Abstract
Brown algae are members of the Stramenopiles and their gametes generally have two heterogeneous flagella: a long anterior flagellum (AF) with mastigonemes and a short posterior flagellum (PF). In this study, swimming paths and flagellar waveforms in free-swimming and thigmotactic-swimming male and female gametes and in male gametes during chemotaxis, were quantitatively analysed in the model brown alga Ectocarpus siliculosus. This analysis was performed using a high-speed video camera. It was revealed that the AF plays a role in changing the locomotion of male and female gametes from free-swimming to thigmotactic-swimming and also in changing the swimming path of male gametes from linear to circular during chemotaxis. In the presence of a sex pheromone, male gametes changed their swimming path from linear (swimming path curvature, 0–0.02 µm–1) to middle and small circular path (swimming path curvature, 0.04–0.20 µm–1). The flagellar asymmetry and the deflection angle of the AF became larger, whereas the oscillation pattern of the AF was stable. However, there was no correlation between the flagellar asymmetry and the deflection angle of the AF and the path curvature when the male gametes showed middle to small circular paths. The PF irregularly changed the deflection angle and the oscillation pattern was unstable depending on the gradient of the sex pheromone concentration. AF waveforms were independent of PF locomotion during chemotaxis. This means that the AF has the ability to change the swimming path of male gametes – for example, from a highly linear path to a circular path – while changes in locomotion from a middle circle path to a small circle path is the result of beating of the PF.
Introduction
Motile gametes of the brown algae usually have two heterogeneous flagella, a long anterior flagellum (AF) and a short posterior flagellum (PF) (Clayton, Citation1989; O’ Kelly, Citation1989; Andersen, Citation2004). The AF is decorated with mastigonemes and generates a force for forward movement of gametes (Jahn et al., Citation1964; Holwill & Sleigh, Citation1967; Bouck, Citation1969). The PF frequently has a basal swelling part (paraflagellar body), and provides rapid lateral beats for changing the direction of the cell movement (Kawai & Kreimer, Citation2000; Maier, Citation1995).
Chemotactic responses of male gametes to a sex pheromone from settled female gametes have been studied for many years in the isogamous brown alga, Ectocarpus siliculosus (Dillwyn) Lyngbye (Müller & Falk, Citation1973; Müller, Citation1978; Geller & Müller, Citation1981). Firstly, female gametes quickly settle on the substratum and secrete the sex pheromone ‘ectocarpen’ (Müller, Citation1967, Müller et al., Citation1971). Secondly, free-swimming male gametes begin to swim in close contact with the surface of the glass slide or coverslip, this response is enhanced by the presence of the sex pheromone from the female gamete, and is regarded as thigmotaxis (Müller, Citation1978; Geller & Müller, Citation1981). The swimming velocity of male gametes decreases and the swimming radius becomes smaller. Thirdly, the male gamete changes the AF waveform, which causes the cell body to move in circular paths in the presence of the sex pheromone. This behaviour of the male gamete is regarded as chemokinesis (Maier, Citation1993, Citation1995). The PF of the male gamete performs fast, unilateral beating when it detects a decrease in the sex pheromone concentration gradient. This PF behaviour results in a reorientation towards the pheromone source and is defined as chemoklinotaxis (Maier, Citation1993, Citation1995). Afterwards, the male gamete anchors itself with the tip of the AF on the surface of the female gamete, and finally, the male gamete fuses with the female (Müller & Falk, Citation1973). Therefore, the AF and PF of male gametes have crucial roles in approach and contact with female gametes.
Geller & Müller (Citation1981) firstly reported that the AF of male gametes in E. siliculosus performs asymmetric bending in the presence of the sex pheromone, which shows a negative linear correlation between the average deflection angle of the AF from the cell axis and the radius of the track of the male gametes during chemokinesis. Their analysis was based on measuring the deflection of the first visible bend of the AF from the cell axis. However, information on the flagellar waveform of brown algae has been limited compared with that of metazoa (Gibbons, Citation1981; Inaba, Citation2003) and the green alga, Chlamydomonas (Mitchell, Citation2000). Analyses of flagellar waveforms in sperm of marine invertebrates using a high-speed camera have revealed that flagellar waveforms became asymmetric when sperm changed swimming direction toward the sex pheromone source. Their flagellar waveforms remained symmetric when sperm swam directly toward the pheromone source (Miller & Brokaw, Citation1970; Miller, Citation1975, Citation1977). It has been reported that Ca2+ is a primary factor regulating the symmetry and asymmetry of flagellar waveforms (Kaupp et al., Citation2008; Yoshida & Yoshida, Citation2011).
In the present study, swimming paths and two heterogeneous flagellar waveforms of male and female gametes of the brown alga E. siliculosus were quantitatively analysed using a high-speed camera, focusing on free- and thigmotactic-swimming male and female gametes and chemotactic-swimming male gametes in order to understand more precisely the relationship between AF and PF waveforms of male gametes during fertilization in brown algae.
