Abstract
Greenland is a continental island in the northern part of the North Atlantic where the foliose Bangiales flora is poorly known. It is an important area for the study of algal biogeography because of the region’s glacial history, in which Greenland has been alternately exposed to or isolated from the North Pacific via the Bering Strait. A molecular study using 3′ rbcL + 5′ rbcL–S sequences was undertaken to assess the diversity of foliose Bangiales on the west coast of Greenland and rbcL sequences were used to study the Greenland flora in a larger phylogenetic and floristic context. New and historic collections document seven species in four genera from the west coast of Greenland. All species had a close link to North Pacific species, being either conspecific with them or North Atlantic–North Pacific vicariant counterparts.
Introduction
The Bangiales include seven filamentous and eight foliose genera of red algae that until recently were classified as Bangia and Porphyra, respectively (Sutherland et al., Citation2011). Members of the foliose Bangiales include the most economically valuable seaweed crop in the world, and the history of harvesting and trading these algae goes back thousands of years in Japan, China, Korea and Southeast Asia (Mumford & Miura, Citation1988). The geographical distribution of foliose Bangiales species is worldwide, ranging from tropical waters to polar seas (Sutherland et al., Citation2011). The gametangial thalli of foliose Bangiales can be monostromatic or distromatic, and they are found in the intertidal and/or the subtidal zones. The sporophyte, known as the conchocelis phase, consists of branched filaments found in shells and other calcareous substrata (Brodie & Irvine, Citation2003).
Recent studies in the northern parts of the North Atlantic, including the Faroe Islands and Iceland, have reported a diverse foliose Bangiales flora (e.g. Klein et al., Citation2003; Brodie & Nielsen, Citation2005; Brodie et al., Citation2007, 2008; Kucera & Saunders, Citation2012; Mols-Mortensen et al., Citation2012). However, until now the foliose Bangiales flora of Greenland has remained poorly known. Greenland () is a northern North Atlantic continental island that is separated from the North American continent by Baffin Bay and David Strait. It stretches from 59°N to 82°N, and its east coast is influenced by the cold East Greenland Current, which originates in the Polar Sea, while the west coast is influenced by both the East Greenland Current and the warmer and more saline Irminger Current, which branches off the North Atlantic Current. The East Greenland Current runs along the entire east coast of Greenland, rounds Cape Farewell, and continues north along the west coast. The Irminger Current meets the East Greenland Current at Cape Farewell, and runs north along the west coast (Merkel et al., Citation2012). The climate of coastal and subtidal West Greenland is subarctic (Dunbar, Citation1954; Wilce, Citation1990).
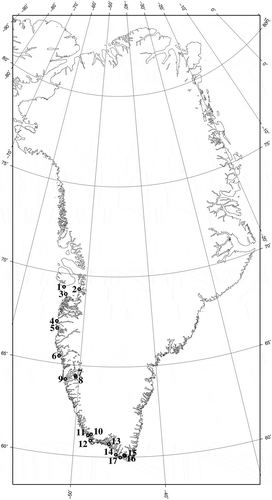
Fig. 1. Map of collecting locations on the west coast of Greenland. 1. Isungua, 2. Ilulissat, 3. Hunde Ejlande, 4. Kumikume, 5. Sisimiut, 6. Maniitsoq, 7. Sulugssugut, 8. Eqalugialik, 9. Nuuk, 10. Kangilinnguit, 11. Arsuk, 12. Asanguit, 13. Qaqortoq, 14. Nanortalik, 15. Anorliuitsup qeqertaa, 16. Umigssat qeqertai, 17. Ikigaat.
Due to biotic interchange through the Bering Strait during interglacial periods, followed by glacial periods when biotas became separated again, the northern areas of the North Atlantic, including boreal (cool temperate) and subarctic thermogeographical regions (Adey & Steneck, Citation2001; Adey & Hayek, Citation2011), are important areas to study biodiversity. Lindstrom (1987, 2001) reported several close links between macroalgal species (from the Chlorophyta, Phaeophyceae and the Rhodophyta) in the Northeast Pacific and the North Atlantic. The geographically separated species were thought of as vicariant counterparts that had evolved in the two oceans due to separation during glacial periods, after the first opening of the Bering Strait in the Late Miocene, c. 5.4 to 5.5 Mya (Gladenkov et al., Citation2002). Several pairs of putative sibling species of foliose Bangiales have also been reported from the Northeast Pacific and the North Atlantic (Lindstrom & Cole, Citation1992, 1993).
