Abstract
The novel phycoerythrin-containing Synechococcus strain CSIRNIO1 belonging to phylogenetic clade II was isolated from the coastal Arabian Sea. Chromophore characteristics of this isolate revealed the presence of phycoerythrin I (PEI), which allows it to utilize green light efficiently. The DNA distribution data indicate a bimodal slow growth model synchronized with the light/dark cycle. The duration of the cell cycle was regulated by spectral wavelength and nutrient concentration. Nitrate and phosphate enrichment shortened G1 phase duration when cells were exposed to equal doses of photosynthetically usable radiation (PUR) of different spectral wavelengths. G2 phase duration was influenced by spectral quality and phosphate concentration. S phase duration was not affected by the spectral wavelength. However, a shorter doubling time corresponding to shortened G1 and S phases was observed under nitrate enrichment. Phosphate enrichment resulted in shortening of all three phases (G1, S and G2). More efficient utilization of green and red light than blue light regulated the duration of the cell cycle as well as the doubling time, suggesting spectral selectivity in this strain. The effects of spectral wavelengths under varying nutrient concentrations will determine the proliferation of Synechococcus and its adaptation to different environmental conditions.
Introduction
Planktonic organisms that pass through 2.0 µm pores and are around one pg (10–12 g) per cell in fresh weight are considered as ‘picoplankton’ (Fogg, Citation1986). Picoplankton includes both chemotrophic and heterotrophic bacteria and photosynthetic forms such as picoeukaryotes and prokaryotic (cyanobacterial and prochlorophyte) phototrophs. The Synecho-coccus group is the most widely distributed photosynthetic picophytoplankton in the world’s oceans (Partensky et al., Citation1999) with a mean annual average of ~7.0 ×1026 cells, contributing 16.7% of global net oceanic primary production (Flombaum et al., Citation2013).
Light and nutrient concentrations influence Synechococcus distribution in coastal as well as open ocean water conditions (Liu et al., Citation1998; Wood et al., Citation1998, Citation1999; Rajaneesh & Mitbavkar, Citation2013). In the marine environment, underwater spectral characteristics vary with depth and water quality. The presence of phytoplankton and high concentrations of humic substances in coastal waters increases turbidity. Thus, the underwater light spectrum of coastal waters is shifted towards the red end, while shorter wavelengths are scattered out due to increased water turbidity (Stomp et al., Citation2007). As a result, phycocyanin-rich (PC) Synechococcus strains are relatively more abundant in coastal mesotrophic waters. In the open ocean, longer wavelengths are rapidly attenuated in surface waters and do not penetrate below ~15 m. Surface open-ocean waters receive blue and green wavelengths whereas only blue light (400–500 nm) penetrates into deeper waters (100–200 m) (Ting et al., Citation2002). Hence, phycoerythrin-rich (PE) Synechococcus strains are ecologically more adapted to blue-green light and thrive in clear open-ocean waters (Stomp et al., Citation2007). Two different types of phycoerythrin (PEI and PEII) are found in all the PE-containing marine Synechococcus strains. PEII always binds to both chromophores, phycoerythrobilin (PEB) and phycourobilin (PUB), whereas PEI binds either to PEB or both PEB and PUB (Ong & Glazer, Citation1991). However, marine strains may contain only one of the types of PE, which only binds to PEB (Everroad & Wood, Citation2006). Chromophore ratio varies with the light niche; open ocean strains have high PUB:PEB whereas a lower ratio or no PUB is observed in coastal strains. It is obvious that Synechococcus does not experience uniform spectral conditions in its natural habitat, and the niche-specific spectral wavelength environment controls growth characteristics, pigment composition and distribution as well as photosynthetic efficiencies (Kana et al., Citation1988; Bidigare et al., Citation1989; Falkowski et al., Citation2004).
Marine Synechococcus proliferate over a wide range of nutrient environments from oligotrophic to mesotrophic waters and efficiently utilize a variety of nitrogen (N) resources. Different marine Synechococcus isolates have been reported to actively uptake nitrate, nitrite, ammonium and urea as their N source (Lindell et al., Citation1998; Collier et al., Citation1999; Moore et al., Citation2002). Of these, ammonium is the preferred source of nitrogen in Synechococcus as it is readily incorporated into amino acids. Growth-regulating factors such as light intensity can influence uptake of nitrogen from different N-sources (Collier et al., Citation2012).
Knowledge of phases of the cell cycle under the influence of spectral wavelength and nutrient (nitrate, phosphate) concentrations provides insights into the growth characteristics of Synechococcus in natural aquatic environments (Binder & Chisholm, Citation1995; Parpais et al., Citation1996; Burbage & Binder, Citation2007). The growth responses of Synechococcus strains to fluctuations in nutrient concentrations and spectral wavelengths differ and can be evaluated through analysis of doubling time and cell cycle. Doubling time is the average time between two successful cell divisions measured in two ways: (1) estimating the change in cell density with time and (2) addition of time fractions of different cell cycle stages (White, Citation1991; Yamaguchi et al., Citation2007). Thus, doubling time and duration of cell cycle phases are closely interdependent. Every successful cell division has a temporal and sequential order of macromolecule (r-protein, rRNA, DNA, cell septum and cell wall) synthesis that is necessary for duplication as well as segregation of cell content. The cell cycle of prokaryotes is categorized into fast and slow growth models (Cooper & Helmstetter, Citation1968). In the fast growth model, generation time is shorter than the total time required for chromosome replication (C) and the time between termination of chromosome replication and cell division (D). Cells with fast growth have several points of origin of chromosome replication at any one time and DNA replication begins with synchronous initiation at all chromosome origin points. As a result, daughter cells inherit a chromosome copy number corresponding to 2n, where n is an integer (Nordström & Austin, Citation1993; Binder & Chisholm, Citation1995). On the contrary, in the slow growth model, the generation time is longer than the sum of the time required for C and D phases. Overlapping of replications does not occur and as a result only one chromosome is inherited by the daughter cell (Helmstetter, Citation1996). DNA cell-cycles and growth rates of Synechococcus strains have been reported in situ as well as under laboratory conditions (Armbrust et al., Citation1989; Liu et al., Citation1995; Asato, Citation2003) and both the fast and slow growth models have been identified in different strains of Synechococcus. For example, the open-ocean strain WH7803 exhibited fast growth characterized by multimodal DNA distribution whereas two other open ocean strains, WH7805 and WH8103, had slow growth with a bimodal DNA distribution pattern (Binder & Chisholm, Citation1995).
Due to the pivotal role of Synechococcus in both the microbial loop and classical food web dynamics (Campbell & Carpenter, Citation1986; Barber, Citation2007; Richardson & Jackson, Citation2007), over the last few decades there has been a great deal of research in both the Atlantic (Zubkov et al., Citation1998; Zinser et al., Citation2006) and Pacific Oceans (Campbell et al., Citation1994; Binder et al., Citation1996). Several studies along the coast of India have focused on the distribution patterns of different Synechococcus groups (Mitbavkar & Anil, Citation2011; Mitbavkar et al., Citation2012; Rajaneesh & Mitbavkar, Citation2013). Lack of detailed physiological characterization of Synechococcus strains in coastal waters is the primary constraint for understanding their ecological importance. Therefore, this paper describes the influence of spectral wavelength and nutrient conditions (N:P ratio), acting individually and synergistically, on cell cycle phase duration of a newly identified Synechococcus strain isolated from the Eastern Arabian Sea. Maximum photosynthetic efficiency and cellular nutrient uptake values in different experimental conditions provide insights into its adaptability and growth characteristics in the marine environment.
