ABSTRACT
Grazing is recognized as one of the selective factors shaping the morphology and physiology of cyanobacteria. A recent study has shown that the filamentous cyanobacterium Aphanizomenon gracile strain SAG 31.79 thickened in the presence of Daphnia (Cladocera) and its exudates. The aims of our study were: (1) to determine whether this type of response to Daphnia cues is common for other strains of A. gracile, and other species of filamentous cyanobacteria, (2) to test whether the response is due to nutrients recycled by Daphnia, or kairomone induced, and (3) whether it is related to toxin production. Prior to the experiment, cyanobacterial strains were inspected using chromatographic methods for the presence of two toxins, cylindrospermopsin (CYN) and three homologues of microcystin (MC-RR, MC-YR, MC-LR). HPLC analyses showed that all strains were free of cylindrospermopsin, whereas microcystins were detected only in one strain (Planktothrix agardhii). We then tested whether Daphnia exudates can cause thickening of cyanobacterial filaments, which would suggest the morphological changes in cyanobacterial filaments are caused by recycled nutrients. Cyanobacteria were also exposed to sodium octyl sulphate (a commercially available Daphnia kairomone). Transmission electron microscopy (TEM) was used to check whether Daphnia exudates and sodium octyl sulphate trigger thickening of cyanobacterial cell walls, which would be a defence mechanism against grazing. The TEM analysis revealed no significant effect of either Daphnia exudates or kairomone (sodium octyl sulphate) on the cell wall thickness of cyanobacteria. However, our study showed that Daphnia exudates triggered filament thickening in nostocalean cyanobacteria, while filaments of the oscillatorialean strain P. agardhii did not show this response. It was also demonstrated that sodium octyl sulphate alone can also cause filament thickening, which suggests that this might be a specific defence response to the presence of grazers.
Introduction
The phytoplankton of many eutrophic lakes is often dominated by colony-forming cyanobacteria, particularly of the genera Aphanizomenon, Anabaena, Cylindrospermopsis, Limnothrix, Microcystis or Planktothrix. Different hypotheses have been proposed in order to explain the success of these prokaryotes (Dokulil & Teubner, Citation2000) and one of them is related to the resistance of these organisms to grazing. Many authors have claimed that filament-forming cyanobacteria are difficult for zooplankton to graze on because they clog filtering apparatuses (Burns, Citation1968; Webster & Peters, Citation1978; Gliwicz & Siedlar, Citation1980; Lynch, Citation1980). However, under some circumstances, cyanobacterial filaments can be effectively grazed by zooplankton. Gliwicz (Citation1990) reported that resistance of filamentous cyanobacteria to grazing can decrease if the abundance of filaments is low or if the filaments are in poor physiological condition (flexible filaments, easily disintegrated, with decreased number of gas vacuoles and a tendency to sink rapidly, and having also an increased number of heterocytes, which are formed to prevent nitrogen deficiency). The importance of the physiological condition of filaments for their grazing resistance was also highlighted in our previous studies, which showed that thin cyanobacterial filaments with signs of autolytic cell destruction are more vulnerable to Daphnia grazing than thick filaments which are in a good physiological state (Wejnerowski et al., Citation2015, Citation2016). Despite a certain amount of resistance to grazing that is offered by filamentous morphology, some of these prokaryotes have also developed various adaptation strategies that can protect them against grazing (for more details see reviews of Van Donk et al., Citation2011; Ger et al., Citation2016). Most of these defence mechanisms are used in response to direct contact with a grazer but there are some examples which show the expression of these strategies in response to chemical stimuli alone (Jang et al., Citation2003; Van Gremberghe et al., Citation2009; Fiałkowska & Pajdak-Stós, Citation2014). Another type of response, such as thickening and shortening of filaments in the presence of Daphnia and its exudates, was reported for the cyanobacterium Aphanizomenon gracile (Lemmermann), strain SAG 31.79 (Cerbin et al., Citation2013). However, the cause and ecological significance of such changes in Aphanizomenon morphology are not fully understood. Perhaps filament thickening in the presence of Daphnia is a defence mechanism against grazing, and cyanobacteria with thicker filaments have a greater resistance to damage during physical contact with daphnids. It seems plausible in light of the fact that Daphnia grazing on filamentous food particles depends on their thickness, and filaments thicker than 2 µm were found to be grazed with lower efficiency by daphnids (Nadin-Hurley & Duncan, Citation1976). On the other hand, filaments might thicken as a result of nutrient recycling by animals. In this scenario, filaments become thicker because they accumulate and utilize nutrients that daphnids supply to the general pool of nutrients. Ammonia and urea constitute a major source of released nitrogen by cladocerans (Elser et al., Citation2001) and availability of ammonium (NH4+) in lake water was found to cause widening of Aphanizomenon flos-aquae (Ralfs ex Bornet & Flahault) filaments (Kohl et al., Citation1985).
A key objective of our study was to determine whether widening and shortening of cyanobacterial filaments in response to Daphnia cues is common in other strains of A. gracile, as well as in other taxonomic groups. We exposed cyanobacterial cultures to either Daphnia exudates or sodium octyl sulphate, a commercially available Daphnia kairomone, which induces colony formation in Scenedesmus gutwinskii (Yasumoto et al., Citation2005). During the experiment, we measured the thickness and the length of filaments. Because one of the proposed hypotheses to explain filament thickening in the presence of Daphnia assumes that it is a defence mechanism against grazing, we also analysed the cell wall thickness in filaments using transmission electron microscopy. The increased cell wall thickness in cyanobacterial filaments exposed to Daphnia exudates or sodium octyl sulphate, if present, might indicate that Daphnia infochemicals stimulate cyanobacteria to defend against grazers through ultrastructural changes. Strengthening of the cell wall in response to grazing pressure among phytoplankton organisms has been discovered in diatoms (Pondaven et al., Citation2007). In order to verify our second hypothesis, which explains filament thickening as a result of nutrient recycling by daphnids, we decided to check the frequency of heterocyte occurrence in nostocalean cyanobacteria, which are able to fix atmospheric nitrogen via heterocytes and convert it into usable forms of nitrogen (Martin-Figueroa et al., Citation2000; Meeks & Elhai, Citation2002). Although the formation and maintenance of heterocytes and the fixation of atmospheric nitrogen by these cells are energetically expensive processes (Kumar et al., Citation2010), the number of heterocytes per filament length increases when the nitrogen resources are poor (lack of nitrogen dissolved in water) (Yema et al., Citation2016). Daphnia can recycle nutrients, especially available forms of nitrogen for cyanobacteria (e.g. ammonium). For this reason, we expected a lower frequency of heterocyte occurrence in nostocalean cyanobacteria in the presence of Daphnia exudates. Additionally, all of the examined strains of cyanobacteria were analysed in terms of their toxicity (cylindrospermopsin and microcystins) in order to discern whether any morphological responses to Daphnia exudates and sodium octyl sulphate were present in only non-toxic strains, only toxic strains or independently of the toxicity of the strains.
