentionally (by hull-fouling or the release of ballast water), as with various other alien marine organisms (Padilla & Williams, 2004; Molnar et al., 2008). Another possibility is that the rbcL marker is not sensitive enough to detect cryptic species of D. marginata. A less conserved barcode marker such as COI-5P could be used to investigate these findings in greater detail (Saunders & MacDonald, Citation2010). Even so, our observations indicate that the separation of any such cryptic D. marginata species (or populations) is evolutionarily recent.
Although some significant and extensive taxonomic studies on the Galaxauraceae have been published (Kjellman, Citation1900; Papenfuss et al., Citation1982), our study has shown some species with terete thalli remain unrecognized. Our molecular analyses showed that Dichotomaria ‘obtusata’ with articulated branches from South Africa in the western Indian Ocean contains two different clades, one that can correctly be referred to as D. obtusata and the other corresponding to D. hommersandii sp. nov. (). Two species of Dichotomaria (D. obtusata and D. papillata) and five terete or subterete Galaxaura species (G. lenta, G. barbata, and G. paschalis, G. articulata and G. infirma) have been reported from the Indo-Pacific region. The five terete or subterete species of Galaxaura were divided into two sections by Kjellman (Citation1900) and in subsequent studies. These are (1) the section Brachycladia, in which the cortex is characterized by having stalk cells between epidermal cells and inner cortex; and (2) the section Vepreculae, in which the cortex possesses numerous club-like papillae derived from the epidermal cells (Børgesen, Citation1924; Tanaka, 1935; Svedelius, Citation1945, Citation1953; Chou, Citation1947; Papenfuss et al., Citation1982). Section Brachycladia includes G. lenta, G. barbata and G. paschalis, while section Vepreculae includes G. articulata and G. infirma. There has been discussion about the correct classification of the species in sections Brachycladia and Vepreculae, and it has been suggested on the basis of molecular evidence that they may belong to the genus Dichotomaria (Huisman et al., Citation2004a ; Kurihara et al., Citation2005). Unpublished rbcL data support the view that some Galaxaura species, but not G. lenta, G. barbata and G. paschalis, which comprise a separate clade, are closely related to Dichotomaria species (A. Kurihara, personal communication). Specifically, G. infirma and G. articulata seem to be phylogenetically close to the D. marginata assemblage (A. Kurihara, personal communication), despite the similarity of G. articulata to D. hommersandii and D. obtusata in external thallus morphology and internal cortical structure. To facilitate the recognition of D. hommersandii, we herein provide a morphological comparison among D. hommersandii, D. obtusata, D. papillata and the five Galaxaura species, and demonstrate that D. hommersandii is morphologically different from these species.
With respect to morphology, Dichotomaria hommersandii can be separated from D. obtusata and G. lenta by having finer terete branches (0.5–1.5 mm, as opposed to 1.5–3.0 mm for D. obtusata and 2.5–3.0 mm for G. lenta: ). Although the branch diameter of D. hommersandii is more similar to D. papillata, G. barbata, and G. infirma (Kjellman, 1900; Tanaka, 1935, 1936; Chou, 1947; Svedelius, 1953), the external thallus morphology and internal cortical structure of D. hommersandii differ from those found in these three species, but are similar to those of D. obtusata and G. articulata (having a moniliform thallus morphology; ). Both G. infirma and G. articulata show a subterete branching pattern (i.e. terete in the lower portion of the thallus but slightly complanate in the upper portion of the thallus: Svedelius, Citation1953; A. Kurihara, personal communication), which was not observed in D. hommersandii. Although the branch width of G. paschalis was not mentioned when it was described (Børgesen, Citation1924), D. hommersandii can easily be distinguished from G. paschalis by its lack of conspicuous whorled assimilatory filaments on the smooth surface of branches ().
