ABSTRACT
Microcystins (MCN), β-N-methylamino-L-alanine (BMAA) and anatoxin-a were investigated in Antarctic cyanobacterial mats collected from Ross Island and the McMurdo Ice Shelf, East Antarctica during Captain Scott’s ‘Discovery’ National Antarctic Expedition (1901–1904). Ultra-performance liquid chromatography-photodiode array detection (UPLC-PDA) and tandem mass spectrometry (MS/MS) analysis were used to quantify the cyanotoxins in seven cyanobacterial mat samples. MCNs were identified in six of the mat samples at concentrations from 0.5 to 16.1 µg g–1 dry weight. BMAA was found in one sample (528 ng g–1 dry weight, total BMAA), as well as two BMAA isomers, 2,4-diaminobutyric acid (DAB) and N-(2-aminoethyl) glycine (AEG) in six samples up to 6.56 and 6.79 μg g–1 dry weight, respectively. No anatoxin-a was detected. The findings confirm that MCNs, BMAA and BMAA isomers are preserved under dry herbarium conditions. The ‘Discovery’ cyanobacterial mat samples represent the oldest polar cyanobacterial samples found to contain cyanotoxins to date and provide new baseline data for cyanotoxins in Antarctic freshwater cyanobacterial mats from prior to human activity in Antarctica, the development of the ozone hole and current levels of climatic change.
Introduction
Cyanobacteria worldwide produce diverse natural toxins known as cyanotoxins. Their mechanisms of toxicity include hepatotoxicity, neurotoxicity, dermatotoxicity, carcinogenicity, tumour promotion and genotoxicity and these compounds are of considerable significance in multiple fields, from ecology to public health (Codd, Citation1995; Metcalf & Codd, 2012; Pearson et al., Citation2016; Meriluoto et al., Citation2017). One group of hepatotoxins is the microcystins (MCNs) that inhibit protein-phosphatases (Mackintosh et al., Citation1990), acute exposure to which can lead to liver failure and death (Falconer et al., Citation1994; Chorus & Bartram, Citation1999). In temperate and tropical environments, planktonic genera including Microcystis and Dolichospermum are the most commonly reported MCN-producers; Phormidium and Nostoc are among the commonest benthic MCN producers (Chorus & Bartram, Citation1999; Izaguirre et al., Citation2007; Wood et al., Citation2010).
Other well-studied cyanotoxins include the bicyclic amine alkaloid, anatoxin-a and its methylene homologue homoanatoxin-a (Dittmann et al., Citation2013). These neurotoxins act by disrupting signal transmission between neurons and muscles which can lead to death by respiratory arrest (Carmichael et al., 1975; Aráoz et al., Citation2010; Metcalf & Codd, Citation2012). They have been found in brackish and freshwater environments in temperate and tropical ecosystems in many countries, and shown to be produced by various species in the genera Dolichospermum, Oscillatoria, Phormidium and Aphanizomenon (Aráoz et al., Citation2010).
In contrast to MCNs and anatoxin-a, which have been investigated in cyanobacteria since the 1970s, β-N-methylamino-L-alanine (BMAA) was more recently found to be produced by a range of cyanobacteria (Cox et al., Citation2003, Citation2005; Esterhuizen & Downing, Citation2008; Downing et al., Citation2011). However, the occurrence of BMAA in cycads has been known for almost 50 years (Vega & Bell, Citation1967; Vega et al., Citation1968; Nunn, Citation2017). Although BMAA can be associated with peptides and proteins, it is a non-encoded amino acid, i.e. one for which no known codon exists (Humphrey & Chamberlin, Citation1997; Nunn & Codd, Citation2017). The ingestion of BMAA has been associated with the human neurodegenerative disease Amyotrophic Lateral Sclerosis/Parkinsonism Dementia Complex (ALS/PDC) and research on causality is in progress (Cox et al., Citation2003, Citation2016; Bradley & Cox, Citation2009; Metcalf & Codd, Citation2009, Citation2012; Cox et al., Citation2016).
