Abstract
A new freshwater diatom, Afrocymbella barkeri Cocquyt & Ryken sp. nov., is described from Lake Challa, a deep and unproductive crater lake near Mt Kilimanjaro in equatorial East Africa, based on light and electron microscopic observations. This tropical diatom is a representative of the rather small genus Afrocymbella with only 12 known species and with a distribution restricted to the African Rift. Taxa belonging to this genus are heteropolar and characterized by dorsiventral valves curved along the pervalvar axis and the presence of small pseudosepta and septa on the open girdle bands. Afrocymbella barkeri was common only towards the end of the dry and windy season corresponding to northern hemisphere summer, when deep water-column mixing caused upwelling of nutrient-rich water from the hypolimnion. This taxon was observed free living in the water column during mixing, but the presence of a small apical pore field at the foot pole, along with some cells with mucilage stalks, suggests that its primary habitat probably involves attachment to a substrate.
Introduction
Species belonging to the genus Gomphocymbella O. Müller are a typical component of the diatom flora from East Africa (Gasse, Citation1986; Cocquyt, Citation2000). However, since the type of the genus Gomphocymbella vulgaris (Kützing) O. Müller (Jahn & Kusber, Citation2015) is actually a Gomphonema Ehrenberg, Krammer (Citation2003) erected the new genus Afrocymbella Krammer with Afrocymbella reichardtii Krammer as type species. A detailed discussion on problems with the genus Gomphocymbella is given in Krammer (Citation2003). In the same publication, Krammer transferred all Gomphocymbella taxa from the African rift to Afrocymbella (Krammer, Citation2003). Representatives of this genus are characterized by distinctly dorsiventral valves which are curved along the pervalvar axis and heteropolar with a head and a foot pole. Afrocymbella taxa produce mucilage stalks at the apical pore field and are solitary or colonial.
Besides Afrocymbella, Krammer (Citation2003) described another related genus, Gomphocymbellopsis Krammer, with the only known species being Gomphocymbellopsis ancyli (Cleve) Krammer, because he put Gomphocymbella ruttneri Hustedt in synonymy with this type species. According to Krammer (Citation2003) this is a widespread taxon in Europe. This genus is similarly characterized by distinctly dorsiventral valves curved along the pervalvar axis and heteropolar with a head and a foot pole. The main difference between them is in the presence or absence of septa and pseudosepta. Small pseudosepta and septa are present on each of the open girdle bands in Afrocymbella, while in Gomphocymbellopsis they are both absent.
Afrocymbella, including the findings of Shinohara et al. (Citation2014), is a small genus. There are only 12 known taxa, consisting of 10 species and two varieties. The present paper deals with the description of a new tropical African representative of this genus.
Lake Challa, a 92-metre deep crater lake at the foot of Mount Kilimanjaro on the border of Kenya and Tanzania, is the object of extensive multidisciplinary studies into the climate and environmental history of East Africa (e.g. Verschuren et al., Citation2009; Barker et al., Citation2011, Citation2013; Wolff et al., Citation2011, Citation2014). The local climate is characterized by two rainy and two dry seasons. The short rains occur from October to December; the major rainfall period of the year occurs during the long rains from March to May. Moreover rainfall in East Africa experiences substantial inter-annual variability linked to the El Niño Southern Oscillation (ENSO), with heavier rains and flooding during El Niño years and intense and prolonged droughts during La Niña years (Wolff et al., Citation2011). The lake is maintained against a strongly negative local water balance by subsurface inflows which are derived from precipitation on the wet forested slopes of Mount Kilimanjaro, and hydrologically open with surface-water conductivity stable at 330–360 µS cm–1. Measured values of surface-water pH vary between 8.4 and 9.3, with no clear seasonal pattern in the available data (Wolff et al., Citation2014). In the stratified Lake Challa diatom growth is, according to Wolff et al. (Citation2014), strongly limited by lack of soluble reactive phosphorus (SRP). However, during the main dry season from July to October there is deep water-column mixing caused by some southern hemisphere winter cooling and increased wind stress. As a result, nutrients are returned to the euphotic zone from the hypolimnion, which results in seasonal diatom blooms (Barker et al., Citation2011; Wolff et al., Citation2011, Citation2014; Buckles et al., Citation2013). Due to a permanently anoxic lower water column (Verschuren et al., Citation2009), sediments accumulating on the bottom of Lake Challa are annually laminated. This renders Lake Challa an ideal study site to investigate past climate variability and its link to ENSO and other drivers of regional climate. Diatoms are the dominant constituent of light-coloured sediment laminae, which are deposited each year during this mixing season (Wolff et al., Citation2011, Citation2014). Besides Nitzschia spp., the new Afrocymbella species described here is the dominant diatom taxon present throughout the sediment record of Lake Challa (Milne, Citation2007; Wolff et al., Citation2014); in these previous studies it was referred to as Gomphocymbella sp.