Materials and methods
Preparation of gametes
Male and female gametophytes (strain Ec32m and Ec25f) of Ectocarpus siliculosus (Dillw.) Lyngbye were gifts from Drs A. F. Peters (Bezhin Rosko, France) and S. M. Coelho (UMR 7139 CNRS-UPMC, Station Biologique de Roscoff, France). These gametophytes were cultured in half-strength PES medium (Provasoli, Citation1968) under cool white fluorescent lamps (30–40 µmol photons m–2 s–1) at 15°C in long day conditions (14 h light:10 h dark). Two days after changing to new medium, gametes were released from plurilocular gametangia within 2 h of light irradiation. They showed a strong negative phototaxis and gathered at the opposite side of culture dishes from the light source.
Observations and recording
30 µl of culture medium containing freshly liberated male and female gametes was added to an observation chamber (90 µm depth) which was made of double-faced adhesive tape between a slide glass and a cover slip. Images of flagellar waveforms were recorded with a phase contrast microscope (BX51, Olympus, Tokyo, Japan) with a 40× objective (UPlan FLN, Olympus) connected to a high-speed CCD camera (HAS220, Ditect, Tokyo, Japan) at 200 or 600 fps (frames per s) and 1/1000 s shutter speed. Observations were carried out using an R-58 red cut-off glass filter (S-058, HOYA, Tokyo, Japan) in a dark room at room temperature (c. 20ºC), to prevent the effect of blue light on phototaxis of E. siliculosus (Kawai et al., Citation1990). Image analyses of flagellar waveforms were executed only on planner bending waveforms.
Data analysis
Swimming velocities, swimming path curvatures, flagellar curvatures and the deflection angle of gametes were analysed with Bohboh software (Bohboh Soft, Tokyo, Japan). Images of the cell body of gametes were automatically tracked and the swimming velocities were measured along the zigzag trajectory of the cell bodies during flagellar beating cycles (trajectory swimming velocity). The path curvature, which is the reciprocal of the radius of curvature (ρ) of the circular swimming path, was also calculated (Fig. S1). Trajectories in a clockwise direction were defined as a plus and trajectories in a counterclockwise direction were defined as a minus. The flagellar waveforms of gametes were automatically traced. The flagellar curvature, which is the reciprocal of the radius of curvature (r) of the flagellar waveform, was calculated based on the method of Baba and Mogami (Citation1985). Flagellar asymmetry was expressed as the ratio of the maximal curvature of P bend (Principal bend) and that of R bend (Reverse bend) (P bend Max/R bend Max). The ratio should be equal to one when the flagella show completely symmetric bending, and it should be greater than one when they show an asymmetric waveform. This ratio is referred to as the asymmetric index (Shiba et al., Citation2008).
The angle of the tangent to the flagellar shaft was measured with respect to the direction of the axis of the cell body. It was referred to the deflection angle (Fig. S2). The right side was defined as plus and the left side was defined as minus, when the AF was localized to the direction of forward movement and viewed from the ventral side of the gametes. The anterior end of the cell body was defined as zero with respect to the deflection angle of the AF and the posterior end of cell body was defined as zero with respect to the deflection angle of the PF. The points which tended to take on large values in maximum and minimum of the deflection angle was 8 µm from the tip of the AF and 1 µm from the tip of the PF.
Results
Locomotion of free- and thigmotactic-swimming gametes
Two patterns of swimming were observed in male and female gametes of E. siliculosus. The first observed pattern was that the gametes swam freely with helical rotations of the cell body without touching anything. Trajectories of these gametes showed spiral or undulating lines (). The second observed pattern was that they swam just on the substratum by eliminating the helical rotation of the cell bodies. These thigmotactic-swimming male and female gametes followed highly linear to large circular paths ().
Figs 1–3. The trajectories of free- and thigmotactic-swimming male gametes in E. siliculosus. , . The swimming trajectories of free-swimming male gametes () and thigmotactic-swimming male gametes () are obtained by image processing during a period of 2s. . Frequency of the swimming path curvatures (absolute value) of thigmotactic-swimming male gametes (n = 115). Scale bars = 100 µm.

Just after adding 30 µl of culture medium containing freshly liberated male or female gametes to a chamber, approximately 20% of gametes showed thigmotaxis and 80% of gametes freely swam. After 3 min, approximately 50% of gametes showed thigmotactic-swimming. The number of thigmotactic-swimming gametes gradually increased.
The swimming velocity was significantly different (P < 0.001) in free-swimming (205.2 ± 49.5 µm s–1, n = 95) and thigmotactic-swimming (131.5 ± 24.1 µm s–1, n = 115) male gametes (). Likewise for the velocities of female free-swimming (191.0 ± 48.4 µm s–1, n = 90) and thigmotactic-swimming (151.8 ± 38.6 µm s–1, n = 115) gametes (P < 0.001). No significant differences were observed between the swimming patterns of male and female gametes.
Table 1. Swimming patterns and flagellar waveforms of Ectocarpus siliculosus male and female gametes.
Thigmotactic-swimming male gametes showed highly linear to large circular paths without a pheromone source, and 66% of the path curvature values ranged from –0.02–0.02 µm–1 (n = 115) (). Similar tendencies were also observed in female gametes.
