Abstract
Macroalgae have played an important role in coastal communities for centuries. In the past, they have been harvested and gathered from shorelines around the world for traditional uses such as food, animal feed and a crude fertilizer (marine manure). Today, seaweeds are used in a multitude of applications with expanding global industries based on hydrocolloids, cosmetics and food supplements, and also as a potential biofuel source. However, of the approximately 10 000 algal species reported to exist, only a small number are commercially utilized. While representing only a small fraction of total global seaweed production, harvesting and gathering ‘wild’ seaweeds has had, and continues to have, an integral role in many coastal societies, often being intrinsically linked to the cultural identity of those coastal communities. Today, 32 countries actively harvest seaweeds from wild stocks, with over 800 000 t harvested annually from natural beds. It is vitally important that seaweeds are utilized sustainably and that natural resources are effectively managed by coastal communities with vested interests around the world. As the popularity of seaweeds increases and the use of less traditional species with novel applications comes to the fore, it is critically important to make certain that the sustainability of the resource is ensured given the increased pressures of harvesting. Issues exist regarding ownership of the resource and its over-exploitation, and the implementation of environmentally damaging harvesting techniques must be avoided. Resource scientists, managers, conservationists, governments, and other stakeholders need to be proactive in the sustainable management of these vulnerable, yet valuable, resources.
Introduction
The increasing popularity of seaweed-based products, coupled with seemingly endless industrial possibilities, may lead to and in some cases has led to, the exploitation putting a strain on natural resources around the world (Avila & Seguel, Citation1993; Feeney, Citation2001; Khan & Satam, Citation2003). Seaweeds are multicellular, macroscopic, marine algae, and their harvesting has played a crucial role in the development of coastal communities for centuries (Rebours et al., Citation2014), providing sources of food, fuel, feed, and fertilizer to those who harvest or gather the plants. As examples, the harvesting of the brown fucoid, Ascophyllum nodosum, which dominates the rocky intertidal of the North Atlantic, has taken place for hundreds of years (Hallsson, Citation1961; Sharp, Citation1987; Hession et al., Citation1998), while Laminaria digitata, Chondrus crispus and Palmaria palmata are species which share equally rich histories of utilization by humans (Kain & Dawes, Citation1987; Vea & Ask, Citation2011; Mouritsen et al., Citation2013; Collen et al., Citation2014).
However, over-harvesting of natural resources to meet commercial demand has led to the deterioration of seaweed beds in some regions (Buschmann et al., Citation2014) and has given rise to genuine concerns regarding over-exploitation of these natural resources (Ugarte & Sharp, Citation2001), thereby highlighting the need for management strategies and stakeholder accountability to be adopted and monitored.
The increasing uses of seaweeds in agriculture, animal feeds and as human food, are expected to maintain the growth of the seaweed industry in the long term. The commercial availability of seaweeds falls into two categories: resource-based, wild-collected enterprises and, similar to commercial agricultural production activities, cultivated seaweeds (Bixler & Porse, Citation2011; Hafting et al., Citation2012). Seaweeds have experienced a renaissance in popularity, prompted in part by the media’s take on their applications as ‘superfood’, with newspapers asking: ‘Is seaweed the new kale?’, or ‘the next superfood?’ (Goodyear, Citation2015; Sbhimani, Citation2016). This review aims to explore current human utilization of selected examples of wild resources and different harvesting strategies adopted, and will examine the sustainable management practices and initiatives driving further expansion of the wild seaweed harvest.
The commodity-based seaweed industry
Escalating global demand for seaweeds and their products fuels the expansion of industrialized processing of these resources, with 42 countries reporting commercial seaweed activity prior to 2005 (Khan & Satam, Citation2003). White & Wilson (Citation2015) estimated the value of the seaweed industry to be US$10.1–16.1 billion, with some projecting that the market will reach US$17.59 billion by 2021 (www.marketsandmarkets.com, Citation2016). Total annual global seaweed production in 2014 was 28.5 million tonnes (FAO, Citation2014) with cultivation accounting for 96% of this figure (1.2 million t harvested wild versus 27.3 million t from aquaculture). The increasing demand for seaweeds as food products can only be adequately met by cultivation, and the high production and cultivation costs are offset by the higher market prices achieved for algal foodstuffs than for other algal products (Hafting et al., Citation2012, Little et al., Citation2016).
Wild harvest of seaweeds
Globally, total macroalgal production has increased by approximately 5.7% per annum (Critchley et al., Citation1993; FAO, Citation2014; Rebours et al., Citation2014). Global harvesting production from natural beds or wild stocks remains relatively stable, fluctuating between 1–1.3 million t per year since 2000 (–; FAO, Citation2014). Global fisheries capture statistics for 2014 reported that there were 20 countries worldwide which were involved in the harvesting of brown seaweeds, totalling 624 136 t, with landings of Chilean and Norwegian kelp alone accounting for 60% of global brown seaweed harvest (FAO, Citation2014). In the mid-1980s, Chile also annually supplied one-third to one-half of the world’s demand for Gracilaria spp. (Santelices & Ugarte, Citation1987). Alginates extracted from brown algae have been used in a wide range of applications such as thickening and gelling agents in the food and feed processing industry, in the pharmaceutical industry as stabilizers of colouring agents, for waterproofing in the textile industry, paper coating and in wastewater treatment (Lee & Mooney, Citation2012; Mesnildrey et al., Citation2012; Gao et al., Citation2017). Today, Chile supplies approximately 10% of the raw materials for alginates, primarily through the annual harvesting and collection of 314 661 dry t of kelp from natural stocks (Buschmann et al., Citation2014).
Table 1. Global seaweed production from wild stocks 1950–2014 (FAO, Citation2014).
Table 2. Global red seaweed production from wild stocks 1950–2014 (FAO, Citation2014).
Table 3. Global green seaweed production from wild stocks 1950–2014 (FAO, Citation2014).
Table 4. Global brown seaweed production from wild stocks 1950–2014 (FAO, Citation2014).
Comprising only a small portion of the global seaweed market today, the harvesting of seaweed from wild stocks still plays an important role in many cultures. For example, European macroalgal production accounted for 1% of the worldwide biomass supply, with a total of 275 390 t produced in 2014 (FAO, Citation2014). Harvesting of wild stocks supplied approximately 99% of the biomass during this period highlighting the importance of wild seaweed resources particularly to the European seaweed industry. Meanwhile the rest of the world heavily relies on biomass derived from aquaculture (see Buschmann et al., Citationin press). The largest producers of seaweed in Europe are France, Norway, Ireland, Iceland and the Russian Federation, accounting for 98% of total biomass supplied in 2014. Smaller scale production occurred in Spain, Italy, Denmark and Portugal (see www.algaplus.com). In Europe, commercial harvesting of kelps is currently carried out in Norway and France (Frangoudes & Garineaud, Citation2015; Steen et al., Citation2016), with some smaller scale harvesting taking place on the southern coast of Ireland (1400 t in 2014). This figure will probably rise in the coming years as an experimental licence to mechanically harvest some 1800 acres (c. 730 hectares) of kelp (Laminaria hyperborea) from Bantry Bay, Cork, has been granted to the Irish biotechnology company BioAtlantis Ltd (O’Sullivan, Citation2017). The granting of this licence by the Marine Licence Vetting Committee was considered ‘not likely to have a significant negative impact on the marine environment, would not adversely impact in marine Natura 2000 sites’. The granting of a licence to mechanically harvest seaweed in Ireland, however, has divided opinion amongst local people and environmental groups (Robinson, Citation2017). Norway leads the harvest and production from wild stocks in Europe with 154 230 t of brown seaweeds harvested (primarily Laminaria hyperborea but also Ascophyllum nodosum), which corresponded to 56% of the European macroalgal biomass production in 2014 (Stévant et al., Citation2017). Annual landings from almost exclusively wild harvested stocks of North European kelps in France in 2013 and 2014 were 17 891 and 33 919 t, respectively (FAO, Citation2014).
Red seaweeds, meanwhile, are wild-harvested from 32 countries, indicating their widespread popularity, with a total of 216 456 t harvested worldwide (FAO, Citation2014). Chile and Indonesia are the world’s largest harvesters of red seaweeds, producing 76% of total global capture (i.e. >165 000 t in 2014). In Europe, the harvesting of red seaweeds from wild stocks has continued to decline since its peak of over 500 000 t harvested between 1960–1969, down to <80 000 t harvested between 2000 and 2009. Spain is now the largest producer of red seaweeds from wild stocks (i.e. 1643 t harvested annually) (FAO, Citation2014). Globally, the wild harvest of green seaweeds was by far the lowest, with 11 countries producing 1660 t annually, the majority of which was green laver (Ulva spp.) harvested in Korea (FAO, Citation2014).
‘Wild’ harvesting of seaweed resources generally occurs by the selective cutting from monospecific stands of seaweed (e.g. rockweeds and kelps) or alternatively, the gathering of storm-cast fronds (which would result in multiple mixed species, along with contaminating flotsam and jetsam). Whether using nets, horses, bulldozers or tractors, the gathering of storm-cast material from beaches, although highly variable and unreliable, can constitute the use of an important, passive local commodity on shores all around the world. In Ireland, for example, 10 000 wt (wet tonnes) of opportunistic macroalgal bloom biomass (Ulva spp.) is annually cleared from a single beach in west Cork and disposed of (Tabassum et al., Citation2017; Wan et al., Citation2017), while in Namibia 15 000 wt of Gracilaria were regularly washed ashore at Lüderitz (Critchley et al., Citation1993); South Africa has built its seaweed industry on the beach collection of both Gracilaria and kelps (Amosu et al., Citation2013). Beach wash-ups of Gracilaria in southern Africa, as a whole, have declined considerably and the industry was significantly reduced as of 2017 (Rothman et al., Citation2009; H. Rotmann, personal communication). Similar declines in Gracilaria wash-ups were also noted at traditional Argentinian collection sites, but the causes of this decline remain unknown (G. Soriano, personal communication).
Historical utilization of wild seaweeds
While the utilization of seaweeds has probably been carried out by coastal dwellers since time immemorial, only a few categorical accounts exist. Gathering of seaweeds as food, for example, has occurred in Iceland for at least 1000 years, with reference made in the Icelandic sagas of the gathering of ‘Sol’ (Palmaria palmata – as Rhodymenia palmata in the text) for food (Hallsson, Citation1961). Pliny meanwhile noted in AD 79 the gathering of ‘Margo’ (thought to be maërl) by ‘peoples of Britain and Gaul’ in order to fertilize their soils (Augris & Berthou, Citation1990; Grall & Hall-Spencer, Citation2003), while in the 600s, Scottish written records made reference to the collection of dulse (Palmaria palmata) by the monks of the small Hebridean island of Iona (www.ambaile.org.uk). Similarly, the Welsh delicacy laver (bara lawr) (Porphyra/Pyropia) has been consumed since at least 1600. In Portugal, the gathering of seaweed species washed up along the shore, collectively called ‘sargago’, has occurred since at least 1308 and was regulated under King D. Dinis (Veiga de Oliveira et al., Citation1975; Santos & Duarte, Citation1991). In Asia, the gathering and trade of seaweeds has taken place for centuries. In the 18th century, shipping documents recorded how Japanese merchants traded raw sugar in return for ‘Konbu’ (Laminaria japonica) along the so-called ‘konbu road’ for trade with Chinese merchants (Sho, Citation2001). In China, the use of ‘Tsu-Tsai’ (Porphyra) as a food and pharmaceutical was first recorded by Si Zuo in the book Odes of Wu Capital, written some 1700 years ago (Yang et al., Citation2017). Dillehay et al. (Citation2008), meanwhile, reported the remains of nine species of seaweed recovered from hearths in a human settlement at Monte Verde II, Chile, dated to approximately 14 000 years ago, assumed to be used for food and medicinal purposes.
Present-day utilization of wild seaweeds
The utilization of wild-harvested macroalgal biomass largely depends on the species, some being used by the food-processing industry and sold as ‘sea-vegetables’ preserved dry, fresh, frozen, canned or salted (Mesnildrey et al., Citation2012; Schreiber, Citation2014). Consumption of wild-gathered seaweeds forms part of the traditional, staple diet of some cultures, particularly throughout Asia. Currently, China and the Republic of Korea are the largest consumers of edible seaweeds. Although more than 10 000 species of macroalgae are reported to exist (Guiry & Morrison, Citation2013; Guiry & Guiry, Citation2017) as few as 200 species are consumed worldwide, mainly as sea vegetables (Pereira & Neto, Citation2015). Currently, following the introduction of regulation EC 258/97, 21 macroalgal species are considered edible in Europe (Mesnildrey et al., Citation2012; CEVA, Citation2014).
