Abstract
Calliblepharis hypneoides Díaz-Tapia, Bárbara & Hommersand, sp. nov., is described based on plants collected in sand-covered rocks from southern France to Portugal. Calliblepharis hypneoides is distinguished by a terete thallus, forming an extensive basal system of entangled prostrate axes that bear few irregularly branched upright axes, an inner structure consisting of a central axial filament surrounded by 5–7 filaments of elongated cells, a continuous outer cortex, spermatangial parent cells transformed from outer cortical cells and bearing spermatangial filaments three or four cells long, a single gonimoblast initial cut off from the inner side of the auxiliary cell, with the young carposporophyte consisting of a central reticulate network of interconnected cells linked inwardly to a cluster of basal nutritive filaments and forming gonimoblasts outwardly that bear chains of carposporangia in non-ostiolate cystocarps arising on main axes and branches, and tetrasporangia terminal in sori on ultimate branchlets. These features are characteristic of Calliblepharis and molecular analyses confirm this relationship. In all phylogenetic analyses of cox1, rbcL and SSU sequences, C. hypneoides was consistently distinct from congeners. In both rbcL and SSU trees, its sister relationship to other species was unresolved, probably due to missing species or the exclusion of undescribed species. This is the first report on the systematics of Calliblepharis using three molecular markers.
Introduction
The family Cystocloniaceae, which produces the economically important carbohydrate polymer carrageenan, includes 13 genera and c. 89 species from cold- to warm-temperate waters (Chiovitti et al., Citation1998; Guiry & Guiry, 2013). Most genera are monospecific or contain less than five species, except Hypnea (c. 54 species), Rhodophyllis (c. 12 species) and Craspedocarpus (c. 7 species).
Kylin (Citation1930) suggested a close relationship between the genera Calliblepharis and Hypnea, based primarily on similarities in their procarp structure. At first, Kylin (Citation1930) placed both genera in the Hypneaceae, but he later moved Calliblepharis to the Cystocloniaceae (Kylin, Citation1932, as Rhodophyllidaceae), based on its carposporophyte structure. While only terminal cells of the carposporophyte form carposporangia in H. musciformis, several layers of gonimoblast filaments are converted into carposporangia in Calliblepharis jubata and the carposporangia are borne terminally in chains. Moreover, the cystocarp of H. musciformis contains numerous filaments that link the gonimoblast filaments and the pericarp (Kylin, Citation1930), which are absent in C. jubata (Kylin, Citation1928). In vegetative structure and other reproductive details Hypnea is similar to other Cystocloniaceae (Womersley, Citation1994). Recent molecular phylogenies do not support the recognition of the Hypneaceae as distinct from Cystocloniaceae and the two families are currently merged (Hommersand & Fredericq, Citation2003; Saunders et al., Citation2004). While most molecular studies have focused on the large genera, such as Hypnea (Yamagishi & Masuda, 2000; Geraldino et al., Citation2009, Citation2010), the diversity and phylogeny of the smaller genera remain unclear.
Calliblepharis, established upon C. ciliata from England (Kützing, Citation1843; Dixon & Irvine, Citation1977), is presently a genus that includes six species with a distribution extending from England to Australia. Calliblepharis ciliata and C. jubata are distributed from the British Isles through the Iberian Peninsula to Mauritania (Dixon & Irvine, Citation1977; Bárbara et al., Citation2005; Araújo et al., Citation2009); Calliblepharis fimbriata was originally described from South Africa and the Falkland Islands and has subsequently been recorded from other localities (Silva et al., Citation1996); C. planicaulis and C. celatospora occur in Australia (Womersley, Citation1994; Chiovitti et al., Citation1998); while C. jolyi is recorded from Brazil (Guimarães & Pereira, Citation1993). The genus Hypnea is much more species-rich, containing about 53 species from tropical and warm temperate regions (Geraldino et al., Citation2009). The only species of this genus reported from the Atlantic coasts of the Iberian Peninsula are H. musciformis, widely recorded worldwide from temperate coasts, and H. coccinea, a poorly documented species reported only from its type locality (Cádiz, Southern Spain).
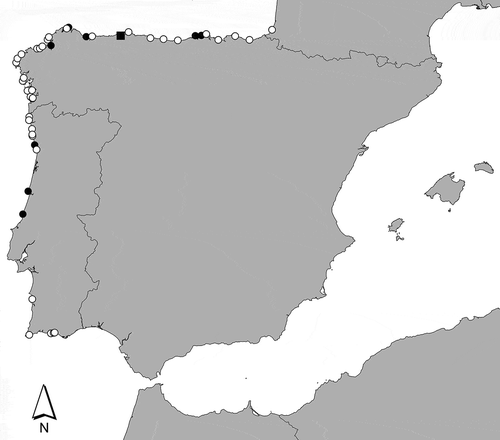
In 1997 and 1999, during a survey of algal diversity in northern Portugal and Asturias (northern Spain), we found a terete gigartinalean rhodophyte on sand-covered rocks from five sites that could not be identified, based on local floristic or monographic references (e.g. Ardré, Citation1970; Dixon & Irvine, Citation1977). Since 2002, we have extended sampling of this species on sand-covered rocks around the entire Atlantic Iberian Peninsula and accumulated more than 60 collections. Our goal in the present study is to characterize this species morphologically and to address its phylogenetic relationships, in particular its relationship to Hypnea and Calliblepharis.
Materials and methods
Collections and morphological studies
An extensive collection of seaweeds from sand-covered rocks was made in the intertidal and upper subtidal from about 80 sites along the Atlantic Iberian Peninsula, between 2002 and 2011. In addition, we also investigated material of this species that had been previously collected and housed in the herbarium of the Universidade de Santiago de Compostela (SANT). The new species was collected from 48 sites () on 63 sampling dates. Materials for DNA extraction were obtained from nine sites (, Table S1) and were preserved in silica gel. Plants for morphological study were preserved in 4% formalin seawater at 4°C and stored in the dark. Some specimens were mounted in 20% Karo® Syrup (ACH Foods, Memphis, TN, USA) and 80% distilled water. Sections for microscopical observations were made by hand using a razor blade and mounted in a mixture of 1% aniline blue, 1% acetic acid, 50% Karo® Syrup and 48% distilled water. Voucher specimens have been deposited in SANT, BM, LD, GENT, MA, MICH, PC and TCD (herbarium abbreviations follow Thiers, Citation2013).
DNA extraction and sequencing
One mg of dried thalli was frozen in liquid nitrogen and individually ground into a fine powder using disposable steel beads and a Mini-Bead Beater (Biospec Products, Bartlesville, OK, USA). Total DNA was extracted immediately afterwards using the Promega Wizard Magnetic 96 DNA Plant System kit following the manufacturer´s instructions. The extracted DNA was stored at −20°C until amplification of rbcL, cox1 and SSU genes.
Primer pairs for the amplification and sequencing reaction of each gene were: F7–R753 and F645–RrbcSstart for rbcL (Freshwater & Rueness, Citation1994; Lin et al., Citation2001; Gavio & Fredericq, Citation2002); 43F–cox11594R for cox1 (Geraldino et al., Citation2006); G01–G14 and G04–G07 for SSU (Saunders & Kraft, Citation1994). PCR amplifications were carried out following Geraldino et al. (Citation2009). After removing excess primers and nucleotides with shrimp alkaline phosphatase and exonuclease I enzymes, fragments were sequenced on an ABI Prism 3730xl DNA Analyzer™ (Perkin–Elmer, Waltham, Massachusetts, U.S.A.) using BigDyeTM terminator kit according to the manufacturer’s recommendations. Sequences were checked and edited with CodonCode Aligner software (CodonCode, Centerville, Massachusetts, U.S.A.).