AF movements of free- and thigmotactic-swimming gametes
Flagellar waveforms of free- and thigmotactic-swimming male and female gametes which showed typical swimming velocities and path curvatures, were quantitatively analysed. The AF showed periodic oscillations, but the PF showed only irregular oscillations in Ectocarpus gametes. Therefore, flagellar curvature, asymmetric index and beat frequency were analysed only in the AF.
AF movements of free-swimming gametes were composed of symmetric and asymmetric waveforms (–, , Movie S1). The AF of thigmotactic-swimming gametes usually showed asymmetric waveforms (–, , Movie S2). In the case of free-swimming male gametes, which showed highly linear paths (path curvature −0.01–0.009 µm–1) (n = 5), maximum values of P-bend and R-bend in the AF were 1.13 ± 0.09 and 1.08 ± 0.06, respectively. The asymmetric index (P bend/R bend) of the AF was 1.05 ± 0.03 for the whole region (, S3). The slightly curved, free-swimming male gametes (> 0.012 µm–1) had an asymmetric waveform which was similar to those of thigmotactic-swimming gametes (not shown). The AF of thigmotactic-swimming male gametes, which showed highly linear paths (from −0.008 to 0.008 µm–1, n = 7), displayed asymmetric waveforms (). The maximum values of P-bend and R-bend were 1.16 ± 0.13 and 0.98 ± 0.12, respectively (Fig. S4). Next, we measured separately the asymmetric index at the base, middle and tip regions of AF () in order to examine which part of AF showed a conspicuous asymmetry. The asymmetric indices were 1.05 ± 0.07 at the base (0–5 µm), 1.19 ± 0.045 at the middle (6–10 µm), 1.42 ± 0.15 at the tip (11–14 µm), and 1.24 ± 0.075 for the whole region (0–14 µm). The AF waveform of these two types of gametes, which showed highly linear paths, were significantly different in asymmetric index, especially between the tip region and the whole region (P < 0.001) (). Female gametes also showed similar patterns of AF waveforms. The asymmetric waveform in AF was observed in both free- and thigmotactic-swimming gametes; however, the symmetric waveform in the AF was observed only in free-swimming gametes during straight swimming.
Figs 4–13. The anterior flagellar (AF) waveforms of free- and thigmotactic-swimming male gametes. –. A typical flagellar waveform of free-swimming gametes (symmetrical waveform of the AF) recorded at 1.6 ms intervals. –. A typical flagellar waveform of thigmotactic-swimming male gametes recorded at 1.6 ms intervals. , . Trace of AF waveforms in free-swimming male gametes () and thigmotactic-male gametes (). Twenty frames were chosen every 1.6 ms and traces of the AF were merged. Black bars show 5 µm reference line and grey dots indicate the anterior end of the cell body. . Flagellar asymmetric indices of free- and thigmotactic- swimming male gametes. I. Basal region of the AF; II. Middle region; III. Tip region; IV. Whole region. Free- vs. thigmotactic-swimming male gametes, *P < 0.001. . Average value of the deflection angle at 8 µm from the tip of the AF. I. Free-swimming male gametes; II. Thigmotactic-male gametes. Free- vs. thigmotactic-swimming male gametes, *P < 0.001. Scale bars = 5 µm.
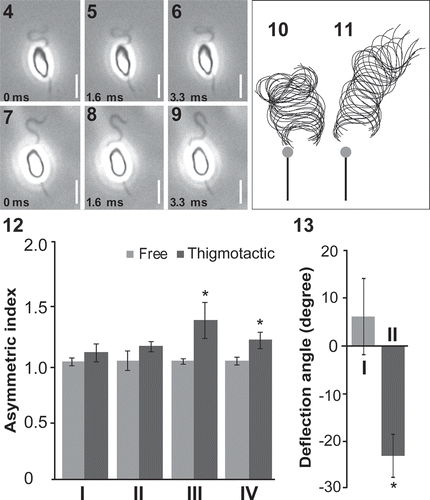
The deflection angle between the flagellum and the axis of cell body was obtained. The average values of the deflection angle in the AF of free- and thigmotactic-swimming male gametes, which showed a highly linear path, were 6.3° ± 8.0° (max 102.6°, min 80.8°) and −22.9° ± 4.5° (max 45.2°, min −106.6°), respectively (, ). There was a statistically significant difference between these two values (P < 0.001). The deflection angle of the AF, of thigmotactic-swimming male gametes moving in an almost linear direction, was similar to that of slightly curved free-swimming gametes (not shown). Beat frequencies of free- and thigmotactic-swimming gametes were nearly the same (53–54 Hz) and there was no difference between male and female gametes (). While, maximum amplitudes of AF, which were measured from the traces of AF waveforms, were 6.3 ± 0.6 µm (n=5) in free-swimming male gametes and 5.2 ± 0.6 µm (n=5) in thigmotactic-swimming ones.