Seaweed extracts and powders made from these natural resources are used widely in organic farming as feed supplements, biofertilizers and biostimulants for soils in agriculture and horticulture (Wang et al., Citation2016). The main algal species used in Europe as fertilizer are the brown seaweeds Ascophyllum nodosum, Fucus spp., Laminaria (including Saccharina) spp. and maërl (free-living calcareous red algae) (Mesnildrey et al., Citation2012). Products derived from these seaweeds are considered to promote improved rates of seedling success, increased crop yields and resistance towards diseases and insect pests (Raghavendra et al., Citation2007; Sathya et al., Citation2010; Vijayanand et al., Citation2014).
Wild harvested seaweed species are also popularly used in biostimulant formulations (Khan et al., Citation2009), feed formulations (Evans & Critchley, Citation2014; Makkar et al., Citation2015), for hydrocolloid production (Porse & Rudolph, Citation2017), or food supplements (Forster & Radulovich, Citation2015), cosmetics (Balboa et al., Citation2015; Sarkar et al., Citation2016), bioremediation (Volesky, Citation2001; Cechinel et al., Citation2016) and as a potential biofuel source (Smith & Ross, Citation2016; Tabassum et al., Citation2017).
The harvesting of the wild seaweed resource
Wild-harvesting techniques
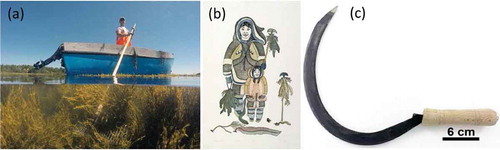
Judged on their catch per unit effort and chosen as a consequence of the target seaweed species, a range of techniques and cutting implements are at the disposal of commercial harvesters. A greater income can be made by harvesting the seaweed using boats, rakes or by diving, than by hand harvesting from the shore at low tide (Rebours et al., Citation2014). The techniques, intensity of exploitation and homogeneity of the harvest all influence the regenerative and recovery capacity of the cut seaweed beds and their associated communities (Kelly et al., Citation2001). Whilst the first commercial harvesting of seaweeds in the USA appears to have been initiated by the Irish fishers, Daniel Ward and Miles O’Brien of Scituate, Massachusetts, between 1848 and 1850, this was short-lived (see www.stmaryscituate.org/aboutus_history.html). Coinciding with the increased demand for seaweed biomass in the middle of the 20th century, came the evolution of harvesting methods and tools. The next wave of commercial harvesting of wild seaweeds in the Western world began in the early 1940s on the shores of the North Atlantic, particularly along Nova Scotian and Irish coasts, and the first traincar load of Chondrus crispus from Canada to the USA came out of Nova Scotia (10 000 lb = 4500 kg) in 1940 (Humm, Citation1951). A drag-rake was the preferred harvesting tool of the day for the delicate carrageen moss (C. crispus) but ultimately its use caused immense damage to the standing stock. The use of appropriate and well-maintained tools markedly influences the health and sustainability of a resource. A hand-held seaweed cutting implement is generally small and lightweight, such as the sickle used to cut Palmaria palmata (dileasc) in Scotland, the small kombu cutting Nejiri tool (Japan), or the Corrán (Irish hook) used to cut an Feamainn bhuí (A. nodosum) in Ireland ().
Fig. 1 (a) A rockweed harvester in the Canadian Maritimes; (b) Image of indigenous Canadian family gathering kelp; (c) Corrán; Irish rockweed cutting implement.
Prior to the harvesting of natural seaweed beds, or purchasing of the rights to harvest seaweed beds from landowners, harvesters are required to estimate the amount of time and materials necessary to successfully extract the resources (Salo et al., Citation2014). There are certain additional costs, such as transportation and extra labour, which must also be taken into account. Productivity on any given day can be influenced by a number of factors. When gathering by foot, access is important, whereas when harvesting by boat, productivity may be affected by tide times, wave height and current, weather, access to wharves, transportation to processing, even the sharpness of the cutter blade and bottom conditions including substratum and shore geomorphology.
Mechanical harvesting of seaweed beds has been successfully carried out in a number of northern Atlantic countries for decades. Using a range of custom-built devices and boats, mechanical harvesting has been the method of choice in Iceland, Norway, Brittany and Maine, USA (Hallsson, Citation1992; Ugarte & Sharp, Citation2001; Vea & Ask, Citation2011; Mesnildrey et al., Citation2012). Recent collapses of some important fisheries in Atlantic Canada have created strong public concern regarding management policies for marine resources in general. Accordingly, a precautionary approach has been urged for these resources and as a consequence of its important role as habitat for invertebrates and vertebrates, a new approach to the management of rockweed was applied (Ugarte & Sharp, Citation2001).
In 1995, under a four-year pilot plan, the A. nodosum harvest expanded from Nova Scotia to the previously unexploited areas of southern New Brunswick. A new joint federal/provincial management strategy for rockweed was implemented after reviewing existing biological information covering 30 years of harvesting history and experience in Nova Scotia. The maximum exploitation rate, cutting height, gear restrictions, and protected areas were management measures employed within a precautionary pilot-harvest plan. A research and monitoring programme involving the industry, universities and the provincial and federal governments was simultaneously initiated to evaluate the effect of the harvest on the resource and associated species, and to provide information on improving the management of rockweed. A scientific peer committee carried out a review of this information in April 1998 and 1999. The consensus was that the impact of harvest on the habitat architecture was minimal and of short duration, and therefore it was advised that the harvest could continue, but to clearly maintain the precautionary approach to management.
The overall objective of efficient mechanical harvesting is to improve the catch per unit effort over hand-harvesting methods. The ‘seaweed trawler’, the first purpose-built boat for seaweed harvesting, was launched in Norway in 1969. Today, 11 seaweed trawlers annually harvest 130 000–180 000 t of brown seaweed every year on Norway’s south-western shore, with a peak harvest of 192 426 t in 2000 (FAO, Citation2014). This high exploitation rate was made possible by the use of trawlers capable of operating in shallow water (>2 m) and with an increased hull capacity, allowing for harvests of 50–150 t per day (Vea & Ask, Citation2011). Based on studies by Per Svendsen of the Biological Station at the University of Bergen, Norway in 1972, an initial 4-year harvest rotation was implemented. Following further investigations, this period was increased to a 5-year rotation in 1992. The sustainability of the Norwegian resource is ensured by The Continental Shelf Act (1994) and an appointed management committee, comprised of seaweed industry representatives, harvesters, fishermen’s associations and marine research institutes. This Committee previously concluded that: ‘so far, it is not shown that seaweed harvesting represents unacceptable or irreversible injury on other organisms or ecosystems’ (Vea & Ask, Citation2011).
Mechanical harvesting of wild seaweeds seems to have reached its peak during the 1980s and 1990s worldwide. Again, using Nova Scotia as an example, since it is well documented, mechanical harvesting of rockweed peaked between 1986 and 1992. During this time, the use of highly efficient Norwegian suction harvesters was in place, capable of exploitation rates of 40–60% (Sharp et al., Citation2006; Vea & Ask, Citation2011). These suction harvesters increased the catch per unit effort and rockweed landings rose from 9448 t in 1985 to a peak of 30 000 t in 1989 (Sharp et al., 2006). In France, the scoubidou trawl has been used since 1974 (Mesnildrey et al., Citation2012); this method of harvesting Saccharina latissima uses a crochet-hook-like implement, which rotates around the fronds and uproots them to be pulled on board. The scoubidou has played a key role in the fresh seaweed industry, annually harvesting 60 000–80 000 t (FAO, Citation2014). The use of suction-based mechanical harvesters in the Canadian Maritimes ceased in 1994 as a result of uncontrolled over-harvesting (Sharp et al., Citation2006). Conversely, the introduction of mechanical harvesters in Norway resulted in the decline of rake harvesting of Laminaria hyperborea and L. digitata (Vea & Ask, Citation2011). Kelly et al. (Citation2001) reported that mechanical harvesters were indeed not suitable for operation in all areas. As an alternative, boat and rake harvesting was implemented in the early 1970s in the Canadian Maritimes and now accounts for 100% of seaweed landed in Eastern Canada (Ugarte & Sharp, Citation2011, Citation2012). The rake, with its long handle and specially designed serrated cutting head with steel guards, is deployed by the harvester from the side of a suitable boat and slowly drawn through and thereby cutting the floating seaweed canopy, which is then landed (example shown in ). This method of harvesting removes large clumps at the upper, distal-end of the canopy where the majority of the biomass is to be found, whilst leaving behind some meristematic tissue to allow for regrowth of the canopy which generally happens within a year or two (Sharp et al., Citation2006). It has a similar effect to pruning of terrestrial crops by encouraging fuller, more robust regrowth. Rockweed rake harvesters can harvest 3–5 t of rockweed per tide (rockweed harvester, personal communication) with some harvesters cutting on ‘two-tides’ when daylength permits in summer.
Over-exploitation – including criticisms of mechanical harvesting
Over-exploitation, i.e. the removal of seaweed biomass beyond its annual or seasonal rate of renewal, of wild seaweed beds could lead to potentially significant, negative ecological responses (Rebours et al., Citation2014). It is important that lessons are learned from past mistakes. Maërl (mostly composed of Lithothamnion corallioides and Phymatolithon calcareum) has a long history of harvest along the Atlantic coast of France. Historically, this assemblage of coralline algae was used as a soil conditioner and replacement for lime in agriculture. However, maërl beds are also valuable, biodiverse marine habitats. The calcareous seaweeds effectively present as large subtidal beds which can be mined mechanically via a sablier, which dredges the seafloor. Unfortunately, this method of harvesting has negatively affected maërl beds to such an extent that the assemblage of calcareous algae are now a Convention for the Protection of the Marine Environment of the North-East Atlantic (OSPAR)-listed ‘seaweed’ as a result of declining natural status and abundance (Barbera et al., Citation2003; Hall-Spencer et al., Citation2010).
It is well known that over-exploitation can result in significant reductions in the biomass of marine biota (Buschmann et al., Citation2001). Over-harvesting can lead to a reduced density of seaweed thalli, skewing the population mix and increasing impurities (i.e. other, unwanted seaweed species) in the harvested seaweed loads. If the biomass continues to be used ‘as is’, reduced purity of the harvest thereby leads to an impaired quality of the finished product (Kelly et al., Citation2001; Werner & Kraan, Citation2004).
The Norwegian suction harvester in the Canadian Maritimes was discontinued in 1994 following widespread over-exploitation in the early 1990s (Ugarte & Sharp, Citation2012), only eight years after its introduction. In the USA and Canada, harvesters reverted to manual, rake harvesting (from boats) for three of the most economically important seaweeds, e.g. Irish moss, rockweed and kelps, in a bid to restore resource balance.
Registration of Natura 2000 sites in Europe (Fock, Citation2011) resulted in the restriction of mechanical harvesting practices in the Basque Country, while in Ireland local NGOs opposed the introduction of mechanical harvesting techniques due to their being considered ‘not compatible with the conservation objectives of Natura 2000 sites’ (Netalgae, Citation2012; Baweja et al., Citation2016), although as stated above, some concessions have been made. Northern Ireland took a similar stance, with the Environment and Heritage Service (EHS) declaring its requirement for long-term studies to ‘demonstrate[d] that it (mechanical harvesting) will not have an adverse impact on the environment’ (EHS, Citation2007).
Impacts of over-harvesting on resources
As is the case with the use of all natural resources, the wild harvest of seaweeds inevitably has ecological implications for the species targeted, and the associated community of flora and fauna, leading to varying degrees of change (Lorentsen, Citation2010; Phillippi et al., Citation2014; Salo et al., Citation2014). As foundational species and important contributors to primary production, the large canopy-forming fucoids and laminarians provide food, habitat, nursery refugia and shelter to a wide range of intertidal species, thereby supporting complex food webs in coastal habitats (Sharp et al., Citation2006). The large structural seaweeds offer protection from predation to some species while allowing refuge from desiccation at low tide and they can also be involved in reducing tidal surge and waves affecting coastline erosion and sedimentation rates, by dampening the incoming energy (Mendez & Losada, Citation2004; Phillippi et al., Citation2014).