Despite the impact of cyanotoxins on human and animal health, and in freshwater ecology, their ecological and physiological functions are not yet well understood. Environmental conditions have been shown to influence cyanotoxin production (Neilan et al., Citation2013). Some of the potential functions of MCNs are protection against grazers, quorum-sensing, gene regulation, cell-signalling, iron-scavenging and maintenance of homeostasis, or alternatively they could be an ancestral relict (Codd, Citation1995; Kaebernick & Neilan, Citation2001; Rantala et al., Citation2004; Dittmann et al., Citation2013; Pearson et al., Citation2016).
For Antarctic freshwater ecosystems, we know of no investigations to date on BMAA and anatoxin-a, but several studies have found MCNs in freshly collected samples of benthic cyanobacterial mats from meltwater ponds on the McMurdo Ice Shelf (Hitzfeld et al., Citation2000; Jungblut et al., Citation2006), from ponds, lakes and hydro-terrestrial environments in the McMurdo Dry Valleys (Wood et al., Citation2008) and from ponds and lakes in the Antarctic Peninsula (Kleinteich et al., Citation2012, Citation2013). Common planktonic MCN producers such as Microcystis are not known from polar freshwater ecosystems and although the genus Phormidium is part of Antarctic cyanobacterial assemblages, Nostoc has been proposed as the most likely MCN producer based on morphological and DNA analysis (Jungblut et al., Citation2006; Wood et al., Citation2008; Cirés et al., Citation2017).
Climate models predict that Antarctica will warm more rapidly than many other parts of the globe. Substantial warming has already been documented for the Western Antarctica Peninsula, and more widespread warming of continental Antarctica is expected to accelerate (Turner et al., Citation2005, Citation2014). In Antarctica, warming is especially important for freshwater ecosystems, as it has 70% of the freshwater supplies on Earth and the continent, once regarded as lifeless, is now known to have a rich and diverse biology, dominated by microbial diversity, and to be an important contributor to carbon and nutrient cycling (Jungblut & Vincent, Citation2017). Much of the productivity and biodiversity of Antarctica is linked to benthic cyanobacteria and cyanobacteria-based microbial mat communities. However, to identify and forecast biological responses to environmental change, baseline data are essential to understand how, and over what time scale, environmental change affects cyanobacterial biology such as cyanotoxin production in freshwater ecosystems. This is especially important as recent laboratory studies by Kleinteich et al. (Citation2012) and co-workers suggested that MCNs are not only present in Polar freshwater ecosystems but also could increase with prolonged elevated water temperatures due to either preferential growth of toxigenic species or increased MCN biosynthesis.
As a previous study by Metcalf et al. (Citation2012) showed that MCNs were detectable in herbarium specimens collected 60–170 years ago from temperate and tropical environments, we carried out an investigation of MCNs, anatoxin-a and BMAA in Antarctic freshwater cyanobacterial mats collected in 1902–1903 during Captain Scott’s ‘Discovery’ expedition to Ross Island and the McMurdo Ice Shelf (1901–1904). This expedition not only had the geographic exploration of Antarctica as its core mission but also a diverse scientific research programme in biology, zoology, geology, meteorology and magnetism. A large collection of specimens was made during the two-year stay on Ross Island, Eastern Antarctica, many of which were deposited at the Natural History Museum in London, including samples of freshwater cyanobacterial mats. The aims of our study were to evaluate the presence of cyanotoxins in samples collected over 100 years ago from Antarctica before it was impacted by human activity, the ozone hole and the current rapid, climatic-driven environmental change.
Materials and methods
Captain Scott’s ‘Discovery’ cyanobacterial mat samples and preservation
Six cyanobacterial mat samples () collected from ponds and sediment accumulated on the ice (also called eskers) during the ‘Discovery’ National Antarctic Expedition led by Captain Robert Falcon Scott between February 1902 and December 1903 in Southern Victoria Land (Fritsch, Citation1912) were investigated. The dried herbarium specimens of cyanobacterial mats are stored in archive quality, acid-free paper folders at room temperature in the dark in the herbarium of the Natural History Museum, London, UK. No records are available concerning how the specimens were dried and stored for the first half-century after receipt, but based on expedition records (Scott, Citation1907), herbarium specimens were likely to have been dried and pressed directly after collection by Dr R. Koettlitz (Jungblut & Hawes, Citation2017).