Materials and methods
Organisms
Eight strains of filamentous cyanobacteria were used, including Aphanizomenon gracile Lemmermann (strains: CCALA8, GOP1, SAG 31.79, ZHL1B), Cylindrospermopsis raciborskii (Woloszynska) Seenayya & Subba Raju (strains: BUSZ3, SAG 1.97), and Planktothrix agardhii (Gomont) Anagnostidis & Komárek (strains: ZHJ1B, SAG 6.89). Strains of CCALA and SAG were obtained from the Culture Collection of Autotrophic Organisms of the Institute of Botany of the Academy of Science of the Czech Republic (strain CCALA 8), and The Culture Collection of Algae at Göttingen University in Germany (strains: SAG 31.79, SAG 1.97, SAG 6.89). The remaining strains were newly isolated from Western Poland lakes, using the procedure described by Zapomělová et al. (Citation2007). These strains are currently maintained in the collection at the Department of Hydrobiology, Adam Mickiewicz University. Identification of cyanobacteria isolates was performed based on the morphological criteria provided by Komárek (Citation2013). Two of the examined strains, CCALA 8 and SAG 6.89, have molecular confirmation of their taxonomic position (Wejnerowski et al., Citation2017b; Christiansen et al., Citation2008, respectively). Detailed information about the origin and year of isolation of examined strains is presented in Supplementary table 1.
Cultures were maintained in 75 cm3 Nunc cell-culture flasks (Thermo Scientific, San Jose, California, USA) filled with 50 ml of WC culture medium (Guillard & Lorenzen, Citation1972) at 20°C ± 0.5°C, with a photoperiod of 16:8 light-dark cycle, and light intensity of 50 µmol photons m–2 s–1 measured with a light meter LI-192 quantum sensor LI-COR (Bio-Sciences, Lincoln, Nebraska, USA). Flasks with cyanobacteria were gently and continuously shaken on an orbital shaker 358S (Elpan, Lubawa, Poland). These growth conditions were maintained during all the experiments.
From 10-day-old stock culture of each strain, we collected 100 ml samples in order to evaluate the presence or absence of cylindrospermopsin (CYN) and microcystins (MC-RR, -YR and -LR) using HPLC-diode-array UV detection (HPLC-DAD) and HPLC-MS/MS analysis. The preparation of samples and determination of cylindrospermopsin and microcystins were performed according to Kokociński et al.(Citation2013) and Hautala et al. (Citation2013), respectively.
A laboratory clone (Bdem2) of Daphnia magna Straus was used as a source of grazer exudates in the experiment. Daphnids were cultured according to Wejnerowski et al. (Citation2017a).
Preparation of culture media filtrates and nutrient analysis
Control medium filtrate was obtained by filtration of WC medium. Daphnia exudates medium filtrate was obtained by filtration of WC medium from a beaker in which adult daphnids (500 individuals per litre) were present for 48 hours. Daphnids were removed from the WC medium before the filtration. The concentrated Daphnia filtrate (5 ml) was added to 45 ml of experimental culture. The final concentration of Daphnia exudates in experimental flasks corresponded to 50 individuals per litre. Similarly, the sodium octyl sulphate medium filtrate was prepared by filtration of WC medium containing sodium octyl sulphate (CAS Number: 142-31-4; Sigma Aldrich, Inc., Saint Louis, USA) and the volume of 5 ml was added to 45 ml of experimental culture. The final concentration of sodium octyl sulphate in the experimental flasks was 1 mg l–1. To minimize the risk of contamination of experimental cultures, each type of medium was filtered in a laminar airflow cabinet CLEAN FLUX O/130 (FoLabo Instruments S.r.l., Buccinasco, Italy) using syringe filters RC with membrane pore size 0.2 µm (Carl Roth GmbH + Co. KG, Karlsruhe, Germany).
Nutrient analyses in culture media filtrates were performed in order to assess whether the Daphnia exudates medium filtrate and/or the sodium octyl sulphate medium filtrate had different nutrient concentration to the control medium filtrate. Each culture medium filtrate was replicated three times. During the analyses, we measured the concentration of ammonia (NH3), ammonium (NH4+), nitrate (NO3–) and orthophosphates (PO43–). Measurement of the nutrients concentration was made using a Hach DR/2010 spectrophotometer (Hach Company, Loveland, Colorado, USA). Prior to the analyses, samples were filtered through 1.2 µm Whatman GF/C filters. Ammonia and ammonium were determined using the Nessler Method (Hach Method 8038, kit: Nitrogen-Ammonia Reagent Set, Nessler, Detection Range: 0.02–2.50 mg l–1). Nitrate was determined using the cadmium reduction method (Hach Method 8039, kit: NitraVer® 5 Nitrate Reagent Powder Pillows, DR: 0.1–10.0 mg l–1). Orthophosphates were determined using PhosVer® 3 Ascorbic Acid method (Hach Method 8048, kit: PhosVer® 3 Phosphate Reagent Powder Pillows, DR: 0.02–2.5 mg l–1). Sample volumes used for determination of ammonia, ammonium, nitrate and orthophosphates were, respectively, 25 ml, 25 ml, 10 ml and 10 ml.