Seaweeds exert a strong influence on intertidal and subtidal community structures (Thompson et al., Citation2010). Large-scale canopy removal of marine macroalgae has a direct influence on marine biodiversity, particularly the abundance and biomass of associated organisms from other trophic levels such as mobile megafaunal invertebrates, fish and apex predators (Kelly et al., Citation2001; Migné et al., Citation2014; Phillippi et al., Citation2014). This can negatively affect recruitment (Levitt et al., Citation2002) and reduce contributions to the marine carbon cycle (Thiel et al., Citation2007). These habitats also provide several other important ecological services to coastal areas, such as the transfer of organic materials between ecosystems (Krumhansl & Scheibling, Citation2012), natural, temporary carbon sequestration (Thiel et al., Citation2007, Hill et al., Citation2015; Raven, Citationin press), removal of dissolved nutrients, thereby decreasing eutrophication of coastal waters, and coastal protection from erosion and hazardous waves (Arkema et al., Citation2013).
Analysis of data for a single year from 10 sites around Nova Scotia suggested that the removal of biomass of Ascophyllum nodosum from coastal environments by harvesting was associated with a reduction in the amount of detrital material entering the food web (Halat et al., Citation2015). This detritus is typically released through epidermal shedding, and if not consumed by herbivores or microbes before reaching the upper intertidal zone, it contributes to coastal, terrestrial fertility. However, the actual amount and impact has been debated (Garbary et al., Citation2017; Ugarte et al., Citation2017). Repeated intensive removal of the seaweed canopy can also have a gradual negative effect on population dynamics, altering the availability of resources such as light and space (Vásquez, Citation1995) and potentially changing the overall structure/architecture of the beds (Kelly et al., Citation2001; Thompson et al., Citation2010).
Following long-term, extensive rake harvesting of Irish moss on Prince Edward Island and Nova Scotia, once-extensive Chondrus crispus beds have gradually transformed from domination by Chondrus to Furcellaria lumbricalis (Sharp et al., Citation2006). Using ecological models, Rinde et al. (Citation2006) calculated that trawling in Norwegian kelp forests had a substantial effect on primary and secondary production, calculating that primary production could be reduced by 45% and secondary production by 70–98% within trawled areas (recovery rates unknown).
Himanthalia elongata has been harvested in Europe for centuries for use as fertilizer, food and for hydrocolloid extraction. It is currently harvested in France, Ireland and Spain mainly for human consumption (Stagnol et al., Citation2016). In Brittany, between 2009 and 2013, the annual harvesting of H. elongata increased by 35% (Stagnol et al., Citation2016). In France, the collection of seaweeds for personal consumption is not regulated, nor managed, and the situation is similar in Portugal, where H. elongata populations are suffering from reduced local abundance and even some local extinctions (Lima et al., Citation2007, Araujo, personal observations).
Some natural biotic influences, such as ice scouring and grazing pressure by sea urchins (Echinoidea) or top shells (Trochidae) have been reported to exert severe strains on certain seaweed resources. Extensive grazing of kelp beds by sea urchins created the phenomenon of ‘sea urchin barrens’; stretches carved through kelp forests characterized by areas of low primary productivity, which may extend for thousands of kilometres within a given kelp bed (Filbee-Dexter & Scheibling, Citation2014). For example, Nova Scotian and Norwegian kelp beds have undergone several cycles of over-grazing by sea urchins creating barren grounds that may take decades to recover (Sharp et al., Citation2006; Norderhaug & Christie, Citation2009; Rinde et al., Citation2014).
Constraints to the wild harvesting industry
Unlike the customary cultivation of high-value seaweeds in Asia for use in food, medicine and as raw materials for the pharmaceutical industry (Hurtado et al., Citation2014; Chellaram et al., Citation2015; Liu et al., Citation2016), harvesting of seaweeds in western countries is typically rooted in providing biomass for industrial processes, primarily as animal feed, fertilizers and thickening agents (Kılınç et al., Citation2013). The harvesting of wild seaweed resources, particularly the larger fucoids and laminarians has, to date, been used to supply animal food supplements, soil conditioners and biostimulant formulations (e.g. Hebridean Seaweeds Ltd, Stornoway, Isle of Lewis, The Outer Hebrides, Scotland; see www.hebrideanseaweed.co.uk). Numerous smaller cottage industries based upon wild collected seaweeds are also appearing in the entrepreneurial landscape, and these will play important educational/accessibility roles at local levels, although care must be taken not to over-exploit here as well.
A lack of adequate resource supply could result in a country not reaching its capacity for seaweed production and associated processing, and would likely be a serious hindrance to the further development and investment required for a sophisticated, sustainable seaweed industry (Kelly et al., Citation2001; Hafting et al., Citation2012). As a consequence of the requirements for industrial amounts of raw material, demand in many regions has far outstripped the capacity that traditional harvesting of wild stocks can supply. Indeed, there is already industrial and commercial concern regarding the inability of traditional harvesting methods to adequately meet current, and especially future, global demand for seaweed products. It is a demand that is expected to grow as the broad range of benefits derived from seaweeds become more universally appreciated, and their human health benefits are more widely known and exploited. Grounds for these concerns relate primarily to the efficiency of traditional harvesting methods, and secondarily to an increasing harvester age profile, lack of recruitment of young harvesters and corresponding reduction in the number of seaweed harvesters (i.e. manual labourer) workforce (Kelly et al., Citation2001).
To exploit seaweed species commercially, it is necessary to have available suitable labour and harvesting technologies (Hafting et al., Citation2012). There are three main constraints to the development of commercial wild harvesting operations. Firstly, the presence of an accurately quantified seaweed resource is essential. A major hindrance to the large-scale economic exploitation of seaweeds results from knowledge gaps relating to a lack of basic data on standing stocks. Knowledge gaps exist from the ground up, with a chronic lack of long-term biomass data in most countries for even the most popular species of seaweed which are already exploited. Estimating seaweed standing stocks is difficult and there is often a large margin of error in estimates, in some cases of ±40%. However, acquiring accurate data on standing stocks is an essential foundation for the building of robust harvest management plans (Bruton et al., Citation2009). Secondly, reliable access to the resource is of great importance, and there may be confusion regarding seaweed exploitation and ownership, often exacerbated by ambiguous laws. Thirdly, the not insignificant costs associated with the seaweed biomass, including drying and transportation to the point of processing, need to be accurately assessed as they can be a hurdle to development of an economically viable industry (Tabassum et al., Citation2017). Significant costs such as drying and transportation can be mitigated by a warm climate and good location of the processing factories (i.e. near to the resource; Buschmann et al., Citation2014).
The role of seaweed gathering and community
Considering the importance of seaweed gathering and harvesting solely in monetary terms does not adequately express the importance of the harvest. Harvesting from wild seaweed beds is a key component of the culture and tradition of many countries, playing an important role in the identity of its harvesters and rural coastal communities. The gathering of seaweeds has traditionally been a domestic task, carried out by multiple members of a family, with basic processing occurring near or within the home. Both men and women may carry out the harvesting (, ). The role of women is central to many harvesting societies (Marinho-Soriano, Citation2016; Msuya & Hurtado, Citationin press).
Fig. 2 (a) Loading trailers on the shore; (b) Beach collection of Gracilaria after wash up, Bahia Bustamante, Argentina, (1960); (c) women of Praia collecting seaweed, Portugal.

In Brazil, women comprise a significant portion of the harvester workforce (estimated at 80%), and in Japan, the picking of nori (Pyropia spp.) is customarily carried out by women, while the famous Ama ladies have a long history of free-diving for fish, pearl oysters and seaweed (Nakuda, Citation1965). In Hawai’i wild seaweed gathering is traditionally considered to be the role of women and children (Hart et al., Citation2014), and in coastal communities around Portugal, women typically controlled seasonal seaweed harvests (Cole, Citation1991) (). Similarly, in South Africa, the majority of seaweed harvesters are women (Amosu et al., Citation2013) whose average annual income was cited as US$5000. Women of the British Columbian (Canada) and Alaskan First Peoples often travelled together in their hand-crafted canoes to the seaweed beds, both for companionship and safety (Turner, Citation2003).
The 19th century historian Robin Flower recounted how Irish women kept a supply of duileasc in their pockets and a Dr Browne, visiting Co. Mayo (Ireland) in the 1880s, described how women ‘attended to all of the housework and the needs of their children, helped in the fields and on the bog and gathered and dried carraigin and dileasc’. This was sold in the neighbouring towns – to which the women walked barefoot, as they were expected to save their boots for market days and holidays – for two shillings a stone. The men on the other hand suffered no such hardship (Rhatigan, Citation2009).
Fundamentally, the long-term, sustainable harvesting of wild seaweeds is a societal issue. As such, sustainable management plans can result from self-imposed harvesting restrictions brought down from the community level, e.g. in the kombu harvesting villages of the Hidaka District, Japan, harvesters followed the instructions of the hatamochi, i.e. the person authorized to define harvesting times and periods (Lida, Citation1998). In Ireland, harvesting practices for A. nodosum have remained relatively unchanged for centuries. Harvesting of familial patches of the foreshore or ‘stripes’ has been practiced since the 19th century, with strategically placed rocks, ‘mearing stones’, marking the margins of individual stripes helping to regulate rotational cutting (McErlean, Citation2007; Skeffington et al., Citation2013). In fact, it was the common practice of many historic estates to give shore rights to those tenants holding land on the adjoining shore and thus mearing stones were placed on the foreshore, as there was a requirement to provide an unambiguous demarcation (McErlean, Citation2007). Harvesting of the stripes may have occurred within some families for multiple generations. In Ireland, as in Japan, the principle of equality and reciprocity (Lida, Citation1998) is evident amongst harvesters in the face of a lack of regulation. The self-imposition of sustainable harvesting practices by local harvester communities is commendable and has played an important role in the maintenance of continuous, sustainable harvesting of natural seaweed beds around the world.
Employment
The seaweed industry provides significant income and support to coastal and remote rural communities worldwide (Guiry & Morrison, Citation2013; Hart et al., Citation2014), particularly those classified as historically populated by disadvantaged persons, such as in southern Africa (Amosu et al., Citation2013). Employment figures for those in the wild harvest seaweed industry are notoriously difficult to decipher, with only a small fraction of those who work gathering seaweeds employed in a full-time role.
The first and most direct economic benefit of gathering wild species is connected to subsistence (Salo et al., Citation2014). Harvesting seaweed rarely accounts for the main income of the household, rather it is an additional income for members of coastal communities, and seaweed collection can be a good alternative to fishing in over-exploited fisheries or where terrestrial resources are limited (Rebours et al., Citation2014). The selling of locally derived products helps rural communities earn supplementary income where limited revenue sources may be available (Salo et al., Citation2014). A study of kelp harvests at two experimental sites in British Columbia indicated that the small-scale harvest of Macrocystis pyrifera had minimal impact on the seaweed and the local fish populations (Krumhansl et al., Citation2016). These results suggest that these benign activities could support economic growth and local livelihoods without having a negative impact on biodiversity of the associated ecosystem benefits (services) (Krumhansl et al., Citation2016).
Seasonal and part-time employment is common for those working in the seaweed collecting industry. For example, in Brittany (France), once the seaweed harvesting season ends, half of the harvesting fleet then turn their attention to alternative fishing activities (Alban & Boncoeur, Citation2004). The onset of winter prevents any commercial seaweed harvesting in Norway and it forces reduced efforts in Canada, resulting in a shorter harvesting season (generally May–October), after which harvesters return to lobster fishing and processing (Sharp, Citation1987; Rebours et al., Citation2014). However in France and Spain, the harvesting of seaweed is an occupation which can be practised year-round, and harvesters supplement their income from fishing or farming with small-scale harvesting and as such is compatible with the subsistence of many coastal communities (Alban & Boncoeur, Citation2004). In Portugal, for example, six different harvesting areas were defined and annual licenses issued to enable harvesting for commercial purposes (Santos & Duarte, Citation1991). The maximum number of boats and divers per boat was fixed per harvesting area. The harvesting period was also restricted to a defined period each year. However, the harvesting of seaweeds for non-commercial purposes is not subject to Portuguese government regulations, but collection of macroalgal biomass from beach-cast using tractors is subject to authorization from local authorities.