Table 1. Original descriptions of cyanobacterial mat samples collected during Captain Scott’s ‘Discovery’ expedition.
Of the six cyanobacterial mat samples, one is from the expedition’s winter quarters on Ross Island. Winter quarters were located in the bay where the RRS Discovery was ice-locked for over two years, within the current vicinity of McMurdo Station, Antarctica (). The other five mat samples are from the McMurdo Ice Shelf between Brown and Black Peninsula (Fritsch, Citation1912) (). The McMurdo Ice Shelf is located south-south-west of the winter quarters and was probably reached on foot or by dog sledges. Cyanobacterial mat specimens were most probably collected by Dr R. Koettlitz. Dr Koettlitz remained at the winter quarters carrying out scientific work including phycological studies, while Dr Wilson joined Captain Scott on the geographic explorations towards the Polar Plateau over the winter of 1902–1903.
Fig. 1. Locations of the cyanobacterial mat specimens collected during Captain Scott’s “Discovery” National Antarctic Expedition at their winter quarters on Ross Island and eskers on the McMurdo Ice Shelf halfway between Black Island (BI) and Brown Peninsula (BP) in 1902–1903, with (inset) geographic location of Ross Island (RI) and McMurdo Ice Shelf (MIS) in Antarctica; black square.
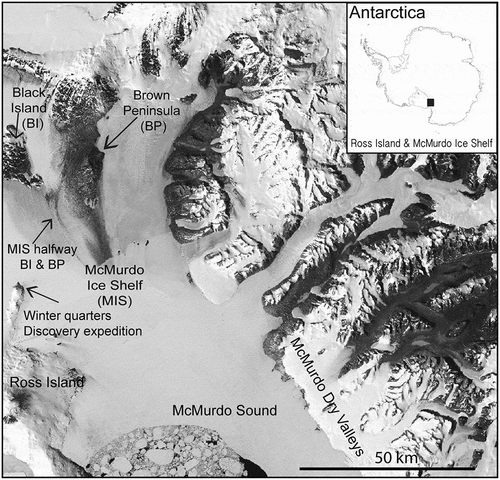
Fig. 2. Herbarium specimen (BM001062583) collected on the McMurdo Ice Shelf, Antarctica during Captain Scott’s Discovery Expedition in December 1902.

Cyanobacterial 16S rRNA analysis of the genetic diversity of the microbial mats has indicated the presence of the cyanobacterial genera Leptolyngbya, Phormidium, Pseudanabaena, Nodularia, Nostoc and Chamaesiphon with a total of 267 16S rRNA gene Operational Taxonomic Units (OTUs) using pyrosequencing (Jungblut & Hawes, Citation2017).
Microcystin, anatoxin-a and BMAA extraction and analysis
The dried cyanobacterial mat specimens were sampled directly. Three sets of sub-samples were taken for each of the seven mat samples: one set for MCNs, one for anatoxin-a and one for BMAA. In addition sample BM001062589 was analysed in duplicate for MCN, anatoxin-a and BMAA. For the anatoxin-a samples, 1 ml 100% methanol was added, the samples were vortexed and ultrasonicated, and then left to extract at room temperature for 1 h. The supernatant was dried, resuspended in 100 µl of Direct-Q® purified (DG) water and analysed for anatoxin-a by UPLC-PDA. To the weighed samples for MCNs, 70% methanol was added at a concentration of 50 mg ml–1, again the samples were vortexed and ultrasonicated, and then left to extract for 1 h at room temperature. The suspension was centrifuged and the supernatant was analysed for MCNs by UPLC-PDA. The remaining supernatant (70% methanol) was dried and resuspended with 100 µl of 70% methanol (10× concentration) and again analysed by UPLC-PDA for MCNs. Triplicate injections were performed on each sample and the spectra compared with MCN-LR as a standard (Sigma Chemical Co., St. Louis, MO). Once analysed, the chromatograms were assessed for peaks that had MCN-like spectra, and the concentration of these peaks was calculated, with reference to the MCN-LR standard (Metcalf et al., Citation2012).