Overall, it should be straightforward to distinguish D. hommersandii from other terete or subterete species by external and internal morphology. To check further that the new species has not been described previously or passed by because of broad species definitions (or under a wrong name), we compared the branch width of D. hommersandii with that of species placed in the section Dichotomaria by Kjellman (Citation1900), because Kjellman (Citation1900) probably placed most terete members of Dichotomaria in this section (Huisman et al., Citation2004a ). summarizes the relevant data and shows that most species of section Dichotomaria in Kjellman (Citation1900) possess wider branches than those of D. hommersandii (), but information on the branch width of three of Kjellman's species, ‘G. obtusata’, ‘G. umbellata’ and ‘G. decaisnei’ (all considered to be synonyms of Dichotomaria obtusata: Guiry & Guiry, Citation2012), is lacking in the original descriptions. The type locality (West Indies) of these three different species are remote from that of D. hommersandii. The branch width of D. obtusata from the Atlantic Ocean is much greater than that of D. hommersandii (Wynne, Citation2011) and the partial rbcL sequence of a D. obtusata specimen from North Carolina clusters with the D. obtusata assemblage (S.-L. Liu, unpublished data), indicating that D. hommersandii differs from D. obtusata from the Atlantic Ocean. Our literature review did not reveal any evidence that D. hommersandii has been described under another name in the past.
Table 2. Thallus width of species under the section Dichotomaria in Kjellman (Citation1900)
Considering that the taxonomy of the calcified genera of Galaxauraceae has received much attention and been studied extensively (Kjellman, Citation1900; Papenfuss et al., Citation1982), why has D. hommersandii not been described in the literature? One explanation is that the thallus branching pattern and cortical features of gametophytes of D. hommersandii and D. obtusata are highly similar. For instance, both species often show a moniliform branching pattern with a 3 (4)-celled cortex in the gametophytes (), which may have led to the two being merged by Papenfuss et al. (Citation1982), despite their very different branch widths. Another explanation is the lack of consensus in defining species. At one extreme is the very restricted species concept of Kjellman (Citation1900), who erected many Galaxaura species on the basis of small variations in external morphology. The other extreme is the liberal species concept proposed by Papenfuss et al. (Citation1982), which was subsequently promoted by Huisman & Borowitzka (Citation1990). They allowed single species Galaxaura to possess a wider range of external variation and sunk many Kjellman's species name into a few representative species. When Papenfuss et al. (Citation1982) reported a specimen from Riet River, South Africa, in which our new species was found, they identified it as Galaxaura obtusata (= D. obtusata) because, according to their broad species definition, thallus width of G. obtusata could range between 1.0 and 3.5 mm. An examination of this specimen from the University of California Herbarium at Berkeley (PR-XLVIII-159, collected 1962) clearly showed a very narrow terete thallus morphology (branches 0.5–1.5 mm in diameter) with obvious constrictions at the nodes and internodes, as in our specimens. Furthermore, the anatomy of the cortex and the reproductive structures are also congruent with D. hommersandii. Thus we conclude that Papenfuss et al. confused G. obtusata and D. hommersandii based on the similarity of their thallus morphology. More generally, it is now clear from molecular analyses that the diversity of Galaxauraceae is much higher than previously appreciated and many of Kjellman's species that were sunk by Papenfuss et al. (Citation1982) and Huisman & Borowitzka (Citation1990) now require re-examination; we advocate a much narrower species definition in Galaxauraceae. However, one caveat is that it should be safe to sink some of Kjellman's species into synonymy because the dimorphic cortical structures of gametophyte and tetrasporophyte in Galaxaura and Dichotomaria were considered as different species by Kjellman (Citation1900), an interpretation shown to be wrong based on molecular analyses (Huisman et al., Citation2004b ; Kurihara et al., Citation2005; Liu et al., Citation2013).
When Wang et al. (Citation2005) and Liu & Wang (Citation2009) examined cystocarp development in Actinotrichia, Dichotomaria and Galaxaura, they proposed that the size of fusion cells might be an important character for delineating relationships among different genera of Galaxauraceae, the fusion cell being much larger in Galaxaura than in either Actinotrichia or Dichotomaria. However, compared with D. obtusata and the D. marginata assemblage, D. hommersandii possesses a much larger fusion cell, since this incorporates the gonimoblast initial, several inner gonimoblast cells, the hypogynous cell, and the basal cell. It seems, therefore, that the size of the fusion cell may be only a species-level character, instead of a character that can be relied upon for the separation of genera in Galaxauraceae.