In Ireland, seaweed harvesters, Buainteoir feamainne, cut A. nodosum (rockweed) in an ad hoc fashion throughout the year, particularly when duties relating to their main source of income (e.g. fishing, farming and building construction) slow down (Irish rockweed harvester, personal communication). Therefore, seaweed harvesters are informally regarded as sole-traders, harvesting as they choose and independently selling the fruits of their labour. The annual Irish landings of rockweed were reported as 28 000 t (FAO, Citation2014), although landings are said to have peaked during the mid-2000s, coinciding with the worldwide economic recession (Guiry & Morrison, Citation2013). In some locations, a history of hardship and a lack of available work options inevitably leads to coastal dwellers counting on the security of the resource that has served their communities for centuries, and especially in times of economic distress. This is clearly expressed by a quote from Donal Hickey, then director of a seaweed factory in Connemara, Ireland, in Mouritsen’s (Citation2013) informative book on seaweeds. Hickey’s words, as he described the relationship the local harvesters have with the seaweed, resonate with a deeply rooted attachment to the one thing that could be relied upon historically for survival, when all else failed: ‘The seaweeds have to be there, if the children return home’.
The occupation of seaweed harvesting
Due to limitations of space and resource, competition between harvesters (and sometimes within families) can be fierce: ‘The harvest is like a war’ (Lida, Citation1998). There are collective and often unwritten rules and customs within harvester communities (Becker, Citation2001). The legal status of seaweed harvesters, or crofters (Scottish term) differs around the world. In France, those who harvest from boats are considered fishers, and as such, receive health insurance and access to social security funds. Harvesters in Norway and Spain are given a similar status. Many countries, including Ireland, do not recognize harvesters in this way. Generally, those who harvest by hand, on foot, are not granted the same rights as those on board vessels. Hand harvesting of seaweeds is far less controlled by authorities and is often unregulated (Baweja et al., Citation2016).
Seaweed harvesting is physically demanding, repetitive, seasonal and weather-dependent and recruitment to the sector is very low. Studies showed that one of the main concerns for the vitality of the wild seaweed harvesting industry was related to the age of its current workforce, e.g. in Brittany, one study found that the average age of fishers was 43, with 25% of the total over 50 and only 8% under 30 (Alban et al., Citation2004). Similarly, in Ireland, a study on the age profile of harvesters in the Connemara region found that only 3% of harvesters were under 30 and 31% were 51–60 years of age (Kelly et al., Citation2001). The movement of young people from rural, coastal areas into the urban centres and the difficulty in attracting young harvesters highlights a potential issue which confronts the seaweed industry worldwide (Alban et al., Citation2004).
Innovations in technology (resulting in increased income per unit effort) may hold the key to the recruitment of younger harvesters and it would seem that mechanization may be an inevitable consequence of an inability to attract this demographic into the industry. Shortages of supply of resources are often met through mechanization. Where hand harvesting practices have historically been in place, however, there may be reluctance to introduce mechanization, born of a sense of historical ownership, and fear of a loss of traditional customs and income.
Poor harvesting practices and the importance of sustainable techniques
For a viable industry to exist, it is essential that sustainable and ethical harvesting activities are carried out to avoid undue stress being placed on the resource (Hafting et al., Citation2012). Some factors have directly influenced the ecological impacts of seaweed harvesting operations (Vasquez, Citation1995). Harvest impact is not only directly related to the magnitude and the frequency of the harvests, but also timing (seasonality), species identity and obviously, the local climatic conditions.
Natural seaweed resources are vulnerable to poor harvesting practices which may sometimes be carried out by predatory or inexperienced harvesters. Over-harvesting of Gracilaria from wild seaweed beds occurred in central Chile in the 1970s as a consequence of a high market price for the agarophyte and a poor economic situation in the country at that time (Lindstrom & Chapman, Citation1996). Poorly managing resources, such as opportunistic harvesting, excessive removal of holdfast material (reducing regeneration), trampling and enhanced grazing by herbivores all place additional stresses on the resource, while near-denudation of a seaweed bed is perhaps the most extreme case of direct impact on the community (Sharp et al., Citation2006; Thompson et al., Citation2010; Araújo et al., Citation2012; Phillippi et al., Citation2014). Unregulated, predatory harvesting resulted in over-exploitation of Brazilian agarophytes in the 2000s, which led to a declining population and a significant and prolonged decrease in productivity (Marinho-Soriano et al., Citation2006; FAO, Citation2014). Ultimately this was then associated with a decrease in the quality of the raw material. Today, the type of Gracilaria that predominates in that region is popularly known as ‘cisco’ (trash), considered commercially worthless when it washes up on shore (Marinho-Soriano, Citation2016).
Incorporation of comprehensive, sustainable harvesting techniques such as those laid out in Ugarte & Sharp (Citation2001) and Nelson & Conroy (Citation1989) for the harvesting of A. nodosum and Porphyra, respectively, are required. Successful collaborations between the scientific and harvester communities are important to help mitigate the impact of intensive harvesting and to ensure sustainability of the resource.
In implementing best-practice harvesting guidelines, limitations of the exploitation rates of the two most economically important brown seaweeds in Chile, i.e. Macrocystis sp. and Lessonia sp., were applied only after an agreed consensus was reached between fishermen, industry, government and scientists (Buschmann et al., Citation2014). The guidelines focused on the selective harvesting of sporophytes in order to allow maintenance of the reproductive stock. This important collaborative effort helped to protect and sustain Chile’s northern kelp beds, estimated to be worth US$540 million (Vásquez et al., Citation2013).
The effective, sustainable harvesting of wild stocks is important as it relates to the ability of harvested beds and their associated ecologies to persist over time. There is a need to take into account both the recovery of the harvested resource and also to acknowledge the potential ‘knock on’ effects of harvesting from monospecific seaweed beds. It is necessary to consider the biodiversity principle of ‘ensure(ing) that the activity does not cause an unacceptable reduction in biodiversity’ while echoing the 1987 World Commission on Environment and Development (WCED) declaration on sustainable development. This definition, formalized in the report ‘Our Common Future’, identified sustainable development as ‘development that meets the needs of the present without compromising the ability of future generations to meet their own needs’ (Brundtland, Citation1987; Jacquin et al., Citation2014).
Historically, it has been the importance of seaweeds for sustenance which drove local-level sustainable harvesting practices. Today there is an industry-led approach to the sustainable, self-imposed management of wild resources due to the high commercial value of seaweeds. There are numerous examples of harvesting restrictions imposed by governments working jointly with industry. In the Canadian Maritimes, New Brunswick operated a 17% exploitation rate for the harvesting of wild A. nodosum beds, while in Nova Scotia a strict 25% exploitation rate has been in place since 1999 (Ugarte & Sharp, Citation2012). A 5-year (in some cases 4) rotational management plan for Laminaria spp. was implemented in Norway in 1992, with the Ministry of Fisheries and Coastal Affairs – the FKD, regulating the harvest of L. hyperborea with local county authorities responsible for regional management of kelp resources (Meland & Rebours, Citation2011; Vea & Ask, Citation2011).
Although successfully implemented in a number of countries, precautionary objections have been raised regarding the sustainability of harvesting wild beds. Some critics of the harvest may feel management plans do not address the wider ecological impact associated with harvesting (Halat et al., Citation2015). This supposition, however, does not address the broader definition of sustainability as outlined in the Brundtland Report (1987) for the WCED, which affirmed the necessity for the inter-dependence of economic spheres with the social and environmental facets of our common global future (Jacquin et al., Citation2014).
Management plans
Although seaweeds have been harvested since ancient times, in the face of growing commercial interests and pressures it is important that specific management tools are developed and implemented to help maintain the health and integrity of not only seaweeds, but of all resources. There should be a concomitant vigilance with respect to global resource science, management, and accountability. There is definite potential for mis-management of these important resources. A clear distinction must be made between the harvesting of wild stocks for personal and artisanal use and exploitation of seaweed biomass on an industrial commercial scale.
Robust, scientific monitoring of harvesting activities is essential for the assurance of a commensal relationship between the spheres of human economic and social needs and the seaweed resource sphere. A rigorous system of management and accountability will thereby lead to the long-term and continued conservation of a persistent and valuable natural resource. Ethical and sustainable harvesting practices are imperative and they need careful consideration alongside economic evaluations when resource exploitation is considered. It is important also to consider the various seaweed species case by case, and which harvesting technique is best employed for the crop and the location. Fortunately, there are some tools available which help regulate the harvest, including licences, quotas and rotation systems (Baweja et al., Citation2016), which may need some periodic enforcement.
It is imperative to develop and implement ecosystem-based management models, while also ensuring that long-term management studies are put into place. It is also essential that regulators are proactive and vigilant in the stewardship of seaweed resources. Non-traditional seaweeds (those not commonly used to date) may soon enjoy a boom in popularity as the next ‘superfood’, as seedstock for cultivation or even as an eco-friendly insecticide (Tay et al., Citation2017). However, as research and markets highlight their economic value, availability and accessibility, vulnerable seaweed resources may be subjected to increased harvesting pressures. To exploit resources fully and to mitigate against a shrinking and ageing workforce, active consideration of mechanization may need to be carefully examined as well.
Important management strategies are being implemented in many countries as the significance of caring for and sharing various components of our coastal resources becomes increasingly evident. When an appropriate harvesting plan was in place for (rockweed) harvesting, studies showed no evidence of negative impacts on invertebrate populations (Phillippi et al., Citation2014). The sustainable harvesting of natural Sargassum beds in the Philippines has been achieved through the incorporation of practical management practices (Marquez et al., Citation2014). Beds of Sargassum are harvested before most of the plants become fertile (<50% of the population) allowing the species to regenerate and proceed to the reproductive and recruitment phases. A gradual move away from unregulated, opportunistic harvesting is becoming more widespread; in 2014 the Department of Agriculture in the Philippines imposed a ban on the harvesting and gathering of all brown seaweeds in response to blatant over-exploitation of wild stocks as a consequence of high demands for ‘sea vegetables’. A breach of the ban was reported to be punishable by a 2–10 year prison sentence (Valencia, Citation2014). In Vietnam, the high price of free-floating Sargassum spp. has driven non-selective, opportunistic harvesting of the seaweeds in Ninh Van and Ninh Phuoc, resulting in a sharp decline of overall Sargassum biomass in that region (Khanh Hoa News, Citation2012).
There is an obvious requirement for regulators to be proactive, and for close collaborations within a strict code of ethical conduct between local indigenous communities, fisheries, industry and government. Increasing stakeholder interests and demands may result in unforeseen harvesting stresses placed on the wild resources of any new species entering the market. Science must first identify an appropriate and sustainable method compatible with biomass regrowth, productivity and environmental responsibilities; in some cases, species should only be cultivated from seed stock that is carefully selected from wild populations. Without adequate regulation and rules provisioning for the ethical and sustainable use of wild resources, they are at risk.
Ownership of the resource
Possibly the most contentious issue surrounding the expansion of wild harvesting operations in some regions is the issue of access and ownership of the actual resource. Ambiguity surrounds the question of ownership and different rules apply in different countries (Higgins, Citation2017). In the majority of Atlantic European countries (i.e. Ireland, France, Spain, Portugal) ownership of seaweed resources belongs mostly to the State and the foreshore, generally from high to low water mark, and in some cases the seabed out to 8–12 nautical miles, is under the jurisdiction of the State. Private ownership of the foreshore, as is the case in large swathes of Norway and Scotland, results in the need to acquire permissions from coastal communities and also pay a fee to local landowners prior to the commencement of any commercial harvesting operations. In Galicia, harvesters may exploit the natural resource within an allocated territory if justified requests are presented to the regional authorities and approved (Baweja et al., Citation2016). In some countries seaweed rights, or ‘wrack rights’ are granted to those who have historically gathered seaweed in the region (EHS, Citation2007). These are informal rights recognized legally and based on an historical precedence relating to harvesting activities which allowed for the removal of small quantities of seaweed from the shore for personal use. In many areas people also harvested for commercial use. As an example, kelps were harvested and burned in the north-west of Ireland, broken up into manageable sizes and exported out of Mullaghmore Harbour to Scotland. It was a valued income for coastal families who had rights to the shore. In Northern Ireland informal wrack rights were recognized if an individual had been harvesting seaweed consistently for more than 20 years (EHS, Citation2007), whereas in the Republic of Ireland 99% of the foreshore is a State-owned asset under the 1933 Foreshore Act, with the Department of the Marine being responsible for granting harvesting licences. In the Republic of Ireland, traditional rightsFootnote1 originated from the legal framework that existed before independence. There exists appurtenant to coastal property ownership, the right to ‘cut, gather and remove’ seaweed from familial stripes. Many coastal householders have the right to harvest unattached, storm-cast seaweed from above the high-water mark and harvest attached seaweed material from below high water, along the boundary of their property for use as fertilizer, food and extracts for both personal and commercial purposes. However, the issue of seaweed ownership and harvesting rights in Ireland is equivocal. Numerous complex cultural, historical and familial issues, rights and definitions need to be clarified before any substantial progress can be made. At the time of writing, this process had already commenced with the Attorney General examining the issue from the various viewpoints of investor, developer, Government, local seaweed harvesters, licence holders and prospective licence holders (Joint Committee on Environment, Culture and the Gaeltacht, Citation2015).