Experimental design
One-week-old cultures of each strain were used as inocula for the experiment. Cyanobacteria were exposed to the following treatments for 10 days: (i) a control with addition of WC medium filtrate (control), (ii) addition of WC medium filtrate containing Daphnia exudates (exudates), (iii) addition of WC medium filtrate containing sodium octyl sulphate – Daphnia kairomone (SOS). Each treatment was replicated five times. As a result, we had 120 experimental flasks (8 strains × 3 treatments × 5 replicates). The volume of cyanobacterial culture in each flask was 50 ml and 90% of it constituted WC medium with cyanobacterial filaments while 10% was culture medium filtrate (control, exudates or SOS). Cyanobacteria were cultivated using a semi-continuous system, where every second day 5 ml of culture volume (10%) of each experimental flask was replaced by 5 ml of freshly prepared WC medium filtrate (control, exudates or SOS). This procedure was performed because the cultures were not axenic and Daphnia cues could be degraded by microorganisms e.g. bacteria (Lürling & Van Donk, Citation1997). Moreover, the replacement of 10% of experimental cyanobacteria cultures with fresh media allowed us to maintain stable population density and reduce the possibility of nutrient limitation throughout the experiment. The culture monitoring data are given in Supplementary fig. 1. The data on the filament density and morphology at the beginning of the experiment are given in Supplementary table 2. Culture manipulations, including: inoculation, medium supply and sample collection, were performed in a laminar airflow cabinet whilst experimental flasks were gently and continuously shaken on an orbital shaker. The location of all flasks was always random on the platform of the shaker.
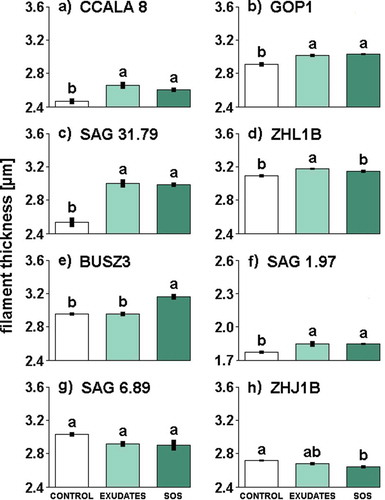
Fig. 1. The influence of different media filtrates (Control, Exudates or SOS) on filament thickness in Aphanizomenon gracile (a, b, c, d), Cylindrospermopsis raciborskii (e, f), Planktothrix agardhii (g, h). Different letters above the bars indicate significant differences highlighted by Tukey’s HSD test at the significance level of P = 0.05.
After 10 days of the experiment, 2 ml samples were taken from cultures for filament morphology measurements (filament length and thickness) and for heterocyte frequency determination (mean number of heterocytes per 1000 µm of filament length). The thickness of 100 filaments and the length of 200 filaments were measured in each sample using a light microscope Axioskop 2 mot plus (Carl Zeiss Light Microscopy, Germany) equipped with a digital camera JENOPTIK ProgRes Speed Xtcore 3 and ProgRes Image Capture Software (JENOPTIK Optical Systems GmbH, Germany). Thickness was measured on vegetative cells located at the central part of the filaments. In addition, we took a randomly selected 10 ml sample from one of five flasks of each treatment for the transmission electron microscopy analysis (TEM) of the cell wall thickness, resulting in 24 samples for this purpose. Samples for TEM were prepared following the same procedure that was described in detail in our previous study of the ultrastructure of cyanobacteria (Wejnerowski et al., Citation2016). Photos of cyanobacterial filaments were taken using a transmission electron microscope JEM 1200 EXII (Jeol, Tokyo, Japan) at an acceleration of 80 kV and magnifications 15 000×–30 000×. The cell wall thickness was analysed in all cyanobacterial strains, except two strains of P. agardhii, whose filaments were covered with white mucilaginous sheaths that prevented us making precise measurements (see Supplementary fig. 2). In each unsheathed strain, a single cell was chosen from 10 filaments of each treatment. The cell wall thickness of each chosen cell was measured in six places (see Supplementary fig. 3) and these measurements were averaged for each specimen. In total, we had 10 measurements for each treatment in each of the examined strains. We also measured the thickness of entire cells, using ProgRes Image Capture Software, and calculated the percentage that was cell wall. These data were used for statistical analyses.
Fig. 2. The influence of different media filtrates (Control, Exudates or SOS) on filament length in Aphanizomenon gracile (a, b, c, d), Cylindrospermopsis raciborskii (e, f), Planktothrix agardhii (g, h). Different letters above the bars indicate significant differences highlighted by Tukey’s HSD test at the significance level of P = 0.05.
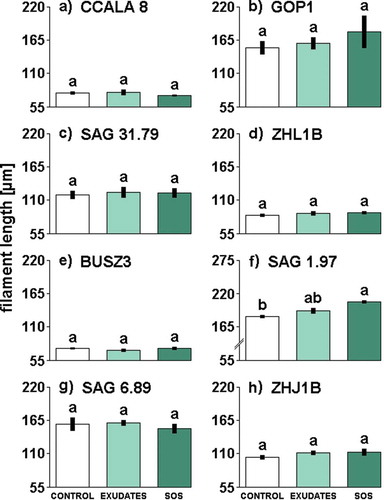
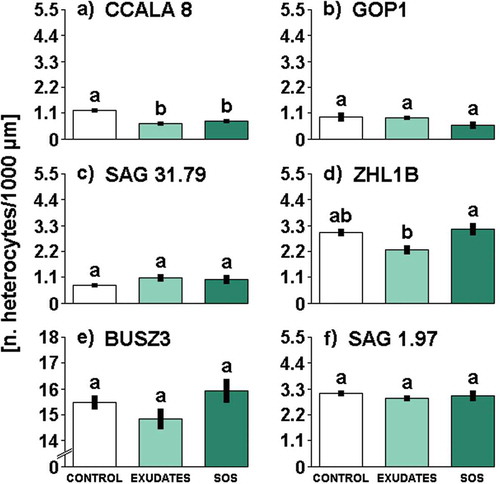
Fig. 3. The influence of different media filtrates (Control, Exudates or SOS) on heterocyte frequency in Aphanizomenon gracile (a, b, c, d), Cylindrospermopsis raciborskii (e, f). Different letters above the bars indicate significant differences highlighted by Tukey’s HSD test at the significance level of P = 0.05.
Statistical analyses
Differences in filament morphology, heterocyte frequency, cell wall thickness among the treatments and in the concentration of nutrients between three culture medium filtrate types were analysed using one-way ANOVA. All the assumptions of ANOVA were met. Whenever the differences were significant, multiple pairwise comparisons were made with post hoc Tukey’s HSD test at the 0.05 significance level. All the analyses were performed using the R statistical software version 3.0.2 (R Core Team, Citation2013).