Kjellman (Citation1883) reported Porphyra abyssicola (now Wildemania abyssicola) from Maniitsoq on the west coast of Greenland, and Rosenvinge (Citation1893) reported Porphyra miniata f. typica (now Wildemania miniata), f. amplissima (now W. amplissima), f. tenuissima, and f. abyssicola (now W. abyssicola), and Porphyra umbilicalis from the west coast; Jónsson (Citation1904) and Christensen (Citation1971) reported P. miniata from the east coast of Greenland. ‘Conchocelis rosea’ was also reported both from the west and east coast (Rosenvinge, Citation1910; Lund, Citation1959; Wilce, Citation1964), but in 1949 the monotypic genus Conchocelis was linked to the Porphyra life history, and it is now known to be the sporophyte (conchocelis-phase) in Bangiales life histories (Drew, Citation1949). Munda & Pedersen (Citation1978) described Porphyra thulaea (now Pyropia thulaea), based on a specimen collected by T. Christensen in 1958 in Nuuk, Greenland, and sequence data were later obtained for the species (Brodie et al., Citation2008; Pedersen, Citation2011; Mols-Mortensen et al., Citation2012). In May and June 2010, one of us (A. Mols-Mortensen) collected foliose Bangiales on the west coast of Greenland from Qaqortoq (60°N) to Ilulissat (69°N). The preliminary results from this effort were published by Pedersen (Citation2011), who reported the occurrence of Boreophyllum birdiae, Porphyra purpurea, P. umbilicalis, Pyropia thulaea, Wildemania miniata, and ‘Porphyra njordii’ (now Pyropia njordii). The identifications were all verified by DNA sequence data.
The aim of the present study was to report on the diversity of foliose Bangiales flora from the west coast of Greenland, based on new and historic collections. Morphological, ecological and molecular characteristics were examined and a dichotomous key to species developed. Finally, the flora was analysed in the broader context of the phylogeny of the group and compared with other floras.
Materials and methods
Collections, identification and molecular methods
Collections were made from the west coast of Greenland () from the intertidal and shallow subtidal at low tide, in Isungua (August 2006), Ilulissat (May and August 2010), Hunde Ejlande and Kumikume (September 2009), Sisimiut (May and June 2010), Maniitsoq (June 2010), Nuuk (March, June and July 2010), Kangilinnguit (July 2008), Arsuk (June 2010), Qaqortoq (July 2005 and June 2010), Nanortalik (October 2007), and Anorliúitsup qeqertaa and Ikigaat (September 2011). A few of the collections from the Qaqortoq area in July 2005 were made by scuba divers. Samples were preserved in silica gel and voucher specimens were dried onto herbarium sheets. Herbarium voucher specimens produced in this work were deposited in the Albion Hodgdon Herbarium (NHA), University of New Hampshire, USA, with duplicates in the Botanical Museum, Copenhagen (C), the Natural History Museum, London (BM), and the Faroese Museum of Natural History, Tórshavn (NGS). Herbarium abbreviations follow Thiers (2012).
Historic collections from Greenland collected by P.M. Pedersen, T. Christensen and L.K. Rosenvinge were made available by the Botanical Museum, Copenhagen. The specimens from which we were able to produce a DNA sequence were from the Qaqortoq area (July and August 1970 and August 1888), Sulugssugut (July 1957), Eqalugialik (July 1957), Nuuk (August 1958), Sisimiut (August 1886), Arsuk (June 1888) and Agsanguit (July 1888).
In total, the collections from Greenland comprised c. 100 specimens, including historical specimens. A segment of the plastid-encoded rbcL gene at its 3′ end and part of the contiguous rbcL–rbcS spacer was selected as a species identification marker. This region, referred to here as 3′ rbcL + 5′ rbcL–S, started from base 1192 in the rbcL gene (the base numbering is based on the sequence of P. umbilicalis, published in GenBank with reference number AB118584) and extended 22 bp into the spacer (298 bp in total; 297 bp in Pyropia thulaea due to a deletion in the spacer). Sequences were generated for 85 specimens (Supplementary ). Mols-Mortensen et al. (Citation2012) found that the suggested standard barcoding marker cox1 (Saunders, Citation2005; Robba et al., 2006) was not able to distinguish between two closely related Bangiales species (Porphyra umbilicalis and P. linearis) and therefore we decided to use the 3′ rbcL + 5′ rbcL–S marker, which we have found to have excellent species-resolving power within the Bangiales (A. Mols-Mortensen & C. Neefus, personal observations). Species identity was verified using the BLAST function on the National Center for Biotechnology Information database and, to ensure correct usage of names, we compared our sequences with sequences deposited in GenBank and, when available, with those from type specimens. A longer region of rbcL, extending from base 218 to base 1398, was generated for one or two specimens of each species identified in the collection. However, Wildemania amplissima, for which there was only one specimen in the collection, was only represented by a 3′ rbcL + 5′ rbcL–S sequence.
Table 1. Pairwise distances for the 3′ rbcL + 5′ rbcL–S marker. The distances (for N specimens) are calculated based on the Tamura–Nei model and presented as patristic distances (sum of branch lengths).
The collection sites were located between 59°N to 69°N, excluding 62°N to 63°N, where no collections were made. It was assumed that if a species was present south of 62°N and north of 63°N, it would also be present at 62°N and 63°N. For most specimens collected, notes were made on where they grew on the coast (the high, mid- or low intertidal, and/or the subtidal), and on which substratum (rock, wood, barnacles or other algae), and the lengths, widths and thicknesses of the blades were measured for three to five specimens of each species.