The lack of clarity regarding ownership is considered to be the main obstacle in further developing the seaweed industry in Ireland, with industry wary of fostering investment/expansion when it exerts little control over either the methods of harvesting, and/or access to the resource. A lack of adequate restrictions on harvesting including robust regulations protecting the resource, the harvester, and processor behaviour has the potential to lead to a ‘tragedy of the commons’ scenario (Hardin, Citation1968).
Seaweeds are often considered a ‘common property resource’, or a resource for which exclusion is difficult and joint use involves sub-tractibility (Feeny et al., Citation1990). Traditional community systems play an essential role in the successful stewarding of common resources sustainably (Lida, Citation1998). Some examples of good community-level resource stewardship and co-management are those of the indigenous Nunavik community of northern Canada and the First Nations Peoples of the Pacific Northeast (Turner, Citation2003; Sharp et al., Citation2008). These examples are situations where community-level research is used to evaluate natural resources and their supply, and the demand and deep cultural respect for the environment are kept in balance.
The lack of clarity about ownership of both intertidal and subtidal resources in some regions has led to clashes among local communities, traditional harvesters and industry, with conflicts arising over competition for space (Baweja et al., Citation2016). However, as the global seaweed industry continues to grow and exert pressures on those resources, it is difficult to envisage that the supply and maintenance of those resources will be held exclusively by numerous individuals claiming historical rights and access to the seaweed. It is more likely that there will be utilization of marine spaces and off-shore seaweed farms in areas where currently no aquaculture exists. While still relatively new in Western regions, the marine offshore cultivation sector is growing rapidly (Troell et al., Citation2009). However, the successful development of offshore aquaculture requires environmental and economic considerations, as well as the ability to add value to the cultured seaweed through biorefinery approaches (see also Buschmann et al., Citationin press).
Climate change and distributional shifts in species
Throughout the last century, the average global surface seawater temperature (SST) increased by approximately 0.6°C and it is predicted to increase by up to 3.2°C in the next century (Simkanin et al., Citation2005; IPCC, Citation2013). Climate variation is seen as a key driver in defining global patterns of distribution and abundance of seaweed species, and is a growing concern for all fisheries around the world (Ugarte et al., Citation2010). Shifts in species’ ranges have been documented for a variety of organisms over the last few decades (Forsman et al., Citation2016; Lehikoinen et al., Citation2016), including marine species (Rilov, Citation2016; Sorte et al., Citation2016) amongst which are some seaweeds (Simkanin et al., Citation2005; Wernberg et al., Citation2010; Brodie et al., Citation2014; Yesson et al., Citation2015; Vergés et al., Citation2016; Araújo et al., Citation2016).
The response of seaweed species to disturbance varies with species identity, location and source of the disturbance and there have been recently reported changes in key structuring seaweed species in response to different disturbance factors (Araújo et al., Citation2016). This is the case for several native European kelp species, some with important economic value, and it was reported that their distribution and abundance in parts of their ranges had drastically changed. For instance, L. digitata, L. hyperborea, L. ochroleuca, L. rodriguezii, Saccharina latissimima and Saccorhiza polyschides showed regression of their populations and/or local extinction in different areas of their distribution (Pehlke & Bartsch, Citation2008; Moy & Christie, Citation2012; Couceiro et al., Citation2013; Oppliger et al., Citation2014; Rinde et al., Citation2014; Araújo et al., Citation2016, Bartsch et al., Citation2016). These changes were related to a multitude of natural and anthropogenic stressors such as over-grazing by sea urchins (Rinde et al., Citation2014), harvesting (Lorentsen et al., Citation2010), decline of water quality (Raybaud et al., Citation2013) and frequently, SST associated with climatic change, especially at distribution limits (Fernández, Citation2011; Assis et al., Citation2014). Other examples include fucoid species that were also commercially exploited in Europe and have undergone distributional changes along the latitudinal or vertical range of the limits of distribution, e.g. Fucus vesiculosus (Nicastro et al., Citation2013) and F. serratus (Araújo et al., Citation2011; Duarte et al., Citation2013). Himanthalia elongata, another structuring species of increased economic interest, suffered significant reductions in its southernmost limits (i.e. northern Spain, 116 km) and central/northern Portugal (230 km) and reduced abundance of the remaining populations, presumably related to ongoing trends of warming SST (Lima et al., Citation2007; Duarte et al., Citation2013; Araújo personal observation).
Other seaweeds, e.g. A. nodosum have maintained their distributional ranges, although they too are showing evidence of differentiated population dynamics with spatial fragmentation of populations towards their southern limits of distribution (Europe: Araújo et al., Citation2011, Citation2014; eastern coast of USA: C. Yarish, personal communication).
Modelling studies have predicted the reduction, or extinction, along stretches of the European shorelines of several structuring species such as L. digitata (Raybaud et al., Citation2013), H. elongata (Martinez et al., Citation2015), F. vesiculosus (Assis et al., Citation2014), L. hyperborea (Assis et al., Citation2016) and L. ochroleuca (Assis et al., Citation2016). In Tasmania, Australia, researchers have warned that a number of seaweed species face extinction as a result of reaching their ‘upper thermal limit’ for SST, with standing stocks of M. pyrifera facing rapid decline (Johnson et al., Citation2011; Mathiesen, Citation2017). Recent studies show that warming SST may affect kelp recovery post-harvest and that warming seawater temperatures may also pose a threat to the viability of kelp resources (Krumhansl et al., Citation2016).
The increasing presence of invasive species, as a result of climatic changes or shipping activities, is also a growing concern (Díez et al., Citation2012),
For most of these species the biomass supply for commercial purposes is assured by the careful harvesting from wild stocks. Some works have examined the effects of harvesting on associated habitats and organisms (Lorentsen et al., Citation2010; Stagnol et al., Citation2013, Citation2016), but empirical studies on this topic are still scarce and controversial for many of the commercially explored species and regions in Europe. This knowledge assumes particular importance at edge locations, where organisms might be ecophysiologically constrained and natural population dynamics might respond differently to the sustainable harvesting practices which have been established for other regions.
Natural disasters such as tsunamis and El Niño have been associated with major losses of inter- and subtidal seaweed species in the past. For example, Chile frequently experiences powerful earthquakes (>7 MW) which can have a major impact on belt-forming sub- and intertidal species, particularly seaweeds. As a consequence of the highly destructive 8.8 MW magnitude Chilean earthquake in 2010, large-scale coastal co-seismic uplifts occurred around the Gulf of Arauco, Santa María Island and the Bay of Concepción. Following the earthquake, investigation into biomarker species indicated coastal uplifts of up to 3.1 m (Castilla et al., Citation2010). This uplift resulted in large-scale mortality of subtidal and intertidal organisms including seaweed species, namely the kelps and coralline algae and resulted in a period of shortages of commercial red species. Similarly in 1985, following a 7.8 MW earthquake in central Chile, coastal uplifts of up to 60 cm were observed, which led to the extensive mortality of kelp (Lessonia nigrescens) near the Estacion Costera de Investigaciones Marinas, Las Cruces (Castilla, Citation1988).
Recent initiatives
There is a recognized requirement to establish a best practice code of conduct for the successful sustainable exploitation of seaweeds (Rebours et al., Citation2014). The sharing of information from government agencies with responsibilities to industry, and education and communication at local levels is important. In France, the agency for food, environment and occupational health and safety (ANSES) integrated seaweeds into their food composition database ‘Ciqual’ which collects, assesses and publishes nutritional composition data of seaweeds typically consumed in France.
The implementation of a global certification programme for seaweed harvesting has been proposed. The Marine Stewardship Council (MSC) and the Aquaculture Stewardship Council (ASC) hope to provide a global standard for the certification of seaweed operations that will ensure the sustainable and responsible exploitation of seaweed resources (see www.msc.org). In the Gulf of Maine, seaweed harvester apprenticeship programmes have been introduced (see www.larchhanson.com), in which apprentices are trained in sustainable harvesting of seaweeds (P. palmata, C. crispus, Laminaria (Saccharina) spp.). Other initiatives such as the ALGMARBIO project have the objective to develop a good practice guide for seaweed producers, as well as regulating the creation of an organically certified seaweed industry (Mesnildrey et al., Citation2012).
Acknowledgements
We would like to thank Arramara Teoranta and Acadian Seaplants for their support and technical expertise during this review. We thank Gonzalo Soriano for useful inputs and use of , Jean-Sebastien Lauzon-Guay (), Dorset Fine Arts (http://www.dorsetfinearts.com) for the permission to reproduce the Kananginak Pootoogook image: ‘Gathering Kelp’ () and Sally Cole for the use of ‘The Women of Praia’ (). Shep Erhardt and Larch Hanson are acknowledged for their inputs and constructive discussions during the writing of this review. Finally, we thank www.marketsandmarkets.com for an insight to their market analysis of the total value of the global seaweed industry (as part of their carrageenan industry report).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
Michéal Mac Monagail
M. MacMonagail: Original concept, writing the manuscript and editing; L. Cornish: Contributing wild harvest and editing; L. Morrison: Concept, figures and editing; R. Araújo: Contributing European update and editing; A. T. Critchley: editing and chivvying team along.
Notes
1 Taken from Irish traditional rights agreement: ‘There is also appurtenant to the lands a right to burn seaweed for kelp … there is also appurtenant to the lands the right to cut gather and remove seaweed whether growing or cast by the sea upon the foreshore and bed of the sea below high water mark of medium tides‘.
References
- Alban, F. & Boncoeur, J. (2004). An assessment of the potential interest of fishermen to engage in boat-chartering in the context of a marine park: the case of the Iroise Sea, Western Brittany, France. In Contesting the Foreshore. Tourism, Society, and Politics on the Coast (Boissevain, J. & Selwyn, T., editors), 185–204. Amsterdam University Press, Amsterdam.
- Alban, F., Le Floc’h, P. & Boncoeur, J. (2004). The impact of economic and regulatory factors on the relative profitability of fishing boats: a case study of the seaweed harvesting fleet of Northwest Brittany (France). Aquatic Living Resources, 17: 185–193.
- Amosu, A.O., Robertson-Andersson, D.V., Maneveldt, G.W., Anderson, R.J. & Bolton, J. J. (2013). South African seaweed aquaculture: a sustainable development example for other African coastal countries. African Journal of Agricultural Research, 8: 5268–5279.
- Araújo, R., Serrão, E.A., Sousa-Pinto, I. & Åberg, P. (2011). Phenotypic differentiation at southern limit borders: the case study of two fucoid macroalgal species with different life-history traits. Journal of Phycology, 47: 451–462.
- Araújo, R., Arenas, F., Åberg, P., Sousa-Pinto, I. & Serrão, E.A. (2012). The role of disturbance in differential regulation of co-occurring brown algae species: interactive effects of sediment deposition, abrasion and grazing on algae recruits. Journal of Experimental Marine Biology and Ecology, 422–423: 1–8.
- Araújo, R.M., Serrão, E.A., Sousa-Pinto, I. & Åberg, P. (2014). Spatial and temporal dynamics of fucoid populations (Ascophyllum nodosum and Fucus serratus): a comparison between central and range edge populations. PLoS ONE, 9(3).
- Araújo, R.M., Assis, J., Aguillar, R., Airoldi, L., Barbara, I., Bartsch, I., Bekkby, T., Christie, H., Davoult, D., Derrien-Courtel, S., Fernandez, C., Fredriksen, S., Gevaert, F., Gundersen, H., Le Gal, A., Leveque, L., Mieszkowska, N., Norderhaug, K.M., Oliveira, P., Puente, A., Rico, J.M., Rinde, E., Schubert, H., Strain, E.M., Valero, M., Viard, F. & Sousa-Pinto, I. (2016). Status, trends and drivers of kelp forests in Europe: an expert assessment. Biodiversity and Conservation, 25: 1319–1348.
- Arkema, K.K., Guannel, G., Verutes, G., Wood, S.A., Guerry, A., Ruckelshaus, M., Kareiva, P., Lacayo, M. & Silver, J.M. (2013). Coastal habitats shield people and property from sea-level rise and storms. Nature Climate Change, 3: 913–918.