Phylogenetic analysis
Thirty-seven rbcL sequences and 28 SSU sequences, including four or three outgroups respectively, and 14 cox1 sequences were collated using the Se-Al version 2.0 a11 software (Rambaut, Citation1996) and aligned visually. The alignments of the SSU and rbcL sequences are available as Supplementary Information.
Maximum likelihood (ML) analyses were performed for the rbcL and SSU datasets using RAxML version 7.2.6 (Stamatakis, Citation2006). Tree likelihoods were estimated using 200 independent replications using an unfixed General Time Reversible + Gamma (GTR+ Γ) model. Automatically optimized Sub-tree Pruning and Reconnection (SPR) rearrangement with rapid hill climbing algorithm was used for the best tree search. The phylogeny obtained using the GTR+ Γ model did not differ from that using GTR, and thus we used GTR model to generate the phylogenetic trees. Statistical support for each branch was obtained from 1000 bootstrap replications using the same substitution model and RAxML program settings.
Maximum-parsimony (MP) trees were constructed for each dataset with PAUP* v.4.0b.10 (Swofford, Citation2002), using a heuristic search algorithm with the following settings: 1000 random sequence additions, tree bisection–reconnection (TBR) branch swapping, MulTrees, all characters unordered and unweighted, and branches with a maximum length of zero collapsed. Bootstrap values for the resulting nodes were assessed using 1000 bootstrapping replicates with 10 random sequence additions, TBR and MulTrees.
Bayesian analyses (BA) were performed with MrBayes v.3.1.1 using the Metropolis-coupled Markov chain Monte Carlo (MC3) with the GTR + Γ + I model. For the rbcL and SSU matrices, four and three million generations, respectively, of two independent runs were performed with four chains and sampling trees every 100 generations. The burn-in period was identified graphically by tracking the likelihoods at each generation to determine whether they reached a plateau. The 67 242 trees for rbcL and 16 931 trees for SSU sampled at the stationary state were used to infer the Bayesian posterior probability.
Results
Though it anticipates the results, we describe the new species first, to facilitate reference to it both while reporting the phylogenetic analyses and in the subsequent discussion.
Calliblepharis hypneoides Díaz-Tapia, Bárbara & Hommersand, Sp. nov.
(–)
Description
Differs from its congeners and Hypnea in forming compact cushion-like turfs up to 7 cm high, attached by a basal system of entangled axes with peg-like projections; upright axes terete, non-percurrent, and irregularly branched; axes with a central axial filament surrounded by 5–7 filaments of elongated cells, two layers of rounded cells, one layer of inner cortical cells, and a thin layer of uniformly distributed outer cortical cells. Spermatangia in sori on branchlets. Young carposporophyte consisting of a central reticulum of elongated cells that are directed inwardly and linked to basal nutritive filaments, together with outwardly directed gonimoblast filaments that terminate in carposporangial chains. Cystocarps on main axes and branches, not ostiolate. Tetrasporangia in sori on branchlets and main axes.
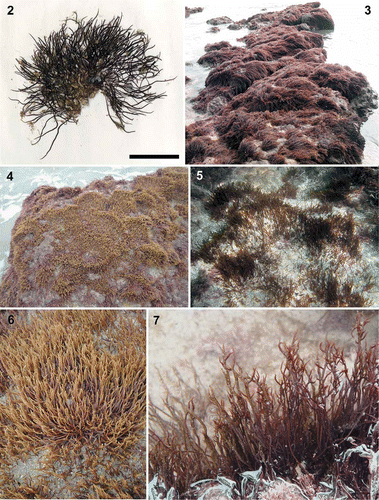
Figs 2–7. Calliblepharis hypneoides. Habit and habitat. 2. Type specimen collected in the Aguilar beach, Asturias, Spain, (SANT-Algae 26428). 3–7. Turf on sand-covered rocks from the lower intertidal at Biarritz, France (3, 7), the low intertidal at Aguilar beach, Asturias, Spain (4, 6); and the upper subtidal of Santa Comba beach, Galicia, Spain (5). Scale bar = 2 cm.
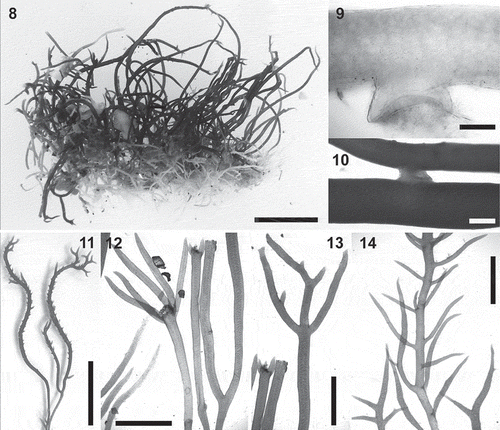
Figs 8–14. Calliblepharis hypneoides. Vegetative morphology. 8. Habit consisting of a fibrous basal system of entangled branches from which upright axes arise. 9. Peg-like projection of an axis of the basal system. 10. Branches connected by a lateral peg-like projection. 11. Vegetative upright axes sparsely and pseudodichotomously branched, that bear numerous spiniform proliferations in median and upper parts. 12. Upright axes with pseudodichotomous and apparent verticillate branchlets growing from a damaged part of the thallus. 13. Upright axes with opposite branches. 14. Upright axes with numerous alternate branchlets bearing tetrasporangial sori. Scale bars = 1 cm (Figs 8, 11), 200 µm (Fig. 9), 400 µm (Fig. 10) and 3 mm (Figs 12–14).
Figs 15–22. Calliblepharis hypneoides. Structure of the thallus. 15, 17. Longitudinal sections at apex, showing the apical cell of a uniaxial tip (a) and axial cells (arrowheads) that are soon obscured by the development of lateral files of cells. 16, 18. Longitudinal sections of median parts, showing two or three central filaments of elongated cells, surrounded by two or three layers of rounded medullary cells, a layer of short, rounded inner cortical cells, and a layer of small outer cortical cells. 19. Cross-section of axis showing a central axial cell surrounded by seven cells that are similar in appearance to the axial cell, two layers of medullary cells, a layer of inner cortical cells, and a layer of outer cortical cells. 20. Cross-section of axis with the central axial cell indistinguishable, resembling a pseudoparenchymatous medulla. 21. Surface view of thallus showing a continuous layer of outer cortical cells. 22. Detail of cortical cells in surface view showing spherical refractive inclusions (arrowheads). Scale bars = 30 µm (Fig. 15), 250 µm (Figs 16, 18), 60 µm (Fig. 17), 150 µm (Fig. 19), 100 µm (Fig. 20), 50 µm (Fig. 21) and 25 µm (Fig. 22).
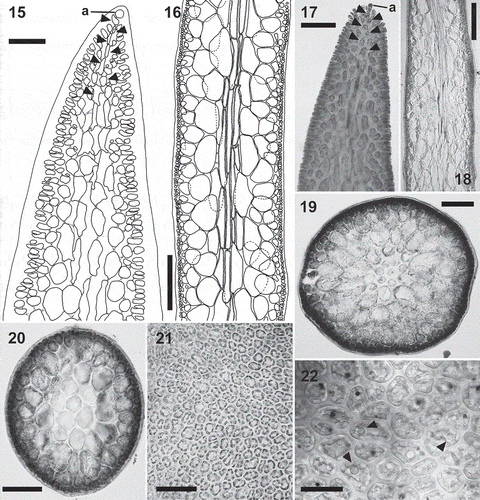
Figs 23–31. Calliblepharis hypneoides. Male reproductive structures. 23, 24. Surface view of spermatangial sorus surrounding upper branchlets. 25, 26. Cross-section of immature (Fig. 25) and mature (Fig. 26) surface spermatangial sori. 27. Primordium of spermatangial filaments after the first oblique division (arrow) and spermatangial filaments with a remnant basal rounded cell (arrowheads showing their pit connections to inner cortical cells). 28. Spermatangial primordium (arrowhead) after the first division leading to the formation of spermatangial filaments from the point of the first separated cell (arrows). 29. Spermatangial chains three or four cells long, which are simple (arrowhead) or branched (arrow). 30. Spermatangial filaments with one to three mature spermatia that have differentiated apical nuclei and basal vacuoles at the tip of each filament (arrowheads). 31. Sorus with mature spermatia and released spermatia with a central nucleus (arrowheads) and elongated cells (arrows), which correspond to primordia that have not cut off spermatangial filaments. Scale bars = 600 µm (Fig. 23), 70 µm (Figs 24, 26), 50 µm (Fig. 25) and 30 µm (Figs 27–31).
