Abstract
Nutrient Induced Fluorescence Transients (NIFTs) have been shown to be a possible way of testing for the limiting nutrient in algal populations. In this study we tested the hypothesis that NIFTs can be used to detect a (co-)limitation for inorganic phosphorus (Pi) and CO2 in the green alga Chlamydomonas acidophila and that the magnitude of the NIFTs can be related to cellular P:C ratios. We show a co-limitation response for Pi and CO2 via traditional nutrient enrichment experiments in natural phytoplankton populations dominated by C. acidophila. We measured NIFT responses after a Pi- or a CO2-spike in C. acidophila batch cultures at various stages of Pi and inorganic C limitation. Significant NIFTs were observed in response to spikes in both nutrients. The NIFT response to a Pi-spike showed a strong negative correlation with cellular P:C ratio that was pronounced below 3 mmol P: mol C (equivalent to 0.2 pg P cell–1). Both cellular P and C content influenced the extent of the Pi-NIFT response. The NIFT response to a CO2-spike correlated to low CO2 culturing conditions and also had a negative correlation with cellular P content. A secondary response within the Pi-NIFT response was related to the CO2 concentration and potentially reflected co-limitation. In conclusion, NIFTs provided a quick and reliable method to detect the growth-limiting nutrient in an extremophile green alga, under Pi-, CO2- and Pi/CO2 (co-)limited growth conditions.
INTRODUCTION
Primary productivity in aquatic ecosystems is frequently limited by nutrient availability. Although it was thought for many years that only one factor can be growth-limiting at any one time, co-limitation by multiple nutrients might be a common phenomenon in natural phytoplankton populations (Elser et al., Citation2007; North et al., Citation2007; Moore et al., Citation2007; Moore et al., Citation2008; Saito et al., Citation2014) and has also been observed in single species cultures of a green alga (Spijkerman et al., Citation2011) and a haptophyte (Buitenhuis et al., Citation2003). Nutrient enrichment experiments can detect such limitations via full factorial design additions of the nutrients and monitoring the change in biomass production (Moore et al., Citation2008; Harpole et al., Citation2011) but do not provide exclusive results on the way the co-limitation acts upon the species or phytoplankton community. Alternatively, models based on Monod kinetics can distinguish between different types of co-limitation (Saito et al., Citation2008; Buitenhuis & Geider, Citation2010) but these experiments are very time consuming. Three different forms of co-limitation have been identified, based on nutrient enrichment experiments: simultaneous, independent and serial co-limitation (Harpole et al., Citation2011). In simultaneous and independent co-limitation the community biomass increases only with the addition of all co-limiting nutrients. In the serial co-limitation response, the synergistic effect of simultaneous nutrient additions only occurs when the primary limitation is surpassed. A serial co-limitation response for Pi and CO2 was found in nutrient enrichment experiments with cultures of the green alga Chlamydomonas acidophila Negoro (Spijkerman, Citation2010), where Pi addition resulted in a large response, CO2 addition gave no response, but the combined addition of Pi and CO2 resulted in a synergistic effect. From a biochemical point of view this can be considered a co-limitation with different nutrients limiting different processes (Wolf-Gladrow & Riebesell, Citation1997; Moore et al., Citation2008), one limiting nutrient biologically substituting with another, either directly within the same macromolecule or indirectly by substituting one macromolecule for another, or when the ability to take up low concentrations may depend on the availability of another nutrient (Moore et al., Citation2013).
Chlamydomonas acidophila is an extremophilic alga which experiences relatively low Pi and CO2 concentrations in its natural environment and is potentially (co-)limited in its growth by these two nutrients (Tittel et al., Citation2005; Spijkerman, Citation2008a). Pi is limiting because the bio-available Pi concentrations are decreased by the typically high total iron concentrations (0.1–10 mM; (Spijkerman, Citation2008a) and inorganic carbon (Ci) concentrations are low because at low pH (2––3.5), > 99% of Ci is CO2 and the total Ci concentration at air equilibrium is only ~14 μM (Stumm & Morgan, Citation1970). Recent work on Ci acquisition in C. acidophila revealed the presence of a CO2 concentrating mechanism (CCM), which was down-regulated under high CO2 and/or Pi-limiting conditions (Spijkerman, Citation2005; Spijkerman, Citation2011). Previous results have suggested that co-limiting conditions for Pi and CO2 resulted in a trade-off in the resources allocated to nutrient transporters on the cytoplasmic membrane, depending on the most limiting nutrient (Spijkerman et al., Citation2011).
When nutrient-limited plants or algae are exposed to a pulse of the limiting nutrient, a change in the chlorophyll a (chl a) fluorescence is often observed (Shelly et al., Citation2010). This has been termed a nutrient-induced fluorescence transient, or NIFT (Wood & Oliver, Citation1995), and has been suggested as a fast and non-invasive monitoring tool for nutrient limitation (Beardall et al., Citation2001) because the chl a fluorescence response is generally not observed if a non-limiting nutrient or distilled water is added to the cells. NIFTs have an advantage over standard enrichment experiments because they give an indication of the immediate nutrient status rather than just indicating potential limiting factors (Beardall et al., Citation2001). The NIFT response to a transient pulse or ‘spike’ in nutrient begins almost immediately and the full response is observed over a few minutes. With this in mind we hypothesized that the serial co-limitation response previously seen in C. acidophila using nutrient enrichment assays could be further unravelled using this type of NIFT approach. Indeed, recent work has demonstrated the use of NIFTs in (co-)limited seagrass (den Haan et al., Citation2013).
To this end, we tested for a (co-)limitation in C. acidophila in its natural environment by conventional bio-assays. We applied NIFTs to batch cultures to test for a limitation in C. acidophila by Pi and CO2, to determine if this limitation can be correlated to the cellular P:C ratio and to ascertain if we can detect a CO2 (co-)limitation in this green alga via a NIFT response.
MATERIALS AND METHODS
Field sampling
Primary productivity in the acid lakes 107 (pH 2.4) and 117 (pH 3.4; both 51º29′ N 13º38′ E), situated in the east part of Germany, has been much studied (Beulker et al., Citation2003; Kamjunke et al., Citation2005) and compared with lakes of similar nutrient status but neutral pH (Nixdorf et al., Citation2003). A mixed water sample from the epilimnion above the deepest point of Lake 107 was taken on 27 June 2010 and from Lake 117 on 24 July 2010. Directly after sampling, water was filtered through a 200 µm zooplankton net. This separation did not eliminate the important predators in these acidic lakes because they consist of small-sized organisms such as rotifers, heliozoans and the chrysophyte Ochromonas sp. (Kamjunke et al., Citation2004). Waters from these lakes were used in a nutrient enrichment experiment as described below. In both lakes C. acidophila is the most important primary producer, whereas Ochromonas sp. often builds up a higher biomass but is predominantly phagotrophic (Kamjunke et al., Citation2004; Spijkerman, Citation2008a).