DNA extraction, PCR amplification, purification and sequencing were carried out as described in Bray et al. (Citation2006) and Mols-Mortensen et al. (Citation2012). The primer pairs used to amplify the 3′ rbcL + 5′ rbcL–S and rbcL regions are listed in Supplementary . All primers were used both to amplify and sequence. The following amplification profile was used for all the primer pairs, with the lid temperature at 105°C: 2.5 min at 95°C; 29 cycles of 45 s at 50°C, 1 min at 72°C and 30 s at 95°C; 45 s at 50°C; 5 min at 72°C; ending with a hold at 4°C. The sequences are deposited in GenBank and listed in Supplementary .
Table 2. The latitudinal distribution of foliose Bangiales species on the west coast of Greenland. ND = no data (no samples from this latitude); + = species present; – = species not recorded.
Phylogenetic analyses
The raw sequence chromatograms were assembled and proofread in Geneious® 6.1.2 (Biomatters, Auckland, New Zealand) and aligned using the Muscle algorithm (Edgar, Citation2004) implemented in Geneious® 6.1.2. The 3′ rbcL + 5′ rbcL–S alignment comprised 85 sequences with a length of 298 bp. Eighty-one sequences were from the Greenland collections, one from the Faroe Islands (from the Pyropia njordii holotype), one from the UK (from the Porphyra umbilicalis neotype) and two Wildemania amplissima sequences were from Iceland (GenBank accession numbers JN847272 and JN847273); the Icelandic specimens were included to enable intraspecific variation analysis in W. amplissima.
The rbcL sequence alignment comprised 78 sequences (Supplementary ) with a length of 1181 bp (the Bangia sp. AF043371 sequence was 1101 bp long). Eight sequences were produced from Greenland material, including GenBank accession numbers JN847258 and JN847268. A sequence from the Porphyra umbilicalis type specimen was also produced and included in the alignment. To place the Greenland foliose Bangiales flora in a wider phylogenetic context (see Sutherland et al., Citation2011), 67 foliose and filamentous Bangiales sequences were downloaded from GenBank (Supplementary ). Erythrocladia sp. (EF660273) and Smithora naiadum (HQ687545) were also downloaded from GenBank to form the outgroup; the overall groupings remained the same when we used florideophyte outgroups (Phycodrys rubens and P. riggii). The 3′ rbcL+ 5′ rbcL–S and rbcL alignments are available in TreeBase (http://treebase.org) as submission ID 14598 and 14577, respectively.
Intra- and interspecific variation was calculated in the 3′ rbcL + 5′ rbcL–S dataset using the Tamura–Nei genetic distance model and neighbour-joining tree building method, implemented in Geneious® 6.1.2. jModelTest 0.1.1 was used to identify the appropriate model of sequence evolution for the rbcL dataset (Posada, Citation2008). Based on a corrected Akaike Information Criterion (AICc) (Hurvich & Tsai, Citation1989) GTR+I+Γ was the preferred model for the rbcL dataset and was implemented in the phylogenetic analyses. Maximum likelihood (ML) searches were carried out using PhyML (Guindon & Gascuel, Citation2003; Guindon et al., Citation2010) implemented in Geneious® 6.1.2, with 1000 bootstrap replicates. Bayesian inference (BI) analysis was also carried out for the rbcL dataset, using MrBayes 3.2.1 (Huelsenbeck & Ronquist, Citation2001), also implemented in Geneious® 6.1.2. The BI analysis was started from random trees and consisted of three heated and one cold chain with temperature set at 0.2, of 1 100 000 generations. The software tool Tracer v1.5 (Rambaut & Drummond, Citation2007) was used to assess whether the stationary phase had been reached, and based on this a burn-in after 150 000 runs was found appropriate.
Results
Diversity, phylogeny and distribution of the foliose Bangiales in West Greenland
Six partial rbcL sequences and 81 3′ rbcL + 5′ rbcL–S sequences were successfully obtained from the Greenland collection of foliose Bangiales (Supplementary ). Based on the sequence data, seven species of foliose Bangiales were recognized in the flora: Boreophyllum birdiae, Porphyra purpurea, P. umbilicalis, Pyropia njordii, P. thulaea, Wildemania amplissima and W. miniata. Tamura–Nei distance analysis of the 298 bp 3′ rbcL + 5′ rbcL–S identification sequence showed intraspecific variation, measured in patristic distance, of 0.000 and 0.006 and interspecific variation of 0.024 and 0.110 (). Pyropia njordii, P. thulaea, W. amplissima (including two samples from Iceland) and W. miniata showed no intraspecific variation in the identification marker, while B. birdiae showed intraspecific variation of 0.000–0.004, Porphyra purpurea 0.000–0.005 and P. umbilicalis 0.000–0.006.