- Assis, J., Serrao, E. A., Claro, B., Perrin, C. & Pearson, G.A. (2014). Climate-driven range shifts explain the distribution of extant gene pools and predict future loss of unique lineages in a marine brown alga. Molecular Ecology, 23: 2797–2810.
- Assis, J., Lucas, A.V., Barbara, I. & Serrao, E. (2016). Future climate change is predicted to shift long-term persistence zones in the cold-temperate kelp Laminaria hyperborea. Marine Environmental Research, 113: 174–182.
- Augris, C. & Berthou, P. (1990). Les gisements de maerl en Bretagne. Institut français de recherche pour l’exploitation de la mer (IFREMER) Internal Report. Retrieved from http://archimer.ifremer.fr/doc/00082/19357/16951.pdf.
- Avila, M. & Seguel, M. (1993). An overview of seaweed resources in Chile. Journal of Applied Phycology, 5: 133–139.
- Balboa, E.M., Conde, E., Soto, M.L., Pérez-Armada, L. & Domínguez, H. (2015). Cosmetics from marine sources. In Springer Handbook of Marine Biotechnology (Kim, S.-K., editor), 1015–1042. Springer-Verlag, Berlin.
- Barbera, C., Bordehore, C., Borg, J.A., Glémarec, M., Grall, J., Hall-Spencer, J.M., de la Huz, C., Lanfranco, E., Lastra, M., Moore, P.G., Mora, J., Ramos-Esplá, A.A., Rizzo, M., Sánchez-Mata, A., Seva, A., Schembri, P.J. & Valle, C. (2003). Conservation and management of northeast Atlantic and Mediterranean maerl beds. Aquatic Conservation: Marine and Freshwater Ecosystems, 13: 65–76.
- Bartsch, I., Paar, M., Fredriksen, S., Schwanitz, M., Daniel, C., Hop, H. & Wiencke, C. (2016). Changes in kelp forest biomass and depth distribution in Kongsfjorden, Svalbard, between 1996–1998 and 2012–2014 reflect Arctic warming. Polar Biology, 39: 1–16.
- Baweja, P., Kumar, S., Sahoo, D. & Levine, I. (2016). Biology of seaweeds. In Seaweed in Health and Disease Prevention (Fleurence, F. & Levine, I., editors), 41–106. Elsevier, Amsterdam.
- Becker, H. (2001). Seaweed Memories: In the Jaws of the Sea. Wolfhound Press, Dublin.
- Bixler, H.J. & Porse, H. (2011). A decade of change in the seaweed hydrocolloids industry. Journal of Applied Phycology, 23: 321–335.
- Brodie, J., Williamson, C.J., Smale, D.A., Kamenos, N.A., Mieszkowska, N., Santos, R., Cunliffe, M., Steinke, M., Yesson, C., Anderson, K.M., Asnaghi, V., Brownlee, C., Burdett, H.L., Burrows, M.T., Collins, S., Donohue, P.J.C., Harvey, B., Foggo, A., Noisette, F., Nunes, J., Ragazzola, F., Raven, J.A., Schmidt, D., Suggett, D., Teichberg, M. & Hall-Spencer, J.M. (2014). The future of the northeast Atlantic benthic flora in a high CO2 world. Ecology and Evolution, 4: 2787–2798.
- Brundtland, G.H. (1987). Report of the World Commission on Environment and Development: Our Common Future. United Nations.
- Bruton, T., Lyons, H., Lerat, Y., Stanley, M. & Rasmussen, M. B. (2009). A review of the potential of marine algae as a source of biofuel in Ireland. Sustainable Energy Ireland Dublin, 88.
- Buschmann, A.H., Camus, C., Infante, J., Neori, A., Israel, Á., Hernández-González, M. C., Pereda, S. V., Gomez-Pinchetti, J. L., Golberg, A.,Tadmor-Shalev, N. & Critchley, A. T. (in press). Seaweed production: overview of the global state of exploitation, farming and emerging research activity. European Journal of Phycology.
- Buschmann, A.H., Prescott, S., Potin, P., Faugeron, S., Vasquez, J.A., Camus, C., Infante, J., Hernandez-Gonzalez, M.C., Gutierrez, A. & Varela, D.A. (2014). The status of kelp exploitation and marine agronomy, with emphasis on Macrocystis pyrifera, in Chile. Advances in Botanical Research, 71: 161–188.
- Buschmann, A.H., Correa, J.A., Westermeier, R., Hernández-González, M. del C. & Norambuena, R. (2001). Red algal farming in Chile: a review. Aquaculture, 194: 203–220.
- Castilla, J.C. (1988). Earthquake-caused coastal uplift and its effects on rocky intertidal kelp communities. Science, 242: 440–443.
- Castilla, J.C., Manríquez, P.H. & Camaño, A. (2010). Effects of rocky shore coseismic uplift and the 2010 Chilean mega-earthquake on intertidal biomarker species. Marine Ecology Progress Series, 418: 17–23.
- Cechinel, M.A.P., Mayer, D.A., Pozdniakova, T.A., Mazur, L.P., Boaventura, R.A.R., de Souza, A.A.U., Guelli U. de Souza, S.M.A. & Vilar, V.J.P. (2016). Removal of metal ions from a petrochemical wastewater using brown macro-algae as natural cation-exchangers. Chemical Engineering Journal, 286: 1–15.
- Centre d’Etude et de valorisation des Algues CEVA. (2014). Edible Seaweed and French Regulation – Synthesis. CEVA, Brittany.
- Chellaram, C., Raja, P. & Raj, K.D. (2015). Medicinal properties of gren seaweeds, Ulva fasciata (Delile, 1813) and U. reeticulate (Forsskal, 1777). International Journal of Research in Pharmaceutical Sciences, 6: 1–6.
- Cole, S.C. (1991). Women of the Praia: Work and Lives in a Portuguese Coastal Community. Princeton University Press, Princeton.
- Collen, J., Cornish, M.L., Craigie, J., Ficko-Blean, E., Herve, C., Krueger-Hadfield, S.A., Leblanc, C., Michel, G., Potin, P., Tonon, T. & Boyen, C. (2014). Chondrus crispus: a present and historical model organism for red seaweeds. Advances in Botanical Research, 71: 53–89.
- Couceiro, L., Robuchon, M., Destombe, C. & Valero, M. (2013). Management and conservation of the kelp species Laminaria digitata: using genetic tools to explore the potential exporting role of the MPA “Parc naturel marin d’Iroise”. Aquatic Living Resources, 26: 197–205.
- Critchley, A.T., Fortes, M.D., Largo, D.B., Kawashima, S., Ohno, M., Oohusa, T., Toma, T. & Trono, G.G. (1993). Seaweed Cultivation and Marine Ranching. Japan International Co-operation Agency, Tokyo.
- Díez, I., Muguerza, N., Santolaria, A., Ganzedo, U. & Gorostiaga, J.M. (2012). Seaweed assemblage changes in the eastern Cantabrian Sea and their potential relationship to climate change. Estuarine, Coastal and Shelf Science, 99: 108–120.
- Dillehay, T.D., Ramirez, C., Pino, M., Collins, M.B., Rossen, J. & Pino-Navarro, J.D. (2008). Monte Verde: seaweed, food, medicine, and the peopling of South America. Science, 320: 784–786.
- Duarte, L., Viejo, R.M., Martínez, B., deCastro, M., Gómez-Gesteira, M. & Gallardo, T. (2013). Recent and historical range shifts of two canopy-forming seaweeds in North Spain and the link with trends in sea surface temperature. Acta Oecologica, 51: 1–10.
- Environment and Heritage Service. (2007). Environmentally Sustainable Seaweed Harvesting in Northern Ireland. Retrieved from http://www.seaweed.ie/irish_seaweed_contacts/doc/seaweedharvestingniehspositionstatement.pdf.
- Evans, F.D. & Critchley, A.T. (2014). Seaweeds for animal production use. Journal of Applied Phycology, 26: 891–899.
- Feeney, M.W. (2001). Regulating seaweed harvesting in Maine: the public and private interests in an emerging marine resource industry. Ocean and Coastal Law Journal, 7: 329–352.
- Feeny, D., Berkes, F., McCay, B.J. & Acheson, J.M. (1990). The tragedy of the commons: twenty-two years later. Human Ecology, 18: 1–19.
- Fernández, C. (2011). The retreat of large brown seaweeds on the north coast of Spain: the case of Saccorhiza polyschides. European Journal of Phycology, 46: 352–360.
- Filbee-Dexter, K. & Scheibling, R.E. (2014). Sea urchin barrens as alternative stable states of collapsed kelp ecosystems. Marine Ecology Progress Series, 495: 1–25.
- Fock, H.O. (2011). Natura 2000 and the European Common Fisheries Policy. Marine Policy, 35: 181–188.
- Food and Agriculture Organisation (FAO). (2014). Fisheries and Aquaculture Information and Statistics Service. FISHSTAT plus: Universal Software for Fishery Statistical Time Series. Food and Agriculture Organization of the United Nations, Rome.
- Forsman, A., Berggren, H., Åström, M. & Larsson, P. (2016). To what extent can existing research help project climate change impacts on biodiversity in aquatic environments? A review of methodological approaches. Journal of Marine Science and Engineering, 4: 75.
- Forster, J. & Radulovich, R. (2015). Seaweed and food security. In Seaweed Sustainability: Food and Non-Food Applications (Tiwari, B.K. & Troy, D.J., editors), 289–313. Elsevier, London.
- Frangoudes, K. & Garineaud, C. (2015). Governability of kelp forest small-scale harvesting in Iroise Sea, France. In Interactive Governance for Small-Scale Fisheries (Jentoft, S. & Chuenpagdee, R., editors), 101–115. Springer, Berlin.
- Gao, C., Pollet, E. & Avérous, L. (2017). Innovative plasticized alginate obtained by thermo-mechanical mixing: effect of different biobased polyols systems. Carbohydrate Polymers, 157: 669–676.
- Garbary, D.J., Galway, M.E. & Halat, L. (2017). Response to Ugarte et al. : Ascophyllum (Phaeophyceae) annually contributes over 100% of its vegetative biomass to detritus. Phycologia, 56: 116–118.
- Goodyear, D. (2015). A New Leaf: Seaweed could be a miracle food – if we can figure out how to make it taste good. Retrieved from http://www.newyorker.com/magazine/2015/11/02/a-new-leaf ( Date accessed 03.04.2017)
- Grall, J. & Hall-Spencer, J.M. (2003). Problems facing maerl conservation in Brittany. Aquatic Conservation: Marine and Freshwater Ecosystems, 13: S55–S64.
- Guiry, M.D. & Guiry, G.M. (2017). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. http://www.algaebase.org.
- Guiry, M. D. & Morrison, L. (2013). The sustainable harvesting of Ascophyllum nodosum (Fucaceae, Phaeophyceae) in Ireland, with notes on the collection and use of some other brown algae. Journal of Applied Phycology, 25: 1823–1830.
- Hafting, J.T., Critchley, A.T., Hubley, S.A. & Archibald, A.F. (2012). On-land cultivation of functional seaweed products for human usage. Journal of Applied Phycology, 24: 385–392.
- Halat, L., Galway, M.E., Gitto, S. & Garbary, D.J. (2015). Epidermal shedding in Ascophyllum nodosum (Phaeophyceae): seasonality, productivity and relationship to harvesting. Phycologia, 54: 599–608.
- Hall-Spencer, J.M., Kelly J. & Maggs, C.A. (2010). Background document for maerl. OSPAR Commission. Retrieved from www.ospar.org/documents%5Cdbase%5Cpublications%5CP00491_maerl.pdf.
- Hallsson, S.V. (1961). The uses of seaweeds in Iceland. Fourth International Seaweed Symposium 1961, France. Pergamon Press, Oxford.
- Hallsson, S.V. (1992). Drying of seaweeds by geothermal heat in Iceland. Geothermics, 21: 717–731.
- Hardin, G. (1968). The tragedy of the commons. Science, 162: 1243–1248.
- Hart, G.M., Ticktin, T., Kelman, D., Wright, A.D. & Tabandera, N. (2014). Contemporary gathering practice and antioxidant benefit of wild seaweeds in Hawai’i. Economic Botany, 68: 30–43.
- Hession, C., Guiry, M.D., McGarvey, S. & Joyce, D. (1998). Mapping and assessment of the seaweed resources (Ascophyllum nodosum, Laminaria spp.) off the West Coast of Ireland. Marine Resource Series, Marine Institute.
- Higgins, A.J. (2017). No one knows who “owns” rockweed in Maine. Bangor Daily News. Retrieved from http://bangordailynews.com/2017/03/09/news/no-one-knows-who-owns-rockweed-in-maine/ (Date accessed 14.03.2017).