Culturing
Batch cultures of C. acidophila (CCAP 11/137; Gerloff-Elias et al. Citation2005) were grown at 20 ± 1 °C in modified Woods Hole medium at pH 2.65 as described in Spijkerman (Citation2010). A multivariate approach with two different CO2 and Pi concentrations was applied in duplicate. Cultures were either non-aerated (low CO2, −C) or gently aerated with 5% CO2 in air (high CO2, +C) and were inoculated from a full strength medium (50 µM P) into a medium containing either a Pi concentration of 0 µM P (Pi-depleted, −P) or 50 µM P (Pi-replete, +P). Cultures started with an optical density (OD; 1 cm pathway at 750 nm) of 0.01 and growth in low CO2 cultures was followed over a 12 d period, though high CO2 cultures were terminated after 8 d because cell densities were very high in the Pi-replete cultures (i.e. 4.6 × 106 cells ml–1) and possibly experienced light limitation. We decided to follow growth by OD as this was the most rapid and reliable method and includes changes in both cell number and cell volume. Light intensity at the surface of the Erlenmeyer flasks (Biospherical Instruments Inc. Model QSL-2101, San Diego, California) was 130–140 µmol m–2 s–1 and was supplied continuously. Every second day, samples were taken for OD, NIFT response, cellular carbon and phosphorus content, and cell density. We checked for a normal distribution of the data, homogeneity of variance and performed statistical tests in SPSS 20.0 (SPSS for Windows, IBM, Chicago, Illinois). All data passed the prerequisites for a parametric test, and therefore differences between groups were examined with ANOVA, paired measurements with a paired t-test and Pearson correlation.
Nutrient enrichment bioassay
To test for the growth limiting factor in the phytoplankton, lake water was enriched with: (a) 5 µM Pi (as K2HPO4), (b) CO2 provided via aeration with 4.5% CO2 in normal air (v/v), and (c) a combination of both Pi and CO2 and incubated at 18 ± 1ºC in a constant temperature room with 16:8 h light:dark cycle. Biomass production was monitored after 8 d via chl a fluorescence (recorded on a TD-700, Turner designs, Bremerhafen, Germany). A control was enriched with de-ionized water and treated similarly. Nutrient enrichments were performed in triplicate.
NIFT measurements
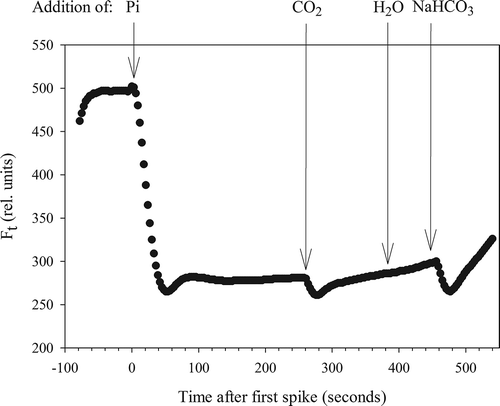
NIFTs were carried out on the batch cultures of C. acidophila using a PhytoPAM fluorometer (Heinz Walz GmbH, Effeltrich, Germany) by recording the chl a fluorescence (Ft) every 3 s without the application of a saturating pulse (Phytowin_v1.47). The actinic light source in the measuring cuvette was set to 120 µmol photons m–2 s–1, approximately matching the light intensity at which the algae were grown. Gain settings on the PAM varied between 8 and 18 during the experiment, as it needed to be adjusted for undiluted culture material. We choose to use undiluted culture material so as not to impose stress on the cells, although this could have affected the response to a nutrient spike as the concentration added per cell differed. Chl a fluorescence was first recorded for at least 1 min to obtain a stable value, after which the response to a spike of Pi, CO2, Ci or distilled water was subsequently monitored for several minutes (). All three additions were performed on sub-samples of each batch culture, but the order was changed between replicates and measuring days. For Pi-addition, unless otherwise stated, we used a final standard concentration of 10 µM KH2PO4 as suggested previously (Holland et al., Citation2004; Roberts et al., Citation2008), which was prepared in acidified water (pH 2.65, not buffered). To test the effect of CO2 we added NaHCO3 to the same acidified water (pH 2.65) and spiked the subculture to a final concentration of 100 µM CO2 after exactly 60 s of reaction time. This time was calculated as sufficient to give 100% conversion of HCO3– to CO2 within 20 s at this low pH (Brinkman et al., Citation1934). Finally, direct HCO3– pulses were given at the end of the run (final concentration 650 µM) to test for a direct response to Ci ().
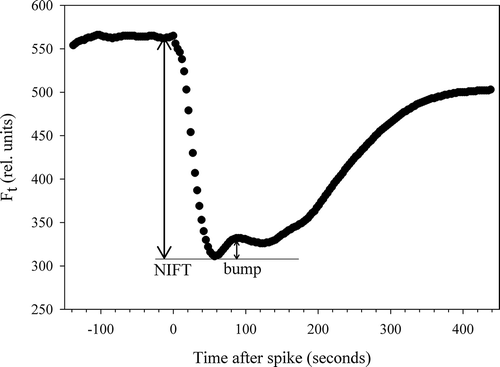
Fig. 1. Example of changes in chlorophyll a fluorescence (Ft) after addition of several nutrients or acidified water.
At the end of the growth experiment (on d 8 for –P+CO2, and d 12 for –P–CO2), NIFT responses were recorded on Pi-depleted cells by the addition of eight different Pi concentrations, ranging from 0.25–7.5 µM P (as KH2PO4). A fresh sample was taken for every measurement to avoid effects from prior additions. An intermediate recovery, or possibly a second NIFT occurred within the NIFT response to a Pi-spike. This intermediate recovery occurred without any external input. After Pi-addition, Ft decreased rapidly, increased again (to a ‘bump’), remained more or less constant or decreased (), and then increased again to regain the starting value of Ft. We calculated the extent of this ‘bump’ (Ft increase) as a percentage of the (initial) NIFT response (Ft decrease) for each Pi concentration.
Chemical analyses and algal density
Cellular P:C ratios were determined by measuring the particulate P and C in the cultures. The particulate P concentration was determined on filtered culture suspension (0.45 μm polysulfon filter, Pall Corporation, Port Washington, New York) after heating in an autoclave for 20 min with 20 mM K2S2O8 and 60 mM H2SO4. Measurements were performed spectrophotometrically using molybdate and ascorbic acid and comparing absorbance with a standard curve generated from similarly treated solutions of KH2PO4 (Murphy & Riley, Citation1962). For particulate C analysis a minimum of 10 µg C culture suspension was filtered on pre-combusted GF/F filters (4 h at 450ºC; Whatman), dried at 50ºC for 2 d and subsequently measured in a carbon analyser (EuroVector CHNS-O Elementaranalysator, Wegberg, Germany). Infrared gas chromatography was calibrated against standards in every run.
The OD of cultures was measured, immediately after sampling, on a Cary UV-Vis spectrophotometer (50 Bio, Varian). Cell numbers were determined after finishing the experiment, in Lugol-fixed samples, using an automatic cell counter (CASY 1, Model TT, Schärfe, Reutlingen, Germany).
RESULTS
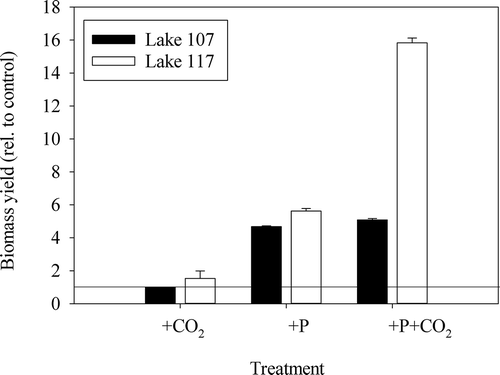
In the nutrient enrichment bioassays in lake water, biomass production, relative to the control after 8 d, was not enhanced by the addition of CO2, but was significantly enhanced when Pi was added (, ANOVA, F2,23 = 91.2, P < 0.001). The phytoplankton of Lake 117 also showed a synergistic enhancement from the addition of CO2 plus Pi in comparison with Pi alone (Tukey, P < 0.001), although this was not significant for Lake 107 samples (P = 0.74; ). Microscopic examination revealed that C. acidophila was the dominant alga in Lake 117 phytoplankton before and after the enrichment, whereas in Lake 107, Ochromonas was present in high densities and likely grazed the potential growth of C. acidophila (biomass densities not shown, but see Spijkerman, Citation2008a for some examples).