Materials and Methods
Isolation and identification of a marine Synecho-coccus strain
Sample collection, isolation and purification of picophytoplankton
A water sample was collected off Goa, Calangute (15°32’35.49”N, 73°45’17.17”E), central western coast of India. It was filtered gravimetrically through 3 µm filter paper and the filtrate was analysed by a flow cytometer (BD FACS Aria II equipped with 480 and 633 nm LASER sources). Picophytoplankton groups were identified based on cell size and accessory (phycocyanin and phycoerythrin) and primary (chlorophyll) pigment fluorescence. To determine the size characteristics of identified groups, 2 µm beads (Bangs Laboratories, Inc.) were used as an internal reference. Two picophytoplankton groups were identified, one PE-rich and the other with low PE (Supplementary fig. 1). The PE-rich group was identified as Synechococcus by its characteristic orange (phycoerythrin) and red (chlorophyll) fluorescence. The second group containing low PE most probably corresponded to a picoeukaryote and was not investigated further. Groups were sorted by a two-way purity cell sorting technique and incubated at 25°C in f/2 media (without Na2SiO3.9 H2O) for ~1 month. Constant 12: 12 h light and dark (L/D) conditions were maintained with photosynthetically active radiation (PAR) of 37 µmol photons m–2 s–1. After sub-culturing the isolate several times, a unialgal culture of the strain was obtained.
A monoclonal culture was obtained by a dilution method (Choi & Noh, Citation2009). One ml culture was diluted into fresh f/2 medium and dispensed into each well of a sterile 48-well plate with a cell concentration of 0.2 cells ml–1. The plate was incubated at 25°C under 37 µmol photons m–2 s–1 (PAR). Cells were pipetted out (500 µl) from each well and transferred into the fresh medium; this procedure was repeated several times to obtain a monoclonal culture of the isolated strain.
Phylogenetic analysis
Genomic DNA was extracted by treating a cellular pellet with a DNA extraction solution (UniFlexTM DNA isolation Kit). The 16S ribosomal RNA gene (rDNA) was amplified using the PCR primer pair F27 (5’-AGAGTTTGATCMTGGCTCAG-3’) and R1492 (5’-TACGGYTACCTTGTTACGACTT-3’) (Robertson et al., Citation2001). The amplified PCR product was purified and sequenced on ABI 3730xl DNA Analyzer by the Sanger method. The near-complete 16S rDNA sequence (1349 bp) was aligned with selected Synechococcus strains from NCBI GenBank and the RDP database. Nucleotide sequences were aligned in Clustal Omega online software (http://www.ebi.ac.uk/Tools/msa/clustalo/), access provided by the European Bioinformatics Institute (EMBL-EBI). Phylogenetic analyses were conducted using MEGA version 6.06 (Tamura et al., Citation2013). A phylogenetic tree was constructed using the neighbour-joining method and subjected to bootstrap analysis with 1000 replications.
Pigment analysis
Two ml of exponentially grown culture was filtered on GF/F filter paper (0.7 µm pore size with 25 mm diameter, Whatman). The filter paper was cut into slivers and disrupted with 3 ml of 100% acetone for 30 s using a homogenizer (PRO250, Pro Scientific Inc. Oxford), on ice to prevent excessive heating. The sample slurry was stored overnight at −20°C. The entire extraction procedure was carried out in dim light and at low temperature to minimize degradation of pigments. High performance liquid chromatography (HPLC) analysis was carried out following the method of Hooker et al. (Citation2005) with minor modifications (Roy, Citation2010).
Chromophore analysis
Determination of phycobilin pigment composition was carried out by filtering 2 ml of exponential phase culture on GF/F filter paper (25 mm diameter, Whatman). An in vivo absorption spectrum was scanned from 400 to 750 nm using a dual beam spectrophotometer (UV-VIS-2550, SHIMADZU). A wet filter paper without sample was used as a reference. The fluorescence emission and excitation spectra (excitation and emission slits: 5 nm) were recorded at room temperature by a spectrofluorophotometer (RF-5310 PC; SHIMADZU). The in vivo emission spectrum was obtained between 500–750 nm at a fixed excitation wavelength of 492 nm; the excitation spectrum was recorded between 400–700 nm whereas emission wavelength was fixed at 573 nm.
Influence of spectral quality and N: P ratio on Synechococcus cell cycle
Culture conditions
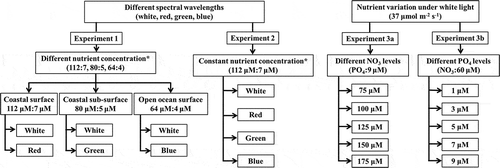
Cells from enriched f/2 media were transferred into various experimental treatments. Desired nitrate and phosphate concentrations of experimental treatments were obtained by using NaNO3 and NaHPO4 (1 mM stock). Initial cell concentration (~106 cells ml–1) was kept constant for all the treatments grown in triplicate under 12:12 h light and dark conditions. Different light conditions were obtained by wrapping experimental chambers with the respective transparent (white) or coloured plastic sheets (red, green and blue) and transmittance spectra of these sheets were recorded by spectrophotometer (UV-VIS-2550, SHIMADZU) (Supplementary fig. 2A).
Experimental light conditions and irradiance measurements
The spectral distribution inside the experimental chambers was measured with a Hyperspectral radiometer (HyperOCR, Satlantic) in terms of energy (W m–2) and values were converted into photon flux density (µmol photons m–2 s–1). The photosynthetic photon flux density values were 32, 35, 45 and 37 µmol photons m–2 s–1 in the blue, green, red and white light treatments, respectively. For each colour, the photosynthetically usable radiation (PUR) of the culture flasks was equated to ~25 µmol photons m–2 s–1 by placing the flasks at different distances from light sources or by covering the experimental set-up with neutral filters. The PUR is defined as
Where, = spectral composition of the incident light;
= a weighted probability function that a photon of a given wavelength will be absorbed by an algal cell. This can be derived from the absorption spectrum of the cells by normalizing the spectrum with respect to its maximum absorbance (amax) (Morel et al., Citation1987). For Synechococcus strain CSIRNIO1 amax occurs at λ = 438 nm (Supplementary fig. 2B–C).
Nutrient and light conditions
Experiments were carried out at three different combinations of nutrient and light conditions:
(1) The Synechococcus culture was maintained in different nutrient concentrations (with a fixed N:P ratio) under varying light environments (equal PUR). In preliminary culture experiments with this strain, the minimum required nutrient concentration (NO3: PO4; all values are µM) under white, red, green and blue light was measured as 60:1, 112:7, 80:5 and 64:4, respectively. The nutrient treatments were designed to mimic the Redfield ratio in three different marine conditions (coastal surface, sub-surface and open ocean surface). Low nutrient concentrations (64:4) under blue light represented the open ocean at greater depth (~254 m). A ratio of 80:5 was used in a green light treatment that represented coastal nutrient-rich subsurface waters (~113 m). The highest nutrient concentration of 112:7 was used in red light conditions representing surface coastal waters (Garrison, Citation2011). One set of controls was maintained in white light with similar nutrient concentrations as experimental treatments to determine spectral influences.