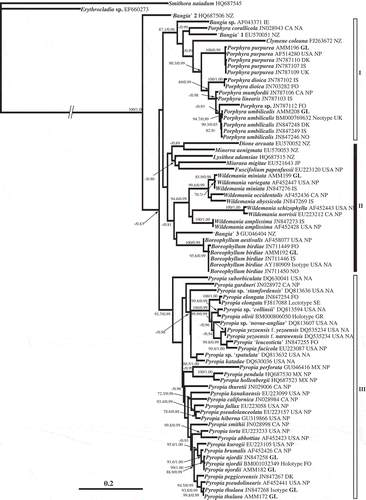
The ML phylogram included 76 Bangiales sequences, with bootstrap values ≥ 70% and posterior probabilities (PP) ≥ 0.8 (). PhyML analysis suggested a division of the foliose and filamentous Bangiales sequences into three major groups (Groups I–III), with the filamentous ‘Bangia’ 2 from New Zealand on its own branch. Group I was supported by a bootstrap of 87.1% and 0.96 PP; Porphyra formed a well-supported clade within it, with Clymene coleana as the sister taxon. However, Porphyra corallicola did not group with the other Porphyra species but with the filamentous ‘Bangia’ 1 from New Zealand. Of the two Porphyra species found in the Greenland material, P. purpurea was represented by specimens from both the North Atlantic and the North Pacific while P. umbilicalis was sister to the North Pacific P. mumfordii. Group II comprised ‘Bangia’ 3, Dione and Minerva, which are all filamentous, and Boreophyllum, Fuscifolium, Lysithea, Miuraea and Wildemania, which are foliose. There was no bootstrap or PP support for Group II but Wildemania and Boreophyllum were well-supported clades within the group. Wildemania miniata, W. amplissima and B. birdiae were the three species in Group II found in the material from Greenland. The North Atlantic Wildemania miniata and North Pacific W. variegata could not be distinguished from each other based on the rbcL gene. The intraspecific variation in W. miniata was 0.006 and the interspecific variation between W. miniata and W. variegata was 0.002–0.008, measured in patristic distances (data not shown). Wildemania amplissima was present in both the North Atlantic and the North Pacific, while the sister taxon to North Atlantic B. birdiae was the North Pacific B. aestivalis. Group III was supported by a bootstrap of 93.7% and 0.99 PP and comprised the foliose genus Pyropia; P. njordii and P. thulaea were the two Pyropia species found in the material collected from Greenland. The North Pacific P. kurogii and P. brumalis formed a well-supported clade with North Atlantic P. njordii, while the North Atlantic P. peggicovensis and North Pacific P. pseudolinearis formed a well-supported clade with North Atlantic P. thulaea.
Fig. 2. Maximum likelihood (ML) phylogram based on rbcL sequences, placing foliose Bangiales species from Greenland in a wider phylogenetic context. ML bootstrap values (> 70%) and Bayesian Inference (BI) posterior probabilities (> 0.80) are indicated on the branches (MLBI). Abbreviations: CA = Canada, DK = Denmark, FO = Faroe Islands, GL = Greenland, GR = Greece, IE = Ireland, IS = Iceland, JP = Japan, Mx = Mexico, NA = North Atlantic, NO = Norway, NP = North Pacific, NZ = New Zealand, SE = Sweden.
Pyropia njordii and P. thulaea had the longest latitudinal distribution in Greenland, stretching over 11° of latitude ( and ). Pyropia njordii occurred between Ikigaat (59°N) and Isungua (69°N), and P. thulaea between Qaqortoq (60°N) and Ilulissat (69°N), P. njordii being the only foliose Bangiales species found south of 60°N. Boreophyllum birdiae and W. miniata were both distributed from Qaqortoq (60°N) to Sisimiut (66°N), and the northernmost distribution of Porphyra umbilicalis was also Sisimiut but it had a much more restricted southward distribution, reaching only Nuuk (64°N). Porphyra purpurea was distributed between Qaqortoq and Sulugssugut (64°N) and showed less northward distribution compared with B. birdiae, P. umbilicalis and W. miniata. Wildemania amplissima was reported only from Uppernaviarsuk in the Qaqortoq area (60°N).
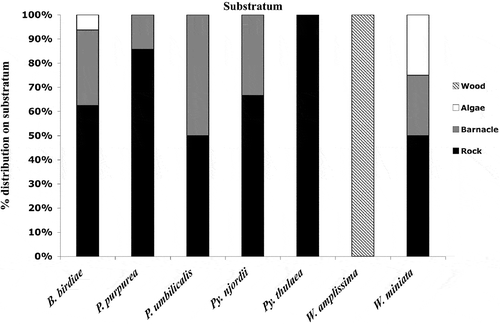
All species collected from the west coast of Greenland, except for W. amplissima, were growing on rock (), and this was the overall most important substratum for foliose Bangiales growth. The single W. amplissima specimen occurred on a wooden piling. Pyropia thulaea was found only on rock but P. njordii, Porphyra purpurea and P. umbilicalis also grew on barnacles. In addition to rock and barnacles, B. birdiae and W. miniata also grew on other algae.
Fig. 3. Distribution of the species on different substrata based on 75 specimens; Boreophyllum birdiae (N = 16), Porphyra purpurea (N = 7), Porphyra umbilicalis (N = 6), Pyropia njordii (N = 30), Pyropia thulaea (N = 7), Wildemania amplissima (N = 1) and Wildemania miniata (N = 8).