The sub-sample for total BMAA analysis was hydrolysed in 6 M HCl at 110ºC for 16 h. After hydrolysis, the supernatant was centrifuged through 0.22 μm centrifuge filters and the filtrate dried in a speedvac (Thermo Savant Speedvac Plus SC250DDA). The filtrate was resuspended with 200 μl 20 mM HCl and derivatized with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (AQC) for UPLC-MS/MS according to Masseret et al. (Citation2013) for the presence of BMAA and its isomers with reference to analytical standards as previously described.
Results
Microcystins (MCNs) were identified in three of the six samples (BM001062583, BM001062587, BM001062589) with at least one MCN variant in cyanobacterial mats collected from ponds and ice eskers between December 1902 and February 1903 in Southern Victoria Land (). Sample BM001062583 had most variants with up to five being identified. MCN concentrations varied between 0.4 µg g–1 and 16.1 µg g–1 dry weight microbial mat, depending on the sample and extraction method. The highest concentration of MCN was detected in sample BM001062583 using analysis of the 70% methanol extract at 50 mg ml–1. Anatoxin-a was not detected in any mat samples analysed at a detection limit of 2 µg g–1 by UPLC-PDA.
Table 2. Determination of microcystins from cyanobacterial mat samples collected during Captain Scott’s ‘Discovery’ expedition in 1902 and 1903.
BMAA was identified in sample BM001062590, with N-(2-aminoethyl)-glycine (AEG) and 2,4-diaminobutyric acid (DAB) in five out of the six samples (, ). Concentrations were determined based on the transition of 459 > 171 in the MS/MS (n=3), with BMAA present at a concentration of 528 ng g–1 dry weight in sample BM001062590. AEG ranged from 737 ng g–1 in 590 and up to 6791 ng g–1 in sample 587. The highest concentration of 6562 ng g–1 was identified in BM001062589 for DAB.
Fig. 3. UPLC-MS/MS analysis of BMAA and isomers in 100-year old Antarctic cyanobacterial mat material. Individual panels show MS transformations of parent to daughter ion of BMAA isomers. AEG, N-(2-aminoethyl)glycine, BMAA, β-N-methylamino-L-alanine; DAB, 2,4-diaminobutyric acid.
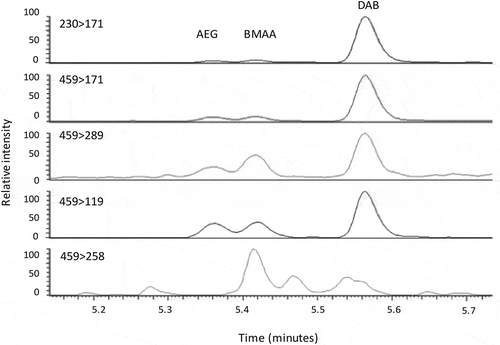
Table 3. Determination of total AEG, BMAA and DAB (ng g–1) in cyanobacterial mat samples collected during Captain Scott’s ‘Discovery’ Expedition in 1902 and 1903.
Discussion
We found that MCNs, BMAA and its isomers AEG and DAB are present in samples of Antarctic benthic cyanobacterial mat communities collected over 100 years ago during Captain Scott’s ‘Discovery’ expedition from the Ross Island and McMurdo Ice Shelf regions. They are among the oldest herbarium cyanobacterial mat material found to contain MCNs (Metcalf et al., Citation2012) and the oldest polar cyanobacteria with confirmed detection of BMAA, AEG and DAB to date.
The detected MCN concentrations of up to 16.3 µg g–1 per dry weight were considerably lower than commonly found in cyanobacterial blooms from temperate latitudes, which can have concentrations as high as 10 mg MCN g–1 dry mass (Chorus & Bartram, Citation1999), but within the range of MCN concentrations reported from cyanobacterial mat communities that were processed for cyanotoxin analysis shortly after recent collection in Antarctic (11.4 µg MC-LR g–1 dry mass, Jungblut et al., Citation2006; 1–16 µg g–1 dry mass, Wood et al., Citation2008; 11–303 ng g–1 organic mass, Kleinteich et al., Citation2014) and Arctic habitats (106 ng MC g–1 dry mass, Kleinteich et al., Citation2013). The Antarctic cyanobacterial communities in the 1902–1903 Discovery Expedition samples comprised the common Polar benthic genera Leptolyngbya, Phormidium, Pseudanabaena, Nodularia, Nostoc and Chamaesiphon (Jungblut & Hawes, Citation2017) similar to recent collections by Jungblut et al. (Citation2006, Citation2010) and Kleinteich et al. (Citation2012). As previous studies on Antarctic MCNs have suggested, the low concentrations of MCNs may be due to the abundance of only one MCN-producing species in the collected material, and/or to low levels of MCN biosynthesis by more dominant members of the community in the material.