(2) To investigate the influence of spectral wavelength on growth characteristics, cells were grown in a fixed nutrient concentration under varying light conditions (white, red, green and blue). Nitrate and phosphate concentrations of 112 and 7 µM, respectively, were fixed under white, red, green and blue light treatments.
(3) To determine the role of nutrients (nitrate and phosphate) in growth characteristics, cells were maintained in different nitrate and phosphate concentrations (with different N: P ratios) under white light. We altered the N: P ratio of the growth media by either (i) changing the nitrate concentration while phosphate was kept constant or (ii) keeping the nitrate concentration unchanged while phosphate was varied ().
Cell cycle analysis
Cells grown in different experimental treatments were used for analysing the DNA frequency distribution. One ml of culture (105–107 cells ml–1) was preserved in glutaraldehyde solution (0.2% final concentration) and placed in liquid nitrogen. Samples were stored at −80°C until analysis. Prior to analysis, the samples were thawed at 37°C, 0.1 g l–1 mixture of RNase A and B (1:1) (Sigma) was added and the samples were incubated for 30 min to improve the precision of the DNA histogram. SYBR green I (30 µl; 10–4 × concentration of commercial stock solution; Invitrogen) was used to stain nucleic acid material which was incubated in the dark for 20 min (Marie et al., Citation1997). DNA fluorescence was detected at 530/30 nm band pass filter against chlorophyll fluorescence (695/45 nm). Flow cytometric data was further analysed by the Mod Fit LT ver 3.2 (Verity Software House) software package to compute the fraction of cells in G1, S and G2 phases.
The duration of each cell cycle was calculated using equations in Slater et al. (Citation1977).
The doubling time (tD) was calculated from 0.6931/µ; where µ is growth rate (Supplementary figs 3 & 4). P(G1), P(G2) and P(S) are the fractions of the cell population in G1, G2 and S phases, respectively.
Maximum photosynthetic efficiency (Fv/Fm) measurement
Triplicate experimental treatment samples were collected during the light period and incubated in complete darkness for 15 min. Subsequently, samples were analysed in a FIRe-Fluorometer (Fluorescence Induction and Relaxation Fluorometer, Satlantic, Halifax, Canada) to measure maximum photosynthetic efficiency. The analyses of all experimental samples were completed sequentially in dark conditions and took around 30 min. Fv/Fm was calculated according to the following equation (Schreiber, Citation1986):
Where F0 is the dark-adapted minimum chlorophyll fluorescence, and Fm is the maximum fluorescence after application of a single turnover flash (STF) with a duration of 120 μs (Hung et al., Citation2013).
Nutrient analysis of batch cultures
Cellular uptake of nitrate and phosphate (molar concentration) was monitored by measuring the initial and final nutrient concentration in growth media during the experimental period. Final nutrient concentrations were obtained at the end of the exponential phase of the batch cultures. The cells were separated from the growth media by using 0.2 µm PTFE syringe filter and nutrient concentration was measured by SKALAR SAN ++Analyzer system. Nitrate and phosphate uptake per cell was calculated by using two formulae
Initial and final nitrate and phosphate concentrations in growth media are denoted by I and F subscripts, respectively. Molar mass of nitrate and phosphate was used as 62.0 and 94.9 g mol–1 in the equation. C represents cell concentration in a litre and the nutrient uptake is presented as fg cell–1.
Results
Identification of Synechococcus – pigment and phylogenetic analyses
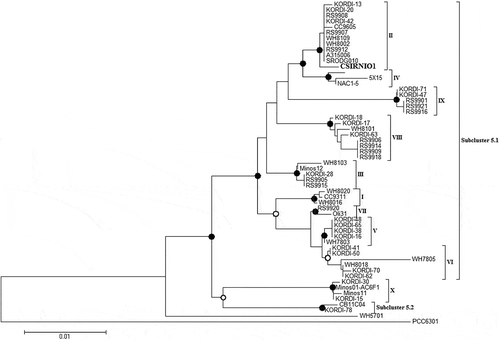
Pigment analysis revealed that the isolated strain was rich in zeaxanthin (a marker pigment for Synechococcus sp.), as well as chlorophyll a and beta carotene (Supplementary fig. 5). The absorbance and fluorescence spectra of whole cells demonstrated maximum PEB absorbance and PE emission at slightly red-shifted wavelengths 567 nm and 578 nm, respectively. The excitation peak of PEB was detected at 568 nm (–). Nucleotide homology and phylogenetic analysis confirmed that the isolated strain was novel and it was named as Synechococcus sp. CSIRNIO1 (GenBank accession number KJ130543). Based on phylogenetic analysis, this isolated strain clustered in clade II of subcluster 5.1 (with GC content of 55.1%) along with other strains (Fuller et al., Citation2003; Choi & Noh, Citation2009; ).
Fig. 2. In vivo absorption (A), excitation (B) and emission spectra (C) of Synechococcus sp. CSIRNIO1. Absorption spectrum was scanned from 400 to 700 nm; excitation spectrum was recorded between 400 and 575 nm; emission wavelength was fixed at 573 nm. Emission spectrum was obtained between 530 to 750 nm at a fixed excitation wavelength of 492 nm.
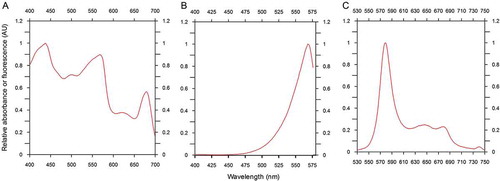
Fig. 3. Neighbour-joining phylogenetic tree of 16S rDNA gene sequences of Synechococcus strains. Clade designations follow the nomenclature of Fuller et al. (Citation2003) and Choi & Noh (Citation2009). Bootstrap values above 40% are shown. Closed circles represent bootstrap values of 70-100%, open symbols represent 40–70%. Synechococcus sp. CSIRNIO1 has 99% similarity to other strains of the clade II Synechococcus PCC6301 used as the root. Scale bar, 0.01 nucleotide substitutions per site.
Response to simultaneous changes in nutrient and light
When Synechococcus was exposed to different concentration of nutrients and light simultaneously, a significantly shorter doubling time (tD) (F709, 2; P < 0.05) was observed in red light (with N:P of 112:7) than in blue (80:5) and green light (64:4) conditions (). A similar trend was observed for the pre-replication (G1) and post-replication (G2) phase duration. However, duration of the S phase did not vary significantly (P > 0.05) among these treatments (). Cellular nitrate uptake differed significantly among these three conditions (F15, 2; P < 0.05) while cellular phosphate uptake did not (P > 0.05). The highest cellular N: P ratio (16:1) was obtained in blue light followed by green and red light (–). The highest photosynthetic efficiency was measured in blue light followed by red and green light (F40, 2; P < 0.05). However, in the case of green light, the Fv/Fm ratio was comparable (P > 0.05) to white light (–).
Fig. 4. Cellular doubling time (tD) measured in different experimental treatments. (A) Cells were grown in different nutrient concentrations (with a fixed N: P ratio) under varying light environments. (B) Cells were grown in a fixed nutrient concentration under varying light conditions. Cells were maintained in different nitrate and phosphate concentrations (with different N: P ratios) under white light condition (C) nitrate variations; (D) phosphate variation.
*, +, ▲ and ● depict significantly different (P < 0.05) from first, second, third and fourth bar, respectively, in each treatment condition.