Results
Morphological and ultrastructural analyses
Daphnia exudates and sodium octyl sulphate had significant effects on the filament thickness of all examined strains of A. gracile and both C. raciborskii strains (see Supplementary table 3 for statistics). Filament thickness in all strains of A. gracile increased in the presence of exudates (). Sodium octyl sulphate triggered the same significant response as exudates in all strains of A. gracile, except ZHL1B (). Although a difference between SOS treatment and control for ZHL1B was not detected, the trend was the same as in the SOS sensitive strains of A. gracile. The strongest increase of filament thickness was found in A. gracile strain SAG 31.79 (). In C. raciborskii, both types of grazer signals (exudates and SOS) triggered an increase in the thickness of filaments in the strain SAG 1.97 (), whereas, in the strain BUSZ3, only SOS caused an increase in filament thickness (). Strains of P. agardhii belonging to the order of Oscillatoriales reacted to exudates or SOS differently to Nostocales (–). Filaments of the strain ZHJ1B exposed to SOS were significantly thinner in comparison to controls. The filaments of this strain grown with exudates also had a tendency to get thinner but it was not statistically significant. The effect of exudates and SOS on the second oscillatorialean strain SAG 6.89 was not significant (Supplementary table 3), but the mean thickness in both treatments was similarly lower than in controls.
Daphnia exudates and sodium octyl sulphate did not affect significantly the length of filaments in almost all examined strains (see Supplementary table 3 for statistics). The only exception was one strain of C. raciborskii, SAG 1.97, in which ANOVA showed a significant effect of Daphnia cues on filament length. The filaments of SAG 1.97 strain exposed to SOS were significantly longer in comparison to those from the control treatment (). However, when supplied with exudates this strain had the tendency to increase in length, but the difference in filament length between exudates and control treatments were statistically non-significant ().
Daphnia cues affected significantly the frequency of heterocytes occurrence in two strains of A. gracile (see Supplementary table 3 for statistics). The frequency of heterocytes in the strain CCALA 8 decreased in exudates and SOS treatments (), whereas the strain ZHL1B only in the exudates (). Daphnia exudates and SOS had no significant effect on heterocytes frequency in other examined nostocalean strains of cyanobacteria.
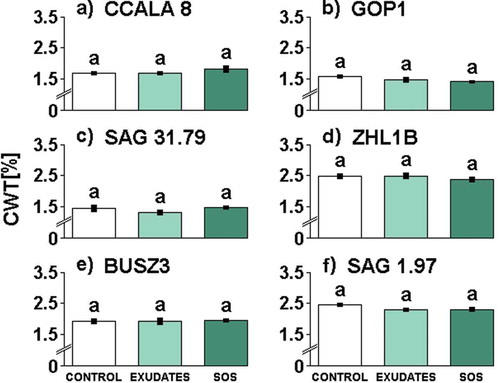
Cell wall thickness of cyanobacterial cells was examined in all investigated strains of A. gracile and C. raciborskii. The cell wall in all strains of these two species constituted a similar percentage of filament thickness (CWT) in all treatments (; see Supplementary table 3 for statistics). Irrespective of the treatment, nostocalean strains exhibited high variability in CWT that ranged from 1.36 to 2.51% (A. gracile) and from 1.90 to 2.39% (C. raciborskii). In each species of cyanobacterium, we found a strain that had a markedly higher CWT. These were the strains ZHL1B with CWT that ranged from 2.38 to 2.53% () and SAG 1.97 with CWT from 2.19 to 2.39% ().
Fig. 4. The influence of different media filtrates (Control, Exudates or SOS) on relative cell wall thickness (CWT) in Aphanizomenon gracile (a, b, c, d), Cylindrospermopsis raciborskii (e, f). The CWT values are expressed in % of the filament thickness. Different letters above the bars indicate significant differences highlighted by Tukey’s HSD test at the significance level of P = 0.05.
Nutrient and toxins analyses
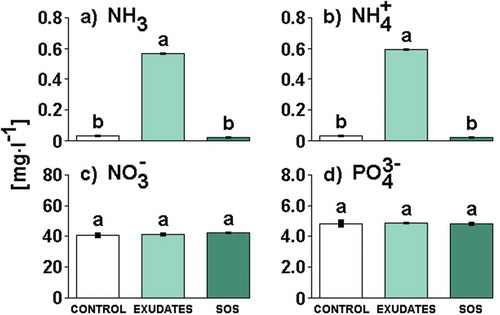
Culture media filtrates differed significantly in the concentration of ammonia and ammonium (see Supplementary table 4 for statistics). Tukey’s tests showed that the concentrations of these nutrients significantly increased in the Daphnia exudates medium filtrate in comparison to the control or SOS medium filtrate (). The concentration of ammonia and ammonium did not differ between the SOS and control medium filtrate. Culture media filtrates also did not differ in the concentration of nitrate and orthophosphates (; see Supplementary table 4 for statistics).
Fig. 5. The concentration of ammonia (a), ammonium (b), nitrate (c) and orthophosphates (d) in culture media filtrates (Control, Exudates or SOS). Different letters above the bars indicate significant differences highlighted by Tukey’s HSD test at the significance level of P = 0.05.
None of the examined strains contained cylindrospermopsin in the cells, as confirmed by HPLC-DAD and the more sensitive HPLC-MS/MS. Concerning microcystins, both methods revealed the presence of MC-RR, -YR and -LR in P. agardhii strain SAG 6.89 (Supplementary table 2). HPLC-MS/MS analyses also revealed the presence of a demethyl group of MC-RR and demethyl group of MC-LR. Other examined strains did not contain these forms of microcystins.