The ‘Discovery’ expedition samples were stored at room temperature for over 100 years and it is probable that degradation of the biomass and of the cyanotoxins had occurred (Metcalf et al., Citation2012): thus, the original concentrations of the toxins might have been higher when the samples were collected in 1902 and 1903. In the current study, we determined concentrations of up 16.3 µg g–1 per dry weight microbial mat which is in the upper range of concentrations determined to date for fresh Antarctic microbial mats (Cirés et al., Citation2017) and therefore the fresh material from the Discovery samples might have had concentrations higher than currently reported from modern microbial mat samples.
The finding of the cyanotoxin BMAA and its isomers AEG and DAB in Antarctic freshwater cyanobacterial mats expands the known geographic range of these neurotoxins. Until now, BMAA had only been described from temperate, desert and tropical aquatic and terrestrial environments (Cox et al., Citation2005). Concentrations of BMAA determined from the historic Antarctic mats were considerably lower than for cultured cyanobacterial isolates (Cox et al., Citation2005) and recent environmental samples (Metcalf et al., Citation2008). However, as with the MCNs, it is not known whether the differences are due to degradation of the samples during storage at room temperature for over 100 years, or to lower concentrations at the time of collection. The results are consistent with the finding of BMAA in 50-year-old dried, stored museum specimens of flying foxes (Banack & Cox, Citation2003) and from flying fox museum specimens stored both dried and fluid-preserved for 20 or more years (Banack et al., Citation2006). It is not possible to determine which Antarctic cyanobacterial genera contributed to the measured BMAA, AEG and DAB concentrations, but strains from recently collected material and extant laboratory cultures of several genera within the mats such as Nostoc, Phormidium and Nodularia have previously been shown to produce BMAA (Cox et al., Citation2005).
The role of BMAA in Antarctic benthic ecosystems is not yet known. As BMAA production is likely to be an ancient trait, its ecological function is probably unrelated to its neurotoxicity, and the presence of this amino acid instead may function in aspects of cyanobacterial biochemistry (Cox et al., Citation2005), including nitrogen metabolism (Downing et al., Citation2011; Berntzon et al., Citation2013). It would be important to test if BMAA production increases with increased temperature (Kleinteich et al., Citation2012) or with other proposed environmental changes to simulate a scenario of climate-driven environmental change in Antarctic freshwater ecosystems.
Our study has confirmed that herbarium material can provide unique baseline data (Metcalf et al., Citation2012). These often large collections of cyanobacterial specimens represent an under-utilized resource of baseline data to extend our knowledge of cyanotoxin production into the past, including in Antarctic and potentially other climatic zones, and of cyanobacterial secondary metabolites relevant to toxicology, environmental microbiology and biogeography. Furthermore, our data demonstrated that MCNs, and potentially other secondary metabolites, may be well-preserved in dried herbarium cyanobacterial specimens in comparison to sediments in aquatic freshwater ecosystems, where MCNs can undergo biodegradation within days to weeks (Bourne et al., Citation1996; Hyenstrand et al., Citation2003). The results further demonstrate the value and potential of dried herbarium cyanobacterial collections in investigating potential effects of climatic change on cyanobacteria and cyanotoxins, and the continuing importance of Captain R.F. Scott’s scientific legacy for current science challenges in Antarctica.
Acknowledgements
We would like to thank Dr P.A. Cox for his support of the research and A. Hall for her help. The authors would like to acknowledge the Editor and reviewers for insightful comments and suggestions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Notes on contributors
A.D. Jungblut
A.D. Jungblut, G.A. Codd: conceived the study; J.S. Metcalf, S.A. Banack: performed analysis; J. Wilbraham: curation of herbarium specimens; all contributed to manuscript writing and editing.