Materials and Methods
For the present study two kinds of samples were used: phytoplankton sampled monthly over a period of 16 months between 3 September 2013 and 5 January 2015, and fossil material from sediment cores recovered from the centre of the lake in 2003 and 2005. Besides vertical net sampling in the pelagic zone and horizontal net sampling in the littoral zone (phytoplankton net with 10 µm mesh width), quantitative phytoplankton samples were taken at the surface (0 m) in the pelagic and the littoral, and at four additional depth intervals (5 m, 10 m, 15 m, 20 m) in the pelagic using a UWITEC water sampler. For each quantitative sample, 100 ml lake water was collected and fixed in situ with an alkaline Lugol’s solution and formalin.
For taxonomic diatom analysis, part of the phytoplankton net samples were oxidized with hydrogen peroxide (30%), rinsed five times with distilled water and embedded in Naphrax® mounting medium. Phytoplankton was studied with an inverted Olympus CKX41 microscope equipped with an Olympus UC30 digital camera, following the Utermöhl method (Utermöhl Citation1931, Citation1958) using sedimentation chambers of 10 ml. At least 500 phytoplankton individuals or colonies were counted; for each colony the numbers of cells was enumerated.
The fossil diatom material studied here originates from the uppermost section, representing the last 500 years, of a 21 m long composite sediment sequence covering the last 25,000 years of lacustrine sedimentation in Lake Challa (Verschuren et al., Citation2009; Wolff et al., Citation2011). This material was not oxidized but diluted in distilled water prior to embedding in Naphrax®. Light microscopic (LM) investigations were carried out with an Olympus BX51 microscope (oil immersion objective 100×), equipped with Nomarski differential interference contrast optics (DIC) and an Olympus UC30 digital camera. For scanning electron microscopy (SEM), aliquots of oxidized phytoplankton samples and aliquots of untreated sediment were filtered through 1.0 µm Millipore® filters and fixed onto aluminium stubs, air-dried and sputter-coated with 50 nm of gold. SEM studies were carried out with a JEOL-5800LV operating at 25 kV. All phytoplankton samples and permanent diatom slides are deposited at the herbarium of the Botanic Garden Meise (formerly the National Botanic Garden of Belgium) (BR).
Results
Afrocymbella barkeri Cocquyt & Ryken, sp. nov.
(–)
Figs 1–15. Afrocymbella barkeri sp. nov., holotype BR 4404 from material CCA 3421, Lake Challa, Kenya. –. Valves showing the size range. –. Girdle views. –. Girdle views from the same frustule taken at different foci. . Valve representing the holotype. LM. Scale bar = 10 µm.