Discussion
The first evidence of increased thickness of cyanobacterial filaments in response to the presence of Daphnia grazing or grazer’s exudates was reported for A. gracile strain SAG 31.79 (Cerbin et al., Citation2013). In the present study, we found filament thickening in other strains of A. gracile as well as in another nostocalean cyanobacterium, C. raciborskii. When exposed to Daphnia exudates, all strains of A. gracile as well as one of two C. raciborskii strains, SAG 1.97, had significantly thicker filaments in comparison to controls (). Interestingly, culture enrichment with sodium octyl sulphate triggered the same type of a response in almost all of the strains that responded to the exudates released by daphnids. Yasumoto et al. (Citation2005) showed evidence that sodium octyl sulphate can induce colony formation in the green alga Scenedesmus gutwinskii, and Van Gremberghe et al. (Citation2009) observed the increase of microcystin production in the strain T3 of Microcystis aeruginosa. Oscillatorialean strains of P. agardhii were also sensitive to Daphnia exudates or sodium octyl sulphate but, in contrast to nostocalean strains, they significantly decreased or had a tendency to decrease the filament thickness (–).
These results are interesting and worthy of future investigation to answer the question why Daphnia exudates and sodium octyl sulphate induced thickening of filaments only in nostocalean cyanobacteria. Perhaps, they assimilate nutrients and incorporate them mostly into their carbon skeleton, and thus grow thicker and are simultaneously more resistant to grazing. On the other hand, it might be a defence mechanism evolved during a long-term coevolution of cyanobacteria and their grazers. We consider this scenario because filament thickening was observed in nostocalean strains exposed to sodium octyl sulphate that did not enhance the medium with ammonium (). However, oscillatorialean strains of P. agardhii did not exhibit filament thickening in response to Daphnia cues. A possible explanation is that P. agardhii possesses other unique features which guarantee grazing resistance, such as mucilaginous envelopes or toxins. For example, species of the genus Planktothrix can often form sheaths or mucilaginous envelopes under stress conditions (e.g. grazing, nutrient limitation or unfavourable light conditions) (Komárek & Komárková, Citation2004). Thanks to these mucilaginous sheaths, cyanobacteria can survive grazer gut passage (Kerfoot et al., Citation1988). It was interesting that, in our experiment, strains of P. agardhii often possessed white envelopes around the filaments (Supplementary fig. 2). These structures were found not only in the exudates and SOS treatments but also in controls, which may suggest that constant presence of mucilaginous sheaths in our strains is a constitutive defence against grazing. However, other environmental factors including light and nutrient conditions can also play a role, but these were not tested in our experiments. In contrast, species of the genus Aphanizomenon and Cylindrospermopsis do not produce mucilaginous envelopes as Planktothrix does (Komárek, Citation2013), therefore, they probably defend against grazers by increasing in thickness. In respect to toxin production, we found three homologues of microcystins (MC-RR, MC-YR and MC-LR) as well as a demethyl group of MC-RR and MC-LR in the examined P. agardhii SAG 6.89 strain, whereas cylindrospermopsin was not detected in any of the strains. It should be noted that filament thickening was not observed in strains that possessed other chemical or morphological means of defence against grazing. However, A. gracile, C. raciborskii and P. agardhii are potentially able to produce neurotoxins and toxic lipopolysaccharides (Bláha et al., Citation2009), and it is necessary to analyse strains in terms of these toxic compounds in the future.
Data obtained from TEM allow us to refute the hypothesis that the cell wall thickness in filamentous cyanobacteria directly increases when Daphnia cues are present. Although nostocalean cyanobacteria had thicker filaments in the presence of Daphnia exudates or sodium octyl sulphate, the cell wall constituted a similar percentage of the filament thickness in all treatments of these strains. For this reason, we can state that our strains have not evolved a defence strategy against grazing consisting of the filament being more resistant by direct thickening of the cell walls. However, these microbes can build up the resistance against grazers just by increasing filament thickness. This scenario is possible because thin filaments of A. gracile were found to be more vulnerable to grazing than thick ones (Wejnerowski et al., Citation2015). Moreover, taking into account that the cell wall thickness is positively correlated with filament thickness in A. gracile (Wejnerowski et al., Citation2016) and is also responsible for the filament rigidity in cyanobacteria (Hoiczyk & Hansel, Citation2000), increasing thickness of the filaments causes an increase of cell wall thickness. In our opinion, this phenomenon leads also to the increase of the resistance of filaments against damage from grazing.
Contrary to a previous study by Cerbin et al. (Citation2013), we did not detect filament shortening in the presence of Daphnia exudates or sodium octyl sulphate in any strains of A. gracile, although all of them exhibited thicker filaments in such conditions. The reason for this contradiction might lie in different cultivation methods and, consequently, different growth conditions. In the previous study (Cerbin et al., Citation2013), WC medium was not supplied to the A. gracile cultures during the experiment, whereas in this study we used a semi-continuous cultivation method with 10% medium replacement every second day. A nutrient shortage due to lack of fresh medium supply might be the source of the trade-off between the thickness and length of filaments observed in the previous study. This is in accordance with Kruskopf & Du Plessis (Citation2006), which showed that nutrient scarcity can result in shorter cyanobacterial filaments. Better growth conditions provided in this study by the semi-continuous method of cultivation probably prevented prolonged nutrient depletion in cultures. Strains of the other nostocalean cyanobacterium, C. raciborskii, did not shorten its filaments either. At the end of the experiment, filaments of BUSZ3 strain were of a similar length in all treatments, whereas those of the SAG 1.97 strain tended even to increase in length in the presence of Daphnia cues. This increase was evident and statistically significant in the treatment with sodium octyl sulphate. The tendency to increase filaments length observed for the SAG 1.97 strain exposed to Daphnia cues may also suggest that growth conditions during the experiment were adequate, allowing filaments to increase both in thickness and in length.