PF movements of free- and thigmotactic-swimming gametes
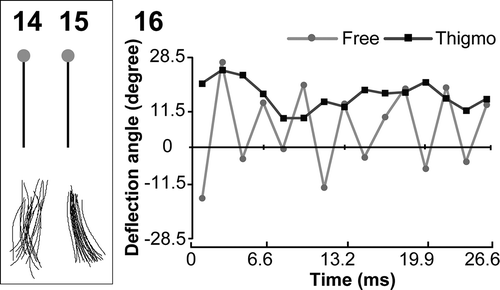
PF waveforms of free-swimming gametes showed continuous oscillations, with the deflection angle ranging from −20.6° to 29.8° (, , ) while those of thigmotactic-swimming ones usually showed no motion or occasional small beats with the deflection angle ranging between 22.3° and 24.0° (, , ). A similar tendency was observed in female gametes (). It was suggested that not only AF waveforms but also PF waveforms were different between free-swimming gametes and thigmotactic-swimming gametes.
Figs 14–16. The posterior flagellar (PF) waveforms of free- and thigmotactic-swimming male gametes. , . Trace of the PF waveforms in free- () and thigmotactic-swimming male gametes (). Twenty forms were chosen every 1.6 ms and traces of the PF were merged. Black bars show 5 µm reference line and grey dots indicate the anterior end of the cell body. . The deflection angle at 1 µm from the tip of PF is plotted against time.
Chemoorientation of male gametes
When adding male gametes to settled female gametes, approximately 50% of male gametes rapidly showed a thigmotactic-swimming pattern. The trajectories of these male gametes were converted from linear paths to middle and small circular paths (). Swimming path curvatures of these male gametes (within 150 µm from settled female gametes) were mainly between 0.04–0.2 µm–1 (n = 115) (). Most of them put their ventral side on the surface of the substratum and swam in clockwise circle. Free-swimming male gametes did not show small circular paths, rather they swam in spirals or undulating lines (). Namely, flagellar behaviours of free-swimming male gametes were not affected by the absence or presence of the sex pheromone from settled female gametes. Swimming velocities of thigmotactic-male gametes during chemotaxis were 78.1 ± 14.3 µm s–1 (n = 108) and significantly decreased compared with the control (P < 0.001). Although, the average swimming velocity of free-swimming male gametes was 199.8 ± 48.4 µm s–1 (n = 83). A significant difference in this value was not observed when compared with the control. The AF beat frequencies of the male gametes were constant at 44–46 Hz despite different distances from the sex pheromone source (within 150 µm from settled female gametes). A significant difference could be detected when compared with the control (53–54 Hz) (P < 0.001).
Figs 17–19. The trajectories of chemotactic male gametes. , . The swimming trajectories of chemotactic gametes are obtained by image processing during the period of 2 s. . Free-swimming male gametes on the upper region from settled female gametes. . Thigmotactic-swimming male gametes near settled female gametes. White arrowheads show settled female gametes. White double arrowheads show the swimming path of chemotactic male gametes shown in . . Frequency of the swimming path curvatures (n = 115). Scale bars = 100 µm.

AF movements of male gametes during chemotaxis
In order to observe the relationship between swimming path curvature and AF waveform, male gametes (0.04–0.28 µm–1 of path curvature, n = 13) were analysed under the sex pheromone (–, Movie S3). We compared these chemotactic male gametes against thigmotactic gametes without the sex pheromone (from −0.008 to 0.008 µm–1 of path curvature, n = 7). The waveforms in the base region (1.05–1.24) and the middle region (1.19–1.30) of AF were almost symmetric, but the tip region sometimes became highly asymmetric during chemotaxis resulting in a decrease in R-bend curvature (, S5). Although a significant difference of the AF to the control in the tip region (, P < 0.05) was observed, a correlation between the path curvature and the AF asymmetric index could not be detected ().
Figs 20–29. The AF beat patterns of chemotactic male gametes. –. Trajectory () and typical flagellar waveforms were recorded at 1.6 ms intervals (–). . The AF asymmetric indices of chemotactic male gametes. I. Base region; II. Middle region; III. Tip region; IV. Whole region. Control vs. Chemotactic gametes, **P < 0.05. . Asymmetric indices of tip region in the AF plotted against path curvatures. . Average value of deflection angle at 8 µm from the tip of AF. I. Control; II. Chemotactic gametes. Control vs. chemotactic gametes, *P < 0.001. . Average value of deflection angles at 8 µm from the tip of AF plotted against path curvatures. Scale bars = 10 µm.
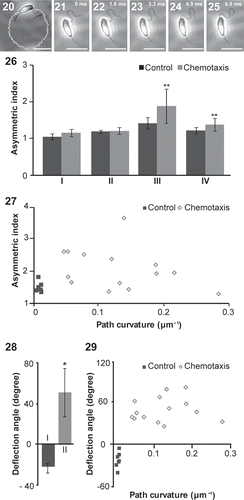
The average value of the AF deflection angle of the chemotactic male gametes was 51.0° ± 23.6° and that of the thigmotactic-swimming male gametes without the sex pheromone was −22.9° ± 4.5° (, ). There was a significant difference between them (P < 0.001). However, there was no correlation between the deflection angle of the AF and the circular path curvature (0.04–0.28 µm–1) (). The maximum amplitude of AF was almost the same in chemotactic (5.4 ± 0.2 µm, n = 7) and thigmotactic (5.2 ± 0.6 µm, n = 7) swimming gametes.