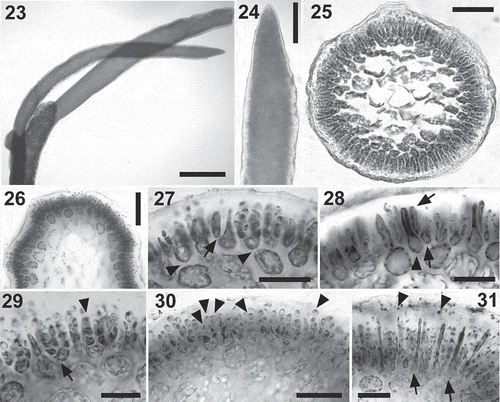
Figs 32–39. Calliblepharis hypneoides. Female stages. 32. Longitudinal section of an apical part of thallus showing the position of carpogonial branches (arrowheads). 33. Detail of a procarp, consisting of a supporting cell (su) bearing a three-celled carpogonial branch towards the inside (1–3), and an auxiliary cell (au). 34. Post-fertilization stage in longitudinal section, showing the supporting cell (su) bearing the disintegrating carpogonial branch (1–3) with the trichogyne elongating towards the thallus surface (arrowheads), gonimoblast initial (gi) cut off inwardly from the auxiliary cell (ac), modified cortical filaments (mcf) and the nutritive filaments (nf). 35. Cross-section showing an enlarged auxiliary cell (ac) and gonimoblast initial (gi), surrounded by modified cortical filaments (mcf) and nutritive filaments (nf). 36, 37. Gonimoblast initial (gi) forming ascending and descending gonimoblast filaments (agf, dgf), linked by secondary pit connections to nutritive filaments (nf). 38. Cross-section showing the auxiliary cell (ac) and the gonimoblast initial (gi) forming ascending (agf) and descending gonimoblast filaments (dgf) that link to basal nutritive filaments (nf). 39. Early branched gonimoblasts (g) growing from a reticulum (r) that consists of a meshwork of small, interconnected cells, and showing apical chains of 3–4 immature carpospores. Scale bars = 70 µm (Fig. 32), 20 µm (Figs 33–35, 37) and 50 µm (Figs 36, 38, 39).
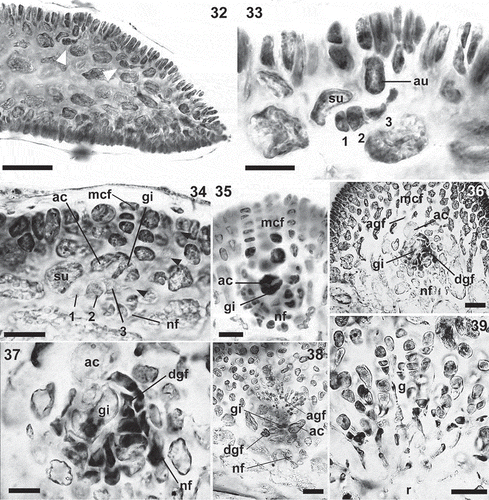
Figs 40–45. Calliblepharis hypneoides. Female structures. 40. Upper part of a female thallus with cystocarps. 41, 42. Cross-section of young cystocarps showing the basal cushion of nutritive filaments (nf), the central reticulum of interconnected cells (r) and the gonimoblasts (g) bearing carposporangia in chains. 43. Cross-section of a mature cystocarp showing the central reticulum surrounded by chains of carposporangia. 44. Cystocarp with a remnant cortical filament (arrowheads). 45. Chains of mature carpospores, with the outermost of them germinating inside the cystocarp. Scale bars = 3 mm (Fig. 40), 50 µm (Figs 41, 42, 44, 45) and 200 µm (Fig. 43).
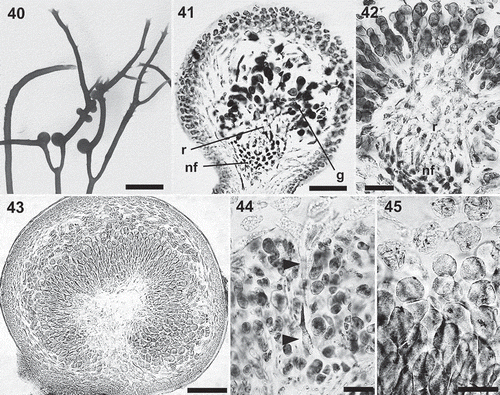
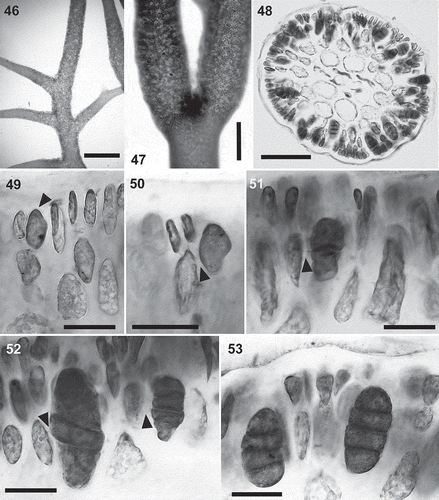
Figs 46–53. Calliblepharis hypneoides. Tetrasporangia. 46, 47. Surface view of tetrasporangial sorus surrounding the branchlets in upper regions of thallus. 48. Cross-section of a tetrasporangial sorus. 49. Longitudinal section of a tetrasporangial sorus showing a terminal tetrasporocyte (arrowhead) formed by the division of an outer cortical cell. 50. Longitudinal section of a tetrasporangial sorus showing an enlarged tetrasporocyte whose pit connection to its bearing cell shifting to a lateral position (arrowhead). 51. Longitudinal section of a tetrasporangial sorus showing a tetrasporocyte after its first division to form two cells, retaining the pit connection to its inner bearing cell (arrowhead). 52. Tetrasporocytes dividing to form tetraspores showing that the pit connection to its bearing cell (arrowheads) remains with the third cell counting from the outermost cell. 53. Cross-section of a tetrasporangial sorus showing two mature tetrasporangia zonately divided into four tetraspores. Scale bars = 700 µm (Fig. 46), 300 µm (Fig. 47), 100 µm (Fig. 48) and 30 µm (Figs 49–53).
Holotype
SANT-Algae 26428 (), from sand-covered rocks in the lower intertidal at Aguilar beach (43°33′28′′N; 6°07′07′′W), Asturias, Spain (), collected by P. Díaz-Tapia and I. Bárbara, 27 May 2010.
Isotypes
BM 001062822, LD 1666782, GENT PDT001, PC 0709136, MA 11086, MICH 1210561, SANT-Algae 27931, TCD 0017768.
Etymology
The species epithet is taken from its similarity in habit to Hypnea.