References
- Aráoz, R., Molgó, J. & Tandeau de Marsac, N. (2010). Neurotoxic cyanobacterial toxins. Toxicon, 56: 813–828.
- Banack, S.A. & Cox, P.A. (2003). Biomagnification of cycad neurotoxins in flying foxes: implications for ALS-PDC in Guam. Neurology, 61: 387–389.
- Banack, S.A., Murch, S.J. & Cox, P.A. (2006). Neurotoxic flying foxes as dietary items for the Chamorro people, Marianas Islands, Journal of Ethnopharmacology, 201: 244–252.
- Berntzon, L., Erasmie, S., Celepli, N., Eriksson, J., Rasmussen, U. & Bergman, B. (2013). BMAA inhibits nitrogen fixation in the cyanobacterium Nostoc sp. PCC 7120. Marine Drugs, 11: 3091–3108.
- Bourne, D.G., Jones, G.J., Blakely, R.L., Jones, A., Negri, A.P. & Riddles, P. (1996). Enzymatic pathway for the bacterial degradation of the cyanobacterial cyclic peptide microcystin-LR. Applied and Environmental Microbiology, 62: 4086–4094.
- Bradley, W.G. & Cox, P.A. (2009). Beyond Guam: cyanobacteria, BMAA and sporadic amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis, 10: 5–6.
- Carmichael, W.W., Biggs, D.F., & Gorham, P.R. (1975). Toxicology and pharmacological action of Anabaena flos-aquae toxin. Science, 187: 542–544.
- Chorus, I. & Bartram, J (1999). Toxic Cyanobacteria in Water. A Guide to their Public Health Consequences, Monitoring and Management. World Health Organization. E. & F.N. Spon, London.
- Cirés, S., Casero, M.C. & Quesada A. (2017). Toxicity at the edge of life: a review on cyanobacterial toxins from extreme environments. Marine Drugs, 15: 233, doi:10.3390/md15070233.
- Codd, G.A. (1995). Cyanobacterial toxins: occurrence, properties and biological significance. Water Science and Technology, 32: 149–156.
- Cox, P.A., Banack, S.A. & Murch, S.J. (2003). Biomagnification of cyanobacterial neurotoxins and neurodegenerative disease among the Chamorro people of Guam. Proceedings of the National Academy of Sciences USA, 100: 13380–13383.
- Cox, P.A., Banack, S.B., Murch, S.J., Rasmussen, U., Tien, U., Bidigare, R.R., Metcalf, J.S., Morrison, L.F., Codd, G.A. & Bergman, B. (2005). Diverse taxa of cyanobacteria produce β-N-methylamino-L-alanine (BMAA), a neurotoxic amino acid. Proceedings of the National Academy of Sciences USA, 102: 5074–5078.
- Cox, P.A., Davis, D.A., Mash, D.C., Metcalf, J.S. & Banack, S.A. (2016). Dietary exposure to an environmental toxin triggers neurofibrillary tangles and amyloid deposits in the brain. Proceedings of the Royal Society B: Biological Sciences, 283: 2015–2397.
- Dittmann, E., Fewer, D.P. & Neilan, B.A. (2013). Cyanobacterial toxins: biosynthetic routes and evolutionary roots. FEMS Microbiology Reviews, 37: 23–43.
- Downing, S., Banack, S.A., Metcalf, J.S., Cox, P.A. & Downing, T.G. (2011). Nitrogen starvation of cyanobacteria results in the production of β-N-methylamino-L-alanine. Toxicon, 58: 187–194.
- Esterhuizen, M. & Downing, T.G. (2008). β-N-methylamino-L-alanine (BMAA) in novel South African cyanobacterial isolates. Ecotoxicology and Environmental Safety, 71: 309–13.
- Falconer, I., Burch, M., Steffensen, D., Choice, M. & Coverdale, O. (1994). Toxicity of the blue-green alga (cyanobacterium) Microcystis aeruginosa in drinking water to growing pigs, as an animal model for human injury and risk assessment. Journal of Environmental Toxicology and Water Quality, 9: 131–139.
- Fritsch, F.E. (1912). Freshwater algae. In British National Antarctic (Discovery) Expedition 1901–04, Natural History, Zoology and Botany (Bell, F.J., editor), Vol. 6, Ch. 2. British Museum (Natural History), London.