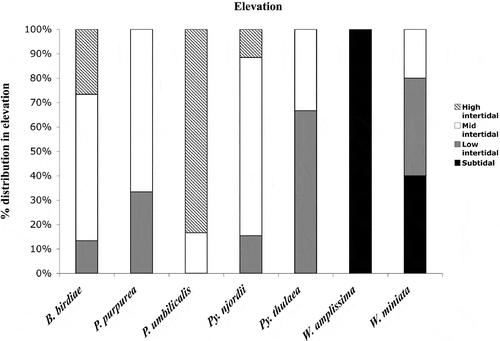
Two of the seven foliose Bangiales species, W. amplissima and W. miniata, were found in the subtidal zone (). Wildemania miniata was found from the mid intertidal zone to a depth of 5–10 m, while the single specimen of W. amplissima was collected in the shallow subtidal. Boreophyllum birdiae and Pyropia njordii were found throughout the intertidal zone, although mostly in the mid intertidal. Porphyra purpurea and Pyropia thulaea were found in the mid and low intertidal zone, with Porphyra purpurea mostly occurring in the mid intertidal and Pyropia thulaea in the low intertidal. Porphyra umbilicalis was mostly found in the high intertidal but extended down into the mid intertidal zone.
Key to the species of foliose Bangiales of West Greenland
Blade distromatic.2
Blade monostromatic3
Sori intermixed around the blade margin; colour pink, blade c. 55 µm thick; latitudinal distribution 60°NWildemania amplissima
2. Sori on separate halves of the blade; colour pale to intense pink; blade c. 45 µm thick; latitudinal distribution 60°N to 66°N……………………..Wildemania miniata
Dioecious, male or female sori around the margin …4
3. Monoecious, male and female sori on separate halves of the blade5
Blade radially symmetrical, c. 65–87.5 µm thick; colour dark to pale brown, greenish brown and pale pink with a greyish tone, attached to rock and barnacles, mostly in the high intertidal zone; latitudinal distribution 64°N to 66°NPorphyra umbilicalis
4. Blade linear to lanceolate, c. 34–64 µm thick; colour pink, purple and brown, attached to rock in the low and mid intertidal zones; latitudinal distribution 60°N to 69°Pyropia thulaea
Blade ovate, typically < 12 cm long, < 4 cm wide and c. 22.5–35 µm thick, attached to rock and barnacles throughout the intertidal zone but mostly in the mid intertidal; latitudinal distribution 59°N to 69°…………………Pyropia njordii
5. Blade round to broad-lanceolate with cordate base, c. 30 – 100 µm thick; colour light brown, greyish purple, pale purple or pale pink……….6
Blade up to 18 cm long, 18 cm broad, and c. 30–37.5 µm thick; colour pale brown and greyish purple; mainly on rock, sometimes epizoic on barnacles within the mid to low intertidal zones; latitudinal distribution 60°N to 61°N ……………Porphyra purpurea
6. Blade up to 12.5 cm long, 14.5 cm broad, and c. 67.5–100 µm thick; colour light pink, light purple or pale brown; mainly on rock and barnacles but also epiphytic on other algae; throughout the intertidal zone; latitudinal distribution 60°N to 66°N ………………..Boreophyllum birdiae
Discussion
The present work represents the first comprehensive study of foliose Bangiales in Greenland. From the west coast we have confirmed the presence of four foliose Bangiales genera and seven species. The preliminary diversity reports based on our work, presented by Pedersen (Citation2011), are confirmed, and in addition we report W. amplissima from the southwest coast of Greenland. Four of the eight foliose Bangiales genera described in Sutherland et al. (Citation2011) are reported from Greenland, and the same four genera have also been reported from other areas in the North Atlantic (Mols-Mortensen et al., Citation2012). However, species diversity of foliose Bangiales in Greenland is less than that reported from other northerly areas in the North Atlantic (), e.g. Iceland and the Faroe Islands (Mols-Mortensen et al., Citation2012). Only seven of the 25 species Mols-Mortensen et al. (Citation2012) recorded from the North Atlantic are reported from Greenland, while 11 species are reported from both Iceland and the Faroe Islands. Six of the foliose Bangiales species in Greenland (B. birdiae, Porphyra purpurea, P. umbilicalis, Pyropia njordii, W. amplissima and W. miniata) occur both in Iceland and the Faroe Islands, while three that occur in Iceland and the Faroe Islands (Porphyra dioica, P. linearis and Pyropia ‘leucosticta’) have not been found in Greenland.
Table 3. Foliose Bangiales species recorded from the North Atlantic, Iceland, Faroe Islands and Greenland. + = present; – = absent.
Porphyra dioica appears to be endemic to the northeast Atlantic, being confined to European coasts, the Faroe Islands and Iceland (Brodie & Irvine, Citation2003; Brodie et al., Citation2008; Mols-Mortensen et al., Citation2012). The species has been sought extensively in the Northwest Atlantic but it has not been found (C.D. Neefus, personal observations; Kucera & Saunders, Citation2012).