Additional specimens examined (from north to south)
France: Pyrenees-Atlantique, Biarritz (43°29′03′′N; 1°33′46′′W), 19 March 2011, SANT-Algae 25425; Spain: Guipúzcoa, Zumaia (43°17′59′′N; 2°15′51′′W), 18 March 2011, SANT-Algae 25138; Vizcaya, Laida (43°24′28′′N ; 2°40′54′′W), 31 March 2006, SANT-Algae 20283; Cantabria, Langre (43°28′37′′N ; 3°41′37′′W), 6 November 2010, SANT-Algae 24626; Cantabria, Virgen del Mar (43°28′40′′N; 3°52′31′′W), 7 November 2010, SANT-Algae 24653; Lugo, Catedrales (43°33′16″N; 7°09′16″W), 20 September 2005, SANT-Algae 16555; Lugo, Bares (43°46′02″N; 7°40′36″W), 31 March 2010, SANT-Algae 24326; A Coruña, Perbes (43°22′33″N; 8°12′56″W), 29 January 2010, SANT-Algae 24107; Pontevedra, Nerga (42°15′19″N; 8°50′07″W), 12 February 2005, SANT-Algae 22817; Portugal: Douro Litoral, Leça de Palmeira (41°12′22″N; 8°43′03″W), 11 June 2010, SANT-Algae 24225; Beira Litoral, Buarcos (40°11′58″N; 8°54′29″W), 11 June 2010, SANT-Algae 24213; Estremadura, Vale Furado (39°41′04″N; 9°03′33″W), 12 June 2010, SANT-Algae 26598; Algarve, Olhos d´Agua (37°5′20″N; 8°11′27″W), 20 February 2011, SANT-Algae 26264.
Distribution, habitat and seasonality
Calliblepharis hypneoides is widely distributed along the Atlantic Iberian Peninsula (). It was collected in 48 out of a total of 88 sampled sites, where it formed turfs on sand-covered rocks from the lower intertidal to the upper subtidal of wave exposed to moderately wave exposed sites. Turfs develop both directly on the sand-covered rocks or overgrowing other turf-forming species, such as Rhodothamniella floridula or Ophidocladus simpliciusculus. In addition, small plants of C. hypneoides were occasionally collected in subtidal maërl beds. The species was collected throughout the year. When several sampling sites were visited on different dates, the populations were often buried by sand, which made observation of the species difficult. Reproductive structures were common throughout the year, especially tetrasporangia, which were observed in 55% of the collections. Female plants were not rare but less common than tetrasporangia (22% of collections), while male plants were only observed in 10% of the collections.
Vegetative morphology
The thalli form compact cushion-like turfs from a few centimetres to metres in extent on sand-covered rocks; they grow up to 7 cm high (–). Plants consist of an extensive basal system of densely entangled creeping branches attached to the substratum by means of numerous peg-like projections, from which 1 (2 or 3) orders of sparsely branched upright axes arise (, –). The thalli are cartilaginous; the basal axes are reddish white and the upright axes reddish brown to bright red.
The basal system consists of much branched, irregularly terete entangled axes that are (170–) 200–300 (–350) µm in diameter and produce numerous peg-like projections (), which are (60–) 100–200 (–250) µm long and (150–) 200–300 (–350) µm in diameter. They connect the axes of the basal system laterally () and attach to the substratum. Upright axes are terete, non-percurrent, (200–) 300–600 (–680) µm in diameter; they taper and end in an apical cell. They are unbranched or irregularly pseudodichotomously branched, the branch angles being narrow, not exceeding 90° (, ); hairs are frequent in apical parts. Damaged parts of thallus often reinitiate growth from 2–5 new whorled branches (). Sparse to dense alternate or opposed branchlets are sometimes also present in the upper parts of erect axes (, ). Spiniform proliferations, when present, have a dense spiral arrangement (); proliferations are generally present only in apical parts of erect axes, but occasionally occur in median parts and rarely in basal regions.
Vegetative growth is uniaxial, initiated by a slightly projecting apical cell (, ), the apical cell dividing obliquely and alternately on two sides, generating a file of axial cells in a zigzag pattern. Numerous secondary pit connections are formed between axial cells and their neighbouring cells, obscuring the development of lateral files shortly below the apex (, ). An axial filament is surrounded by 5–7 filaments of elongated cells of similar length (, ). Axial filaments are indistinguishable in longitudinal sections from surrounding filaments, but they are usually distinguishable in cross-sections owing to their central position (). Central filaments of elongated cells are surrounded by two or three layers of large, inflated cells, sometimes giving the appearance of a completely pseudoparenchymatous medulla (). Lenticular thickenings are occasionally observed in the walls of medullary cells. The cortex consists of a layer of rounded inner cortical cells and a layer of surface cortical cells. Surface views of the cortex show a continuous layer of irregularly polygonal cells (5–) 10–15 (–20) × (5–) 8–13 (–15) µm with surface rosettes absent (). Surface cortical cells contain ovoid rhodoplasts, and usually possess one or two refractive spherical inclusions ().
Reproductive morphology
The spermatangia form sori, which girdle the branchlets in the upper parts of the thallus (–). Soral development begins with the elongation of cortical cells near the apex, which form the primordia of spermatangial chains (, ). Each primordium divides obliquely at its apex, first to one side and then to the other, producing three elongated apical cells and a basal rounded cell (, ). The three resulting apical cells divide successively to form three- or four-celled spermatangial chains (, ), and these may also be branched to produce spermatangial chains in clusters (). Spermatia form inside the spermatangia and are fully differentiated before they are released. Spermatia mature from the apex to the base along the spermatangial chains. Mature spermatia are ovoid and c. 3 µm in length, with the nucleus displaced towards the apex; they contain a basal vacuole (–). At the time of release, spermatia tend to round up and have a central nucleus (). Occasionally, some spermatangial primordia in the sori may elongate and not cut off spermatangial chains. Such elongated primordial cells may separate clusters of spermatangial filaments ().
Carpogonial branches are formed near the apices of main axes and branches (). The supporting cell and distal auxiliary cell are situated in a cortical filament that branches to the outside. The supporting cell is an inner cortical cell that bears a three-celled carpogonial branch, the carpogonium producing a straight to slightly curved trichogyne directed towards the thallus apex (). Sterile cells are absent. The auxiliary cell distal to the supporting cell is linked to it by a primary pit connection and is barely distinguishable in shape from the surrounding cortical cells prior to fertilization (). Mature post-fertilization stages show a broadened trichogyne emerging towards the thallus surface (, arrowheads). Following presumed fertilization, cortical cells distal to the auxiliary cell enlarge and begin to divide to form the modified terminal cortical filaments that will initiate the pericarp (). The auxiliary cell cuts off a single gonimoblast initial inwardly, directly above the carpogonium (). Concurrently, the auxiliary cell extends in length and branched short-celled nutritive filaments develop from the surrounding medullary cells and grow towards the supporting cell (). Subsequently, the auxiliary cell and the gonimoblast initial enlarge () and the gonimoblast initial divides producing ascending and descending branched gonimoblast filaments (, ). Ascending gonimoblast filaments cluster in branched files linked by primary pit connections (, ). The inwardly directed descending gonimoblast filaments unite laterally to form a reticulum of small cells that are interconnected by means of secondary pit connections (, , ) and do not unite into a single fusion cell but link secondarily to the basal cushion of gametophytic nutritive filaments (, ). The basal nutritive filaments and inner gonimoblast filaments form a placenta that is almost completely surrounded by chains of carposporangia at maturity (). Remnant cortical filaments are sometimes seen extending through the gonimoblasts in mature cystocarps (). Mature carposporangia are globose and (20–) 25–35 (–40) µm in diameter, and germinated carpospores are seen frequently inside the cystocarp (). Mature cystocarps are subglobose, (600–) 700–1000 (–1100) µm in diameter, non-ostiolate, and often form dense aggregates ().