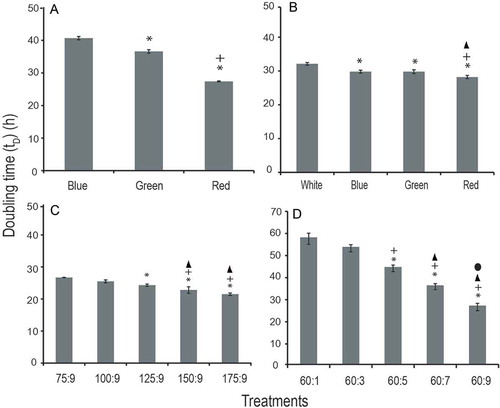
Fig. 5. Cell cycle phase duration compared in different experimental treatments. (A) Experiment 1; cells were grown in different nutrient concentrations (with a fixed N:P ratio) under varying light environments. (B) Experiment 2; cells were grown in a fixed nutrient concentration under varying light conditions. Experiment 3; cells were maintained in different nitrate and phosphate concentrations (with different N:P ratios) under white light condition (C – nitrate variations; D – phosphate variations). Symbols are as for .
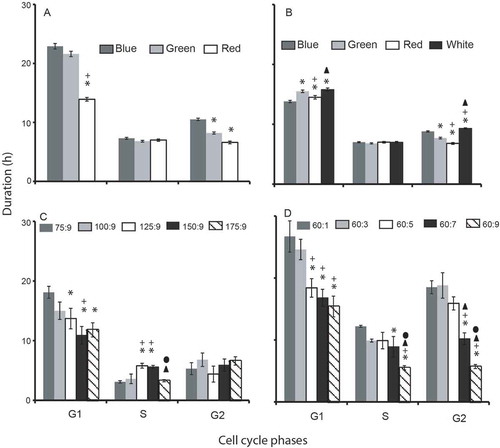
Fig. 6. Cellular nutrient uptake and N: P ratios are measured in different experimental treatments. (A–C) Experiment 1; cells were grown in different nutrient concentrations (with a fixed N: P ratio) under varying light environments. (D–F) Experiment 2; cells were grown in a fixed nutrient concentration under varying light conditions. Experiment 3; cells were maintained in different nitrate and phosphate concentrations (with different N: P ratios) under white light condition (G–I: nitrate variations; J–L: phosphate variations). Symbols are as for .
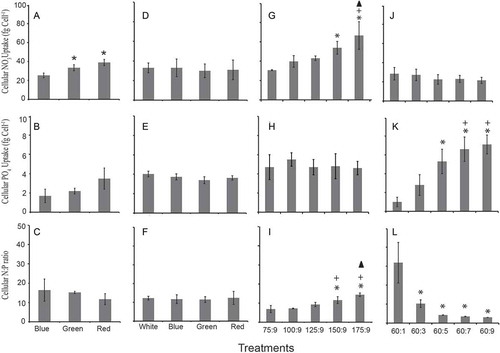
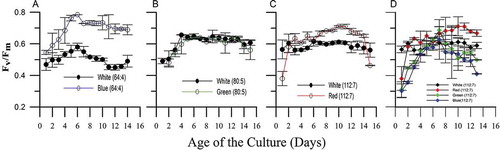
Fig. 7. Maximum photosynthetic efficiency in different nutrient concentrations (with a fixed N: P ratio) under varying light environments. (A) Relatively lower nutrient concentration (64 µM NO3: 4 µM PO4) and blue light environment representing the open ocean waters. (B) Nitrate and phosphate ratio of 80 µM: 5 µM and green light environment representing coastal sub-surface waters. (C) Highest nutrient concentration of 112 µM NO3: 7 µM PO4 and the red light environment representing coastal surface waters. (D) Maximum photosynthesis efficiency measured in a fixed nutrient concentration (112 µM NO3:7 µM PO4) under varying light conditions (white, red, green and blue).
Response to different light conditions under constant nutrient concentrations
A significantly longer doubling time was observed in white light than in other spectral conditions (blue, green and red) (F35, 3; P < 0.05; post hoc Tukey test). Amongst these three spectral conditions, the shortest and longest doubling times were observed in red and blue light, respectively (). The G2 phase duration followed a similar trend as observed with doubling time (white > blue > green > red). A post hoc analysis revealed that the G1 and G2 phase duration significantly differed among red, green and blue wavelengths (P < 0.05; post hoc Tukey test). However, this was not the case with the S phase duration (). The cellular nutrient uptake and N: P ratio was found to be independent of spectral wavelength (–). The highest photosynthetic efficiency was measured in red light followed by green and blue light, and these values were significantly higher than the white light ().
Response to different nutrient concentrations in white light
Doubling time significantly shortened with the enrichment of nitrate (F42.38, 4; P < 0.05) and phosphate (F148.97, 4; P < 0.05) in the growth medium. The shortest doubling times, 21.5 h and 26.7 h, were observed at the highest nitrate (175 µM) and phosphate (9 µM) concentrations, respectively ( & ). The G1 phase duration positively correlated with the doubling time in all nutrient treatments (r2 = 0.95). The three cell cycle phases (G1, S and G2) significantly shortened with phosphate enrichment ( & ). Cellular nitrate and phosphate uptake increased with the nutrient enrichment in the medium. The cellular N:P ratio correlated positively with the nitrate enrichment and negatively with phosphate enrichment (–). The Fv/Fm ratio ranged from 0.64–0.66 and 0.60–0.62 in different nitrate and phosphate concentrations, respectively. When cells were grown in white light, nutrient enrichment had no appreciable effect on the photosynthetic efficiency.
Discussion
Phylogenetic analyses grouped marine Synechococcus strains into three major clusters, MC-A, MC-B and MC-C (Waterbury, Citation1989; Herdman et al., Citation2001). Later, these three clusters were reclassified as 5.1, 5.2 and 5.3. Of these clusters, 5.1 consists of nine clades (I-IX) of PE-containing strains. The PE-lacking strains containing phycocyanin (PC) as major light-harvesting pigments are grouped into cluster 5.2 (Fuller et al., Citation2003; Six et al., Citation2007). The per cent GC content of clusters 5.1 and 5.2 ranges are 55–62% and 63–66%, respectively. In this scenario Synechococcus CSIRNIO1, with a GC content of 55.1%, belongs to clade II.
Based on phycobiliprotein composition (PC, PEI and PEII) of the phycobilisome (PBS) rod substructures, marine Synechococcus is partitioned into three major groups: Types 1, 2 and 3. The PBS rods containing PC belong to type 1. The type 2 strains contain PC and phycoerythrin I (PEI). In type 3, both phycoerythrins (PEI and PEII) are found in the PBS rod substructures along with PC (Six et al., Citation2007). The CSIRNIO1 strain is a type 2 characterized by only PEB attachment (No PUB) to phycoerythrin I (PEI). PEB-rich strains can utilize a broad spectrum (500–700 nm) of photosynthetically active radiation (PAR) and proliferate in turbid coastal waters (Wood et al., Citation1998). Thus, PEB attachment to PEI in CSIRNIO1 is an adaptive mechanism at the cellular level for efficient utilization of the green light in coastal waters.