Fig. 3. Relative increase in biomass to the control (untreated water from each lake) in enrichment experiments with water from Lake 107 and Lake 117 after an 8 day incubation. The straight line at 1 illustrates no response in relation to control. The biomass in untreated water increased by a factor of 2.6 and 2.7 in Lake 107 and Lake 117, respectively. Mean ± SD of triplicates (ANOVA, F2,23 = 91.2, P < 0.001).
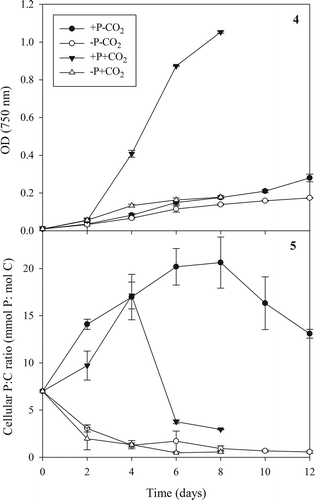
In the batch culture experiments performed to test the NIFTs, growth of C. acidophila was highest over the first 4 d (), with the +P+CO2 treatments growing fastest (0.94 ± 0.02 d–1) followed by –P+CO2 (0.65 ± 0.02 d–1), and then the −CO2 treatments (both ~0.50 d–1). The optical density at d 8 was likewise highest in the +P+CO2 treatments (1.05 ± 0.05, equivalent to 4.6 × 106 cells ml–1), with the other three treatments being about one-sixth as dense, with little difference between them (OD ~0.15, ~1 × 106 cells ml–1). By d 14 there was a clear distinction between the cell density in the +P−CO2 and the −P−CO2 treatments, with the former approximately 60% higher than the latter ().
There were clear differences between the cellular P:C ratio in batch-grown algae over time when comparing cells grown in Pi-replete or Pi-depleted medium (). Independent of the presence of CO2-aeration, the P:C ratio rapidly decreased in cells grown in Pi-depleted medium to 0.57 ± 0.11 mmol P: mol C (mean ± SD of values obtained on the last 2 measuring days, i.e. d 6 and 8 for +CO2 and d 10 and 12 for –CO2 cultures). In contrast, P:C ratio increased in the cells grown in Pi-replete medium, but after 4 d rapidly decreased in the high CO2 cultures because Pi became depleted in the medium. The highest P:C ratio was measured in cells grown in Pi-replete medium at low CO2 on d 8, reaching 22.1 ± 0.5 mmol P: mol C (mean ± SD; after which the medium Pi concentrations were depleted. P:C ratio values can thus vary at least 40-fold in C. acidophila.
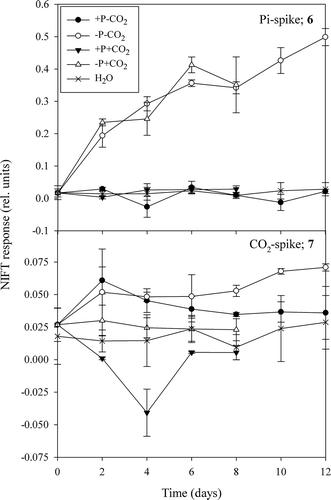
To determine if NIFTs matched responses seen in the nutrient enrichment bioassay in lake water and semi-continuous cultures of C. acidophila (Spijkerman, Citation2010), batch cultures were tested for their NIFT response throughout the growth period. Two days after inoculation in Pi-depleted medium, the cells showed a NIFT in response to the addition of Pi, with an approximately 20% reduction in chl a fluorescence (). This NIFT response coincided with a 2-fold decrease in P:C ratio (). The extent of the response was the same in high and low CO2-grown cells and steadily increased with increasing culturing time and, concomitantly, with decreasing P:C ratio. The maximum effect ranged between 30 and 50% of the initial chl a fluorescence at the end of the growth experiment. The order in which the nutrient spikes were supplied (i.e. whether the Pi-spike came before or after the CO2-spike) had no effect on most NIFT responses (paired t-test, n ≥ 4, P > 0.08), except for the CO2-spike in the Pi-depleted plus CO2 cultures (paired t-test, n = 4, t = 10.4, P < 0.05). In these cultures, if Pi was added first, the NIFT for CO2 was lower than when CO2 was added first. Although the Pi concentration in the medium of ‘Pi-replete’ plus high CO2 cultures had decreased after 4 d and P:C ratio decreased to close to that in Pi-depleted cells, cells did not respond to a Pi-spike over the 8 d of incubation. This possibly resulted from the cells entering the stationary phase and becoming light limited.
Furthermore, in the low CO2-grown batch cultures a reproducible NIFT in response to the addition of CO2 was also observed, as compared with the lack of response in high CO2-grown cells (ANOVA, F4,26 = 13.5, P < 0.001), although it was much less pronounced than the response of Pi-depleted cells to Pi and never exceeded 10% of Ft (). The CO2-NIFT was the same in both the Pi-replete and Pi-depleted cells after 2 and 4 d of batch growth. Against expectations, the CO2-NIFT response after d 2 remained the same or slightly increased in the Pi-depleted cells (−P−CO2), whereas it decreased in the Pi-replete cultures (+P−CO2) to levels measured in the control (H2O spikes). This deviation in NIFT response between the +P and −P low CO2 cultures occurred when the +P−CO2 cultures reached their maximum P:C ratio (at d 6 and 8; ). Although P:C ratio decreased slightly in the +P–CO2 cultures after d 8, it appeared that a high P:C ratio coincided with a diminished NIFT response to CO2 (). The response to CO2 addition in the high CO2, Pi-depleted cells was the same as that in the control. The –P–CO2 was the only treatment whose CO2-NIFTs were significantly different to the response to acidified water (Tukey, P < 0.001), whereas +P–CO2 only differed at the 10% level (Tukey, P = 0.052). In addition, we observed a ‘reverse NIFT‘ (an increase in Ft) in response to a CO2-spike in the +P+CO2 cultures, but only at d 4 (). This effect coincided with a high cellular P:C ratio at that time (), which together with the decreasing NIFT in the +P–CO2 cultures suggests that a high P:C ratio might result in an increase in Ft after a CO2 spike (compare with 7).