Tetrasporangia form sori that surround the branchlets; mature sori are initiated in the basal and median parts of the thallus and extend towards the apex of the branchlets as the thallus grows (–). Occasionally sori occur on the apical parts of main axes. Tetrasporocytes are initially terminal cells on cortical filaments and are attached to their bearing cells by a basal pit connection (). Subsequently, they enlarge and increase in length from their inner sides, such that the pit connection is situated in a lateral position (). As the tetrasporocytes enlarge, adjacent cortical cells continue dividing, extending the cortical filaments outwardly surrounding the tetrasporocytes, which remain buried inside cortical filaments (, ). Tetrasporocytes cleave successively twice transversely and enlarge outwardly, to form regularly zonately arranged tetraspores at maturity. After the first division of each tetrasporocyte, the pit connection to its bearing cell is attached by its inner cell, whereas after the second division it usually remains attached to the third cell, counting inward from the outside (, ). Occasionally, pit connections are seen attached to the innermost tetraspore. Mature tetrasporangia are (40–) 50–65 (–75) µm long and (15–) 20–30 (–40) µm in diameter ().
Phylogeny of SSU, rbcL and cox1
We generated a total of 74 sequences in the present study: 27 rbcL (1389 bp), 9 SSU (1788 bp) and 38 cox1 (1344 bp). All cox1 sequences from 38 specimens of C. hypneoides collected at eight sites along the range of the species were identical. Conversely, they differed by 67 and 55 bp (per cent divergence: 0.16% and 0.13%) from C. jubata and C. ciliata, respectively, which are the two species of the genus previously reported in Southern Europe (tree not shown because of the limited availability of cox1 sequences from Calliblepharis).
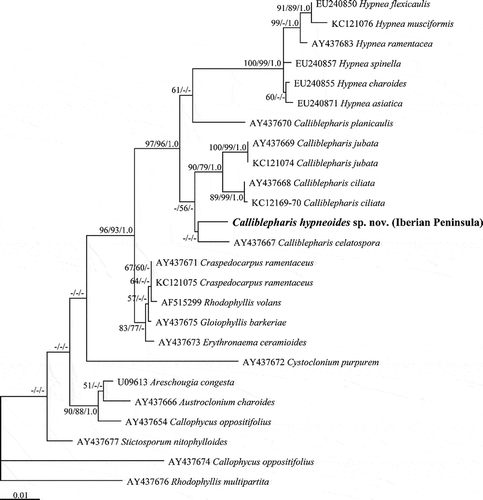
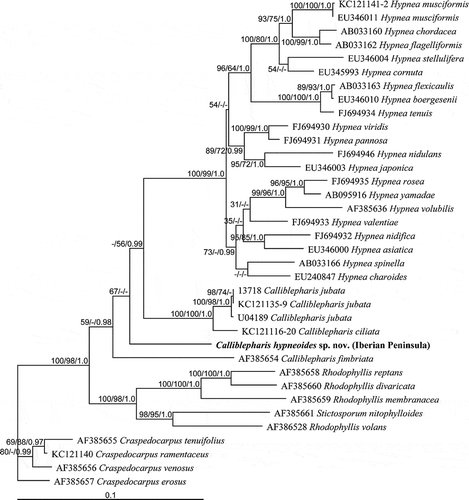
The ML and MP trees () of rbcL sequences from 33 species of the Cystocloniaceae were identical except the bootstrap support values. Calliblepharis hypneoides was consistently placed between C. fimbriata and the C. ciliata–C. jubata clade, although the relationships between these four species of Calliblepharis were not well supported.
Fig. 54. The maximum likelihood phylogeny of Calliblepharis hypneoides based on rbcL data (–lnL = 8552.880744). Support values shown on or below branches are maximum likelihood (MLBt), maximum parsimony (MPBt) and Bayesian posterior probability (BPP) from DNA data.
The SSU sequences from three specimens of Calliblepharis hypneoides from the Atlantic Iberian Peninsula were identical. Calliblepharis together with Hypnea formed a clade with strong support (97% for ML and 93% for MP, ). However, the monophyly of Calliblepharis was not supported, as was seen in the rbcL tree. Calliblepharis hypneoides was a sister to the C. ciliata–C. jubata clade, although this relationship was not well supported. Calliblepharis planicaulis was rarely related to Hypnea (53% for ML only), and C. celastopora was positioned at the base of the genus without support.
Discussion
The new species possesses all the familial characters that Kylin (Citation1932, Citation1956) considered to be diagnostic of the Cystocloniaceae; however, it is difficult to establish its generic assignment based solely on morphological characters. Calliblepharis hypneoides superficially resembles the species of Hypnea but a detailed investigation of its vegetative and reproductive features shows that it is more closely related to Calliblepharis, in agreement with the phylogenetic affinities suggested by SSU and rbcL data.
Calliblepharis hypneoides is the only species of the genus in which the thallus is completely terete (). In this feature, it resembles Hypnea. Even C. planicaulis, which is closely related to Hypnea, has the lower branches flattened (Min-Thein & Womersley, Citation1976). Within the family Cystocloniaceae, only Hypnea, Cystoclonium, Austroclonium and Erythronaema have terete thalli, whereas the other genera are flattened or complanate (). Calliblepharis hypneoides is also characterized by its inner structure, which consists of an axial filament surrounded by 5–7 filaments of elongated cells. This structure is shared with C. jubata and C. ciliata, which have an inner medulla with a few filaments (Kylin, Citation1928; Dixon & Irvine, Citation1977; our unpublished observations). Conversely, the surrounding filaments are absent in the other species of the genus for which the structure is known () and they are also absent in H. musciformis (Kylin, Citation1930). These species have a distinct axial filament surrounded by medullary cells. The absence of vegetative rhizoidal filaments is characteristic of several genera of Cystocloniaceae, including both Hypnea and Calliblepharis (). On the other hand, several genera of Cystocloniaceae have a discontinuous cortex, with the formation of rosettes (). These are absent in C. hypneoides, as well as in C. ciliata, C. jubata and Hypnea, whereas they are weakly developed in the other species of Calliblepharis (, ).
Table 1. Comparison of selected morphological characteristics in the species of the genus Calliblepharis.
Table 2. Comparison of selected morphological characteristics of genera of Cystocloniaceae.
The positions of the reproductive structures in C. hypneoides are the reverse of those in C. jubata and C. ciliata (). The two latter species have the cystocarps restricted to short proliferations, while the tetrasporangia and male structures (where known) are located on the main axes. Conversely, C. hypneoides usually has the male structures and tetrasporangia borne on branchlets, and the cystocarps on both the main axes and lateral branches. Spermatangia of C. hypneoides are formed in sori that surround the branchlets, similar to those described for C. jolyi and C. planicaulis (). Conversely, C. ciliata has spermatangia in surface sori on the younger parts of the plant (Dixon & Irvine, Citation1977). Male structures show great variation in their location among taxa of the family, and spermatangia may form sori or are scattered, as well as being situated on the thallus surface or on proliferations or in rosettes (). Carpogonial branches are formed successively on cortical filaments in the apices of main axes and branches in C. hypneoides and cystocarps occur on the main axes and branches, often forming dense aggregates. These structures are usually restricted to branchlets and proliferations in Calliblepharis () and this seems also to be the prevalent position in Hypnea (Womersley, Citation1994; Chiang, Citation1997; Yamagishi & Masuda, Citation1997). Within the family Cystocloniaceae, several genera have cystocarps on branchlets, whereas others have cystocarps on thallus surfaces or margins of main axes and branches (). The tetrasporangia of C. hypneoides are formed in sori, as in all species of the genus Calliblepharis except C. fimbriata (). The tetrasporangia are usually scattered in species placed in the Cystocloniaceae, and Calliblepharis and Hypnea are the only two exceptions ().