- Hitzfeld, B., Lampert, C.S., Spaeth, N., Mountfort, D., Kaspar, H. & Dietrich, D.R. (2000). Toxin production in cyanobacterial mats from ponds on the McMurdo Ice Shelf, Antarctica. Toxicon, 38: 1731–1748.
- Humphrey, J.M. & Chamberlin, A.R. (1997). Chemical synthesis of natural product peptides: coupling methods for the incorporation of noncoded amino acids into peptides. Chemical Reviews, 97: 2243–2266.
- Hyenstrand, P., Rohrlack, T., Beattie, K.A., Metcalf, J.S., Codd, G.A. & Christoffersen, K. (2003). Laboratory studies of dissolved radiolabelled microcystin-LR in lake water. Water Research, 37: 3299–3306.
- Izaguirre, G., Jungblut, A.-D. & Neilan, B.A. (2007). A benthic Phormidium species that produces microcystin-LR, isolated from four reservoirs in Southern California. Water Research, 41: 492–498.
- Jungblut, A.D. & Hawes, I. (2017). Using Captain Scott’s Discovery specimens to unlock the past: has Antarctic cyanobacterial diversity changed over the last 100 years? Proceedings of the Royal Society B: Biological Sciences, 284: doi: 10.1098/rspb.2017.0833.
- Jungblut, A.D. & Vincent, W.F. (2017). Cyanobacteria in polar and alpine aquatic ecosystems. In Psychrophiles: From Biodiversity to Biotechnology ( Margesin, R., editor), 181–206. Springer, Dordrecht.
- Jungblut, A.D., Hoeger, S.J, Mountfort, D., Hitzfeld, B.C., Dietrich, D.R. & Neilan, B.A. (2006). Characterization of microcystin production in an Antarctic cyanobacterial mat community. Toxicon, 47: 271–278.
- Jungblut, A.D., Lovejoy, C. & Vincent, W.F. (2010). Global distribution of cyanobacterial ecotypes in the cold biosphere. The ISME Journal, 4: 191–202.
- Kaebernick, M. & Neilan, B. A. (2001). Ecological and molecular investigations of cyanotoxin production. FEMS Microbiology Ecology, 35: 1–9.
- Kleinteich, J., Wood, S.A., Küppers, F.C., Camacho, A., Quesada, A., Frickey, T. & Dietrich, D.R. (2012). Temperature-related changes in polar cyanobacterial mat diversity and toxin production. Nature Climate Change, 2: 356–360.
- Kleinteich, J., Wood, S.A., Puddick, J., Schleheck, D., Küppers, F.C. & Dietrich, D.R. (2013). Potent toxins in Arctic environments: presence of saxitoxins and an unusual microcystin variant in Arctic freshwater ecosystems. Chemico-Biological Interactions, 206: 423–431.
- Kleinteich, J., Hildebrand, F., Wood, S.A., Cires, S, Agha, S.R., Quesada, A., Peace, D.A., Convey, P., Küppers, F.C. & Dietrich D.R. (2014). Diversity of toxin and non-toxin containing cyanobacterial mats of meltwater ponds on the Antarctic Peninsula: a pyrosequencing approach. Antarctic Science, 26: 521–532.
- Mackintosh, C., Beattie, K.A., Klumpp, S., Cohen, P. & Codd, G.A. (1990). Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Letters, 264: 187–192.
- Masseret, E., Banack, S.A., Boumediene, F., Abadie, E., Brient, L., Pernet, F., Juntas-Morales, R., Pageot, N., Metcalf, J., Cox, P., Camu, W. & the French network on BMAA/ALS. (2013). Detection of BMAA in the marine environment of an ALS cluster in Southern France. PLoS ONE, 8: e83406.
- Meriluoto, J., Spoof, L. & Codd, G.A., editors (2017). Handbook of Cyanobacterial Monitoring and Cyanotoxin Analysis. Wiley, Chichester.
- Metcalf, J.S. & Codd, G.A. (2009). Cyanobacteria, neurotoxins and water resources: are there implications for human neurodegenerative disease? Amyotrophic Lateral Sclerosis, 10: 74–78.