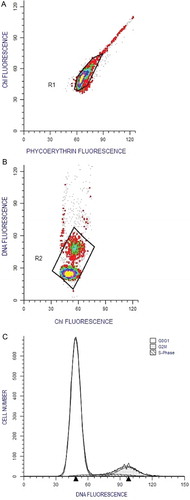
Spectral quality and nutrient concentration regulate cellular photosynthetic efficiency, nutrient uptake, doubling time and cell cycle duration of Synechococcus (Hayashi et al., Citation1969; Morel et al., Citation1993; Lepp & Schmidt, Citation1998). The bimodal DNA frequency distribution of CSIRNIO1 corroborates with a previous study (Binder & Chisholm, Citation1995) which indicated the existence of slow growth model in marine Synechococcus isolates (). The bimodal DNA distribution pattern was observed in all the experimental treatments. A similar mode of the cell cycle was preserved regardless of the nutrient concentration and spectral wavelength. However, the cell cycle phase duration altered proportionally with the doubling time in different experimental treatments. The diel pattern of DNA distribution was strongly synchronized to 12:12 h L/D cycle (Supplementary fig. 6) associated with a highly ordered sequential process of macromolecule synthesis (Asato, Citation1979). The maximum cell fractions in G1 and minimum cell fraction in both S and G2 phases occurred at the onset of the dark period. Cells in the S and G2 phase reached a maximum during the early hours of the light period corresponding to minimum cell fraction of G1 phase. The three cell cycle phases were arrested during the dark phase, and this could be due to inactivation of regulatory factors for macromolecule synthesis in the dark phase (Armbrust et al., Citation1989).
Fig. 8. Flow cytometric plots of Synechococcus sp. CSIRNIO1. (A) Region 1 (R1) selected to avoid outliers in the cell cluster. (B) Region 2 (R2) selected to avoid cell aggregates in DNA distribution. (C) DNA bimodal distribution and features as obtained from ModFit LT ver. 3.2 software (CV and RCS value of shown sample was 9% and 0.96, respectively).
Light availability and spectral wavelength in the water column play a significant role in regulating the cell cycle of Synechococcus (Binder & Chisholm, Citation1990; Vaulot, Citation1995; Asato, Citation2003; Scanlan, Citation2003). The increased photosynthetic efficiency of CSIRNIO1 in different spectral wavelengths (red, green and blue) indicated the efficient utilization of these respective wavelengths in the PS II reaction centre. The relatively faster growth rate in green light suggests spectral selectivity in CSIRNIO1, as this strain was isolated from sub-surface coastal waters. Furthermore, the shortest doubling time in red light also indicated its adaptability to surface water conditions. It has also been observed (Glover et al., Citation1987) that enhanced photosynthetic efficiency results in higher growth efficiency in phycoerythrin-rich Synechococcus culture. The Fv/Fm ratio of CSIRNIO1 was comparable between green and white light at low N: P ratio (80:5). In contrast, a significantly higher Fv/Fm ratio was measured in green rather than white light in higher N: P ratio (112:7) conditions. This observation points out that efficient utilization of green light occurs only when the nutrient concentration is high. Further studies pertaining to this observation are required to unravel the underlying cellular mechanism.
The photophysiology and molecular adaptive mechanism in marine picophytoplankton (Prochlorococcus and Synechococcus) under different spectral environments have been well documented in several studies (Kana & Gilbert, Citation1987; Kana et al., Citation1988; Armbrust et al., Citation1989; Binder, Citation2000; Bhaya et al., Citation2002; Six et al., Citation2004; Burbage & Binder, Citation2007). However, evidence regarding the internal adjustment of the cell division process under different spectral wavelengths in combination with nutrient variations is limited. The present study shows spectral wavelength and nutrients have both individual and synergetic influence on the doubling time as well as cell cycle phase duration of CSIRNIO1. Alteration in duration of any of the specific phases can modulate cellular doubling time significantly (Burbage & Binder, Citation2007). When exposed to varied spectral environments, Synechococcus cells exhibited two discrete sequences for G1 (white = green > red > blue) and G2 phase (white > blue > green > red) duration in fixed nutrient conditions. The shortest G1 and G2 phases were observed in blue and red light, respectively. The S phase duration remained unchanged in all these spectral environments. This constancy in the S phase duration indicates that time required for DNA synthesis does not depend on the spectral wavelength. The doubling time sequence was analogous to the G2 phase sequence among these treatments. This observation suggests that amongst these three cell cycle phases, the G2 phase duration exclusively regulates the doubling time in different spectral conditions (). Choi et al. (Citation2013) reported a similar sequence of doubling time (white > blue > green > red) with Synechococcus KMMCC-314 grown under different spectral wavelengths. These authors concluded that the red spectrum was complementary to the colour of Synechococcus cells and most influenced photosynthesis. Another study (Brunelle et al., Citation2007) revealed that a dinoflagellate (Karenia brevis) cell cycle was responsive to blue light and controlled by the cellular circadian rhythm. This further established that spectral wavelength can alter cell cycle phase duration significantly as the S phase was completed earlier under blue light than in the same intensity of white light.
Table 1. Influence of different spectral wavelength and nutrients (nitrate and phosphate) on cellular nutrient uptake, maximum photosynthetic efficiency, doubling time and cell cycle phase duration of Synechococcus sp. CSIRNIO1.
Concentrations of nutrients and their efficient uptake significantly influence the growth characteristics of Synechococcus in different aquatic environments (Chisholm, Citation1992; Harrison et al., Citation1996). The higher growth rate of Synechococcus in coastal waters than in the open ocean conditions is attributed to differences in nutrient concentration (Glover et al., Citation1988; Liu et al., Citation1998). The small size and high surface to volume ratio of Synechococcus allow efficient utilization of nutrients, particularly nitrate, required for synthesizing the light-harvesting pigment complex in phycobilisomes (Raven, Citation1998). A faster growth rate of CSIRNIO1 under higher nitrate concentration (>125 µM) can be attributed to shortening of S and G2 phase durations. A similar effect of enhanced growth rate with nitrate enrichment (ranging from 98.3–112.8 µM) in chemostat culture was observed with Synechococcus strain WH7803 (Liu et al., Citation1999). Liu et al. (Citation1999) concluded that the faster growth rate corresponded to shortening of G1 phase duration while S and G2 phase remained unchanged. Nitrogen supply is essential for macromolecule synthesis during the G1 phase. The cell division time increases in N-depleted cells, as the prolonged G1 phase delays the onset of S phase. Cellular protein synthesis initiates during G1 phase at the expense of nitrogen and terminates in the S and G2 phases (Vaulot, Citation1995). On the other hand, increased phosphate concentration can shorten the duration of all three cell cycle phases and regulate doubling time significantly (Vaulot et al., Citation1996). This can be attributed to the need for cellular phosphate for replication of nucleic acids and macromolecule synthesis during S and G2 phases (Asato, Citation1979).