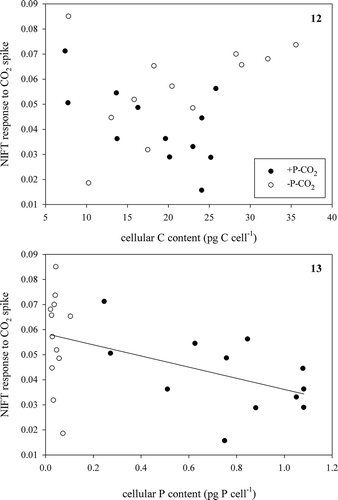
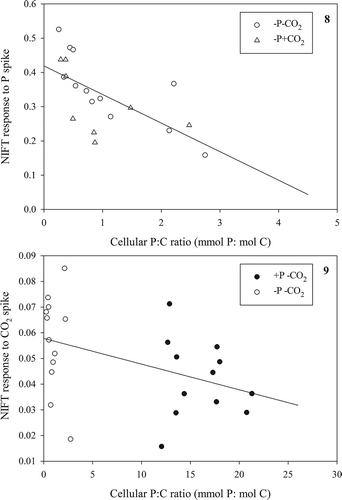
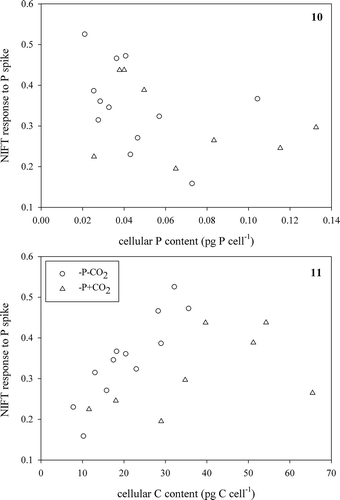
We hypothesized that the NIFT response was directly related to P:C ratio, and this was confirmed when we analysed both the response to a Pi-spike in relation to P:C ratio for Pi-depleted cells (, Pearson correlation, r = −0.65, n = 20, P < 0.005) and the response to a CO2-spike in relation to P:C ratio for the low CO2-grown cultures (, Pearson correlation, r = –0.43, n = 24, P < 0.05). While care has to be taken in interpreting these correlations in the fast changes in P:C ratio occurring in batch cultures, we found no relationship between NIFTs and the cellular P content (focusing on Pi-depleted cells that contained < 0.2 pg P cell–1; Pearson correlation r = −0.40, n = 20, P = 0.08; ) or to their cellular C content (Pearson correlation r = 0.42, n = 20, P = 0.07; ) in response to a Pi-spike (see & for the total data set). These results suggest that, unexpectedly, both the cellular P and C content equally contributed to the NIFT response to Pi.
We came to a different conclusion after comparing the response to a CO2-spike with the cellular contents of the low CO2-grown cells (, ); we found no correlation with the cellular C content (Pearson correlation r = 0.10, n = 24, P = 0.65; ) but a negative correlation with the P content (Pearson correlation r = −0.52, n = 24, P < 0.05; ). These results suggest that the cellular C content had no influence on the NIFT response to CO2, coinciding with the absence of response to CO2 enrichment in the bioassays, but that a higher cellular P content decreased the NIFT.
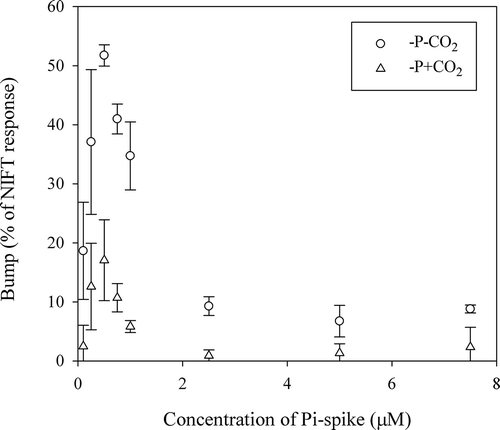
The addition of a range of different concentrations of Pi to Pi-depleted batch-grown cells resulted in a concentration-dependent response that followed Michaelis–Menten kinetics ( & Supplementary Table 1). An intermediate recovery in chl a fluorescence occurred within the NIFT response to a Pi-addition, which we denoted as a ‘bump’ (). This bump was an intrinsic physiological process as it occurred without the further addition of nutrients. The extent of this bump, expressed as a percentage of the total, initial NIFT response, varied with the concentration of the Pi-spike (). In this case it is important to notice that we kept the volume of the spike constant (10 µl), and thereby the applied CO2 concentration was also constant. When higher Pi concentrations were applied (> 2.5 µM P in low CO2 and > 0.75 µM P in high CO2 cultures), then the second decrease in Ft resulted in a lower Ft than before the bump. The extent of the bump as a proportion of the NIFT response directly following Pi-addition first increased with increasing Pi concentration up to a concentration of 0.5 µM Pi and then decreased with higher concentrations (). The extent of the bump was 2-fold higher in low CO2 cells (maximum 53%) than in high CO2 cells (maximum of 22%), a result that could not have been influenced by the different density of the culture, as high CO2, Pi-depleted cultures were only 2.2% more dense than low CO2, Pi-depleted cultures as determined by OD at the time of measurement.
Figs 4, 5. Changes in the optical density (OD; Fig. 4) and cellular P:C ratio (Fig. 5) of Chlamydomonas acidophila grown in batch cultures over time when cultured in four different combinations of Pi and CO2 concentrations. High CO2, Pi-replete cultures ran out of P on day 4 and low CO2, Pi-replete cultures on day 8. All Pi was determined to be inside the cells on day 2 in the Pi-starved cultures. Mean ± SD of duplicates.
Figs 6, 7. Extent of NIFT response to a Pi-spike (Fig. 6) and CO2-spike (Fig. 7) of C. acidophila grown in batch cultures over time. Mean ± SD of duplicates. In addition the response to acidified water is shown in both graphs.
Figs 8, 9. Extent of NIFT response to a Pi-spike (10 µM) as a function of P:C ratio in Pi-depleted batch cultures (Fig. 8) and extent of NIFT response to a CO2-spike (100 µM) as a function of P:C ratio in low CO2 batch cultures (Fig. 9) of Chlamydomonas acidophila. Lines represent significant correlations (Pearson correlation; r = −0.65, P < 0.005: Fig. 8 and r = −0.43, P < 0.05: Fig. 9).
Figs 10, 11. Extent of NIFT response to a Pi-spike (10 µM) as a function of cellular P content (Fig. 10) and cellular C content (Fig. 11) in Pi-depleted batch cultures of Chlamydomonas acidophila.
DISCUSSION
The bioassays indicate that growth of the phytoplankton of Lake 117 was co-limited for Pi and CO2, similar to that observed for semi-continuous cultures of C. acidophila (Spijkerman, Citation2010). The phytoplankton of Lake 117 was dominated by C. acidophila (> 60%), whereas that of Lake 107 was not (< 20%). The phytoplankton of Lake 107 did not show the synergistic response to CO2 and Pi, which can be attributed to the low density of C. acidophila in relation to its predator, Ochromonas sp. (Tittel et al., Citation2003; Spijkerman, Citation2008a). We detected a synergistic response in C. acidophila, which is consistent with a serial co-limitation (Harpole et al., Citation2011) because no effect was observed after a single CO2 addition and a significant primary Pi-limitation was present. Since addition of CO2 alone made no difference to the biomass after 8 days, cells were unlikely to be CO2-limited though there were clear interactions between CO2 and Pi. Bioassays have been criticized in the past for indicating which nutrients may become limiting rather than those that are limiting in situ at the time of sampling (Holland et al., Citation2004). Thus, we applied the potentially more immediate technique of NIFTs and analysed the extent and shape of the response to Pi and CO2 in the extremophile C. acidophila.
NIFT-type responses have been observed in several algal species and phytoplankton communities and in response to several different nutrient limitations. For example, a NIFT response to nitrogen (N) has been observed in the green algae Selenastrum minutum (Birch et al., Citation1986; Turpin & Weger, Citation1988; Holmes et al., Citation1989) and Dunaliella tertiolecta (Turpin, Citation1983; Young & Beardall, Citation2003) and in the cyanobacterium Microcystis aeruginosa (Wood & Oliver, Citation1995). Responses to Pi-additions have been recorded from Dunaliella tertiolecta (Roberts et al., Citation2008) and Selenastrum minutum (Gauthier & Turpin, Citation1997), and even in spinach chloroplasts (Cerovic et al., Citation1991). There have been a limited number of studies showing a NIFT response to Ci additions in Dunaliella tertiolecta (Ihnken et al., Citation2014), Chlamydomonas reinhardtii (Lucker & Kramer, Citation2013) and the cyanobacteria Nostoc sp. (Qiu & Liu, Citation2004) and Synechococcus UTEX 625 (Miller et al., Citation1991). In the latter study, quenching of chl a fluorescence was directly correlated with active CO2 uptake and the size of the internal inorganic carbon pool (Miller et al., Citation1991). There is also some evidence that NIFTs may occur with silicate (Lippemeier et al., Citation2001). However, none of these previous NIFT studies considered co-limitation effects.