PF movements of male gametes during chemotaxis
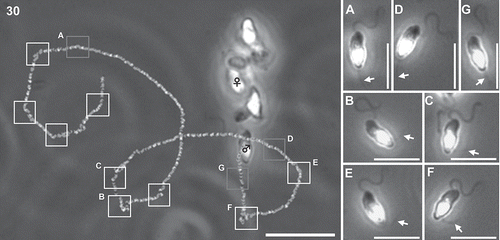
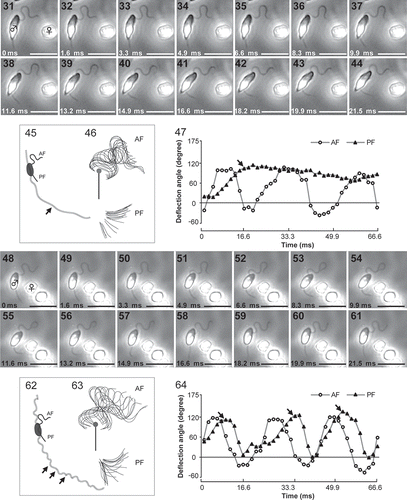
PF unilateral beating in male gametes was occasionally observed when they were close (less than 100 µm) to the pheromone source (settled female gametes) (Fig. S6). When PF showed strong unilateral beating, the swimming-path curvature of male gametes suddenly increased, namely the swimming direction of male gametes strongly changed. Beat frequency of the PF increased when male gametes were closer to the pheromone source. Several beats of the PF were also detected when they were further away from the pheromone source (). However, the exact timing of PF beating was still not known. – show two cases of PF beating of male gametes around settled female gametes, (1) PF bent for a while (–) and (2) PF repeated beating (–). Maximum and minimum values of the PF deflection angles became 151.3° and 12.0° (0.06–0.20 µm–1 of path curvatures, n = 4), (–). The forward stroke of the PF took a longer time (8–16 ms) than the recovery stroke (5–8 ms) (, , Movie S4). It also became clear that the AF waveform was not affected by the PF waveform in chemotactic male gametes (, ).
Fig. 30. A trajectory and PF bending pattern of a chemotactic male gamete (♂) near female gamete (♀). White squares show the timing of lateral PF beating of male gamete. Grey squares show the motionless PF. A-F show detailed images of male gametes at each square, respectively. Arrows indicate the position of the PF. Scale bar = 20 µm in Fig. 30, and µm in Fig. 30A–F.
Figs 31–64. Two patterns of lateral beats of the PF in chemotactic male gametes. PF bent for a while (–) and PF repeated beating (–). Images were recorded at 1.6 ms intervals under the same conditions (– were analysed from –, and – were analysed from –). , . Trajectories of chemotactic male gametes. Arrows indicate the timing of unilateral beats of PF. , . Trace of AF and PF waveforms. Twenty forms were chosen every 1.6 ms and merged. Black bars show 5 µm reference line and grey dots indicate the anterior end of cell body. , . Deflection angles at 8 µm from the tip of the AF and at 1 µm from the tip of the PF are plotted. Arrows indicate the timing of unilateral beats of PF in & . Scale bars = 10 µm.
Discussion
Brown algal swarmers (heterokont algae, Stramenopiles), including gametes and zoospores, have morphologically and functionally heterogeneous flagella. This is quite different from the green alga, Chlamydomonas and the sperm of marine invertebrates. In this study, swimming velocities of E. siliculosus male and female gametes were about 200–240 µm s–1 in the free-swimming case and 130–150 µm s–1 in the thigmotactic-swimming one. This value was similar to a previous observation using 16 mm film (Geller & Müller, Citation1981). Although the number, length and beat frequency of flagella are diverse, swimming speeds of gametes or sperm in aquatic organisms are not so variable. Some of these values are 174 µm s–1 in Chlamydomonas reinhardtii P.A. Dangeard (Goodenough, Citation1983), 165 µm s–1 in sea urchin (Wood et al., Citation2005), 276 µm s–1 in ascidians (Yoshida et al., Citation2002) and 375 µm s–1 in starfish (Shiba et al., Citation2006).
Thigmotactic-swimming gametes of E. siliculosus did not show helical rotations of their cell body and they displayed only two-dimensional waveforms of the AF; however, free-swimming gametes frequently showed helical rotations of their cell body and they displayed two- and three-dimensional waveforms of the AF. It was mathematically shown by Chwang & Wu (Citation1971) and Keller & Rubinow (Citation1976) that three-dimensional (helical) flagellar movement induces rotation of the cell. Helical rotations of Ectocarpus gametes would be induced by a combination of several complex AF waveforms as suggested by Geller & Müller (Citation1981). Although there was no significant difference in beat frequency of the AF between free- and thigmotactic-swimming gametes, swimming velocities between them were different. AF amplitude also did not seem to affect the gamete swimming speed. An increase in swimming speed in free-swimming gametes may be caused by helical rotation of the gamete cell body. When Ishijima (Citation2012) examined the movement characteristics of the sperm and flagella from a lancelet and 35 species of fishes using high-speed video microscopy, he concluded that flagellar beat frequency had the greatest effect on the swimming speed, not the flagellar wavelength nor amplitude.