The synergistic influences of spectral wavelength and nutrients on different cell cycle phase duration were compared when Synechococcus cells were exposed to different N:P ratios and light quality simultaneously. The doubling time significantly varied under these experimental treatments; the shortest doubling time was observed at the highest nutrient (112:7) concentration under red light treatment. In addition to the doubling time, both the G1 and G2 phase duration pursued a comparable trend (blue > green > red) among these experimental treatments. The shortest and longest G1 phase duration was observed in the highest (112:7) and lowest (60:5) nutrient treatments, respectively. This observation reveals that nutrient enrichment can shorten the G1 phase duration irrespective of spectral wavelength. G2 phase duration significantly shortened with phosphate enrichment. The shortest G2 phase duration was observed in red light and highest phosphate concentration, whereas this duration was longest in blue light with minimum phosphate concentration. The shortening of the G2 phase duration in longer spectral wavelengths corroborates observations in Experiment 2, wherein cells were exposed to different spectral conditions in fixed nutrient concentrations. Olson et al. (Citation1986) and Vaulot et al. (Citation1987) reported that the doubling time of marine phytoplankton (diatoms and coccolithophores) was regulated by G1 phase duration in white light treatment and both studies suggested the cell cycle phase duration varied with cellular nitrogen acquisition. A similar observation was made in the dinoflagellate cell cycle (Olson & Chisholm, Citation1986) and authors concluded that light- and nitrogen-dependent processes are heavily concentrated in the G1 phase of the cell cycle, while S and G2 phases are independent of light or nitrogen supply. Vaulot et al. (Citation1996) reported shortening of G1 phase duration of Synechococcus at increased phosphorus concentration while in the case of Prochlorococcus all the three cell cycle phases were arrested in phosphate-starved conditions (Parpais et al., Citation1996). The present study concludes: (1) photosynthetic efficiency of Synechococcus CSIRNIO1 is influenced by the spectral wavelength (red > green > blue) and this, in turn, controls doubling time; (2) G2 phase duration is regulated by spectral wavelength as well as nutrient (especially phosphate) concentration; (3) G1 and S phase durations are independent of spectral wavelength; and (4) nitrate enrichment induces shortening of G1 and S phases while phosphate enrichment shortens all three cell cycle phases (G1, S and G2), resulting in shorter cellular doubling time.
Experiments with Synechococcus CSIRNIO1 presented here emphasize that light quality and nutrients both have marked effects on the cell division cycle as well as the growth rate. Differentiating the influence of spectral wavelength under varying nutrient concentrations will determine the proliferation and adaptability of Synechococcus to different environmental conditions.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://10.1080/09670262.2016.1214881
Supplementary fig. 1. Cytogram of natural water sample collected from Calangute. ‘a’ and ‘b’ clusters denote PE-rich and PE-low picoplankton groups, respectively. Cluster ‘c’ is 2 µm internal size reference bead.
Supplementary fig. 2. (A) Transmittance characteristics of different coloured and transparent filters used for the experimental set-up. (B) Spectral composition of blue, green and red light inside the coloured chambers used in the experiments. The dotted line represents the in vivo cellular absorption spectrum used as the weighing function to obtain Photosynthetically Usable Radiation (PUR). The photosynthetically available radiation (PAR) varied inside the experimental chambers (see the Materials and methods). The PAR:PUR ratios (spectrally integrated values) are 1.22, 1.34, 1.84 and 1.54 for blue, green, red and white light conditions, respectively. (C) Spectral distribution and fraction of PAR for the white fluorescent light.
Supplementary fig. 3: Growth curve obtained in the experiment 1 (A) and experiment 2 (B–D).
Supplementary fig. 4: Growth curve obtained in experiment 3 (a and b) are presented in (A) and (B), respectively.
Supplementary fig. 5:
Characterization of primary pigment composition of Synechococcus sp. CSIRNIO1 by HPLC. ZEA – Zeaxanthin (marker pigment for Synechococcus sp. cyanobacteria), CHL A – Chlorophyll a and BCAR – Beta carotene.
Supplementary fig. 6.
Representative plot of diel DNA cell cycle of Synechococcus CSIRNIO1 in laboratory culture (under white light treatment, NO3:PO4; 64 µM:4 µM)
Acknowledgements
The authors appreciate the help of Dr Lidita Khandeparker for molecular identification. We thank Dr T. Suresh, Mr Ashvesh Gimonkar and Ms Shreya Joshi for the radiometric measurement. We gratefully acknowledge Profs Christine Maggs and Mario Giordano and the two anonymous reviewers for their valuable suggestions and efforts to improve the quality of the paper.
DISCLOSURE STATEMENT
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Suchandan Bemal
A. C. Anil: Original concept, designing of experiments, writing the manuscript; S. Bemal: Designed and carried out the culture experiment, collated the results, writing the manuscript.
Arga Chandrashekar Anil
A. C. Anil: Original concept, designing of experiments, writing the manuscript; S. Bemal: Designed and carried out the culture experiment, collated the results, writing the manuscript.
REFERENCES
- Armbrust, E., Bowen, J., Olson, R. & Chisholm, S. (1989). Effect of light on the cell cycle of a marine Synechococcus strain. Applied and Environmental Microbiology, 55: 425–432.
- Asato, Y. (1979). Macromolecular synthesis in synchronized cultures of Anacystis nidulans. Journal of Bacteriology, 140: 65–72.
- Asato, Y. (2003). Toward an understanding of cell growth and the cell division cycle of unicellular photoautotrophic cyanobacteria. Cellular and Molecular Life Sciences, 60: 663–687.
- Barber, R.T. (2007). Oceans: picoplankton do some heavy lifting. Science, 315: 777–778.
- Bhaya, D., Schwarz, R. & Grossman, A.R. (2002). Molecular responses to environmental stress. In The Ecology Of Cyanobacteria. Springer, Dordrecht. pp. 397–442.
- Bidigare, R.R., Schofield, O. & Prezelin, B.B. (1989). Influence of zeaxanthin on quantum yield of photosynthesis of Synechococcus clone WH7803. Marine Ecology Progress Series, 56: 177–188.
- Binder, B. (2000). Cell cycle regulation and the timing of chromosome replication in a marine Synechococcus (cyanobacteria) during light- and nitrogen-limited growth. Journal of Phycology, 36:120-126.
- Binder, B.J. & Chisholm, S.W. (1990). Relationship between DNA cycle and growth rate in Synechococcus sp. strain PCC 6301. Journal of Bacteriology, 172: 2313–2319.
- Binder, B.J. & Chisholm, S.W. (1995). Cell cycle regulation in marine Synechococcus sp. strains. Applied and Environmental Microbiology, 61: 708–717.
- Binder, B.J., Chisholm, S.W., Olson, R.J., Frankel, S.L. & Worden, A.Z. (1996). Dynamics of picophytoplankton, ultraphytoplankton and bacteria in the central equatorial Pacific. Deep Sea Research Part II: Topical Studies in Oceanography, 43: 907–931.
- Brunelle, S.A., Hazard, E.S., Sotka, E.E. & Dolah, F.M.V. (2007). Characterization of a dinoflagellate cryptochrome blue‐light receptor with a possible role in circadian control of the cell cycle. Journal of Phycology, 43: 509–518.
- Burbage, C.D. & Binder, B.J. (2007). Relationship between cell cycle and light‐limited growth rate in oceanic Prochlorococcus (MIT9312) and Synechococcus (WH8103) (cyanobacteria) Journal of Phycology, 43: 266–274.
- Campbell, L. & Carpenter, E.J. (1986). Estimating the grazing pressure of heterotrophic nanoplankton on Synechococcus spp. using the sea water dilution and selective inhibitor techniques. Marine Ecology Progress Series, 33: 121–129.
- Campbell, L., Nolla, H. & Vaulot, D. (1994). The importance of Prochlorococcus to community structure in the central North Pacific Ocean. Limnology and Oceanography, 39: 954–961.