Effect of P
We showed pronounced NIFT responses after a Pi-addition to Pi-depleted batch-grown cells (those with P:C ratio < 3 mmol P: mol C). After two days of Pi-starvation, the NIFT was already significant, as was the decrease in P:C ratio, and with increasing Pi-starvation the effect increased up to 40% of the Ft at the start of the perturbation. Such responses fall well within those observed in non-acidophilic algal species, such as a maximum quenching of 45% in the green alga Dunaliella tertiolecta (Roberts et al., Citation2008) and 35% in Selenastrum minutum (Gauthier & Turpin, Citation1997). The NIFT response thus is also a valuable tool to detect Pi-limitation in the extremophile green alga C. acidophila. NIFTs are easy to measure, quick and non-invasive, and provide a similar response to that obtained by the more labour-intensive bioassays and/or measurements of photosynthetic or dark respiration rates. The latter, for example, has been shown for Pi-limited Selenastrum minutum, which increased its dark respiration rate 2–2.5-fold after a Pi-addition (Gauthier & Turpin, Citation1994) and exhibited a decrease in net O2 evolution by 26% (Gauthier & Turpin, Citation1997). Pi-limitation has also been detected using delayed chl a fluorescence in Scenedesmus obtusiusculus (Mellvig & Tillberg, Citation1986). In delayed chl a fluorescence (emitted light after a period of illumination) the effect of a nutrient limitation does not primarily affect the extent of the chl a fluorescence response, but rather the timing of the delayed fluorescence peak (Berden-Zrimec et al., Citation2011), as shown in Dunaliella tertiolecta in response to N-limitation (Berden-Zrimec et al., Citation2008).
After two days of growth, Pi-replete cells were growing at high rates (0.94 ± 0.02 d–1 and 0.64 ± 0.03 d–1 for high and low CO2 cultures, respectively) that lie well within the range reported previously (Spijkerman, Citation2005, Citation2008b; Tittel et al., Citation2005). At that time, cells growing in Pi-depleted medium grew at a similarly high rate (0.86 ± 0.07 d–1 and 0.60 ± 0.05 d–1 for high and low CO2 cultures, respectively), but already showed a pronounced NIFT response (). Our observations thus indicate the NIFTs can provide early indications of the onset of limitation when other measurements show no effect. For instance, our data can be contrasted with the absence of photosynthetic suppression in response to additions of the limiting nutrient in ammonium limited Dunaliella tertiolecta when the growth rate exceeded c. 80% of its maximum (Turpin, Citation1983). In C. acidophila the response to the Pi-spike was related to the decrease in its P:C ratio in that the response appeared to be switched on when the P:C ratio fell below 3 mmol P: mol C. Against expectations based on a clear positive relation between maximum photosynthetic rates and P:C ratio (Spijkerman, Citation2010), cellular P and C alone did not contribute to the extent of a Pi-NIFT.
‘The bump’
An interesting phenomenon during the NIFT response to Pi-addition was observed in C. acidophila that has not been previously recorded in other algal species using a similar set-up (): an intermediate recovery, or possibly second NIFT occurred within the NIFT response to Pi-addition. In other green microalgae, Ft steadily increases after reaching the minimum Ft (Holland et al., Citation2004; Roberts et al., Citation2008). However in C. acidophila a second decrease after a short recovery (‘the bump’) was always observed, although it was hardly visible when higher (i.e. 10 µM Pi) Pi-concentrations were applied. The extent of the bump was related to the magnitude of the Pi-spike, with an optimum at 0.5 µM Pi. In addition, the extent of the bump was also related to the CO2 concentration in the medium, as it was two-fold higher in low CO2 cells (maximum 50%) than in high CO2 cells (maximum of 22%).
Possibly, the ‘bump’ is related to similar observations made in isolated chloroplasts from spinach (Cerovic et al., Citation1991). Cerovic and co-authors showed a similar chl a fluorescence rise as we have shown here, which they suggested to be characteristic of photosynthetic induction in leaves. Cerovic et al. (Citation1991) stated that the ‘bump’ was a consequence of an inadequate rate of recycling of Pi by the cytosol. After the first decrease in Ft, the quenching relaxes as the rate of Pi uptake declines (Roberts et al., Citation2008) and the rate of electron transport increases. However, at low Pi concentrations the maximal rate of Pi recycling can only be attained transiently, resulting in a second transient rise in chl a fluorescence. Consequently, the quenching would thus be related to a temporary decrease in cellular ATP content associated with ATP-demanding Pi-uptake processes. Fast changes (occurring within 5 seconds) in cellular ATP pool sizes and adenylate energy charges (lasting for a few minutes) have been nicely illustrated in Pi-limited Selenastrum minutum in response to a Pi-spike (Gauthier & Turpin, Citation1994). Furthermore in transients following ammonium additions to nitrogen limited algae, a marked drop in endogenous ATP has been shown (cited in Turpin, Citation1983), though Turpin points to a range of acclimation processes that might contribute to observed changes in chl a fluorescence. Although the precise physiology behind NIFTs is still poorly understood (Petrou et al., Citation2008), it is believed that the transient change in chl a fluorescence is, in part at least, attributable to a reallocation of energy from photosynthesis to nutrient uptake (Holland et al., Citation2004). For Pi there is a suggestion that if cytosolic Pi concentrations increase during uptake, then rapid ATP export from the chloroplast may deplete stromal metabolite pools, decreasing regeneration of Calvin cycle intermediates and, subsequently, Calvin cycle activity (Heineke et al., Citation1989; Gauthier & Turpin, Citation1997).
There was a clear relationship between the concentration of Pi and the extent of the bump in C. acidophila, and the bump was higher in low CO2 cells, which suggests inorganic carbon transport might also be involved. Similar to what has been observed in isolated chloroplasts (Cerovic et al., Citation1991), the bump probably relates to transporter processes on the chloroplast envelope and the described interaction between Pi supply and CCM activity (Beardall & Giordano, Citation2002; Raven & Beardall, Citation2014). Inorganic carbon transport across the chloroplast membrane towards RUBISCO is thought to be an active process in C. acidophila (Spijkerman, Citation2011) and could in some way thus interact with the P-translocator (Gauthier & Turpin, Citation1997). The concentration at which the bump is at a maximum is, however, the same in high and low CO2 acclimated cells, suggesting that the underlying mechanism might not depend on the activation of a CCM (Spijkerman, Citation2005) but rather on the Pi concentration in the cytosol. Possibly, the latter also regulates the extent of the CCM in C. acidophila (Spijkerman, Citation2011). This requires further investigation.