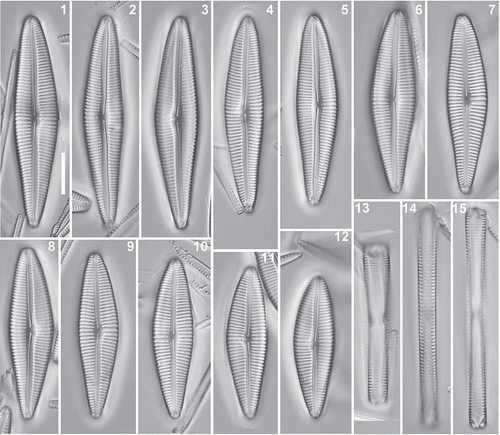
Figs 16–41. Afrocymbella barkeri sp. nov. from subfossil material from Lake Challa, Kenya (at 36.50 m depth in the sediment record, corresponding to around 1700 years AD). Valves showing the size range. LM. Scale bar = 10 µm.
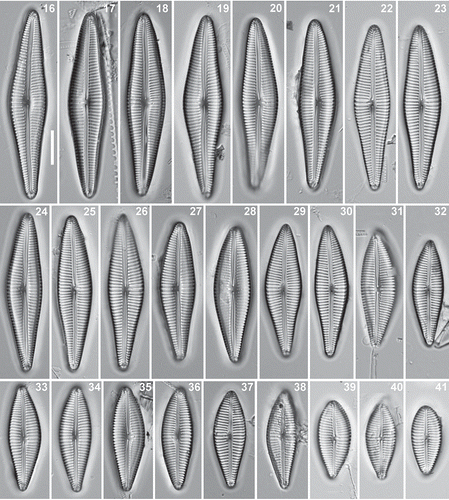
Figs 42–47. Afrocymbella barkeri sp. nov. from Lake Challa, Kenya. External view. . Valve from type material CCA 3421. , –. Valves from subfossil material from Lake Challa, Kenya. –. Valve surface with irregular often comma-shaped areolae (arrow) especially close to the axial area. . Girdle view showing the perforated valvocopula (white arrow) and open girdle bands with a row of small poroids (black arrow). . Eroded valve. SEM. Scale bar 42 = 2 µm; 45–47 = 5 µm; 43, 44 = 10 µm.
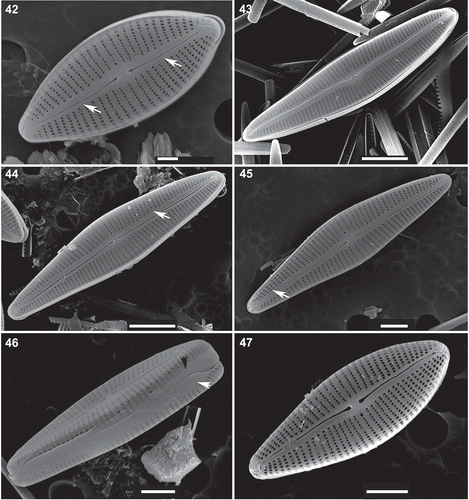
Figs 48–51. Afrocymbella barkeri sp. nov. from Lake Challa, Kenya. Internal view. . Valve from type material CCA 3421. , –. Valves from subfossil material from Lake Challa, Kenya. SEM. Scale bars = 5 µm.
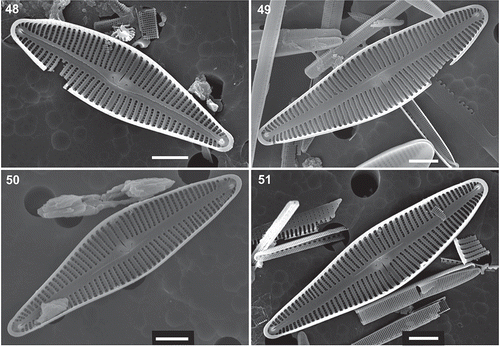
Figs 52–60. Afrocymbella barkeri sp. nov. from Lake Challa, Kenya. External views. –. Details of the apical pore field at the foot pole, external views. . Girdle view with rounded areolae on the lower part of the valve mantle (white arrow) and apically elongated areolae on the girdle band (black arrow). . Detail of the girdle near the head pole and an open girdle band with a septum (white arrow). . Detail of the girdle near the head pole and an open girdle band (arrow). . Detail of the girdle near the head pole and the valvocopula (arrow). SEM. , . Valves from type material CCA 3421. –, . Valves from subfossil material from Lake Challa, Kenya. Scale bars 52–56 = 1 µm; 59–60 = 2 µm; 57–58 = 5 µm.