- Metcalf, J.S., & Codd, G.A. (2012). Cyanotoxins. In Ecology of Cyanobacteria II. Their Diversity in Space and Time (Whitton, B.A., editor), 651–675. Springer, Dordrecht.
- Metcalf, J.S., Banack, S.A., Lindsay, J., Morrison, L.F., Cox, P.A. & Codd, G.A. (2008). Co-occurrence of β-N-methylamino-L-alanine, a neurotoxic amino acid with other cyanobacterial toxins in British waterbodies, 1990–2004. Environmental Microbiology, 10: 702–708.
- Metcalf, J.S., Beattie, K.A., Purdie, E.L., Bryant, J.A., Irvine, L.M. & Codd, G.A. (2012). Analysis of microcystins and microcystin genes in 60–170-year old dried herbarium specimens of cyanobacteria. Harmful Algae, 15: 47–52.
- Neilan, B.A., Pearson, L.A., Muenchoff, J., Moffitt, M.C. & Dittmann, E. (2013). Environmental conditions that influence toxin biosynthesis in cyanobacteria. Environmental Microbiology, 15: 1239–1253.
- Nunn, P.B. (2017). 50 years of research on α-amino-β-methylaminopropionic acid (β-methylaminoalanine). Phytochemistry, 144: 271–281.
- Nunn, P.B. & Codd, G.A. (2017). Metabolic solutions to the biosynthesis of some diaminomonocarboxylic acids in nature: formation in cyanobacteria of the neurotoxins 3-N-methyl-2,3-diaminopropanoic acid (BMAA) and 2,4-diaminobutanoic acid (2,4-DAB). Phytochemistry, 144: 253–270.
- Pearson, L.A., Dittmann, E., Mazouz, R., Ongley, S.E., D’Agostino, P.M. & Neilan, B.A. (2016). The genetics, biosynthesis and regulation of toxic specialized metabolites of cyanobacteria. Harmful Algae, 54: 98–111.
- Rantala, A., Fewer, D., Hisburges, M., Rouhiainen, L., Vaitomaa, J. & Börner, T. (2004). Phylogenetic evidence for the early evolution of microcystin synthesis. Proceedings of the National Academy of Sciences USA, 101: 568–573.
- Scott, F.R. (1907). The South Polar Times. Smith, Elder & Co, London.
- Turner, J., Colwell, S.R., Marshall, G.J., Lachlan-Cope, T.A., Carelton, A.M., Jones, P.D., Lagun, V., Reid, P.A. & Iagovikina, S. (2005). Antarctic climate change during the last 50 years. International Journal of Climatology, 25: 279–294.
- Turner, J., Barrand, N.E., Bracegirdle, T.J., Convey, P., Hodgson, D.A., Jarvis, M., Jenkins, A., Marshall, G., Meredith, M.P., Roscoe, H., Shanklin, J., French, J., Goosse, H., Guglielmin, M., Gutt, J., Jacobs, S., Kennicutt, M.C., Masson-Delmotte, V., Mayewski, P., Navarro, F., Robinson, S., Scambos, T., Sparrow, M., Summerhayes, C., Speer, K. & Klepikov, A. (2014). Antarctic climate change and the environment: an update. Polar Records, 50: 237–259.
- Vega, A. & Bell, E.A. (1967). α-Amino-β-methylaminopropionic acid, a new amino acid from seeds of Cycas circinalis. Phytochemistry, 6: 759–762.
- Vega, A., Bell, E.A. & Nunn, P.B. (1968). L- and D-α-amino-β-methylaminopropionic acids and the identification of the compound isolated from Cycas circinalis as the L-isomer. Phytochemistry, 7: 1885–1887.
- Wood, S.A., Mountfort, D., Selwood, A.I., Holland, P.T., Puddick, J. & Cary, S.C. (2008). Widespread distribution and identification of eight novel microcystins in Antarctic cyanobacterial mats. Applied and Environmental Microbiology, 74: 7243–7251.
- Wood, S.A., Heath, M.W., Holland, P.T., Munday, R., McGregor, G. & Ryan, K.G. (2010). Identification of a benthic microcystin producing filamentous cyanobacterium (Oscillatoriales) associated with a dog poisoning in New Zealand. Toxicon, 55: 897–903.