Effect of C
The NIFT response to a CO2-addition in CO2-limited algae was less pronounced but also significant. CO2 is a potentially growth-limiting nutrient as C. acidophila is an extremophilic algae living in very low pH environments (Tittel et al., Citation2005). At a pH of 2.6, as used for culturing in this study, CO2 is the predominant form of Ci (> 99%; Stumm & Morgan, Citation1970) and thus we assume that CO2 was the sole Ci source used by intact algae. The NIFT response to CO2 (and also to HCO3–) was clearly related to CO2 limitation because it was present in low CO2 grown cells but absent in high CO2 cells. Interestingly there appeared to be a negative relationship between cellular P:C ratio and the response to CO2 in these low CO2 grown cells. In addition, we found an increase in Ft after a CO2 perturbation in cells with a high P:C ratio, as was found for perturbation by NO3– in N-limited algae (Young & Beardall, Citation2003). The response to CO2 spikes in the +P–CO2 cultures thus suggests the presence of compensatory dynamics that dampened the sole effect of CO2 in this treatment. This conclusion was confirmed by the observation in the –P+CO2 cultures that a first Pi-spike (resulting in an increased cellular P content) decreased the response to a CO2 spike. Our observations contrast with those made in the seagrass L. variegata where the order of spike addition did not matter (den Haan et al., Citation2013). As with the addition of Pi to Pi-starved green algae, CO2-uptake in CO2-limited C. acidophila might be an ATP demanding process resulting in a state transition from state 1 to 2 (Gauthier & Turpin, Citation1997; Petrou et al., Citation2008; Roberts et al., Citation2008) and/or an enhancement of cyclic electron transport as shown in Chlamydomonas reinhardtii (Lucker & Kramer, Citation2013). The fast NIFT responses recorded here (the Ft responses to Pi and CO2-spikes were approximately four times faster (100 s to minimum Ft compared with 400–500 s) than those of Dunaliella tertiolecta; Petrou et al., Citation2008), suggest that energy quenching and/or cyclic electron transport is of higher relative importance than state transitions in C. acidophila. Possibly, and especially if high concentrations of P are accumulated in the cell, demands on linear electron transport might dominate Ci-assimilation and result in an increase in Ft after a CO2-spike. An increase in Ft has been shown after a NO3–-spike to N-limiting Dunaliella tertiolecta, and was supposedly related to enhanced linear electron transport (Young & Beardall, Citation2003).
In the cyanobacterium Synechococcus UTEX 625, a large NIFT response to Ci addition was observed that was related to active CO2 uptake (Miller et al., Citation1991) and Na+-dependent HCO3– uptake (Crotty et al., Citation1994). A large NIFT response was also detected in the cyanobacterium Nostoc sp. (Qiu & Liu, Citation2004). In this Nostoc sp. the quenching of chl a fluorescence (both the rate and the extent) after a KHCO3 spike was related to the concentration of the spike and also related to the net photosynthetic activity (Qiu & Liu, Citation2004). These studies concluded that the rate of chl a fluorescence quenching reflects active Ci uptake, whereas the extent of the chl a fluorescence quenching reflects the internal Ci pool (Miller et al., Citation1991). The small extent of the response in C. acidophila coincides with the results of a recent study showing that the extent of a CCM (measured as the accumulation of Ci internally, relative to external concentrations) never exceeded an accumulation factor of 10 (Spijkerman, Citation2011; Spijkerman et al., Citation2014), although CO2 uptake was an active process that was > 95% inhibited by incubation in the dark, or in the light but in the presence of DCMU (see supplementary material in Spijkerman, Citation2011). In contrast to expectations, changes in the cellular C content could not explain the extent of the CO2-NIFTs, whereas cellular P had a negative effect on this response.
Effect of co-limitation
Antagonistic responses might result from NIFTs performed on co-limited algae, but in C. acidophila the response to Pi addition in cells limited in Pi and CO2 (Spijkerman, Citation2010) appeared similar to the response in other green algae that were only Pi-limited (Roberts et al., Citation2008). We thought that an enhanced NIFT response might be observed in co-limited algae when both nutrients are added in concert, or close together. When studying the growth response of C. acidophila to the addition of the limiting nutrient, providing CO2 alone had no effect and only the combination of Pi and CO2 resulted in a higher growth response than when only Pi was added (Spijkerman, Citation2010). We therefore expected the NIFT response to a CO2 spike to be higher when provided after the Pi-spike than vice versa. However, in putatively co-limited cultures (Pi-depleted, low CO2) the NIFT response to a CO2-spike was the same when added before or after the Pi-spike, and only in Pi-depleted, high CO2 cultures was an effect of the CO2-spike observed; thus rejecting our hypothesis.
Another response that might reveal co-limitation is the bump within the NIFT response to Pi-addition, which may be related to the CO2-concentration in the culture. The bump, potentially a second NIFT, might be a response to a physiological acclimation to CO2 in the culture because the bump was higher in low CO2 than in high CO2 cells. The transient response within the NIFT to Pi thus suggests a dependence in CO2-limitation on Pi-limitation, which was confirmed by the correlation between the NIFT response to CO2-addition and cellular P status. More experiments will be necessary to fully reveal the effect of co-limitation on the NIFT response, possibly including delayed chl a fluorescence recordings and quenching analyses in cells grown in well-defined continuous culture conditions.
NIFTs are a quick and reliable method to detect the growth limiting nutrient or nutrients, as seen in in CO2 and Pi/CO2 (co-)limited algae as well as in N/P (co-)limited seagrass (den Haan et al., Citation2013). Intermediate concentrations of a Pi-spike might provide information about additional effects of CO2 in co-limited algae after further testing on CO2-(co-)limited phytoplankton (Jansson et al., Citation2012). Although the synergistic growth response to Pi and CO2 in C. acidophila suggests a serial co-limitation, the NIFT response reveals that the time scale of this response lies within a minute. We show that NIFTs after a spike of the limiting nutrient did not solely depend on the cellular content of that particular nutrient but were a response to a complex interaction between different nutrients and that the ability to take up low CO2 concentrations may depend on the availability of Pi. The possibility that high CO2 simply down-regulated CCM activity, freeing up additional energy for Ci fixation and Pi acquisition, needs to be tested but since CO2 addition alone (Fig 4) did not support a large increase in biomass, we think this is unlikely.
Thus in summary, we show with traditional bioassays that growth of the phytoplankton of Lake 117, which was dominated by C. acidophila (> 60%), was co-limited for Pi and CO2. In batch cultures we showed pronounced NIFT responses after Pi-addition when cells had a cellular P:C ratio < 3 mmol P: mol C. Also in response to CO2 a NIFT response was recorded, that could not be explained by the cellular C content but was inversely related to the cellular P content. The additional observation that medium CO2 concentration affected the shape of the response to a Pi-addition in a second transient, suggests that the cellular P content mainly controls a co-limitation for Pi and CO2. However, care must be taken when interpreting the correlations between cellular contents and the NIFT response in the fast changing environment of batch cultures.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
SUPPLEMENTARY INFORMATION
The following supplementary material is accessible via the supplementary Content tab on the artivle’s online page at http://dx.doi.org/10.1080/09670262.2015.1095355
Supplementary Table 1. Pi-uptake kinetics of Chlamydomonas acidophila grown in Pi-limiting semi-continuous cultures (33P uptake) or Pi-limited batch cultures (fluorescence; this study) at 2 different CO2 concentrations
Supplementary Figs 1-2. Extent of NIFT response to a Pi-spike (Fig. 1: 10 μM) and CO2-spike (Fig. 2: 100 μM) plotted versus the cellular P content (Fig. 1) and cellular C content (Fig. 2) of Chlamydomonas acidophila cultured under 4 different combinations of Pi and CO2 conditions.