Many studies have shown that nutrients can affect the morphology and physiology of phytoplankton (Kohl et al., Citation1985; Sommer, Citation1985; Komárková et al., Citation1999; Zapomělová et al., Citation2008), and that grazers can regenerate nutrients by excretion, and hence they can positively affect phytoplankton growth, especially when nutrients are limited (Lehman & Sandgren, Citation1985; Sterner, Citation1986; Elser et al., Citation1987). Attention to the influence of nutrient recycling by zooplankton on cyanobacteria has revealed that simultaneous nutrient enrichment and zooplankton presence can promote the growth of these organisms (Wang et al., Citation2010; Hong et al., Citation2015). In the face of these reports, we cannot exclude that the results obtained in our study (i.e. the filament thickening in nostocalean cyanobacteria) were due to the presence of either ammonia or ammonium recycled by D. magna into the medium. Our suspicion is in accordance with the results provided by Kohl et al. (Citation1985) who revealed that availability of ammonium causes filament thickening in Aphanizomenon species. Moreover, the protonated form of ammonium is the preferred source of nitrogen for cyanobacteria (Ohmori et al., Citation1977; Tandeau de Marsac & Houmard, 1993) and can be readily incorporated into cell biomass (Flores & Herrero, Citation1994). These forms of nitrogen can favour non-heterocytous cyanobacteria rather than N2-fixers (Donald et al., Citation2011), and hence, these cyanobacteria (especially of the Planktothrix genus) can often successfully outcompete N2-fixing cyanobacteria in natural reservoirs (Ferber et al., Citation2004; Bugajev & Koreiviene, Citation2015). Taking into account these reports, the lack of filament thickening observed in our two strains of non-heterocytous cyanobacterium P. agardhii was perhaps due to these cyanobacteria exploiting nutrients recycled from zooplankton in a different way than nostocalean cyanobacteria. For example, they might utilize nutrients to accelerate growth. Availability of ammonium can inhibit heterocyte differentiation and also can result in a reduced frequency of heterocyte occurrence in filaments of some N2-fixers (Bothe, Citation1982; Guerrero & Lara, Citation1987; Adams & Duggan, Citation1999). In our study, only one strain (CCALA 8) had a markedly reduced frequency of heterocytes in the presence of Daphnia exudates and sodium octyl sulphate, whereas for other strains Daphnia cues had a weak influence on this parameter. The results obtained for A. gracile indicate that changes in the frequency of heterocyte occurrence in response to Daphnia exudates and sodium octyl sulphate might be strain specific.
However, daphnids do not only excrete ammonia and ammonium. They can also exude aliphatic sulphates and sulfamates that can be perceived by phytoplankters as alarm substances triggering defensive responses (Jang et al., Citation2003; Yasumoto et al., Citation2005, Citation2008; Van Gremberghe et al., Citation2009). As was mentioned earlier, sodium octyl sulphate triggered filament thickening in nostocalean cyanobacteria, exactly as Daphnia exudates did. In addition, sodium octyl sulphate and standard WC medium used for the experiment had the same concentrations of nutrients. For these reasons, our results did not allow us to unambiguously state that filament thickening observed in the presence of Daphnia exudates is due to assimilation and accumulation of a nutrient surplus (NH3 and NH4+) supplied by daphnids. This finding indicates clearly that the ‘nutrient regeneration’ hypothesis alone cannot explain the phenomenon of filament thickening in cyanobacteria. Some compounds such as ammonia and ammonium are perhaps assimilated and incorporated into cyanobacterial cells and promote the growth of cyanobacteria, which also results in filament thickening. However, the same response was caused by sodium octyl sulphate, which carries information concerning the threat of grazing. It indicates that the same morphological response might be caused by two independent factors (nutrients and kairomones).
To conclude, we found that Daphnia exudates (containing a large amount of ammonium) as well as commercially available Daphnia kairomone, sodium octyl sulphate (free of ammonium), induced filament thickening in nostocalean cyanobacteria. It suggests that filament thickening in response to Daphnia exudates and sodium octyl sulphate might be a chemical defence against grazing. In future studies, we will attempt to test whether the induction of filament thickening in cyanobacteria by Daphnia cues reduces the fitness of animals as well as if Daphnia cues stimulate faster growth of cyanobacteria.
Author contributions
L. Wejnerowski and S. Cerbin designed the study; L. Wejnerowski carried out the experiments, analysed data, drafted and finalized the manuscript; S. Cerbin and M.K. Dziuba revised the manuscript; L. Wejnerowski and M.K. Wojciechowicz prepared samples for TEM and analysed ultrastructure of filaments; M. Glama prepared ultra-thin sections for TEM; T. Jurczak performed HPLC-DAD analyses; L. Wejnerowski and J. Meriluoto performed HPLC-MS/MS analyses; M.K. Dziuba was responsible for Daphnia cultivation. All authors read and approved the final manuscript.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2018.1442585
Supplementary table 1. Basic data on examined strains of cyanobacteria.
Supplementary table 2. The size of inoculum (filament density; mean ± standard deviation), filament morphology (mean ± standard deviation), and toxicity of each cyanobacterial strain in the experiments.
Supplementary table 3. Results of one-way ANOVA for filament thickness, filament length, heterocyte frequency, and relative cell wall thickness of cyanobacteria at the end of the experiment.
Supplementary table 4. Results of one-way ANOVA for concentrations of ammonium, ammonium ions, nitrate ions, and phosphates measured in culture media filtrates.
Supplementary fig. 1. Measurements of turbidity in cultures of A. gracile, C. raciborskii, P. agardhii grown in semi-continuous growth conditions.
Supplementary fig. 2. TEM showing longitudinal sections of filaments of P. agardhii strain SAG 6.89 and strain ZHJ1B.
Supplementary fig. 3. Scheme of cyanobacterial filament showing locations measurements of cell wall thickness and filament thickness. TEM showing longitudinal sections of specimens of CCALA8, GOP1, SAG 31.79, ZHL1B, BUSZ3, SAG 1.97, showing ultrastructure of cells.
Supplementary_material.zip
Download Zip (1.5 MB)Acknowledgements
The authors would like to thank Markéta Bohunická (Research and Breeding Institute of Pomology Holovousy Ltd., Holovousy, Czech Republic) for providing the cyanobacterial strain CCALA8, Anna Kozłowska (Adam Mickiewicz University) for her help in maintaining of the cultures, and two anonymous reviewers for their valuable criticism and suggestions, which helped to improve the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adams, D.G. & Duggan, P.S. (1999). Tansley Review no. 107. Heterocyst and akinete differentiation in cyanobacteria. New Phytologist, 144: 3–33.
- Bláha, L., Babica, P. & Maršálek, B. (2009). Toxin produced in cyanobacterial water blooms – toxicity and risks. Interdisciplinary Toxicology, 2: 36–41.
- Bothe, H. (1982). Nitrogen fixation. In The Biology of Cyanobacteria (Carr, N.G. & Whitton, B.A., editors), 87–104. Blackwell, Oxford.
- Bugajev, A.O. & Koreiviene, J. (2015). Determining optimal growth conditions for the highest biomass microalgae species in Lithuanian part of the Curonian Lagoon for further cultivation. International Journal of Environmental Research, 9: 233–246.