After Müller (Citation1967; Müller et al., Citation1971) first discovered the brown algal pheromone ‘ectocarpene’ in Ectocarpus siliculosus, many other sex pheromones in brown algae were identified (see the reviews of Maier & Müller, Citation1986 and Maier, Citation1993). As a result of these studies, detailed observations of the behaviour of male and female gametes during the fertilization process of E. siliculosus were carried out. Ectocarpus male and female gametes are morphologically isomorphic. Female gametes settle onto the substratum more quickly than male gametes. When settled, the female gametes begin to secrete the sex pheromone. However, the locomotion and flagellar waveforms of male and female gametes were observed to be almost same after adding them into the chamber.
During chemotaxis in the presence of sex pheromone, the AF deflection angle of male gametes significantly changed in comparison to the control, with decreasing beat frequency of the AF. Geller & Müller (Citation1981) showed a correlation between the average deflection angle of the AF and the radius of the track. In addition to their results, we clarified that the deflection angle of the AF was significantly changed when male gametes altered their swimming pattern from highly linear paths to small or medium circular paths. However, the deflection angle did not significantly change when the gametes switched from medium to small circular paths. Therefore, it was suggested that changing the swimming paths of male gametes from a highly linear path to a circular path was affected by the AF, while altering the trajectory from a medium circular to a small circular path was attributed to the PF.
Geller & Müller (Citation1981) and Maier & Calenberg (Citation1994) showed that the PF performed fast and unilateral beats when the cell sensed decreasing concentration of pheromone. Unilateral beats of the PF were usually observed for reorientation of male gametes towards the sex pheromone source (settled female gametes). In this study, it became clear that, regardless of unilateral beating of the PF, the waveform of the AF remained stable (, ). It was suggested that the AF and PF would move independently in the presence of sex pheromone.
Matsunaga et al. (Citation2010) showed the rapid lateral beats of the PF during phototactic-orientation of gametes in the brown alga, Scytosiphon lomentaria. Those studies reveal that the forward stroke of PF took a longer time (6–8 ms) than the recovery stroke (2–4 ms). When the gametes showed phototactic turning, AF undulation ceased. Therefore, AF motion during phototactic turning of gametes is clearly different from the case of the chemotactic one, although unilateral beat patterns of the PF seem to be similar.
It was revealed that the AF plays a role in changing gamete locomotion from free- to thigmotactic-swimming and also from straight to circular swimming. Moreover, the AF changes the flagellar asymmetry and deflection angle during these steps but it does not change these properties in the presence of the sex pheromone. The PF shows irregular changes in the deflection angle and large bending are dependent on the concentration gradient of the sex pheromone. The exact timing and mechanism of large unilateral beats in the PF during chemotaxis remain unclear. In an ascidian, Yoshida & Yoshida (Citation2011) report that sperm attractants appear to induce Ca2+ entry from extracellular spaces into sperm cells. This increases the intracellular Ca2+ concentration which mediates the beating patterns of sperm flagella. This increase in Ca2+ concentration results in the observed chemotactic turn. Maier & Calenberg (Citation1994) reported on the effects of calcium on flagellar movements of E. siliculosus male gametes during chemotaxis. They surveyed several calcium antagonists and channel blockers including lanthanum, ruthenium red, verapamil, nifedipine and trifluoperazine, and showed that: (1) the asymmetric bending of the AF of male gametes will be strongly inhibited by La3+, (2) nefedipine might interact with Ca2+ channels which are involved in the action of PF, and (3) trifluoperazine might bind to and activate the pheromone receptors in Ectocarpus via hydrophobic interactions. Recently, Fu et al. (2014) conducted a proteomics analysis of flagella of the brown alga, Colpomenia bullosa (Saunders) Yamada and suggested that 8% of the 495 flagellar proteins were related to proteins with calcium-binding function. Further investigation on the roles of calcium in flagellar bending using a high-speed camera will clarify the characteristic beat patterns of the heterogeneous AF and PF of brown algal gametes during chemotaxis.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://dx.doi.org/10.1080/09670262.2015.1109144
Supplementary Figs S1, S2. Definitions of the terms. 1. Definition of the swimming path curvature (Г) and the flagellar curvature (γ). Blue dots and lines show the centre of cell body and zigzag trajectory of the cell body. 2. Definition of the deflection angle (θ).
Supplementary Figs S3–S5. AF curvature plotted against distance from near the base of the flagellum. The basal part of AF was hidden by the cell body, therefore the distance of the base of AF was obtained by adding 4 um (as the half width of the cell body) to the AF length which was detected under microscopy. Data from 20 waveforms were merged. 3. Free-swimming male gametes. 4. Thigmotactic-swimming male gametes. 5. Chemotactic-male gametes.