The spermatangia of Calliblepharis hypneoides are cut off from a primordium that is a transformed outer cortical cell that divides obliquely forming filaments composed of three or four small rounded cells that mature basipetally into spermatia. Similar male reproductive structures were previously described for the genus only in C. jolyi (Guimarães & Pereira, Citation1993). By contrast, the male structures in C. jubata seem also to be formed from a primordium that produces only three cells (Kylin, Citation1928), whereas they are described as coming from spermatangial mother cells derived from outer cortical cells in C. planicaulis (Min-Thein & Womersley, Citation1976). Male reproductive structures are unknown or were not described in detail in other species of the genus (). With regard to Hypnea, spermatangia were not described in detail in most species; however, several species appear to have spermatangia borne in chains (Womersley, Citation1994; Yamagishi & Masuda, Citation1997) similar in appearance to those described in C. hypneoides. The spermatangia found in C. hypneoides resemble those described for Acanthococcus (Fredericq et al., Citation1992a), suggesting that this type of spermatangial sorus is more widespread in the Cystocloniaceae than previously thought. In species having rosettes, the spermatangia initials are cut off from outer cortical cells ().
The procarp of Calliblepharis hypneoides consists of a supporting cell, which is an inner cell of a cortical filament that bears an auxiliary cell distally and a three-celled carpogonial branch inwardly, with a trichogyne extending outward, oriented in the direction of the cortical branch. This structure resembles that seen in other members of Calliblepharis and in most genera of the Cystocloniaceae (Kylin, Citation1928, Citation1930, Citation1932; Hansen, Citation1980; Schneider, Citation1988; Fredericq et al., Citation1992a; Womersley, Citation1994). Variations occur in the family in Cystoclonium, in which a lateral branch is borne on the first cell of the carpogonial branch (Kylin, Citation1923; Thrainsson & Hommersand, Citation2012); in Stictosporum, which has filaments borne on the supporting cell (Searles, Citation1968); and in Craspedocarpus blepharicarpus, which has a three (or four)-celled carpogonial branch (Min-Thein & Womersley, Citation1976). By contrast, carposporophyte development and its structure show great morphological variation among taxa. After presumed fertilization, a gonimoblast initial is cut off inwardly from the auxiliary cell in C. hypneoides. This occurs in most species of Cystocloniaceae (), with the exception of Fimbrifolium (Hansen, Citation1980) and Stictosporum (Searles, Citation1968), which form gonimoblast initials outwardly. Although inwardly formed, the gonimoblast initial is located distal to the auxiliary cell in H. musciformis, C. jolyi and C. planicaulis (Kylin, Citation1930; Min-Thein & Womersley, Citation1976; Guimarães & Pereira, Citation1993), whereas it is formed between the carpogonial branch and the auxiliary cell in C. hypneoides and C. jubata (Kylin, Citation1928).
The carposporophyte of Cystocloniaceae either contains a large fusion cell or several central cells, with radiating gonimoblast filaments terminating in carposporangia (Womersley, Citation1994). The second type occurs in both Calliblepharis and Hypnea, which in turn differ in that only terminal cells of the carposporophyte form carposporangia in H. musciformis whereas they are borne terminally in chains in Calliblepharis (Kylin, Citation1928, Citation1930). In addition, the carposporophyte of C. hypneoides, as in other species of the genus, has a central cluster of cells from which the fertile filaments issue outwardly and inwardly with the inwardly directed filaments linked secondarily to a cushion of basal nutritive filaments (Kylin, Citation1928; Min Thein & Womersley, Citation1976; Guimarães & Pereira, Citation1993; Chiovitti et al., Citation1998; our unpublished observations). In contrast, H. musciformis is characterized by the presence of elongated cells, which cut off numerous clusters of small cells that produce radially directed gonimoblast filaments bearing terminal carposporangia (Kylin, Citation1930). Moreover, according to Kylin (Citation1930), the gonimoblasts produce filaments that attach to the outer walls of the pericarp in Hypnea. The current concept of the genus Hypnea is largely based on Kylin´s work with H. musciformis; however, this genus currently contains about 53 species whose female structures have not been studied in detail. The structure of the central cluster of cells varies within the genus Calliblepharis. It consists of a network of inwardly directed, interconnected small cells in C. hypneoides, C. jubata and C. ciliata. By contrast, the inwardly directed gonimoblasts consist of a series of parallel elongate cells oriented perpendicular to the floor of the cystocarp cavity in C. fimbriata, C. celatospora and C. jolyi (Thrainsson, Citation1986; Guimarães & Pereira, Citation1993; Chiovitti et al., Citation1998). Further studies on the female structure of C. planicaulis are required in order to clarify the morphology of its carposporophyte. In general, members of Cystocloniaceae have non-ostiolate cystocarps (Womersley, Citation1994), as in Calliblepharis hypneoides. Conversely, although reputedly absent, an ostiole is formed in cystocarps of C. jubata and C. ciliata (Kylin, Citation1928; Dixon & Irvine, Citation1977; our unpublished observations).
Tetrasporocytes are formed terminally from an outer cortical cell in Calliblepharis hypneoides, as in most Cystocloniaceae (). In contrast, intercalary tetrasporangia have been described for C. celatospora (Chiovitti et al., Citation1998) and Fimbrifolium (Hansen, Citation1980).
The morphological features found in Calliblepharis hypneoides clearly differ from those reported for other species currently assigned to this genus. Moreover, C. hypneoides was consistently distinct from congeners in all of our phylogenetic analyses of cox1, rbcL and SSU sequences. These analyses placed C. hypneoides among species presently assigned to the genus Calliblepharis, although in both rbcL and SSU trees, its sister relationship to other species was unresolved, probably due to undiscovered or incompletely described taxa. In addition, phylogenetic trees support the relationship between Hypnea and Calliblepharis. However, while the first genus forms a distinct clade, Calliblepharis is not monophyletic indicating that the genus requires reinvestigation. Calliblepharis planicaulis forms an independent clade that is more closely related to Hypnea than to the generitype C. ciliata. Nevertheless, the available morphological data are insufficient to distinguish this species from Hypnea and Calliblepharis and place it in a separate genus. Moreover, further work, involving additional molecular data and comparative study of the morphology of C. fimbriata and C. celatospora, especially of female structures, is required to solve their generic assignment, as well as that of the new species. Interestingly, while molecular data are uncertain regarding the placement of C. hypneoides, our morphological observations, especially those performed on female structures, suggest that it is more closely related to C. ciliata and C. jubata than to any other species of the genus.
The Hypnea-like habit of Calliblepharis hypneoides plus the fact that carposporophyte structure has rarely been investigated in Hypnea species, lead us to think that some other species placed in Hypnea based on vegetative morphology could belong in Calliblepharis. However, 23 species of Hypnea have been studied molecularly to date and all have been placed in the Hypnea group with strong bootstrap support (Geraldino et al., Citation2009). Accordingly, an extensive bibliographic revision was performed in order to compare the morphology of C. hypneoides with that of previously described species of Hypnea. In our attempt to identify materials of C. hypneoides as a previously known species, we found that H. arbuscula is the species most similar to C. hypneoides. It is a poorly documented species originally described from Senegal (Dangeard, Citation1952) and subsequently reported from Western Africa and the Macaronesian Islands (Lawson & John, Citation1987; Fredericq et al., Citation1992b; Afonso-Carrillo & Sansón, Citation1999; Parente et al., Citation2000; Haroun et al., Citation2002). Calliblepharis hypneoides does not differ morphologically from H. arbuscula, as it was originally described by Dangeard (Citation1952), but subsequent studies of H. arbuscula (based on examination of material from Senegal and other localities: Bodard, Citation1968, Lawson & John, Citation1987; Afonso-Carrillo & Sansón, Citation1999) have indicated that portion of the thallus are flattened, unlike C. hypneoides. Although a review of the type material of H. arbuscula would be desirable, despite considerable effort we could not find material in Dangeard´s collection. Another collection similar to both H. arbuscula and C. hypneoides was described from Namibia as ‘Hypnea sp.’ (Rull Lluch, Citation2002). We have examined these Namibian specimens (BCF-A 1167) and observed that, in addition to the morphological features described by Rull Lluch (Citation2002), they also have refractive inclusions in the outer cortical cells, as in C. hypneoides. The vegetative and sporangial morphology of these specimens also apparently agree with C. hypneoides; however, female structures, which provide the most reliable diagnostic characters, were not present. Additional morphological and molecular studies are needed in order to verify the apparent similarities between C. hypneoides and specimens of Hypnea spp. from south-west Africa.