Supplementary Fig. 3 The rate of change in fluorescence (ΔF% min-1) of Chlamydomonas acidophila grown in Pi-limited batch cultures and 2 different CO2 concentrations in relation to the concentration of the Pi-spike.
Supplementary material
Download MS Word (294.1 KB)ACKNOWLEDGEMENTS
ES & SL thank Sabrina Ryl and Barbara Schmitz for practical assistance. Two anonymous reviewers provided helpful comments on the manuscript.
Additional information
Funding
Notes on contributors
Daryl Holland
E. Spijkerman, S. Stojkovic, D. Holland, J. Beardall: designing experiments; E. Spijkerman, S. Stojkovic, D. Holland: culture experiments; E. Spijkerman, S.C. Lachmann, J. Beardall: data analysis and interpretation; E. Spijkerman, S. Stojkovic, D. Holland, S.C. Lachmann, J. Beardall: writing manuscript.
REFERENCES
- Beardall, J., Berman, T., Heraud, P., Kadiri, M.O., Light, B.R., Patterson, G., Roberts, S., Sulzberger, B., Sahan, E., Uehlinger, U. & Wood, B. (2001). A comparison of methods for detection of phosphate limitation in microalgae. Aquatic Sciences, 63: 107–121.
- Beardall, J. & Giordano, M. (2002). Ecological implications of microalgal and cyanobacterial CO2 concentrating mechanisms, and their regulation. Functional Plant Biology, 29: 335–347.
- Berden-Zrimec, M., Drinovec, L., Molinari, Il., Zrimec, A., Umani, S.F. & Monti, M. (2008). Delayed fluorescence as a measure of nutrient limitation in Dunaliella tertiolecta. Journal of Photochemistry and Photobiology B–Biology, 92: 13–18.
- Berden-Zrimec, M., Drinovec, L. & Zrimec, A. (2011). Delayed fluorescence. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications (Suggett, D.J. & Govindjee, editors), 293–309. Springer, Dordrecht.
- Beulker, C., Lessmann, D. & Nixdorf, B. (2003). Aspects of phytoplankton succession and spatial distribution in an acidic mining lake (Plessa 117, Germany). Acta Oecologica, 24: S25–S31.
- Birch, D.G., Elrifi, I.R. & Turpin, D.H. (1986). Nitrate and ammonium induced photosynthetic suppression in N-limited Selenastrum minutum. 2. Effects of NO3– and NH4+ addition on CO2 efflux in the light. Plant Physiology, 82: 708–712.
- Brinkman, R., Margaria, R. & Roughton, F.J.W. (1934). The kinetics of the carbon dioxide-carbonic acid reaction. Philosophical Transactions of the Royal Society, B: Biological Sciences, 232: 65–97.
- Buitenhuis, E.T. & Geider, R.J. (2010). A model of phytoplankton acclimation to iron-light colimitation. Limnology and Oceanography, 55: 714–724.
- Buitenhuis, E.T., Timmermans, K.R. & de Baar, H.J.W. (2003). Zinc-bicarbonate colimitation of Emiliania huxleyi. Limnology and Oceanography, 48: 1575–1582.
- Cerovic, Z.G., Vucinic, Z. & Walker, D.A. (1991). Photosynthetic oxygen evolution and chlorophyll fluorescence in intact isolated chloroplasts on a solid support: the influence of orthophosphate. Planta, 184: 248–253.
- Crotty, C.M., Tyrrell, P.N. & Espie, G.S. (1994). Quenching of chlorophyll a fluorescence in response to Na+-dependent HCO3– transport-mediated accumulation of inorganic carbon in the cyanobacterium Synechococcus UTEX 625. Plant Physiology, 104: 785–791.
- den Haan, J., Huisman, J., Dekker, F., ten Brinke, J.L., Ford, A.K., van Ooijen, J., van Duyl, F.C., Vermeij, M.J.A. & Visser, P.M. (2013). Fast detection of nutrient limitation in macroalgae and seagrass with nutrient-induced fluorescence. PLoS ONE, 8: e68834.
- Elser, J.J., Bracken, M.E.S., Cleland, E.E., Gruner, D.S., Harpole, W.S., Hillebrand, H., Ngai, J.T., Seabloom, E.W., Shurin, J.B. & Smith, J.E. (2007). Global analysis of nitrogen and phosphorus limitation of primary producers in freshwater, marine and terrestrial ecosystems. Ecology Letters, 10: 1135–1142.
- Gauthier, D.A. & Turpin, D.H. (1994). Inorganic phosphate (Pi) enhancement of dark respiration in the Pi-limited green alga Selenastrum minutum. Interactions between H+/Pi cotransport, the plasmalemma H+-ATPase, and dark respiratory carbon flow. Plant Physiology, 104: 629–637.
- Gauthier, D.A. & Turpin, D.H. (1997). Interactions between inorganic phosphate (Pi) assimilation, photosynthesis and respiration in the Pi-limited green alga Selenastrum minutum. Plant Cell and Environment, 20: 12–24.
- Gerloff-Elias, A., Spijkerman, E. & Pröschold, T. (2005). Effect of external pH on the growth, photosynthesis and photosynthetic electron transport of Chlamydomonas acidophila Negoro, isolated from an extremely acidic lake (pH 2.6). Plant Cell and Environment, 28: 1218–1229.
- Harpole, W.S., Ngai, J.T., Cleland, E.E., Seabloom, E.W., Borer, E.T., Bracken, M.E., Elser, J.J., Gruner, D.S., Hillebrand, H., Shurin, J.B. & Smith, J.E. (2011). Nutrient co-limitation of primary producer communities. Ecology Letters, 14: 852–862.
- Heineke, D., Stitt, M. & Heldt, H.W. (1989). Effects of inorganic phosphate on the light dependent thylakoid energization of intact spinach chloroplasts. Plant Physiology, 91: 221–226.
- Holland, D., Roberts, S. & Beardall, J. (2004). Assessment of the nutrient status of phytoplankton: a comparison between conventional bioassays and nutrient-induced fluorescence transients (NIFTs). Ecological Indicators, 4: 149–159.
- Holmes, J.J., Weger, H.G. & Turpin, D.H. (1989). Chlorophyll a fluorescence predicts total photosynthetic electron flow to CO2 or NO3–/NO2– under transient conditions. Plant Physiology, 91: 331–337.
- Ihnken, S., Kromkamp, J.C., Beardall & J. Silsbe, G.M. (2014). State-transitions facilitate robust quantum yields and cause an over-estimation of electron transport in Dunaliella tertiolecta cells held at the CO2 compensation point and re-supplied with DIC. Photosynthesis Research, 119: 257–272.
- Jansson, M., Karlsson, J. & Jonsson, A. (2012). Carbon dioxide supersaturation promotes primary production in lakes. Ecology Letters, 15: 527–532.
- Kamjunke, N., Gaedke, U., Tittel, J., Weithoff, G. & Bell, E.M. (2004). Strong vertical differences in the plankton composition of an extremely acidic lake. Archiv für Hydrobiologie, 161: 289–306.
- Kamjunke, N., Tittel, J., Krumbeck, H., Beulker, C. & Poerschmann, J. (2005). High heterotrophic bacterial production in acidic, iron-rich mining lakes. Microbial Ecology, 49: 425–433.
- Lippemeier, S., Hintze, R., Vanselow, K.H., Hartig, P. & Colijn, F. (2001). In-line recording of PAM fluorescence of phytoplankton cultures as a new tool for studying effects of fluctuating nutrient supply on photosynthesis. European Journal of Phycology, 36: 89–100.