- Hill, R., Bellgrove, A., Macreadie, P.I., Petrou, K., Beardall, J., Steven, A. & Ralph, P.J. (2015). Can macroalgae contribute to blue carbon? An Australian perspective. Limnology and Oceanography, 60: 1689–1706.
- Humm, H.J. (1951). The red algae of economic importance. Agar and related phycocolloids. In Marine Products of Commerce (Tressler, D.K. & Lemon, J. Mc. W., editors), 31–47. Reinhold Publishing Corporation, New York.
- Hurtado, A.Q., Gerung, G.S., Yasir, S. & Critchley, A.T. (2014). Cultivation of tropical red seaweeds in the BIMP-EAGA region. Journal of Applied Phycology, 26: 707–718.
- Intergovernmental Panel on Climate Change (IPCC). (2013). IPCC Fifth Assessment Report (AR5). IPCC.
- Jacquin, A.G., Brulé-Josso, S., Cornish, M.L., Critchley, A.T. & Gardet, P. (2014). Selected comments on the role of algae in sustainability. Advances in Botanical Research, 71: 1–30.
- Johnson, C.R., Banks, S.C., Barrett, N.S., Cazassus, F., Dunstan, P.K., Edgar, G.J., Frusher, S.D., Gardner, C., Haddon, M., Helidoniotis, F., Hill, K.L., Holbrook, N.J., Hosie, G.W., Last, P.R., Ling, S.D., Melbourne-Thomas, J., Miller, K., Pecl, G.T., Richardson, A.J., Ridgway, K.R., Rintoul, S.R., Ritz, D.A., Ross, D.J., Sanderson, J.C., Shepard, S.A., Slotwinski, A., Swadling, K.M. & Taw, N. (2011). Climate change cascades: shifts in oceanography, species’ ranges and subtidal marine community dynamics in eastern Tasmania. Journal of Experimental Marine Biology and Ecology, 400: 17–32.
- Joint Committee on Environment, Culture and the Gaeltacht. (2015). Report of the Committee on Developing the Seaweed Industry in Ireland.
- Kain, J.M. & Dawes, C.P. (1987). Useful European seaweeds: past hopes and present cultivation. Hydrobiologia, 151–152: 173–181.
- Kelly, L., Collier, L., Costello, M.J., Diver, M., McGarvey, S., Kraan, S., Morrissey, J. & Guiry, M.D. (2001). Impact assessment of hand and mechanical harvesting of Ascophyllum nodosum on regeneration and biodiversity. Marine Resource Series, 19. Retrieved from http://oar.marine.ie/bitstream/10793/207/1/No 19 Marine Resources Series.pdf.
- Khan, S.I. & Satam, S.B. (2003). Seaweed mariculture: scope and potential in India. Aquaculture Asia, 8: 26–28.
- Khan, W., Rayirath, U.P., Subramanian, S., Jithesh, M.N., Rayorath, P., Hodges, D.M., Critchley, A.T., Cragie, J.S., Norrie, J. & Prithiviraj, B. (2009). Seaweed extracts as biostimulants of plant growth and development. Journal of Plant Growth Regulation, 28: 386–399.
- Khanh Hoa News. (2012). Over-exploitation leads to Sargassum loss. Retrieved from http://baokhanhhoa.com.vn/english/socio_politic/201206/over-exploitation-leads-to-sargassum-loss-2166392/(Date accessed 15.01.2017)
- Kılınç, B., Cirik, S., Turan, G., Tekogul, H. & Koru, E. (2013). Seaweeds for food and industrial applications. In Food Industry (Kılınç, B., Cirik, S., Turan, G., Tekogul, H. & Koru, E., editors), 735–748. InTech publishers.
- Krumhansl, K.A. & Scheibling, R.E. (2012). Production and fate of kelp detritus. Marine Ecology Progress Series, 467: 281–302.
- Krumhansl, K.A., Bergman, J.N. & Salomon, A.K. (2016). Assessing the ecosystem-level consequences of a small-scale artisanal kelp fishery within the context of climate-change. Ecological Applications, 27: 799–813.
- Lee, K.Y. & Mooney, D.J. (2012). Alginate: properties and biomedical applications. Progress in Polymer Science, 37: 106–126.
- Lehikoinen, A., Foppen, R.P.B., Heldbjerg, H., Lindstrom, A., van Manen, W., Piirainen, S., van Turnhout, C.A.M. & Butchart, S.H.M. (2016). Large-scale climatic drivers of regional winter bird population trends. Diversity and Distributions, 22: 1163–1173.
- Levitt, G.J., Anderson, R.J., Boothroyd, C.J.T. & Kemp, F.A. (2002). The effects of kelp harvesting on its regrowth and the understorey benthic community at Danger Point, South Africa, and a new method of harvesting kelp fronds. South African Journal of Marine Science, 24: 71–85.
- Lida, T. (1998). Competition and communal regulations in the kombu kelp (Laminaria angustata) harvest. Human Ecology, 26: 405–423.
- Lima, F.P., Ribeiro, P.A., Queiroz, N., Hawkins, S.J. & Santos, A.M. (2007). Do distributional shifts of northern and southern species of algae match the warming pattern? Global Change Biology, 13: 2592–2604.
- Lindstrom, S.C. & Chapman, D.J. (1996). Future uses of marine algae: science, technology and economics at work. Hydrobiologia, 13: 1–13.
- Little, D.C., Newton, R.W. & Beveridge, M.C.M. (2016). Aquaculture: a rapidly growing and significant source of sustainable food? Status, transitions and potential. Proceedings of the Nutrition Society, 75: 274–286.
- Liu, B., Kongstad, K.T., Wiese, S., Jager, A.K. & Staerk, D. (2016). Edible seaweed as future functional food: identification of α-glucosidase inhibitors by combined use of high-resolution α-glucosidase inhibition profiling and HPLC–HRMS–SPE–NMR. Food Chemistry, 203: 16–22.
- Lorentsen, S.H., Sjøtun, K. & Grémillet, D. (2010). Multi-trophic consequences of kelp harvest. Biological Conservation, 143: 2054–2062.
- Makkar, H.P.S., Tran, G., Heuzé, V., Giger-Reverdin, S., Lessire, M., Lebas, F. & Ankers, P. (2015). Seaweeds for livestock diets: a review. Animal Feed Science and Technology, 212: 1–17.
- Marinho-Soriano, E. (2016). Historical context of commercial exploitation of seaweeds in Brazil. Journal of Applied Phycology, 29: 665–671.
- Marinho-Soriano, E., Moreira, W.S.C. & Carneiro, M.A.A. (2006). Some aspects of the growth of Gracilaria birdiae (Gracilariales, Rhodophyta) in an estuary in northeast Brazil. Aquaculture International, 14: 327–336.
- Marketsandmarkets.com. (2016). Commercial seaweeds market by type (red, brown, green), form (liquid, powdered, flakes), application (agriculture, animal feed, human food, and others), and by region - global forecasts to 2021. Retrieved from http://www.marketsandmarkets.com/Market-Reports/commercial-seaweed-market-152763701.html (Date accessed 19.02.2017)
- Marquez, G.P.B., Santiañez, W.J.E., Trono, G.C., Montaño, M.N.E., Araki, H., Takeuchi, H. & Hasegawa, T. (2014). Seaweed biomass of the Philippines: sustainable feedstock for biogas production. Renewable and Sustainable Energy Reviews, 38: 1056–1068.
- Martinez, B., Arenas, F., Trilla, A., Viejo, R.M. & Carreno, F. (2015). Combining physiological threshold knowledge to species distribution models is key to improving forecasts of the future niche for macroalgae. Global Change Biology, 21: 1422–1433.
- Mathiesen, K. (2017). 100+ species face extinction as warming hits Australia’s southern waters. http://www.climatechangenews.com/2017/02/21/more-than-100-species-face-extinction-as-warming-hits-australias-southern-waters/ (Date accessed 15.03.2017).
- McErlean, T.C. (2007). Archaeology of the Strangford Lough kelp industry in the eighteenth- and early nineteenth centuries. Historical Archaeology, 41: 76–93.
- Meland, M. & Rebours, C. (2011). Introduction to the management and regulation of the Norwegian seaweed industry. Bioforsk, 7: 278–279.
- Mendez, F.J. & Losada, I.J. (2004). An empirical model to estimate the propagation of random breaking and nonbreaking waves over vegetation fields. Coastal Engineering, 51: 103–118.
- Mesnildrey, L., Jacob, C., Frangoudes, K., Reunavot, M. & Lesueur, M. (2012). Seaweed industry in France. Report. Interreg program NETALGAE. Les publications du Pole halieutique AGROCAMPUS OUEST, no. 9, 34 pp.
- Migné, A., Golléty, C. & Davoult, D. (2014). Effect of canopy removal on a rocky shore community metabolism and structure. Marine Biology, 162: 449–457.
- Mouritsen, O.G. (2013). Seaweeds : Edible, Available, And Sustainable. The University of Chicago Press (Vol. 1). https://doi.org/10.1017/CBO9781107415324.004
- Mouritsen, O.G., Dawczynski, C., Duelund, L., Jahreis, G., Vetter, W. & Schröder, M. (2013). On the human consumption of the red seaweed dulse (Palmaria palmata (L.) Weber & Mohr). Journal of Applied Phycology, 25: 1777–1791.
- Moy, F.E. & Christie, H.C. (2012). Large-scale shift from sugar kelp (Saccharina latissima) to ephemeral algae along the south and west coast of Norway. Marine Biology Research, 8: 357–369.
- Msuya, F. & Hurtado, A. (in press). The role of women in seaweed aquaculture in West Indian Ocean and Southeast Asia. European Journal of Phycology.
- Nakuda, M. (1965). Historical development of the Ama’s diving activities. In Physiology of Breath-Hold Diving and the Ama of Japan (Rahn, H & Yokoyama, T., editors), 25–40. National Academy of Sciences National Research Council, Washington, DC.
- Nelson, W.A. & Conroy, A.M. (1989). Effect of harvest method and timing on yield and regeneration of Karengo (Porphyra spp.) (Bangiales, Rhodophyta) in New Zealand. Journal of Applied Phycology, 1: 277–283.
- Netalgae. (2012). Seaweed Industry in Europe: A Guide to Better Practice. Retrieved from www.netalgae.eu (Date accessed 29.12.2016).
- Nicastro, K.R., Zardi, G.I., Teixeira, S., Neiva, J., Serrao, E.A. & Pearson, G.A. (2013). Shift happens: trailing edge contraction associated with recent warming trends threatens a distinct genetic lineage in the marine macroalga Fucus vesiculosus. BMC Biology, 11: 1–13.
- Norderhaug, K.M. & Christie, H.C. (2009). Sea urchin grazing and kelp re-vegetation in the NE Atlantic. Marine Biology Research, 5: 515–528.
- O’Sullivan, C. (2017). Commercial harvesting of native seaweed forest in Bantry Bay allowed. The Irish Examiner, 2 May. Retrieved from http://www.irishexaminer.com/ireland/commercial-harvesting-of-native-seaweed-forest-in-bantry-bay-allowed-449156.html (Date accessed 02.05.2017).
- Oppliger, L.V., von Dassow, P., Bouchemousse, S., Robuchon, M., Valero, M., Correa, J. A., Mauger, S. & Destombe, C. (2014). Alteration of sexual reproduction and genetic diversity in the kelp species Laminaria digitata at the southern limit of its range. PLoS ONE, 9(7).
- Pehlke, C. & Bartsch, I. (2008). Changes in depth distribution and biomass of sublittoral seaweeds at Helgoland (North Sea) between 1970 and 2005. Climate Research, 37: 135–147.
- Pereira, L. & Neto, J.M. (2015). Marine Algae: Biodiversity, Taxonomy, Environmental Assessment, and Biotechnology. CRC Press, Boca Raton, FL.
- Phillippi, A., Tran, K. & Perna, A. (2014). Does intertidal canopy removal of Ascophyllum nodosum alter the community structure beneath? Journal of Experimental Marine Biology and Ecology, 461: 53–60.
- Porse, H. & Rudolph, B. (2017). The seaweed hydrocolloid industry: 2016 updates, requirements, and outlook. Journal of Applied Phycology, online early. https://doi.org/10.1007/s10811-017-1144-0.
- Raghavendra, V.B., Lokesh, S. & Prakash, H.S. (2007). Dravya, a product of seaweed extract (Sargassum wightii), induces resistance in cotton against Xanthomonas campestris pv. malvacearum. Phytoparasitica, 35: 442–449.