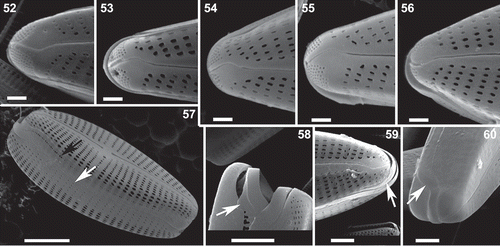
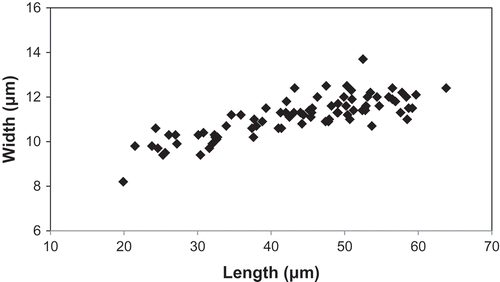
Description: Valves dorsiventral and heteropolar with rounded apices, the head pole broader than the narrowly rounded foot pole; 19.9–63.8 µm long and 8.2–13.7 µm wide. Length to width ratio: 2.3–4.8. Striae slightly radiate, 10.5–14 in 10 µm in the middle portion of the valve and of the same density on the dorsal and the ventral side, becoming denser towards the poles. Striae composed of 20–28 areolae in 10 µm, rarely between 30 and 32 in 10 µm. Axial area linear and narrow, slightly curved. Central area narrow lanceolate. A single distinct stigma present on the dorsal side of the narrow central area, in the prolongation of the middle stria. Raphe complex and slightly curved; portion of the raphe between the head pole and the central nodule shorter than the part between the foot pole and the central nodule. Apical pole field near the foot pole small, divided into a dorsal and a ventral part by the terminal raphe fissure.
Morphological observations based on SEM
Striae composed of more or less rounded areolae mid-valve, becoming transapically elongated on the rest of the valve face. Regularly these elongated areolae become irregular and often comma-shaped (–), especially close to the axial area; rarely they are divided in two near both apical poles (). The striae continue on the upper half of the valve mantle where they are also composed of transapical elongated areolae, even those located mid-valve. On the lower half of the valve mantle, two to four rounded areolae are present in the prolongation of the striae and separated by a hyaline zone of variable width (, ).
The terminal raphe fissures on the exterior valve face on both ends of the valve are comma-shaped, not enlarged and ventrally deflected (–). The central raphe endings are slightly expanded and dorsally curved and the raphe slit is slightly undulating (–).
The stigma is seen as a large pore on the external valve face (–) while internally it is composed of a large, transapically elongated and very narrow furrow, which is distally connected with the middle dorsal alveolus and broadened proximally near the central nodule (–). The intermissio connecting the internal central raphe fissures is complex and composed of an antler-shaped part with enlarged crochet-hooked ends that is connected to the main intermissio by a short transapically oriented slit (–). A small helictoglossa is present on both internal terminal raphe endings (–). Internally near both apical poles, a small pseudoseptum is present (–). The apical pole field at the foot pole is small and divided by the terminal raphe fissure into a dorsal and a ventral part (–). The distance between the pore field and the raphe slit is variable and can be linear to wedge-shaped (–). The delimitation of the apical pore field towards the last stria on the valve face can be more or less straight (, ) or in two steps, with two shorter rows of small poroids near the last striae, shorter by one () to two () lines of poroids.
The girdle is composed of several open bands (–). The valvocopula () and the other girdle bands bear a single row of apically elongated areolae, around 40–46 in 10 µm (, ). The girdle bands have a small septum ().