Supplementary Fig. S6. Distribution of deflection angles of the PF of the control (n = 148 plots from five male gametes) and chemotactic-male gametes (n = 148 plots from four male gametes).
Supplementary Movie S1. Free-swimming male gametes.
Supplementary Movie S2. Thigmotactic-swimming male gametes.
Supplementary Movie S3. Chemotactic-male gametes.
Supplementary Movie S4. Rapid lateral beats of the PF of male gametes.
Supplementary Movie S4
Download Microsoft Video (AVI) (6.6 MB)Supplementary Movie S3
Download Microsoft Video (AVI) (14 MB)Supplementary Movie S2
Download Microsoft Video (AVI) (961.5 KB)Supplementary Movie S1
Download Microsoft Video (AVI) (1.8 MB)Supplementary Fig. S6
Download TIFF Image (6.2 MB)Supplementary Figs S3–S5
Download TIFF Image (1 MB)Supplementary Figs S1, S2
Download TIFF Image (7.1 MB)DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Nana Kinoshita
N. Kinoshita: designing and conducting experiments and writing manuscript; K. Shiba and K. Inaba: teaching, suggestions and advises on analysis of the flagellar waveforms; G. Fu: suggestions culture experiments; C. Nagasato and T. Motomura: designing experiments, discussing and editing manuscript.
Kogiku Shiba
N. Kinoshita: designing and conducting experiments and writing manuscript; K. Shiba and K. Inaba: teaching, suggestions and advises on analysis of the flagellar waveforms; G. Fu: suggestions culture experiments; C. Nagasato and T. Motomura: designing experiments, discussing and editing manuscript.
Kazuo Inaba
N. Kinoshita: designing and conducting experiments and writing manuscript; K. Shiba and K. Inaba: teaching, suggestions and advises on analysis of the flagellar waveforms; G. Fu: suggestions culture experiments; C. Nagasato and T. Motomura: designing experiments, discussing and editing manuscript.
Gang Fu
N. Kinoshita: designing and conducting experiments and writing manuscript; K. Shiba and K. Inaba: teaching, suggestions and advises on analysis of the flagellar waveforms; G. Fu: suggestions culture experiments; C. Nagasato and T. Motomura: designing experiments, discussing and editing manuscript.
Chikako Nagasato
N. Kinoshita: designing and conducting experiments and writing manuscript; K. Shiba and K. Inaba: teaching, suggestions and advises on analysis of the flagellar waveforms; G. Fu: suggestions culture experiments; C. Nagasato and T. Motomura: designing experiments, discussing and editing manuscript.
Taizo Motomura
N. Kinoshita: designing and conducting experiments and writing manuscript; K. Shiba and K. Inaba: teaching, suggestions and advises on analysis of the flagellar waveforms; G. Fu: suggestions culture experiments; C. Nagasato and T. Motomura: designing experiments, discussing and editing manuscript.
References
- Andersen, R.A. (2004). Biology and systematics of heterokont and haptophyte algae. American Journal of Botany, 91: 1508–1522.
- Baba, S.A. & Mogami, Y. (1985). An approach to digital image analysis of bending shapes of eukaryotic flagella and cilia. Cell Motility, 5: 475–489.
- Bouck, G.B. (1969). Extracellular microtubules: the origin, structure, and attachment of flagellar hairs in Fucus and Ascophyllum antherozoids. Journal of Cell Biology, 40: 446–460.
- Chwang, A.T. & Wu, T.Y. (1971). A note on the helical movement of micro-organisms. Proceedings of the Royal Society of London. Series B, Biological Sciences, 178: 327–346.
- Clayton, M. N. (1989). Brown algae and chromophyte phylogeny. In The Chromophyte Algae: Problems and Perspectives (Green, J.C., Leadbeater, B.S.C. & Diver, W.L. editors), Systematics Association Special, 38: 230–253. Clarendon Press, Oxford.
- Geller, A. & Müller, D.G. (1981). Analysis of the flagellar beat pattern of male Ectocarpus siliculosus gametes (Phaeophyta) in relation to chemotactic stimulation by female cells. Journal of Experimental Biology, 92: 53–66.
- Fu, G., Nagasato, C., Oka, S., Cock, J. M. & Motomura, T. 2014. Proteomics analysis of heterogeneous flagella in brown algae (Stramenopiles). Protist 165: 662–675.
- Gibbons, I.R. (1981). Cilia and flagella of eukaryotes. Journal of Cell Biology, 91(suppl): 107s–124s.
- Goodenough, U.W. (1983). Motile detergent-extracted cells of Tetrahymena and Chlamydomonas. Journal of Cell Biology, 96: 1610–1621.
- Holwill, M.E.J. & Sleigh, M.A. (1967). Propulsion by hispid flagella. Journal of Experimental Biology, 47: 267–276.
- Inaba, K. (2003). Molecular architecture of the sperm flagella: molecules for motility and signaling. Zoological Science, 20: 1043–1056.
- Ishijima, S. (2012). Comparative analysis of movement characteristics of lancelet and fish spermatozoa having different morphologies. Biological Bulletin, 222: 214–221.