Our study has revealed a few features that can be used to circumscribe D. hommersandii with respect to other Dichotomaria species, namely: (1) a thallus morphology showing a very narrow terete branching pattern with a smooth surface and sometimes moniliform shape, and (2) a large fusion cell. The first morphological feature is unique to D. hommersandii compared with other flattened (or even terete/subterete) members of Dichotomaria. The size of the fusion cell has been examined in only a limited number of Dichotomaria species and so the usefulness of this character for distinguishing Dichotomaria species requires further works.
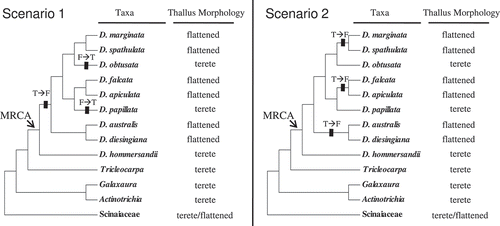
Our phylogenetic analyses reveal that D. hommersandii is sister to all other Dichotomaria species. This, together with the terete nature of the next most closely related genera (Tricleocarpa, Galaxaura and Actinotrichia: ), indicates that the terete thallus morphology is probably ancestral in Dichotomaria. Accordingly, we can propose two possible evolutionary progressions of thallus morphology in Dichotomaria based on the most parsimonious criterion (). In both of these, at least three thallus transitions occurred. In the first scenario, the first transition, from a terete to a flattened form, occurred after the split between D. hommersandii and the remaining Dichotomaria species. The second and third transitions, both from the flattened to the terete form, occurred on the branches leading to the D. obtusata assemblage and D. papillata, respectively. In contrast, in the second scenario three independent transitions, all from the terete to the flattened form, occurred in the branches leading to the D. marginata–spathulata lineage, the D. falcata–apiculata lineage and the D. australis–diesingiana lineage. Better taxon sampling may reveal which of these scenarios is closer to the truth.
Figs 5. Two competing most-parsimonious speculative evolutionary progressions of thallus morphology in Dichotomaria. Rectangular lines on the branch indicate the transition events between the terete and flattened thallus morphology over the evolution of Dichotomaria. Abbreviation: T, the terete form; F, the flattened form.
Supplementary material
Download MS Word (58.5 KB)Acknowledgements
We are grateful to Dr Max H. Hommersand for providing the galaxauraceous specimens worldwide, as well as his comments on this manuscript. We also thank Dr Kathy Miller for helping with the specimen loan from University of California Herbarium at Berkeley, Dr Akira Kurihara for his critical comments during the preparation of this manuscript, and Dr John Huisman and an anonymous reviewer for their constructive comments for improving this manuscript. This study was partly supported by a NSC grant (NSC-101-2621-B-029-004) and a start-up fund of Tunghai University to S.-L. Liu.
References
- Børgesen , F. 1924 . “ Marine algae from Easter Island ” . In The Natural History of Juan Fernandez and Easter Island, vol. 2 , Edited by: Skottsberg , C. 247 – 309 . Uppsala, Sweden. : Almqvist & Wiksells Boktryckeri .
- Børgesen , F. 1932 . A revision of Forsskål's algae mentioned in Flora Aegyptiaco-Arabica and found in his herbarium in the Botanical Museum of the University of Copenhagen . Dansk Botanisk Arkiv , 8 : 1 – 16 .
- Chou , R.C.Y. 1947 . Pacific species of Galaxaura II. Sexual type . Papers from the Michigan Academy of Science, Arts and Letters , 31 : 3 – 24 .
- Edgar , R.C. 2004 . MUSCLE: multiple sequence alignment with high accuracy and high throughput . Nucleic Acids Research , 32 : 1792 – 1797 .