Porphyra linearis is a common winter annual in the North Atlantic (Brodie & Irvine, Citation2003), and its northernmost confirmed report is from Iceland (Mols-Mortensen et al., Citation2012). The foliose phase of P. linearis is recorded from the British Isles between October and May (Brodie & Irvine, Citation2003), and it has been found in the Faroe Islands from October to April, and in New Hampshire, U.S.A. from November to May (A. Mols-Mortensen, personal observations). Even though collections were made in Nuuk in late March and in Nanortalik in early October, P. linearis was not observed.
Foliose Bangiales species with spermatangial sori arranged in pale patches or streaks, as in Pyropia ‘leucosticta’, have not been found in Greenland, and this type of sorus seems to be rare in cold-water areas of the North Atlantic. The northernmost reports of P. ‘leucosticta’ are from Newfoundland (Kucera & Saunders, Citation2012) and Iceland (Mols-Mortensen et al., Citation2012), where it is confined to the west and southwest coasts, which have warmer sea temperatures (Astthorsson et al., Citation2007). The northernmost record of P. elongata, which also has this type of sorus arrangement, is in the Faroe Islands (Brodie et al., Citation2008; Mols-Mortensen et al., Citation2012), but at the northern limit no reproductive specimens of the species have been observed (A. Mols-Mortensen, personal observations).
Pyropia njordii is widely distributed and a common species on the west coast of Greenland. Using DNA sequence analysis it has been possible to verify this species in historic collections (Supplementary ). It was collected by Rosenvinge (in 1888) in Arsuk, Asanguit and Qaqortoq, and identified as Porphyra umbilicalis f. laciniata. Current work confirms that Pyropia njordii was included in Rosenvinge’s concept of P. umbilicalis f. laciniata. Pyropia thulaea is a cold-water species that has so far been reported only from western Greenland and eastern Iceland (Munda & Pedersen, Citation1978; Brodie et al., Citation2008; Pedersen, Citation2011; Sutherland et al., Citation2011; Mols-Mortensen et al., Citation2012). It is widespread on the west coast of Greenland (see ), but in Iceland it is confined to the east coast (Munda & Pedersen, Citation1978), where the cold East Icelandic Current influences the climate. Pyropia thulaea seems to be rare in Iceland, and it was not observed by Mols-Mortensen et al. (Citation2012).
Rosenvinge (Citation1893) reported Wildemania amplissima (as Porphyra miniata var. amplissima) from Qaqortoq in southwest Greenland, but the taxon has not been reported again until now. Wildemania amplissima seems to be rare in Greenland and confined to the Qaqortoq area (60°N) in the southwest, where both Rosenvinge’s and our observations were made, 117 years apart. Wildemania abyssicola, which Kjellman (Citation1883) reported (as P. abyssicola) from the deep waters at Maniitsoq and Rosenvinge (Citation1893) reported (as P. miniata var. abyssicola) from several locations in the subtidal zone in West Greenland, was not found in our study. It is possible however, that we overlooked the species due to limited subtidal sampling. Wildemania abyssicola was originally described by Kjellman (Citation1883) from deep waters in the Norwegian Arctic Sea, Murman Sea, White Sea, as well as the west coast of Greenland at Sukkertoppen (Maniitsoq). Mols-Mortensen et al. (Citation2012) reported W. abyssicola in Iceland from 17 m depth, but it is also found in the shallow subtidal (J. Brodie & K. Gunnarsson, unpublished observations).
Our observations support the hypothesis of dispersal of macroalgal species through the Bering Strait followed by vicariant speciation due to subsequent isolation, as proposed by Lindstrom (Citation2001). All of the foliose Bangiales species found in Greenland have a North Pacific–North Atlantic link, either as closely related sibling species or conspecific populations. Boreophyllum birdiae and B. aestivalis, Porphyra umbilicalis and P. mumfordii, Pyropia njordii and P. brumalis, and Pyropia thulaea and P. pseudolinearis are each North Atlantic and North Pacific vicariant counterparts. Porphyra purpurea and W. amplissima have populations in both the North Atlantic and North Pacific (Bray et al., Citation2007; Kucera & Saunders, Citation2012). Wildemania miniata and W. variegata have been regarded as North Atlantic and North Pacific vicariant counterparts (Lindstrom & Cole, Citation1992), but observations from current work and Mols-Mortensen et al. (Citation2012) show that the two species are very closely related and should possibly be regarded as the same species, with populations in the two oceans.
Acknowledgements
The authors are grateful for the grant awarded to Agnes Mols-Mortensen by the Faroese Research Council (Granskingarráðið) and the additional funding provided by a Hatch Grant and Sea Grant to Christopher D. Neefus from the New Hampshire Agricultural Experiment Station and New Hampshire Sea Grant College Program, respectively. We thank Dr Michael Lynch, University of Waterloo, Canada for helpful advice with technical phylogenetic questions; Dr Susse Wegeberg, Aarhus University, Denmark for providing samples; Heðin I. Abrahamsen, p/f Fiskaaling, Faroe Islands for drawing the map of the collecting locations; p/f Fiskaaling for providing office and laboratory space to Agnes Mols-Mortensen; and Lindsay A. Green for helping out with a scan and cross section. We also would like to thank Dr Arthur Mathieson, Dr Janet Sullivan and Dr Anita Klein from the University of New Hampshire, and the Associate Editor and two anonymous reviewers for thorough and helpful comments on the manuscript.