- Burns, C.W. (1968). Direct observations of mechanisms regulating feeding behaviour of Daphnia, in lakewater. International Revue der Gesamten Hydrobiologie und Hydrographie, 53: 83–100.
- Cerbin, S., Wejnerowski, L. & Dziuba, M. (2013). Aphanizomenon gracile increases in width in the presence of Daphnia. A defence mechanism against grazing? Journal of Limnology, 72: 505–511.
- Christiansen, G., Molitor, C., Philmus, B. & Kurmayer, R. (2008). Nontoxic strains of cyanobacteria are the result of major gene deletion events induced by a transposable element. Molecular Biology and Evolution, 25: 1695–1704.
- Dokulil, M.T. & Teubner, K. (2000). Cyanobacterial dominance in lakes. Hydrobiologia, 438: 1–12.
- Donald, D.B., Bogard, M.J., Finlay, K. & Leavitt, P.R. (2011). Comparative effects of urea, ammonium, and nitrate on phytoplankton abundance, community composition, and toxicity in hypereutrophic freshwaters. Limnology and Oceanography, 56: 2161–2175.
- Elser, J.J., Goff, N.C., MacKay, N.A., Amand, A.L.St., Elser, M.M. & Carpenter, S.R. (1987). Species-specific algal responses to zooplankton: experimental and field observations in three nutrient-limited lakes. Journal of Plankton Research, 9: 699–717.
- Elser, J.J., Gudex, L., Kyle, M., Ishikawa, T. & Urabe, J. (2001). Effects of zooplankton on nutrient availability and seston C: N: P stoichiometry in inshore waters of Lake Biwa, Japan. Limnology, 2: 91–100.
- Ferber, L.R., Levine, S.N., Lini, A. & Livingston, G.P. (2004). Do cyanobacteria dominate in eutrophic lakes because they fix atmospheric nitrogen? Freshwater Biology, 49: 690–708.
- Fiałkowska, E. & Pajdak-Stós A. (2014). Chemical and mechanical signals in inducing Phormidium (Cyanobacteria) defence against their grazers. FEMS Microbiology Ecology, 89: 659–669.
- Flores, E. & Herrero, A. (1994). Assimilatory nitrogen metabolism and its regulation. In The Molecular Biology of Cyanobacteria (Bryant, D.A., editor), 487–517. Kluwer Academic, Dordrecht.
- Ger, K.A., Urrutia-Cordero, P., Frost, P.C., Hansson, L.A., Sarnelle, O., Wilson, A.E. & Lürling, M. (2016). The interaction between cyanobacteria and zooplankton in a more eutrophic world. Harmful Algae, 54: 128–144.
- Gliwicz, Z.M. (1990). Why do cladocerans fail to control algal blooms? Hydrobiologia, 200/201: 83–97.
- Gliwicz, Z.M. & Siedlar, E. (1980). Food size limitation and algae interfering with food collection in Daphnia. Archiv für Hydrobiologie, 88: 155–177.
- Guerrero, M.G. & Lara, C. (1987). Assimilation of inorganic nitrogen. In The Cyanobacteria (Fay, P. & Van Baalen, C., editors), 163–185. Elsevier Science, Amsterdam.
- Guillard, R.R.L. & Lorenzen, C.J. (1972). Yellow-green algae with chlorophyllide c. Journal of Phycology, 8: 10–14.
- Hautala, H., Lemminmäki, U, Spoof, L., Nybom, S., Meriluoto, J. & Vehniäinen, M. (2013). Quantitative PCR detection and improved sample preparation of microcystin‐producing Anabaena, Microcystis and Planktothrix. Ecotoxicology and Environmental Safety, 87: 49–56.
- Hoiczyk, E. & Hansel, A. (2000). Cyanobacterial cell walls: news from an unusual prokaryotic envelope. Journal of Bacteriology, 182: 1191–1199.
- Hong, Y., Burford, M.A., Ralph, P.J. & Doblin, M.A. (2015). Subtropical zooplankton assemblage promotes the harmful cyanobacterium Cylindrospermopsis raciborskii in a mesocosm experiment. Journal of Plankton Research, 37: 90–101.
- Jang, M.H., Ha, K., Joo, G.J. & Takamura, N. (2003). Toxin production in cyanobacteria is increased by exposure to zooplankton. Freshwater Biology, 48: 1540–1550.
- Kerfoot, W.C., Levitan, C. & DeMott, W.R. (1988). Daphnia-phytoplankton interactions: density-dependent shifts in resource quality. Ecology, 69: 1806–1825.
- Kohl, J.G., Dudel, G., Schlangstedt, M. & Kühl, H. (1985). On the morphological and ecological distinction of Aphanizomenon flos-aquae Ralfs ex Born et Flah and Aphanizomenon gracile (Lemm) Lemm. Archiv für Protistenkunde, 130: 119–131.
- Kokociński, M., Mankiewicz-Boczek, J., Jurczak, T., Spoof, L., Meriluoto, J., Rejmonczyk, E., Hautala, H., Vehniäinen, M., Pawełczyk, J. & Soininen, J. (2013). Aphanizomenon gracile (Nostocales), a cylindrospermopsin-producing cyanobacterium in Polish lakes. Environmental Science and Pollution Research, 20: 5243–5264.
- Komárek, J. (2013). Cyanoprokaryota: 3. Teil/3rd part: Heterocytous genera. In Süßwasserflora von Mitteleuropa, Bd. 19/3 (Büdel, B., Gärtner, G., Krienitz, L. & Schagerl, L., editors). Springer Spectrum, Berlin, Heidelberg.
- Komárek, J. & Komárková, J. (2004). Taxonomic review of the cyanoprokaryotic genera Planktothrix and Planktothricoides. Czech Phycology, 4: 1–18.
- Komárková, J., Laudares-Silva, R. & Senna, P.A.C. (1999). Extreme morphology of Cylindrospermopsis raciborskii (Nostocales, Cyanobacteria) in the Lagoa do Peri, a freshwater coastal lagoon, Santa Catarina, Brazil. Algological Studies, 94: 207–222.
- Kruskopf, M. & Du Plessis, S. (2006). Growth and filament length of the bloom forming Oscillatoria simplicissima (Oscillatoriales, Cyanophyta) in varying N and P concentrations. Hydrobiologia, 556: 357–362.