- Lucker, B. & Kramer, D.M. (2013). Regulation of cyclic electron flow in Chlamydomonas reinhardtii under fluctuating carbon availability. Photosynthesis Research, 117: 449–459.
- Mellvig, S. & Tillberg, J.E. (1986). Transient peaks in the delayed luminescence from Scenedesmus obtusiusculus induced by phosphorus starvation and carbon dioxide deficiency. Physiologia Plantarum, 68: 180–188.
- Miller, A.G., Espie, G.S. & Canvin, D.T. (1991). The effects of inorganic carbon and oxygen on fluorescence in the cyanobacterium Synechococcus UTEX 625. Canadian Journal of Botany, 69: 1151–1160.
- Moore, C.M., Hickman, A.E., Poulton, A.J., Seeyave, S. & Lucas, M.I. (2007). Iron-light interactions during the CROZet natural iron bloom and EXport experiment (CROZEX): II – Taxonomic responses and elemental stoichiometry. Deep-Sea Research Part II–Topical Studies in Oceanography, 54: 2066–2084.
- Moore, C., Mills, M.M., Langlois, R., Milne, A., Achterberg, E.P., La Roche, J. & Geider, R.J. (2008). Relative influence of nitrogen and phosphorus availability on phytoplankton physiology and productivity in the oligotrophic sub-tropical North Atlantic Ocean. Limnology and Oceanography, 53: 291–305.
- Moore, C.M., Mills, M.M., Arrigo, K.R., Berman-Frank, I., Bopp, L., Boyd, P.W., Galbraith, E.D., Geider, R.J., Guieu, C., Jaccard, S.L., Jickells, T.D., La Roche, J., Lenton, T.M., Mahowald, N.M., Maranon, E., Marinov, I., Moore, J.K., Nakatsuka, T., Oschlies, A., Saito, M.A., Thingstad, T.F., Tsuda, A. & Ulloa, O. (2013). Processes and patterns of oceanic nutrient limitation. Nature Geoscience, 6: 701–710.
- Murphy, J. & Riley, J.P. (1962). A modified single solution method for determination of phosphate in natural waters. Analytica Chimica Acta, 27: 31–36.
- Nixdorf, B., Krumbeck, H., Jander, J. & Beulker, C. (2003). Comparison of bacterial and phytoplankton productivity in extremely acidic mining lakes and eutrophic hard water lakes. Acta Oecologica, 24: 281–288.
- North, R.L., Guildford, S.J., Smith, R.E.H., Havens, S.M. & Twiss, M.R. (2007). Evidence for phosphorus, nitrogen, and iron colimitation of phytoplankton communities in Lake Erie. Limnology and Oceanography, 52: 315–328.
- Petrou, K., Doblin, M.A., Smith, R.A., Ralph, P.J., Shelly, K. & Beardall, J. (2008). State transitions and nonphotochemical quenching during a nutrient-induced fluorescence transient in phosphorus-starved Dunaliella tertiolecta. Journal of Phycology, 44: 1204–1211.
- Qiu, B.S. & Liu, J.Y. (2004). Utilization of inorganic carbon in the edible cyanobacterium Ge-Xian-Mi (Nostoc) and its role in alleviating photo-inhibition. Plant Cell and Environment, 27: 1447–1458.
- Raven, J.A. & Beardall, J. (2014). CO2 concentrating mechanisms and environmental change. Aquatic Botany, 118: 24–37.
- Roberts, S., Shelly, K. & Beardall, J. (2008). Interactions among phosphate uptake, photosynthesis, and chlorophyll fluorescence in nutrient-limited cultures of the chlorophyte microalga Dunaliella tertiolecta. Journal of Phycology, 44: 662–669.
- Saito, M.A., Goepfert, T.J. & Ritt, J.T. (2008). Some thoughts on the concept of colimitation: three definitions and the importance of bioavailability. Limnology and Oceanography, 53: 276–290.
- Saito, M.A., McIlvin, M.R., Moran, D.M., Goepfert, T.J., DiTullio, G.R., Post, A.F. & Lamborg, C.H. (2014). Multiple nutrient stresses at intersecting Pacific Ocean biomes detected by protein biomarkers. Science, 345: 1173–1177.
- Shelly, K., Holland, D. & Beardall, J. (2010). Assessing nutrient status of microalgae using chlorophyll a fluorescence. In Chlorophyll a Fluorescence in Aquatic Sciences: Methods and Applications(Suggett, D.J., Borowitzka, M.A. & Prášil, O., editors), 223–235. Springer Science+Business Media B.V., Heidelberg.
- Spijkerman, E. (2005). Inorganic carbon acquisition by Chlamydomonas acidophila across a pH range. Canadian Journal of Botany, 83: 872–878.
- Spijkerman, E. (2008a). Phosphorus limitation of algae living in iron-rich, acidic lakes. Aquatic Microbial Ecology, 53: 201–210.
- Spijkerman, E. (2008b). What physiological acclimation supports increased growth at high CO2 conditions? Physiologia Plantarum, 133: 41–48.
- Spijkerman, E. (2010). High photosynthetic rates under a colimitation for inorganic phosphorus and carbon dioxide. Journal of Phycology, 46: 658–664.
- Spijkerman, E. (2011). The expression of a carbon concentrating mechanism in Chlamydomonas acidophila under variable phosphorus, iron, and CO2 concentrations. Photosynthesis Research, 109: 179–189.
- Spijkerman, E., de Castro, F. & Gaedke, U. (2011). Independent colimitation for carbon dioxide and inorganic phosphorus. PLoS ONE, 6: e28219.
- Spijkerman, E., Stojkovic, S. & Beardall, J. (2014). CO2 acquisition in Chlamydomonas acidophila is influenced mainly by CO2, not phosphorus, availability. Photosynthesis Research, 121: 213–221.
- Stumm, W. & Morgan, J.J. (1970). Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters. 1st ed. Wiley-Interscience, New York.
- Tittel, J., Bissinger, V., Zippel, B., Gaedke, U., Bell, E., Lorke, A. & Kamjunke, N. (2003). Mixotrophs combine resource use to outcompete specialists: implications for aquatic food webs. Proceedings of the National Academy of Sciences USA, 100: 12776–12781.
- Tittel, J., Bissinger, V., Gaedke, U. & Kamjunke, N. (2005). Inorganic carbon limitation and mixotrophic growth in Chlamydomonas from an acidic mining lake. Protist, 156: 63–75.
- Turpin, D.H. (1983). Ammonium induced photosynthetic suppression in ammonium limited Dunaliella tertiolecta (Chlorophyta). Journal of Phycology, 19: 70–76.
- Turpin, D.H. & Weger, H.G. (1988). Steady state chlorophyll a fluorescence transients during ammonium assimilation by the N-limited green alga Selenastrum minutum. Plant Physiology, 88: 97–101.
- Wolf-Gladrow, D. & Riebesell, U. (1997). Diffusion and reactions in the vicinity of plankton: a refined model for inorganic carbon transport. Marine Chemistry, 59: 17–34.
- Wood, M.D. & Oliver, R.L. (1995). Fluorescence transients in response to nutrient enrichment of nitrogen limited and phosphorus limited Microcystis aeruginosa cultures and natural phytoplankton populations: a measure of nutrient limitation. Australian Journal of Plant Physiology, 22: 331–340.
- Young, E.B. & Beardall, J. (2003). Rapid ammonium- and nitrate-induced perturbations to chl a fluorescence in nitrogen-stressed Dunaliella tertiolecta (Chlorophyta). Journal of Phycology, 39: 332–342.