Among Hypnea and Calliblepharis species from the Iberian Peninsula, Hypnea coccinea is a poorly documented species that has only been reported from its type locality: Cádiz, Spain. Its habit is similar to that of Calliblepharis hypneoides in that both species have a fibrous holdfast and irregularly arranged pseudodichotomous branches. Conversely, H. coccinea is much thinner than C. hypneoides and the cross-section shows fewer layers of medullary and cortical cells, with the latter similar in size to the medullary cells. The tetrasporangia also appear to be smaller and the carposporangia are apparently solitary (Cremades & Pérez-Cirera, Citation1990). Moreover, the cystocarps of H. coccinea have a central fusion cell that differs from both that found in Calliblepharis and in Hypnea musciformis. Hypnea musciformis, the other species of the genus previously reported from the Atlantic Iberian Peninsula, shows obvious morphological differences with C. hypneoides, some of which have been commented on above.
Calliblepharis hypneoides is widely distributed along the Atlantic Iberian Peninsula and is a common species on sand-covered rocks. Curiously, we could not find evidence of collections of this species along the Atlantic Iberian Peninsula prior to 1997. In our opinion, it remained overlooked until recently due to: (1) the great specificity of its habitat – sand-covered rocks in the lower intertidal and upper subtidal of moderately to highly exposed coasts (98% of collections) – which is scarcely ever studied in detail; (2) the likelihood that populations are often buried by sand, especially during the summer months because of seasonal sand movements, in which sand typically builds up in spring and is washed into the subtidal in autumn (Lobban & Harrison, Citation1997); (3) the small size of the species, which can be easily confused with other species having a similar habit; and (4) the patches it forms are usually small and therefore easily overlooked: we found extensive populations in only four sites. Calliblepharis hypneoides has also probably been overlooked on other coasts and it is probably more widespread than our collections imply. Similar examples of poorly known species from sand-covered rocks include Ptilothamnion sphaericum and Erythroglossum lusitanicum, species that are also widely distributed in this habitat along the Atlantic Iberian Peninsula but which have remained unnoticed for long periods (Díaz et al., Citation2009; Díaz Tapia & Bárbara, Citation2011).
Supplementary information
The following supplementary material is available for this article, accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org/10.1080/09670262.2013.844860
Table S1. Cystocloniaceae species used in this study and GenBank accession numbers.
Supplementary file 1. SSU alignment (fasta format).
Supplementary file 2. RbcL alignment (fasta format).
Supplementary material
Download PDF (27.5 KB)Acknowledgements
We thank B. de Reviers from PC for his help in locating the type material of Hypnea arbuscula and SANT for sending collections. Thanks also go to J. Rull Lluch, A. Secilla and J. Cabioch for providing materials of Hypnea and Calliblepharis; and two anonymous reviewers for their suggestions and comments on the manuscript. This work was partially funded by a PhD grant from Xunta de Galicia to P. Díaz-Tapia; the project CGL2009-09495/BOS (Ministerio de Ciencia e Innovación, FEDER); NSF grant DEB0937978 to J.M. Lopez Bautista, M.H. Hommersand and S. Fredericq; a Korean Research Foundation grant (2012-0704) to S.M. Boo; and CHED-BARD (CEB 307-2009) to P. Geraldino.
References
- Afonso-Carrillo, J. & Sansón, M. (1999). Algas, hongos y fanerógamas marinas de las Islas Canarias. Clave analítica. Universidad de La Laguna, Tenerife.
- Araújo, R., Bárbara, I., Tibaldo, M., Berecibar, E., Diaz-Tapia, P., Pereira, R., Santos, R. & Sousa Pinto, I. (2009). Checklist of benthic marine algae and cyanobacteria of northern Portugal. Botanica Marina, 52: 24–46.
- Ardré, F. 1970. Contribution à l'étude des algues marines du Portugal. I. La Flore. Portugaliae Acta Biologica, Série B, 10: 137–555.
- Bárbara, I., Cremades, J., Calvo, S., López Rodríguez, M.C. & Dosil, J. (2005). Checklist of the benthic marine and brackish Galician algae (NW Spain). Anales del Jardín Botánico de Madrid, 62: 69–100.
- Bodard, M. (1968). Les Hypnea au Sénégal. Bulletin de l'Institut Fondamental Afrique Noire, Série A, 28: 811–825.
- Chiang, Y.M. (1997). Species of Hypnea Lamouroux (Gigartinales, Rhodophyta) from Taiwan. In Taxonomy of economic seaweeds with reference to some Pacific species (Abbott, I.A., editor), 163–177. California Sea Grant College System, La Jolla, CA.
- Chiovitti, A., Kraft, G.T., Bacic, A., Craik, D.J., Munro, S.L.A. & Liao, M.L. (1998). Carrageenans from Australian representatives of the family Cystocloniaceae (Gigartinales, Rhodophyta), with description of Calliblepharis celatospora sp. nov., and transfer of Austroclonium to the family Areschougiaceae. Journal of Phycology, 34: 515–535.
- Cremades, J. & Pérez-Cirera, J.L. (1990). Nuevas combinaciones de algas bentónicas marinas, como resultado del estudio del herbario de Simón de Rojas Clemente y Rubio (1777–1827). Anales del Jardín Botánico de Madrid, 47: 489–492.
- Dangeard, P. (1952). Algues de la presqu’ile du Cap Vert (Dakar) et de ses environs. Le Botaniste, 36: 195–329.
- Díaz Tapia, P. & Bárbara, I. (2011). Sexual structures in Ptilothamnion sphaericum and Pterosiphonia complanata (Ceramiales, Rhodophyta) from the Atlantic Iberian Peninsula. Botanica Marina, 54: 35–46.
- Díaz, P., Berecibar, E., Bárbara, I., Cremades, J. & Santos R. (2009). Biology and taxonomic identity of Erythroglossum lusitanicum (Delesseriaceae, Rhodophyta) from the Iberian Peninsula. Botanica Marina, 52: 207–216.
- Dixon, P.S. & Irvine, L.M. (1977). Seaweeds of the British Isles. Volume 1. Rhodophyta. Part 1. Introduction, Nemaliales, Gigartinales. British Museum (Natural History), London.
- Fredericq, S., Hommersand, M.H. & Leister, G.L. (1992a). Morphology and systematics of Acanthococcus antarcticus (Cystocloniaceae, Rhodophyta). Phycologia, 31: 101–118.
- Fredericq, S., Serrão, E. & Norris, J.N. (1992b). New records of marine red algae from the Azores. Arquipélago, 10: 1–4.
- Freshwater, D.W. & Rueness, J. (1994). Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species, based on rbcL nucleotide sequence analysis. Phycologia, 33: 187–194.
- Gavio, B. & Fredericq, S. (2002). Grateloupia turuturu (Halymeniaceae, Rhodophyta) is the correct name of the non-native species in the Atlantic known as Grateloupia doryphora. European Journal of Phycology, 37: 349–359.