Holotype: slide BR 4404 from sample CCA 3421, collected from phytoplankton in the pelagic region of Lake Challa on 7 September 2013, deposited in the Botanic Garden Meise, Belgium (BR). The valve representing the holotype is illustrated in .
Isotype: slide Zu 10/28 in the Friedrich Hustedt Diatom Collection, Alfred-Wegener-Institut für Polar- und Meeresforschung, Bremerhaven, Germany (BRM).
Type locality: Lake Challa (Kenya/Tanzania), plankton.
Etymology: The name barkeri is in honour of Prof. Philip A. Barker (Lancaster Environment Centre, Lancaster University, United Kingdom), a diatomist with a long-term research interest in the palaeolimnology of East Africa’s lakes, including Lake Challa.
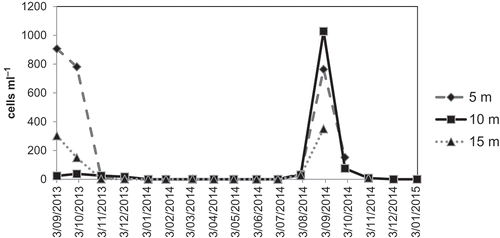
Ecology: Afrocymbella barkeri is a dominant component of the phytoplankton of Lake Challa in September, towards the end of the period of deep water-column mixing in the long dry season coinciding with southern hemisphere winter. A maximum of more than 1000 frustules per ml was observed at 10 m depth in early September 2014, and 900 frustules per ml at 5 m depth at the beginning of September 2013 (). During this peak blooming period of Afrocymbella barkeri in September 2013, the diatom community comprised more than 90% of the phytoplankton biovolume at 5 m depth in the water column. In early September 2014 the diatom community comprised 78% of the phytoplankton biovolume at 5 m and 10 m depth. Afrocymbella barkeri was responsible for 86% of the diatom biovolume in early September 2014 at 5 m, and 75% at 10 m. In September 2013 this was 53% and 35% respectively. Besides A. barkeri, a planktonic Nitzschia sp. is important at this time. The rest of the year, during both wet seasons and the first part of the long dry season, the phytoplankton is mainly composed of small chlorophytes (e.g. Tetraedron minimum (A. Braun) Hansgirg) followed by dinophytes and cyanobacteria (C. Cocquyt, unpublished data).
Discussion
Afrocymbella barkeri differs from the closely related A. beccarii (Grunow) Krammer and A. kociolekii Krammer in the shape of the valve and the shape of the central area, which is narrow lanceolate in A. barkeri while not well-defined and often slightly irregular and wider on the dorsal side in A. beccarii, and very small and not well-defined in A. kociolekii (Krammer, Citation2003). The smallest valves observed in A. barkeri (length 19.9 µm) are much smaller than in the related taxa A. beccarii, A. brunii (Fricke) Shinohara, Maruyama, Rusuwa & Ohtsuka, A. kociolekii, A. reichardtii and its var. procera Krammer, A. muelleri (Kociolek & Stoermer) Krammer and its var. acuminata Krammer () with a minimum of 28 µm for A. kociolekii. The variation in valve width is rather narrow (8.2–13.7 µm), compared with other taxa such as A. brunii and A. kociolekii (8–20 µm) but comparable to A. beccarii (11–14.1 µm) and A. muelleri (11–15.4 µm). The large variation in valve length (19.9–63.8 µm; ) is probably due to the large number of recent and fossil valves measured (n = 84). The smallest observed valves have a somewhat different shape compared with the largest and medium valves. All intermediate shapes could be observed (–) confirming that all valves belong to the same species. The range of stria density (between 10.5 and 14 in 10 µm) is comparable to that of A. brunii, but the areolae composing the striae are denser in A. barkeri (20–28 in 10 µm) than in A. brunii (13–26 in 10 µm); however the latter cited range (Shinohara et al., Citation2014) is very large and should be verified. An overview of some LM characteristics of Afrocymbella barkeri and related taxa is given in .