Supplementary information
The following supplementary material is available for this article, accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org/10.1080/09670262.2013.871062
Supplementary Table 1. Taxa used in the analysis with collecting details and GenBank accession numbers. NA = not available.
Supplementary Table 2. List of amplification and sequencing primers used in this study.
Supplementary material
Download Zip (32.1 KB)References
- Adey, W.H. & Hayek, L.A.C. (2011). Elucidating marine biogeography with macrophytes: quantitative analysis of the North Atlantic supports the thermogeographic model and demonstrated a distinct subarctic region in the Northwestern Atlantic. Northeastern Naturalist, 18 (Monograph 8): 1–128.
- Adey, W.H. & Steneck, R.S. (2001). Thermogeography over time creates biogeographic regions: a temperature/space/time-integrated model and an abundance-weighted test for benthic marine algae. Journal of Phycology, 37: 677–698.
- Astthorsson, O.S., Gislason, A. & Jonsson, S. (2007). Climate variability and the Icelandic marine ecosystem. Deep-Sea Research II, 54: 2456–2477.
- Bray, T.L., Neefus, C.D. & Mathieson, A.C. (2006). Morphological and molecular variability of Porphyra purpurea (Roth) C. Agardh (Rhodophyta, Bangiales) from the Northwest Atlantic. Nova Hedwigia, 82: 1–22.
- Bray, T.L., Neefus, C.D. & Mathieson, A.C. (2007). A morphological and molecular investigation of the Porphyra purpurea complex in the Northwest Atlantic. Nova Hedwigia, 84: 277–298.
- Brodie, J.A. & Irvine, L.M. (2003). Seaweeds of the British Isles Volume 1 3B: The Bangiophycidae. Intercept, Hampshire.
- Brodie, J. & Nielsen, R. (2005). The diversity of the Bangiophycidae (Rhodophyta) of the Faroes in the context of the northern Atlantic. Biofar Proceedings Fróðskaparrit (Annales Societatis Scientarum Færoensis Supplementum), 41: 53–62.
- Brodie, J., Bartsch, I., Neefus, C., Orphanidis, S., Bray, T. & Mathieson, A.C. (2007). New insights into the cryptic diversity of the North Atlantic – Mediterranean ‘Porphyra leucosticta’ complex: P. olivii sp. nov. and P. rosengurttii (Bangiales, Rhodophyta). European Journal of Phycology, 20: 939–949.
- Brodie, J., Mols Mortensen, A., Ramirez, M. E., Russell, S. & RInkel, B. (2008). Making the links: towards a global taxonomy for the red algal genus Porphyra (Bangiales, Rhodophyta). Journal of Applied Phycology, 20: 939–949.
- Christensen, T. (1971). Havbundens planter. In Danmarks natur 10, Grønland og Færøerne (Nørrevang, A., Meyer T.J. & Christensen, S., editors), 184–192. Politikens, Copenhagen.
- Drew, K.M. (1949). Conchocelis-phase in the life-history of Porphyra umbilicalis (L.) Kütz. Nature, 164: 748–749.
- Dunbar, M.J. (1954). Arctic and subarctic marine ecology: immediate problems. Arctic, 7: 213–228.
- Edgar, R.C. (2004). MUSCLE: multiple sequence alignments with high accuracy and high throughput. Nucleic Acids Research, 32: 1792–1797.
- Gladenkov, A.Y., Oleinik, A.E., Marincovich Jr, L. & Barinov, K.B. (2002). A refined age for the earliest opening of Bering Strait. Palaeoecology, 183: 321–328.
- Guindon, S. & Gascuel, O. (2003). A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Systematic Biology, 52: 696–704.
- Guindon, S., Dufayard, J.F., Lefort, V., Anisimova, M., Hordijk, W. & Gascuel, O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology, 59: 307–321.
- Huelsenbeck, J.P. & Ronquist, F. (2001). MRBAYES: Bayesian inference of phylogeny. Bioinformatics, 17: 754–755.
- Hurvich, C.M. & Tsai, C.L. (1989). Regression and time series model selection in small samples. Biometrika, 76: 297–307.
- Jónsson, H. (1904). The marine algae of East Greenland. Meddelelser om Grønland, 30: 1–73.
- Kjellman, F. (1883). The algae of the Arctic sea. Kongliga Svenska Vetenskaps-Akademiens Handlingar, 20: 1–350.
- Klein, A.S., Mathieson, A.C., Neefus, C.D., Cain, D.F., Taylor, H.A., Teasdale, B.W., West, A.L., Hehre, E.J., Brodie, J., Yarish, C. & Wallace, A.L. (2003). Identification of north-western Atlantic Porphyra (Bangiaceae, Bangiales) based on sequence variation in nuclear SSU and plastid rbcL genes. Phycologia, 42: 109–122.