- Jahn, T.L., Landman, M.D. & Fonseca, J.R. (1964). The mechanism of locomotion of flagellates. II. Function of the mastigonemes of Ochromonas. Journal of Protozoology, 11: 291–296.
- Kaupp, U.B., Kashikar, N.D. & Weyand, I. (2008). Mechanisms of sperm chemotaxis. Annual Reviews Physiology, 70: 93–117.
- Kawai, H. & Kreimer, G. (2000). Sensory mechanisms: light perception and taxis in algae. In The Flagellates: Unity, Diversity and Evolution (Leadbeater, B. & Green, J., editors), 124–146. Taylor and Francis, London.
- Kawai, H., Müller, D.G., Folster, E. & Häder, D.P. (1990). Phototactic responses in the gametes of the brown alga, Ectocarpus siliculosus. Planta, 182: 292–297.
- Keller, J.B. & Rubinow, S.I. (1976). Swimming of flagellated microorganisms. Biophysical Journal, 16: 151–170.
- Maier, I. (1993). Gamete orientation and induction of gametogenesis by pheromone in algae and plants. Plant, Cell & Environment, 16: 891–907.
- Maier, I. (1995). Brown algal pheromones. In Progress in Phycological Research (Round, F.E. & Chapman, D.J., editors), 11: 51–102. Biopress, Bristol.
- Maier, I. & Müller, D.G. (1986). Sexual pheromones in algae. Biological Bulletin, 170: 145–175.
- Maier, I. & Calenberg, M. (1994). Effect of extracellular Ca2+ and Ca2+ antagonists on the movement and chemoorientation of male gametes of Ectocarpus siliculosus (Phaeophyceae). Botanica Acta, 107: 451–460.
- Matsunaga, S., Uchida, H., Iseki, M., Watanabe, M. & Murakami, A. (2010). Flagellar motions in phototactic steering in a brown algal swarmer. Photochemistry and Photobiology, 86: 374–381.
- Miller, R.L. (1975). Chemotaxis of the spermatozoa of Ciona intestinalis. Nature, 254: 244–245.
- Miller, R.L. (1977). Chemotactic behavior of the sperm of chitons (Mollusca: Polyplacophora). Journal of Experimental Zoology, 202: 203–211.
- Miller, R.L. & Brokaw, C.J. (1970). Chemotactic turning behaviour of Tubularia spermatozoa. Journal of Experimental Biology, 52: 699–706.
- Mitchell, D.R. (2000). Chlamydomonas flagella. Journal of Phycology, 36: 261–273.
- Müller, D.G. (1967). Ein leicht flüchtiges Gyno-Gamon der Braunalge Ectocarpus siliculosus. Naturwissenschaften, 54: 496–497.
- Müller, D.G. (1978). Locomotive responses of male gametes to the species-specific sex attractant in Ectocarpus siliculosus (Phaeophyta). Archiv für Protistenkunde, 120: 371–377.
- Müller, D.G. & Falk, H. (1973). Flagellar structure of the gametes of Ectocarpus siliculosus (Phaeophyta) as revealed by negative staining. Archiv für Mikrobiologie, 91: 313–322.
- Müller, D.G., Jaenicke, L., Donike, M. & Akintobi, T. (1971). Sex attractant in a brown alga: chemical structure. Science, 171: 815–817.
- O’ Kelly, C.J. (1989). The evolutionary origin of the brown algae: information from studies of motile cell structure. In The Chromophyte Algae: Problems and Perspectives (Green, J.C., Leadbeater, B.S.C. & Diver, W.L., editors), Systematics Association Special, 38: 256–278. Clarendon Press, Oxford.
- Provasoli, L. (1968). Media and prospects for the cultivation of marine algae. In Cultures and Collections of Algae (Watanabe, A. & Hattori, A., editors), 63–75. Japanese Society of Plant Physiology, Hakone.
- Shiba, K., Tagata, T., Ohmuro, J., Mogami, Y., Matsumoto, M., Hoshi, M. & Baba, S.A. (2006). Peptide-induced hyperactivation-like vigorous flagellar movement in starfish sperm. Zygote, 14: 23–32.
- Shiba, K., Baba, S.A., Inoue, T. & Yoshida, M. (2008). Ca2+ bursts occur around a local minimal concentration of attractant and trigger sperm chemotactic response. Proceedings of the National Academy of Sciences USA, 105: 19312–19317.
- Wood, C.D., Nishigaki, T., Furuta, T., Baba, S.A. & Darszon, A. (2005). Real-time analysis of the role of Ca2+ in flagellar movement and motility in single sea urchin sperm. Journal of Cell Biology, 169: 725–731.
- Yoshida, M. & Yoshida, K. (2011). Sperm chemotaxis and regulation of flagellar movement by Ca2+. Molecular Human Reproduction, 17: 457–465.
- Yoshida, M., Murata, M., Inaba, K. & Morisawa, M. (2002). A chemoattractant for ascidian spermatozoa is a sulfated steroid. Proceedings of the National Academy of Sciences USA, 99: 14831–14836.