- Raven, J. (in press). The possible roles of algae in restricting the increase in atmospheric CO2 and global temperature. European Journal of Phycology.
- Raybaud, V., Beaugrand, G., Goberville, E., Delebecq, G., Destombe, C., Valero, M., Davoult, D., Morin, P. & Gevaert, F. (2013). Decline in kelp in West Europe and climate. PLoS ONE, 8(6).
- Rebours, C., Marinho-Soriano, E., Zertuche-Gonzalez, J.A., Hayashi, L., Vásquez, J.A., Kradolfer, P., Soriano, G., Ugarte, R., Abreu, M.H., Bay-Larsen, I., Hovelsrud, G., Rodven, R. & Robledo, D. (2014). Seaweeds: an opportunity for wealth and sustainable livelihood for coastal communities. Journal of Applied Phycology, 26: 1939–1951.
- Rhatigan, P. (2009). Prannie Rhatigan’s Irish Seaweed Kitchen: The Comprehensive Guide to Healthy Everyday Cooking with Seaweeds. Booklink.
- Rilov, G. (2016). Multi-species collapses at the warm edge of a warming sea. Scientific Reports, 6: 36897. https://doi.org/10.1038/srep36897.
- Rinde, E., Christie, H. & Bekkby, T. (2006). Økologiske effekter av taretråling. Analyser basert på GIS-modellering og empiriske data. Norwegian Institute for Water Research.
- Rinde, E., Christie, H., Fagerli, C.W., Bekkby, T., Gundersen, H., Norderhaug, K.M. & Hjermann, D. (2014). The influence of physical factors on kelp and sea urchin distribution in previously and still grazed areas in the NE Atlantic. PLoS ONE, 9(6).
- Robinson, A. (2017). Community outcry over plans to harvest kelp forest in Bantry Bay. Retrieved from http://coastmonkey.ie/bantry-bay-plan-harvest-kelp-forest-bioatlantis/ (Date accessed 01.07.2017).
- Rothman, M.D., Anderson, R.J., Boothroyd, C.J.T., Kemp, F.A. & Bolton, J.J. (2009). The gracilarioids in South Africa: long-term monitoring of a declining resource. Journal of Applied Phycology, 21: 47–53.
- Salo, M., Sirén, A. & Kalliola, R. (2014). Diagnosing Wild Species Harvest: Resource Use and Conservation. Academic Press, London.
- Santelices, B. & Ugarte, R. (1987). Production of Chilean Gracilaria: problems and perspectives. Hydrobiologia, 41: 295–299.
- Santos, R. & Duarte, P. (1991). Marine plant harvest in Portugal. Journal of Applied Phycology, 3: 11–18.
- Sarkar, S.I.S., Kamal, M., Hasan, M.M., Hossain, I., Shikha, F.H. & Rasul, G. (2016). Manufacture of different value added seaweed products and their acceptance to consumers. Asian Journal of Medical and Biological Research, 2: 639–645.
- Sathya, B., Indu, H., Seenivasan, R. & Geetha, S. (2010). Influence of seaweed liquid fertilizer on the growth and biochemical composition of legume crop, Cajanus cajan (L). Mill sp. Journal of Phytology, 2: 50–63.
- Sbhimani [blogger name] (2016). Seaweed: The New Kale? Retrieved from https://www.citymarket.coop/blog/2016/02/05/seaweed-new-kale (Date accessed 16.12.2016).
- Schreiber, L. (2014). Edible Vegetables from the Sea. Maine News Index – MaineBiz. 6221., pp. 14–16. Retrieved from http://digitalcommons.portlandlibrary.com/news_mainebiz/6221.
- Sharp, G. (1987). Ascophyllum nodosum and its harvesting in Eastern Canada. FAO Technical Report, 281: 3–46.
- Sharp, G., Allard, M., Lewis, A., Semple, R. & Rochefort, G. (2008). The potential for seaweed resource development in subarctic Canada; Nunavik, Ungava Bay. Journal of Applied Phycology, 20: 491–498.
- Sharp, G.J., Ugarte, R. & Semple, R. (2006). The ecological impact of marine plant harvesting in the Canadian maritimes, implications for coastal zone management. ScienceAsia, 32: 77–86.
- Sho, H. (2001). History and characteristics of Okinawan longevity food. Asia Pacific Journal of Clinical Nutrition, 10: 159–164.
- Simkanin, C., Power, A., Myers, A., McGrath, D., Southward, A., Mieszkowska, N., Leaper, R. & O’Riordan, R. (2005). Using historical data to detect temporal changes in the abundances of intertidal species on Irish shores. Journal of the Marine Biological Association of the United Kingdom, 85: 1329–1340.
- Skeffington, M.S., Scott, N.E. & Gosling, P. (2013). Numbered seaweed mearing stones on Island Eddy and the adjoining mainland at Carrowmore townland, Ballinacourty, Galway Bay. Journal of Irish Archaeology, 22: 93–109.
- Smith, A.M. & Ross, A.B. (2016). Production of bio-coal, bio-methane and fertilizer from seaweed via hydrothermal carbonisation. Algal Research, 16: 1–11.
- Sorte, C.J.B., Davidson, V.E., Franklin, M.C., Benes, K.M., Doellman, M.M., Etter, R.J., Hannigan, R.E., Lubchenco, J. & Menge, B.A. (2016). Long-term declines in an intertidal foundation species parallel shifts in community composition. Global Change Biology, 23: 341–352.
- Stagnol, D., Michel, R. & Davoult, D. (2016). Population dynamics of the brown alga Himanthalia elongata under harvesting pressure. Estuarine, Coastal and Shelf Science, 174: 65–70.
- Stagnol, D., Renaud, M. & Davoult, D. (2013). Effects of commercial harvesting of intertidal macroalgae on ecosystem biodiversity and functioning. Estuarine, Coastal and Shelf Science, 130: 99–110.
- Steen, H., Moy, F.E., Bodvin, T., & Husa, V. (2016). Regrowth after kelp harvesting in Nord-Trøndelag, Norway. Ices Journal of Marine Science, 73: 2708–2720.
- Stévant, P., Rebours, C. & Chapman, A. (2017). Seaweed aquaculture in Norway: recent industrial developments and future perspectives. Aquaculture International, 25: 1373–1390.
- Tabassum, M.R., Xia, A. & Murphy, J.D. (2017). Potential of seaweed as a feedstock for renewable gaseous fuel production in Ireland. Renewable and Sustainable Energy Reviews, 66: 136–146.
- Tay, J.W., Hoddle, M.S., Mulchandani, A. & Choe, D.H. (2017). Development of an alginate hydrogel to deliver aqueous bait for pest ant management. Pest Management Science (epub ahead of print). https://doi.org/10.1002/ps.4616.
- Thiel, M., Macaya, E. & Acuña, E. (2007). The Humboldt current system of northern-central Chile oceanographic processes, ecological interactions. Oceanography and Marine Biology: An Annual Review, 45: 195–344.
- Thompson, S.A., Knoll, H., Blanchette, C.A. & Nielsen, K.J. (2010). Population consequences of biomass loss due to commercial collection of the wild seaweed Postelsia palmaeformis. Marine Ecology Progress Series, 413: 17–31.
- Troell, M., Joyce, A., Chopin, T., Neori, A., Buschmann, A.H. & Fang, J.G. (2009). Ecological engineering in aquaculture – potential for integrated multi-trophic aquaculture (IMTA) in marine offshore systems. Aquaculture, 297: 1–9.
- Turner, N.J. (2003). The ethnobotany of edible seaweed (Porphyra abbottae and related species; Rhodophyta: Bangiales) and its use by First Nations on the Pacific Coast of Canada. Canadian Journal of Botany, 81: 283–293.
- Ugarte, R.A. & Sharp, G. (2001). A new approach to seaweed management in Eastern Canada: the case of Ascophyllum nodosum. Cahiers de Biologie Marine, 42: 63–70.
- Ugarte, R. & Sharp, G. (2011). An evaluation of the mortality of the brown seaweed Ascophyllum nodosum (L.) Le Jol. produced by cutter rake harvests in southern New Brunswick, Canada. Journal of Applied Phycology, 23: 401–407.
- Ugarte, R. & Sharp, G. (2012). Management and production of the brown algae Ascophyllum nodosum in the Canadian maritimes. Journal of Applied Phycology, 24: 409–416.
- Ugarte, R., Lauzon-Guay, J.S. & Critchley, A.T. (2017). Comments on Halat L., Galway M.E., Gitto S. & Garbary D.J. 2015. Epidermal shedding in Ascophyllum nodosum (Phaeophyceae): seasonality, productivity and relationship to harvesting. Phycologia 54 (6):599–608. Phycologia, 56: 114–115.
- Ugarte, R.A., Craigie, J.S. & Critchley, A.T. (2010). Fucoid flora of the rocky intertidal of the canadian maritimes: implications for the future with rapid climate change. In Cellular Origin, Life in Extreme Habitats and Astrobiology (Seckbach, J. editor), 69–90. Springer, Berlin.
- Valencia, C. (2014). DA bans trade of brown algae and sea grass in the wild. The Philippine Star. Retrieved from http://www.philstar.com/business/2014/04/25/1315756/da-bans-trade-brown-algae-and-sea-grass-wild ( Date accessed 04.04.2017).
- Vásquez, J.A. (1995). Ecological effects of brown seaweed harvesting. Botanica Marina, 38: 251–258.
- Vásquez, J.A., Zuñiga, S., Tala, F., Piaget, N., Rodríguez, D.C. & Vega, J.M.A. (2013). Economic valuation of kelp forests in northern Chile: values of goods and services of the ecosystem. Journal of Applied Phycology, 26: 1–8.
- Vea, J. & Ask, E. (2011). Creating a sustainable commercial harvest of Laminaria hyperborea, in Norway. Journal of Applied Phycology, 23: 489–494.
- Veiga de Oliveira, E., Pereira, B. & Galhano, F. (1975). Actividades Agro-Maritimas em Portugal. Instituto de Alta Cultura. Lisboa.
- Vergés, A., Doropoulos, C., Malcolm, H.A., Skye, M., Garcia-Pizá, M., Marzinelli, E.M., Campbell, A.H., Ballesteros, E., Hoey, A.S., Vila-Concejo, A., Bozec, Y.M. & Steinberg, P.D. (2016). Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, and loss of kelp. Proceedings of the National Academy of Sciences of the United States of America, 113: 13791–13796.
- Vijayanand, N., Ramya, S.S. & Rathinavel, S. (2014). Potential of liquid extracts of Sargassum wightii on growth, biochemical and yield parameters of cluster bean plant. Asian Pacific Journal of Reproduction, 3: 150–155.
- Volesky, B. (2001). Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy, 59: 203–216.
- Wan, A.H.L., Wilkes, R.J., Heesch, S., Bermejo, R., Johnson, M.P. & Morrison, L. (2017). Assessment and characterisation of Ireland’s green tides (Ulva species). PLoS ONE, 12(1).
- Wang, Y., Fu, F., Li, J., Wang, G., Wu, M., Zhan, J., Chen, X. & Mao, Z. (2016). Effects of seaweed fertilizer on the growth of Malus hupehensis Rehd. seedlings, soil enzyme activities and fungal communities under replant condition. European Journal of Soil Biology, 75: 1–7.
- Wernberg, T., Thomsen, M.S., Tuya, F., Kendrick, G.A., Staehr, P.A., & Toohey, B.D. (2010). Decreasing resilience of kelp beds along a latitudinal temperature gradient: potential implications for a warmer future. Ecology Letters, 13: 685–694.
- Werner, A. & Kraan, S. (2004). Review of the potential mechanisation of kelp harvesting in Ireland. Marine Environment and Health Series, No. 17. Marine Environment and Health, 17: 56.
- White, W.L. & Wilson, P. (2015). World seaweed utilization. In Seaweed Sustainability: Food and Non-Food Applications (Tiwari, B.K. & Troy, D.J., editors), 7–25. Elsevier, London.
- Yang, L.E., Lu, Q.Q. & Brodie, J. (2017). A review of the bladed Bangiales (Rhodophyta) in China: history, culture and taxonomy. European Journal of Phycology, 52: 1–13.
- Yesson, C., Bush, L.E., Davies, A.J., Maggs, C.A. & Brodie, J. (2015). Large brown seaweeds of the British Isles: evidence of changes in abundance over four decades. Estuarine, Coastal and Shelf Science, 155: 167–175.