- Felsenstein , J. 1985 . Confidence limits on phylogenies: an approach using bootstrap . Evolution , 39 : 783 – 791 .
- Freshwater , D.W. and Rueness , J. 1994 . Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide sequence analysis . Phycologia , 33 : 187 – 194 .
- Guiry, M.D. & Guiry, G.M. (2012). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. ; searched on 27 November 2012.) http://(http://www.algaebase.org (http://(http://www.algaebase.org)
- Hall , T.A. 1999 . BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT . Nucleic Acids Symposium Series , 41 : 95 – 98 .
- Howe , M.A. 1917 . A note on the structural dimorphism of sexual and tetrasporic plants of Galaxaura obtusata . Bulletin of the Torrey Botanical Club , 43 : 621 – 624 .
- Howe , M.A. 1918 . Further note on the structural dimorphism of sexual and tetrasporic plants in the genus Galaxaura . Memoirs of the Brooklyn Botanic Garden , 1 : 191 – 197 .
- Huelsenbeck , J.P. and Ronquist , F.R. 2001 . MrBayes. Bayesian inference of phylogeny . Biometrics , 17 : 754 – 755 .
- Huisman , J.M. 2006 . Algae of Australia: Nemaliales , Canberra. : Australian Biological Resources Study .
- Huisman , J.M. and Borowitzka , M.A. 1990 . A revision of the Australian species of Galaxaura (Rhodophyta, Galaxauraceae), with a description of Tricleocarpa gen. nov . Phycologia , 29 : 150 – 172 .
- Huisman , J.M. , Harper , J.T. and Saunders , G.W. 2004 . Phylogenetic study of the Nemaliales (Rhodophyta) based on large subunit ribosomal DNA sequences supports segregation of the Scinaiaceae fam. nov. and resurrection of Dichotomaria Lamarck . Phycological Research , 52 : 224 – 234 . a
- Huisman , J.M. , Sherwood , A.R. and Abbott , I.A. 2004 . Studies of Hawaiian Galaxauraceae (Nemaliales, Rhodophyta): large subunit rDNA gene sequences support conspecificity of G. rugosa and G. subverticillata . Cryptogamie: Algologie , 25 : 337 – 352 . b
- Kjellman , F.R. 1900 . Om floridé-slägtet Galaxaura dess organografi och systematic . Kungliga Svenska Vetenskapsakademiens Handlingar , 33 : 1 – 109 .
- Kurihara , A. , Arai , S. , Shimada , S. and Masuda , M. 2005 . The conspecificity of Galaxaura apiculata and G. hystrix (Nemaliales, Rhodophyta) inferred from comparative morphology and rbcL and ITS1 sequences . European Journal of Phycology , 40 : 39 – 52 .
- Lee , Y.P. and Lee , I.K. 1989 . Notes on Galaxaura (Rhodophyta) from Cheju Island . Korean Journal of Phycology , 4 : 1 – 9 .
- Lin , S.M. , Fredericq , S. and Hommersand , M.H. 2001 . Systematics of the Delesseriaceae (Ceramiales, Rhodophyta) based on large subunit rDNA and rbcL sequences, including the Phycodryoideae, subfam. nov . Journal of Phycology , 37 : 881 – 899 .
- Liu , S.L. and Wang , W.L. 2009 . Molecular systematics of the genus Actinotrichia (Galaxauraceae, Rhodophyta) from Taiwan, with a description of Actinotrichia taiwanica sp. nov . European Journal of Phycology , 44 : 89 – 105 .
- Liu , S.L. , Liao , L.M. and Wang , W.L. 2013 . “ Botanical Studies ” . In Conspecificity of two morphologically distinct calcified red algae from the northwest Pacific Ocean: Galaxaura pacifica and G. filamentosa (Galaxauraceae, Rhodophyta)
- Magruder , W.H. 1984 . Reproduction and life history of the red alga Galaxaura oblongata (Nemaliales, Galaxauraceae) . Journal of Phycology , 20 : 402 – 409 .