- Kornmann, P. (1986). Porphyra yezoensis bei Helgoland – eine entwicklungsgeschichtliche Studie. Helgoländer Meeresuntersuchungen, 40: 327–342.
- Kucera, H. & Saunders, G.W. (2012). A survey of Bangiales (Rhodophyta) based on multiple molecular markers reveals cryptic diversity. Journal of Phycology, 48: 869–882.
- Lindstrom, S.C. (1987). Possible sister groups and phylogenetic relationships among selected North Pacific and North Atlantic Rhodophyta. Helgoländer Meeresuntersuchungen, 41: 245–260.
- Lindstrom, S.C. (2001). The Bering Strait connection: dispersal and speciation in boreal macroalgae. Journal of Biogeography, 28: 243–251.
- Lindstrom, S.C. & Cole, K.M. (1992). Relationships between some North Atlantic and North Pacific species of Porphyra (Bangiales, Rhodophyta): evidence from isozymes, morphology, and chromosomes. Canadian Journal of Botany, 70: 1355–1363.
- Lindstrom, S.C. & Cole, K.M. (1993). The systematics of Porphyra: character evolution in closely related species. Hydrobiologia, 261: 151–157.
- Lund, S. (1959). The marine algae of East Greenland II. Geographic distribution. Meddelelser om Grønland, 156(2): 1–70.
- Merkel, F., Boertmann, D., Mosbech, A. & Ugarte, F. [ editors] (2012). Davis Stræde. En foreløbig, strategisk miljøvurdering af aktiviteter forbundet med olieefterforskning og udvinding I den østlige del af Davis Stræde. Videnskabelig rapport fra DCE – Nationalt Center for Miljø og Energi 26. DCE – Nationalt Center for Miljø og Energi, Aarhus University, Denmark. http://www.dmu.dk/Pub/SR26.pdf
- Mols-Mortensen, A., Neefus, C.D., Nielsen, R., Gunnarsson, K., Egilsdóttir, S., Pedersen, P.M. & Brodie, J. (2012). New insights into the biodiversity and generic relationships of foliose Bangiales (Rhodophyta) in Iceland and the Faroe Islands. European Journal of Phycology, 47: 146–159.
- Mumford, T.F. & Miura, A. (1988). Porphyra as food: cultivation and economics. In Algae and human affairs (Lembi, C.A. & Waaland, J.R., editors), 87–117. Cambridge University Press, Cambridge.
- Munda, I.M. & Pedersen, P.M. (1978). Porphyra thulaea sp. nov. (Rhodophyceae, Bangiales) from East Iceland and West Greenland. Botanica Marina, 21: 283–288.
- Pedersen, P.M. (2011). Grønlands Havalger. Epsilon, Copenhagen.
- Posada, D. (2008). jModelTest: phylogenetic model averaging. Molecular Biology and Evolution, 25: 1253–1256.
- Rambaut, A. & Drummond, A.J. (2007). Tracer v1.5. available from http://beast.bio.ed.ac.uk/Tracer
- Robba, L., Russell, S.J., Barker, G.L. & Brodie, J. (2006). Assessing the use of the mitochondrial cox1 marker for use in DNA barcoding of red algae (Rhodophyta). American Journal of Botany, 93: 1101–1108.
- Rosenvinge, L.K. (1893). Grønlands Havalger. Meddelelser om Grønland, 3: 765–981.
- Rosenvinge, L.K. (1910). On the marine algae from North-East Greenland, collected by the ‘Danmark Expedition’. Meddelelser om Grønland, 43(4): 91–133.
- Saunders, G.W. (2005). Applying DNA barcoding to red macroalgae: a preliminary appraisal holds promise for future applications. Philosophical Transactions of the Royal Society of London, series B, 360: 1879–1888.
- Sutherland, J., Lindstrom, S.C., Nelson, W., Brodie, J., Lynch, M., Hwang, M.S., Choi, H.G., Miyata, M., Kikuchi, N., Oliveira, M., Farr, T., Neefus, C., Mols-Mortensen, A., Milstein, D. & Müller, K. (2011). A new look at an ancient order: generic revision of the Bangiales (Rhodophyta). Journal of Phycology, 47: 1131–1151.
- Teasdale, B., West, A., Taylor, H. & Klein, A. (2002). A simple restriction fragment length polymorphism (RFLP) assay to discriminate common Porphyra (Bangiophyceae, Rhodophyta) taxa from the Northwest Atlantic. Journal of Applied Phycology, 14: 293–298.
- Thiers, B. [continuously updated]. Index herbarium: a global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih
- Wilce, R.T. (1964). Studies of attached marine algae in northwest Greenland. In Proceedings of the 4th International Seaweed Symposium, 4 (Davy de Virville, A. & Feldmann, J., editors), 280–287. Pergamon Press, Oxford.
- Wilce, R.T. (1990). Role of the Arctic Ocean as a bridge between the Atlantic and Pacific Oceans: facts and hypothesis. In Evolutionary biogeography of the marine algae of the North Atlantic (Garbary, D.J. & South, G.R., editors), 323–348. Springer, Berlin.