- Kumar, K.R.A., Herrera, M. & Golden, J.W. (2010). Cyanobacterial heterocysts. Cold Spring Harbor Perspectives in Biology, 2/4/a000315.
- Lehman, J.T. & Sandgren, C.D. (1985). Species-specific rates of growth and grazing loss among freshwater algae. Limnology and Oceanography, 30: 34–46.
- Lürling, M. & Van Donk, E. (1997). Morphological changes in Scenedesmus induced by infochemicals released in situ from zooplankton grazers. Limnology and Oceanography, 42: 783–788.
- Lynch, M. (1980). Aphanizomenon blooms: alternate control and cultivation by Daphnia pulex. In Evolution and Ecology of Zooplankton Communities (Kerfoot, W.C., editor), 299–304. University Press of New England, Hanover.
- Martin-Figueroa, E., Navarro, F. & Florencio, F.J. (2000). The GS-GOGAT pathway is not operative in the heterocysts. Cloning and expression of glsF gene from the cyanobacterium Anabaena sp. PCC 7120. FEBS Letters, 476: 282–286.
- Meeks, J.C. & Elhai, J. (2002). Regulation of cellular differentiation in filamentous cyanobacteria in free-living and plant-associated symbiotic growth states. Microbiology and Molecular Biology Reviews, 66: 94–121.
- Nadin-Hurley, C.M. & Duncan, A. (1976). A comparison of daphnid gut particles with the sestonic particles present in two Thames Valley reservoirs throughout 1970 and 1971. Freshwater Biology, 6: 109–123.
- Ohmori, M., Ohmori, K. & Strotmann, K. (1977). Inhibition of nitrate uptake by ammonia in a blue-green alga, Anabaena cylindrica. Archives of Microbiology, 114: 225–229.
- Pondaven, P., Gallinari, M., Chollet, S., Bucciarelli, E., Sarthou, G., Schultes, S. & Jean, F. (2007). Grazing-induced changes in cell wall silicification in a marine diatom. Protist, 158: 21–28.
- R Core Team (2013). R: A language and environment for statistical computing. R. Foundation for Statistical Computing, Vienna. Available from: http://www.R-project.org/
- Sommer, U. (1985). Comparison between steady state and non-steady state competition: experiments with natural phytoplankton. Limnology and Oceanography, 30: 335–346.
- Sterner, R.W. (1986). Herbivores’ direct and indirect effects on algal populations. Science, 231: 605–607.
- Tandeau de Marsac, N. & Houmard, J. (1993). Adaptation of cyanobacteria to environmental stimuli: new steps towards molecular mechanisms. FEMS Microbiology Letters, 104: 119–189.
- Van Donk, E., Ianora, A. & Vos, M. (2011). Induced defences in marine and freshwater phytoplankton: a review. Hydrobiologia, 668: 3–19.
- Van Gremberghe, I., Vanormelingen, P., Van der Gucht, K., Mancheva, A., D’Hondt, S., De Meester, L. & Vyverman, W. (2009). Influence of Daphnia infochemicals on functional traits of Microcystis strains (Cyanobacteria). Hydrobiologia, 635: 147–155.
- Wang, X., Qin, B., Gao, G. & Paerl, H.W. (2010). Nutrient enrichment and selective predation by zooplankton promote Microcystis (Cyanobacteria) bloom formation. Journal of Plankton Research, 32: 457–470.
- Webster, K.E. & Peters, R.H. (1978). Some size-dependent inhibitions of larger cladoceran filters in filamentous suspensions. Limnology and Oceanography, 23: 1238–1245.
- Wejnerowski, L., Cerbin, S. & Dziuba, M.K. (2015). Thicker filaments of Aphanizomenon gracile are more harmful to Daphnia than thinner Cylindrospermopsis raciborskii. Zoological Studies, 54: 2.
- Wejnerowski, L., Cerbin, S., Wojciechowicz, M.K. & Dziuba, M.K. (2016). Differences in cell wall of thin and thick filaments of cyanobacterium Aphanizomenon gracile SAG 31.79 and their implications for different resistance to Daphnia grazing. Journal of Limnology, 75: 634–643.
- Wejnerowski, L., Cerbin, S. & Dziuba, M.K. (2017a). Setae thickening in Daphnia magna alleviates the food stress caused by the filamentous cyanobacteria. Aquatic Ecology, 51: 485–498.
- Wejnerowski, L., Wojciechowicz, M.K., Glama, M., Olechnowicz, J., Dziuba, M.K. & Cerbin, S. (2017b). Solitary terminal cells of Aphanizomenon gracile (Cyanobacteria, Nostocales) can divide and renew trichomes. Phycological Research, doi: 10.1111/pre.12182.
- Yasumoto, K., Nishigami, A., Yasumoto, M., Kasai, F., Okado, Y., Kusumi, T. & Ooi, T. (2005). Aliphatic sulfates released from Daphnia induce morphological defense of phytoplankton: isolation and synthesis of kairomones. Tetrahedron Letters, 46: 4765–4767.
- Yasumoto, K., Nishigami, A., Aoi, H., Tsuchihashi, C., Kasai, F., Kusumi, T. & Ooi, T. (2008). Isolation and absolute configuration determination of aliphatic sulfates as the Daphnia kairomones inducing morphological defense of a phytoplankton – Part 2. Chemical and Pharmaceutical Bulletin, 56: 129–132.
- Yema, L., Litchman, E., de Tezanos Pinto, P. (2016). The role of heterocytes in the physiology and ecology of bloom-forming harmful cyanobacteria. Harmful Algae, 60: 131–138.
- Zapomělová, E., Řeháková, K., Znachor, P. & Komárková, J. (2007). Morphological diversity of coiled planktonic types of the genus Anabaena (cyanobacteria) in natural populations – taxonomic consequences. Cryptogamie Algologie, 28: 353–371.
- Zapomělová, E., Hrouzek, P., Řeháková, K., Šabacká, M., Stibal, M., Caisová, L., Komárková, J. & Lukešová, A. (2008). Morphological variability in selected heterocystous cyanobacterial strains as a response to varied temperature, light intensity and medium composition. Folia Microbiologica, 53: 333–341.