- Molnar , J.L., Gamboa, R.L., Revenga, C. & Spalding, M.D. 2008 . Assessing the global threat of invasive species to marine biodiversity . Frontiers in Ecology and the Environment , 6 : 485 – 492 .
- Padilla , D.K. and Williams, , S.L. 2004 . Beyond ballast water: aquarium and ornamental trades as sources of invasive species in aquatic ecosystems . Frontiers in Ecology and the Environment , 2 : 131 – 138 .
- Papenfuss , G.F. , Mshigeni , K.E. and Chiang , Y.M. 1982 . Revision of the red algal genus Galaxaura with special reference to the species occurring in the western Indian Ocean . Botanica Marina , 25 : 401 – 444 .
- Saunders , G.W. 2005 . Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications . Philosophical Transactions of the Royal Society B: Biological Sciences , 306 : 1879 – 1888 .
- Saunders , G.W. and MacDonald , B. 2010 . DNA barcoding reveals multiple overlooked Australian species of the red algal order Rhodymeniales (Florideophyceae), with resurrection of Halopeltis J. Agardh and description of Pseudohalopeltis gen. nov . Botany , 88 : 639 – 667 .
- Svedelius , N. 1942 . Zytologisch-entwicklungsgeschichtliche Studien über Galaxaura, eine diplobiontische Nemalionales-Gattung . Nova Acta Regiae Societatis Scientiarum Upsaliensis, series 4 , 13 : 1 – 154 .
- Svedelius , N. 1945 . Critical notes on some species of Galaxaura from Ceylon . Arkiv för Botanik , 32A : 1 – 74 .
- Svedelius , N. 1953 . Critical studies on some species of Galaxaura from Hawaii . Nova Acta Regiae Societatis Scientiarum Upsaliensis, series 4 , 15 : 1 – 92 .
- Swofford , D.L. 2003 . PAUP*: Phylogenetic Analysis using Parsimony (*And Other Methods). Version 4.0b10 , Sunderland, MA. : Sinauer Associates .
- Tamura , K. and Nei , M. 1993 . Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees . Molecular Biology and Evolution , 10 : 512 – 526 .
- Tanaka , T. 1935 . Four new species of Galaxaura from Japan . Scientific Papers of the Institute of Algological Research, Faculty of Science, Hokkaido Imperial University , 1 : 51 – 57 .
- Tanaka , T. 1936 . The genus Galaxaura from Japan . Scientific Papers of the Institute of Algological Research, Faculty of Science, Hokkaido Imperial University , 1 : 141 – 173 .
- Tamura , K. , Peterson , D. , Peterson , N. , Stecher , G. , Nei , M. and Kumar , S. 2011 . MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods . Molecular Biology and Evolution , 28 : 2731 – 2739 .
- Wang , W.L. and Chiang , Y.M. 2001 . The reproductive development of the red alga Actinotrichia fragilis (Galaxauraceae, Nemaliales) . European Journal of Phycology , 36 : 377 – 383 .
- Wang , W.L. , Liu , S.L. and Lin , S.M. 2005 . Systematics of the calcified genera of the Galaxauraceae (Nemaliales, Rhodophyta) with an emphasis on Taiwan species . Journal of Phycology , 41 : 685 – 703 .
- Wittmann , W. 1965 . Aceto-iron-haematoxylin-chloral hydrate for chromosome staining . Stain Technology , 40 : 161 – 164 .
- Wynne , M.J. 2011 . The benthic marine algae of the tropical and subtropical Western Atlantic: changes in our understanding in the last half century . Algae , 26 : 109 – 140 .
- Yang , Z. and Rannala , B. 2012). . Molecular phylogenetics: principles and practice . Nature Review Genetics , 13 : 303 – 314 .
Supplementary information
The following supplementary material is available for this article, accessible via the Supplementary Content tab on the article's online page at http://dx.doi.org/10.1080/09670262.2013.797110
Supplementary Table. List of taxa used in the rbcL phylogenetic analysis, GenBank accessions and collection information.