- Chisholm, S.W. (1992). Phytoplankton size. In Primary Productivity and Biogeochemical Cycles in the Sea. Springer, Dordrecht. pp. 213–237.
- Choi, C.Y., Kim, N.N., Shin, H.S., Park, H.G., Cheon, S.-G. & Kil, G.-S. (2013). The effect of various wavelengths of light from light-emitting diodes on the antioxidant system of marine cyanobacteria, Synechococcus sp. Molecular and Cellular Toxicology, 9: 295–302.
- Choi, D.H. & Noh, J.H. (2009). Phylogenetic diversity of Synechococcus strains isolated from the East China Sea and the East Sea. FEMS Microbiology Ecology, 69: 439–448.
- Collier, J.L., Brahamsha, B. & Palenik, B. (1999). The marine cyanobacterium Synechococcus sp. WH7805 requires urease (urea amiohydrolase, EC 3.5. 1.5) to utilize urea as a nitrogen source: molecular-genetic and biochemical analysis of the enzyme. Microbiology, 145: 447–459.
- Collier, J.L., Lovindeer, R., Xi, Y., Radway, J.C. & Armstrong, R.A. (2012). Differences in growth and physiology of marine Synechococcus (cyanobacteria) on nitrate versus ammonium are not determined solely by nitrogen source redox state. Journal of Phycology, 48: 106–116.
- Cooper, S. & Helmstetter, C.E. (1968). Chromosome replication and the division cycle of Escherichia coli. British Journal of Molecular Biology, 31: 519–540.
- Everroad, R.C. & Wood, A.M. (2006). Comparative molecular evolution of newly discovered picocyanobacterial strains reveals a phylogenetically informative variable region of β‐phycoerythrin. Journal of Phycology, 42: 1300–1311.
- Falkowski, P.G., Katz, M.E., Knoll, A.H., Quigg, A., Raven, J.A., Schofield, O. & Taylor, F.J.R. (2004). The evolution of modern eukaryotic phytoplankton. Science, 305: 354–360.
- Flombaum, P., Gallegos, J.L., Gordillo, R.A., Rincón, J., Zabala, L.L., Jiao, N., Karl, D.M., Li, W.K., Lomas, M.W. & Veneziano, D. (2013). Present and future global distributions of the marine Cyanobacteria Prochlorococcus and Synechococcus. Proceedings of the National Academy of Sciences USA 110: 9824–9829.
- Fogg, G. (1986). Review lecture: picoplankton. Proceedings of the Royal Society of London B: Biological Sciences, 228: 1–30.
- Fuller, N.J., Marie, D., Partensky, F., Vaulot, D., Post, A.F. & Scanlan, D.J. (2003). Clade-specific 16S ribosomal DNA oligonucleotides reveal the predominance of a single marine Synechococcus clade throughout a stratified water column in the Red Sea. Applied and Environmental Microbiology, 69: 2430–2443.
- Garrison, T. (2011). Essentials of Oceanography. Cengage Learning.
- Glover, H.E., Keller, M.D. & Spinrad, R.W. (1987). The effects of light quality and intensity on photosynthesis and growth of marine eukaryotic and prokaryotic phytoplankton clones. Journal of Experimental Marine Biology and Ecology, 105: 137–159.
- Glover, H.E., Prézelin, B.B., Campbell, L., Campbell, M. & Garside, C. (1988). A nitrate-dependent Synechococcus bloom in surface Sargasso Sea water. Nature, 331: 161–163.
- Harrison, W., Harris, L. & Irwin, B. (1996). The kinetics of nitrogen utilization in the oceanic mixed layer: nitrate and ammonium interactions at nanomolar concentrations. Limnology and Oceanography, 41: 16–32.
- Hayashi, F., Ishida, M. & Kikuchi, T. (1969). Macromolecular synthesis in a blue-green alga, Anacystis nidulans, in dark and light phases. Annual Reports, Research Reactor Institute, Kyoto University, 2: 56–66.
- Helmstetter, C.E. (1996). Timing of synthetic activities in the cell cycle. In Escherichia coli and Salmonella: Cellular and Molecular Biology, 2nd ed. ASM Press, Washington, DC. pp. 1627–1639.
- Herdman, M., Castenholz, R., Waterbury, J. & Rippka, R. (2001). Form-genus XIII. In Synechococcus. Bergey’s Manual of Systematic Bacteriology Volume 1. Springer, Dordrecht.
- Hooker, S.B., Van Heukelem, L., Thomas, C.S., Claustre, H., Ras, J., Barlow, R., Sessions, H., Schlüter, L., Perl, J. & Trees, C. (2005). Second SeaWiFS HPLC Analysis Round-robin Experiment (SeaHARRE-2). National Aeronautics and Space Administration, Goddard Space Flight Center, Greenbelt, MD.
- Hung, S.-H., Chung, C.-C., Liao, C.-W., Gong, G.-C. & Chang, J. (2013). Sequence diversity and expression levels of Synechococcus phosphate transporter gene in the East China Sea. Journal of Experimental Marine Biology and Ecology, 440: 90–99.
- Kana, T.M. & Glibert, P.M. (1987). Effect of irradiances up to 2000 μE m−2 s−1 on marine Synechococcus WH7803—I. Growth, pigmentation, and cell composition. Deep Sea Research Part A. Oceanographic Research Papers, 34: 479–495.
- Kana, T.M., Gilbert, P.M., Goericke, R. & Welschmeyer, N.A. (1988). Zeaxanthin and ß‐carotene in Synechococcus WH7803 respond differently to irradiance. Limnology and Oceanography, 33: 1623–1626.
- Lepp, P.W. & Schmidt, T.M. (1998). Nucleic acid content of Synechococcus spp. during growth in continuous light and light/dark cycles. Archives of Microbiology, 170: 201–207.
- Lindell, D., Padan, E. & Post, A.F. (1998). Regulation of ntcA expression and nitrite uptake in the marine Synechococcus sp. strain WH 7803. Journal of Bacteriology, 180: 1878–1886.
- Liu, H., Campbell, L., Landry, M., Nolla, H., Brown, S. & Constantinou, J. (1998). Prochlorococcus and Synechococcus growth rates and contributions to production in the Arabian Sea during the 1995 Southwest and Northeast Monsoons. Deep Sea Research Part II: Topical Studies in Oceanography, 45: 2327–2352.
- Liu, H., Bidigare, R.R., Laws, E., Landry, M.R. & Campbell, L. (1999). Cell cycle and physiological characteristics of Synechococcus (WH7803) in chemostat culture. Marine Ecology Progress Series, 189: 17–25.
- Liu, Y., Tsinoremas, N.F., Johnson, C.H., Lebedeva, N.V., Golden, S.S., Ishiura, M. & Kondo, T. (1995). Circadian orchestration of gene expression in cyanobacteria. Genes and Development, 9: 1469–1478.
- Marie, D., Partensky, F., Jacquet, S. & Vaulot, D. (1997). Enumeration and cell cycle analysis of natural populations of marine picoplankton by flow cytometry using the nucleic acid stain SYBR Green I. Applied and Environmental Microbiology, 63: 186–193.
- Mitbavkar, S. & Anil, A.C. (2011). Tiniest primary producers in the marine environment: an appraisal from the context of waters around India. Current Science, 100: 986–988.