- Geraldino, P.J.L., Yang, E.C. & Boo, S.M. (2006). Morphology and molecular phylogeny of Hypnea flexicaulis (Gigartinales, Rhodophyta) from Korea. Algae, 21: 417–423.
- Geraldino, P.J.L., Yang, E.C., Kim, M.S. & Boo, S.M. (2009). Systematics of Hypnea asiatica sp. nov. (Hypneaceae, Rhodophyta) based on morphology and nrDNA SSU, plastid rbcL, and mitochondrial cox1. Taxon, 58: 606–616.
- Geraldino, P.J.L., Riosmena-Rodríguez, R., Liao, L.M. & Boo, S.M. (2010). Phylogenetic relationships within the genus Hypnea (Gigartinales, Rhodophyta), with a description of H. caespitosa sp. nov. Journal of Phycology, 40: 336–345.
- Guimarães, S.M.P.D.B. & Pereira, A.P.V. (1993). Rhodofíceas marinhas bentônicas do estado do Espírito Santo, Brasil: gênero Calliblepharis (Cystocloniaceae, Gigartinales). Hoehnea, 20: 35–46.
- Guiry, M.D. & Guiry, G.M. (2012). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org; searched on 19 September 2012.
- Hansen, G.I. (1980). A morphological study of Fimbrifolium, a new genus in the Cystocloniaceae (Gigartinales, Rhodophyta). Journal of Phycology, 16: 207–217.
- Haroun, R., Cruz-Reyes, A., Herrera-López, G., Parente, M.I. & Gil-Rodríguez, M.C. (2002). Flora marina de la Isla de Madeira: Resultados de la expedición “Macaronesia 2000”. Revista de la Academia Canaria de las Ciencias, 14: 37–52.
- Hommersand, M.H. & Fredericq, S. (2003). Biogeography of the marine red algae of the South African West Coast: a molecular approach. In Proceedings of the 17th International Seaweed Symposium (Chapman, A.R.O., Anderson, R.J., Vreeland, V.J. & Davison, I.R., editors), 325–336. Oxford University Press, Oxford.
- Kützing, F.T. (1843). Phycologia generalis oder Anatomie, Physiologie und Systemkunde der Tange ... Mit 80 farbig gedruckten Tafeln, gezeichnet und gravirt vom Verfasser. F.A. Brockhaus, Leipzig.
- Kylin, H. (1923). Studien über die Entwicklungsgeschichte der Florideen. Bihang til Kongliga Svenska Vetenskaps-Akademiens Handlingar, 63: 1–139.
- Kylin, H. (1928). Entwicklungsgeschichtliche Florideenstudien. Lunds Universitets Ärsskrift, 24: 1–127.
- Kylin, H. (1930). Über die Entwicklungsgeschichte der Florideen. Acta Universitatis Lundensis, 26: 1–104.
- Kylin, H. (1932). Die Florideenordnung Gigartinales. Acta Universitatis Lundensis, 28: 1–88.
- Kylin, H. (1956). Die Gattungen der Rhodophyceen. C.W.K. Gleerups Förlag, Lund.
- Lawson, G.W. & John, D.M. (1987). The marine algae and coastal environment of tropical west Africa. Nova Hedwigia, Beiheft 93: 1–415.
- Lin, S.M., Fredericq, S. & Hommersand, M. (2001). Systematics of the Delesseriaceae (Ceramiales, Rhodophyta) based on large subunit rDNA and rbcL sequences, including the Phycodryoideae, subfam. nov. Journal of Phycology, 37: 881–899.
- Lobban, C.S. & Harrison, P.J. (1997). Seaweed Ecology and Physiology. 2nd ed. Cambridge University Press, Cambridge.
- Masuda, M., Yamagishi, Y., Chiang, Y. M., Lewmanomont, K. & Xia, B. (1997). Overview of Hypnea (Rhodophyta, Hypneaceae). In Taxonomy of economic seaweeds with reference to some Pacific species (Abbott, I.A., editor), 127–133. California Sea Grant College System, La Jolla, CA.
- Min-Thein, U. & Womersley, H.B.S. (1976). Studies on southern Australian taxa of Solieriaceae, Rhabdoniaceae and Rhodophyllidaceae (Rhodophyta). Australian Journal of Botany, 24: 1–166.
- Parente, M.I., Gil-Rodríguez, M.C., Haroun, R.J., Neto, A.I., Smedt, G.D., Hernández-González, C.L. & Berecibar Zugasti, E. (2000). Flora marina de las Ilhas Selvagens: resultados preliminares de la expedición “Macaronesia 2000”. Revista de la Academia Canaria de las Ciencias, 12: 9–20.
- Rambaut, A.E. (1996). Se-Al: sequence alignment editor. Available from: http://tree.bio.ed.ac.uk/software/seal/. Accessed 3 June 2012.
- Rull Lluch, J. (2002). Marine benthic algae of Namibia. Scientia Marina, 66 ( Supplement 3): 5–256.
- Saunders, G.W. & Kraft, G.T. (1994). Small-subunit rRNA gene sequences from representatives of selected families of the Gigartinales and Rhodymeniales (Rhodophyta). 1. Evidence for the Plocamiales ord. nov. Canadian Journal of Botany, 72: 1250–1263.
- Saunders, G.W., Chiovitti, A. & Kraft, G.T. (2004). Small-subunit rRNA gene sequences from representatives of selected families of the Gigartinales and Rhodymeniales (Rhodophyta). 3. Recognizing the Gigartinales sensu stricto. Canadian Journal of Botany, 82: 43–74.
- Schneider, C.W. (1988). Craspedocarpus humilis sp. nov. (Cystocloniaceae, Gigartinales) from North Carolina, and a reappraisal of the genus. Phycologia, 27: 1–9.
- Searles, R.B. (1968). Morphological studies of red algae of the order Gigartinales. University of California Publications in Botany, 43: 1–100.
- Silva, P.C., Basson, P.W. & Moe, R.L. (1996). Catalogue of the benthic marine algae of the Indian Ocean. University of California Publications in Botany, 79: 1–1259.
- Stamatakis, A. (2006). RaxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22: 407–416.
- Swofford, D.L. (2002). PAUP*: phylogenetic analysis using parsimony (* and other methods). Version 4.0b10. Sinauer, Sunderland, MA.
- Thiers, B. (2013). (continuously updated) Index herbariorum: a global directory of public herbaria and associated staff. New York Botanical Garden's Virtual Herbarium. http://sweetgum.nybg.org/ih/
- Thrainsson, S.A. (1986). Morphological study of some members of the family Cystocloniaceae. MA Thesis, UNC-CH, Chapel Hill, NC.
- Thrainsson, S.A. & Hommersand, M.H. (2012). New features in the vegetative and reproductive morphology of Cystoclonium purpureum (Cystocloniaceae, Rhodophyta) from the North Atlantic Ocean. European Journal of Phycology, 47: 384–392.
- Womersley, H.B.S. (1994). The marine benthic flora of southern Australia. Rhodophyta. Part IIIA. Bangiophyceae and Florideophyceae (Acrochaetiales, Nemaliales, Gelidiales, Hildenbrandiales and Gigartinales sensu lato). Australian Biological Resource Study, Canberra.
- Yamagishi, Y. & Masuda, M. (1997). Species of Hypnea from Japan. In Taxonomy of economic seaweeds with reference to some Pacific species (Abbott, I.A., editor), 135–162. California Sea Grant College System, La Jolla, CA.
- Yamagishi, Y. & Masuda, M. (2000). A taxonomic revision of a Hypnea charoides-valentiae complex (Rhodophyta, Gigartinales) in Japan, with a description of Hypnea flexicaulis sp. nov. Phycological Research, 48: 27–36.