Table 1. Selected morphological characteristics of Afrocymbella barkeri (own observations) and related taxa (A. beccarii, A. kociolekii, A. reichardtii and its variety procera, A. muelleri and its variety acuminata all according to Krammer, Citation2003; A. brunii according to Shinohara et al., Citation2014). For the related taxa only the maximum length to width ratio (max.) is given.
Although Afrocymbella barkeri frustules have an apical pore field at the foot pole, this taxon was observed free-living in the water column of Lake Challa. Slime stalks were only sporadically present, and then the frustules were attached to each other. SEM investigation showed that the pore field at the foot pole was not always as well-developed in all frustules but often reduced to only a small number of pores (–). The presence of a small pore field at the foot pole is a typical characteristic for representatives of the genus Afrocymbella. The reduced pore field as present in the new species, and probably also in other species such as A. reichardtii Krammer (Krammer Citation2003, pl. 153, ), points to adaptation to a free-living lifestyle. In his description of Afrocymbella, Krammer (Citation2003) mentioned only that “the taxa produce mucilage stalks with the apical pore fields and are solitary or live colonial”. He gives no information on the planktonic or attached way of living. Only Müller (Citation1905) mentioned that A. aschersonii (O. Müller) Krammer occurs also in the plankton. The material of A. brunii (Fricke) Shinohara, Maruyama, Rusuwa & Ohtsuka and A. rossii (Kociolek & Stoermer) Shinohara, Maruyama, Rusuwa & Ohtsuka from Lake Malawi studied by Shinohara et al. (Citation2014) came from epilithic samples. Nonetheless the apical pore field they depicted (Shinohara et al., Citation2014, ) is also rather small, but still much larger compared with the apical pole field of A. barkeri. The epilithic Gomphocymbellopsis ancyli, formerly Gomphocymbella ancyli (Cleve) Hustedt, a widespread taxon from oligotrophic lakes in Europe, has also a reduced apical pole field at the foot pole divided into two parts, comparable to the apical pole field of Afrocymbella. However this genus is characterized by the presence of a small apical pore field at the head pole of the valve (Cantonati & Angeli Citation2003; Krammer, Citation2003), to ensure the cells can live attached to the substratum. A reduced apical pore field at the foot pole, such as in Gomphocymbellopsis and hence as in Afrocymbella, seems insufficient to ensure attachment during mixing periods leading to times of temporary suspension, which could be an advantage for recharging nutrients.
Acknowledgements
The authors wish to thank Caxton Mukhwana Oluseno for the monthly sampling of Lake Challa phytoplankton. Many thanks are due to Myriam de Haan for technical assistance in SEM, and to Dirk Verschuren for the instructive discussion and for putting the fossil material at our disposal.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Christine Cocquyt
C. Cocquyt: original concept, description new species, drafting and editing manuscript; E. Ryken: morphometric data from sediment record samples.
Els Ryken
C. Cocquyt: original concept, description new species, drafting and editing manuscript; E. Ryken: morphometric data from sediment record samples.
References
- Barker, P.A., Hurrell, E.R., Leng, M.J., Wolff C., Cocquyt, C., Sloane, H.J. & Verschuren D. (2011). Seasonality in equatorial climate over the past 25 k.y. revealed by oxygen isotope records from Mount Kilimanjaro. Geology, 39: 1111–1114. doi:10.1130/G32419.1
- Barker, P.A., Hurrell, E.R., Leng, M.J., Plessen, B., Wolff, C., Conley, D.J., Keppens, E., Milne, I., Cumming, B.F., Laird, K.R., Kendrick, C.P., Wynn, P.M. & Verschuren, D. (2013). Carbon cycling within an East African lake revealed by the carbon isotope composition of diatom silica: a 25-ka record from Lake Challa, Mt. Kilimanjaro. Quaternary Science Reviews, 66: 55–63. doi:10.1016/j.quascirev.2012.07.016
- Buckles, L.K., Villanueva, L., Weijers, J.W.H., Verschuren, D. & Sinninghe Damsté, J.S. (2013). Linking isoprenoidal GDGT membrane lipid distributions with gene abundances of ammonia-oxidizing Thaumarchaeota and uncultured crenarchaeotal groups in the water column of a tropical lake (Lake Challa, East Africa). Environmental Microbiology, 15: 2445–2462. doi:10.1111/1462-2920.12118.