- Mitbavkar, S., Rajaneesh, K., Anil, A. & Sundar, D. (2012). Picophytoplankton community in a tropical estuary: detection of Prochlorococcus-like populations. Estuarine, Coastal and Shelf Science, 107: 159–164.
- Moore, L. R., Post, A. F., Rocap, G. & Chisholm, S. W. (2002). Utilization of different nitrogen sources by the marine cyanobacteria Prochlorococcus and Synechococcus. Limnology and Oceanography, 47: 989–996.
- Morel, A., Lazzara, L. & Gostan, J. (1987). Growth rate and quantum yield time response for a diatom to changing irradiances (energy and color). Limnology and Oceanography, 32: 1066–1084.
- Morel, A., Ahn, Y.-H., Partensky, F., Vaulot, D. & Claustre, H. (1993). Prochlorococcus and Synechococcus: a comparative study of their optical properties in relation to their size and pigmentation. Journal of Marine Research, 51: 617–649.
- Nordström, K. & Austin, S.J. (1993). Cell‐cycle‐specific initiation of replication. Molecular Microbiology, 10: 457–463.
- Olson, R.J. & Chisholm, S.W. (1986). Effects of light and nitrogen limitation on the cell cycle of the dinoflagellate Amphidinium carteri. Journal of Plankton Research, 8: 785–793.
- Olson, R.J., Vaulot, D. & Chisholm, S.W. (1986). Effects of environmental stresses on the cell cycle of two marine phytoplankton species. Plant Physiology, 80: 918–925.
- Ong, L. & Glazer, A. (1991). Phycoerythrins of marine unicellular cyanobacteria. I. Bilin types and locations and energy transfer pathways in Synechococcus spp. phycoerythrins. Journal of Biological Chemistry, 266: 9515–9527.
- Parpais, J., Marie, D., Partensky, F., Morin, P. & Vaulot, D. (1996). Effect of phosphorus starvation on the cell cycle of the photosynthetic prokaryote Prochlorococcus spp. Marine Ecology Progress Series, 132: 265–274.
- Partensky, F., Blanchot, J. & Vaulot, D. (1999). Differential distribution and ecology of Prochlorococcus and Synechococcus in oceanic waters: a review. Bulletin-Institut Oceanographique Monaco-Numero Special, 457–476.
- Rajaneesh, K. & Mitbavkar, S. (2013). Factors controlling the temporal and spatial variations in Synechococcus abundance in a monsoonal estuary. Marine Environmental Research, 92: 133–143.
- Raven, J. (1998). The twelfth Tansley Lecture. Small is beautiful: the picophytoplankton. Functional Ecology, 12: 503–513.
- Richardson, T.L. & Jackson, G.A. (2007). Small phytoplankton and carbon export from the surface ocean. Science, 315: 838–840.
- Robertson, B.R., Tezuka, N. & Watanabe, M.M. (2001). Phylogenetic analyses of Synechococcus strains (cyanobacteria) using sequences of 16S rDNA and part of the phycocyanin operon reveal multiple evolutionary lines and reflect phycobilin content. International Journal of Systematic and Evolutionary Microbiology, 51: 861–871.
- Roy, R. (2010). Short-term variability in halocarbons in relation to phytoplankton pigments in coastal waters of the central eastern Arabian Sea. Estuarine, Coastal and Shelf Science, 88: 311–321.
- Scanlan, D.J. (2003). Physiological diversity and niche adaptation in marine Synechococcus. Advances in Microbial Physiology, 47: 1–64.
- Schreiber, U. (1986). Detection of rapid induction kinetics with a new type of high-frequency modulated chlorophyll fluorometer. Photosynthesis Research, 9: 261–272.
- Six, C., Thomas, J., Brahamsha, B., Lemoine, Y. & Partensky, F. (2004). Photophysiology of the marine cyanobacterium Synechococcus sp. WH8102, a new model organism. Aquatic Microbial Ecology, 35: 17–29.
- Six, C., Thomas, J.-C., Garczarek, L., Ostrowski, M., Dufresne, A., Blot, N., Scanlan, D.J. & Partensky, F. (2007). Diversity and evolution of phycobilisomes in marine Synechococcus spp.: a comparative genomics study. Genome Biology, 8: R259.
- Slater, M.L., Sharrow, S.O. & Gart, J.J. (1977). Cell cycle of Saccharomyces cerevisiae in populations growing at different rates. Proceedings of the National Academy of Sciences USA, 74: 3850–3854.
- Stomp, M., Huisman, J., Vörös, L., Pick, F.R., Laamanen, M., Haverkamp, T. & Stal, L.J. (2007). Colourful coexistence of red and green picocyanobacteria in lakes and seas. Ecology Letters, 10: 290–298.
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30: 2725–2729.
- Ting, C.S., Rocap, G., King, J. & Chisholm, S.W. (2002). Cyanobacterial photosynthesis in the oceans: the origins and significance of divergent light-harvesting strategies. Trends in Microbiology, 10: 134–142.
- Vaulot, D. (1995). The cell cycle of phytoplankton: coupling cell growth to population growth. In Molecular Ecology of Aquatic Microbes. Springer, Dordrecht. pp. 303–322.
- Vaulot, D., Olson, R., Merkel, S. & Chisholm, S. (1987). Cell-cycle response to nutrient starvation in two phytoplankton species, Thalassiosira weissflogii and Hymenomonas carterae. Marine Biology, 95: 625–630.
- Vaulot, D., Lebot, N., Marie, D. & Fukai, E. (1996). Effect of phosphorus on the Synechococcus cell cycle in surface Mediterranean waters during summer. Applied and Environmental Microbiology, 62: 2527–2533.
- Waterbury, J. (1989). Subsection I. Order Chroococcales Wettstein 1924, emend. Rippka et al., 1979. In Bergey’s Manual of Systematic Bacteriology, Volume 3. Springer, Dordrecht. pp. 1728–1746.
- White, R. (1991). A theory for analysis of cell populations with non-cycling S phase cells. Journal of Theoretical Biology, 150: 201–214.
- Wood, A. M., Phinney, D. A. & Yentsch, C. S. (1998). Water column transparency and the distribution of spectrally distinct forms of phycoerythrin-containing organisms. Marine Ecology Progress Series, 162: 25–31.
- Wood, A.M., Lipsen, M. & Coble, P. (1999). Fluorescence-based characterization of phycoerythrin-containing cyanobacterial communities in the Arabian Sea during the Northeast and early Southwest Monsoon (1994–1995). Deep Sea Research Part II: Topical Studies in Oceanography, 46: 1769–1790.
- Yamaguchi, M., Ohkusu, M., Biswas, S.K. & Kawamoto, S. (2007). Cytological study of cell cycle of the pathogenic yeast Cryptococcus neoformans. Japanese Journal of Medical Mycology, 48: 147–152.
- Zinser, E.R., Coe, A., Johnson, Z.I., Martiny, A.C., Fuller, N.J., Scanlan, D.J. & Chisholm, S.W. (2006). Prochlorococcus ecotype abundances in the North Atlantic Ocean as revealed by an improved quantitative PCR method. Applied and Environmental Microbiology, 72: 723–732.
- Zubkov, M.V., Sleigh, M.A., Tarran, G.A., Burkill, P.H. & Leakey, R.J. (1998). Picoplanktonic community structure on an Atlantic transect from 50 N to 50 S. Deep Sea Research Part I: Oceanographic Research Papers, 45: 1339–1355.