- Buckles, L.K., Weijers, J.W.H., Verschuren, D., Cocquyt, C. & Sinninghe Damsté, J.S. (2015). Short-term variability in the BIT index of sediments from Lake Challa, East Africa over the past 2,200 years: what does it mean? Climate of the Past. doi:10.5194/cp11-1177-2015.
- Cantonati, M. & Angeli, N. (2003). New findings on the ecology and ultrastructure of Cymbella ancyli Cleve. Diatom Research, 18: 377–384.
- Cocquyt, C. (2000). Biogeography and species diversity of diatoms in the northern basin of Lake Tanganyika. Advances in Ecological Research, 31: 125–150.
- Gasse, T. (1986). East African diatoms – taxonomy, ecological distribution. Bibliotheca Diatomologica, 11: 1–202 +44 pl.
- Jahn, R. & Kusber, W.-H. (eds) (2015). AlgaTerra Information System [online]. Botanic Garden and Botanical Museum Berlin-Dahlem, Freie Universität Berlin. [July 27, 2015]. Available from http://www.algaterra.org.
- Krammer, K. (2003). Cymbopleura, Delicata, Navicymbula, Gomphocymbellopsis, Afrocymbella. In Diatoms of Europe (Lange-Bertalot, H., editor), Volume 4, 530 pp. A.R.G. Gantner Verlag, Königstein.
- Milne, I. (2007). Climate and environmental change inferred from diatom communities in Lake Challa (Kenya–Tanzania). Unpublished thesis, Queen’s University Kingston, Ontario, Canada.
- Müller, O. (1905). Bacillariaceen aus dem Nyassalande und einigen benachbarten Gebieten. III. Botanische Jahrbücher für Systematik, Pflanzengeschichte und Pflanzengeographie, 36: 137–205.
- Shinohara, K., Maruyama, A., Rusuwa, B. & Ohtsuka, T. (2014). Taxonomic revision of three diatoms found in Lake Malawi: Afrocymbella brunii (Fricke) comb. nov., Afrocymbella rossii (Kociolek & Stoermer) comb. nov., and Aulacoseira euareolata (O. Müller) comb. nov et nom. nov. Phycological Research, 62: 9–15.
- Utermöhl, H. (1931). Neue Wege in der quantitativen Erfassung des Planktons. Mitteilungen Internationale Vereinigung für Theoretische und Angewandte Limnologie, 5: 567–595.
- Utermöhl, H. (1958). Zur Vervollkommung der quantitative phytoplankton-methodik. Mitteilungen Internationale Vereinigung für Theoretische und Angewandte Limnologie, 9: 1–38.
- Verschuren, D., Sinninghe Damsté, J.S., Moernaut J., Kristen, I., Blaauw, M., Fagot, M., Haug, G.H. & CHALLACEA project members (2009). Half-precessional dynamics of monsoon rainfall near the East African Equator. Nature, 462: 637–641. doi:10.1038/nature08520.
- Wolff, C., Haug, G.H., Timmerman, A., Sinninghe Damsté, J.S., Brauer, A., Sigman, D.M., Cane, M.A. & Verschuren, D. (2011). Reduced international rainfall variability in East Africa during the Last Ice Age. Science, 333: 743–747. doi:10.1126/science.1203724.
- Wolff, C., Kristen-Jenny, I., Schettler, G., Plessen, B., Meyer, H., Dulski, P., Naumann, R., Brauer, A., Verschuren, D. & Haug, G.H. (2014). Modern seasonality in Lake Challa (Kenya/Tanzania) and its sedimentary documentation in recent lake sediments. Limnology and Oceanography, 59: 1621–1636. doi:10.4319/lo.2014.59.5.1621.