ABSTRACT
Laminaria is an abundant kelp genus in temperate nearshore ecosystems that grows with a circannual ‘stop-start’ pattern. Species of Laminaria play important ecological roles in kelp forests worldwide and are harvested commercially as a source of food and valuable extracts. In order to evaluate seasonal differences in tissue properties and composition, we compared the material properties, histology and cell-wall composition of overwintering blades with newly synthesized, actively growing blades from Laminaria setchellii. We found that overwintering blades were fortified with a thicker cortex and increased cell wall investment, leading to increased material strength. Overwintering tissues were composed of higher proportions of cellulose and fucose-containing polysaccharides (i.e. FCSPs, fucoidans) than newly formed blades and were found to possess thicker cell walls, likely to withstand the waves of winter storms. Chemical cell wall profiling revealed that significant proportions of fucose were associated with cellulose, especially in overwintering tissues, confirming the association between cellulose and some fucose-containing polysaccharides. Changes in material properties during the resting phase may allow these kelps to retain their non-growing blades through several months of winter storms. The results of this study demonstrate how one species might regulate its material properties seasonally, and at the same time shed light on the mechanisms that might control the material properties of kelps in general.
Introduction
Seasonal variability in tissue characteristics and morphology is a widespread phenomenon among plant species (e.g. Blue & Jensen, Citation1988; Caritat et al., Citation2000; Ferreira et al., Citation2000). For perennial plants, circannual weather patterns and changes in light availability can impose varying constraints and affect important biological functions throughout the year. For instance, the growth rates and reproductive phenology of many plant species are closely tied to physical factors such as day length, light quality, rainfall and nutrient availability (e.g. Schweingruber & Briffa, Citation1996; Briffa et al., Citation1998, Citation2004; Skormarkova et al., Citation2006). For long-lived plants, a strong seasonality can increase fitness by helping fine-tune physiological, biomechanical and functional properties to meet the demands associated with survival and reproductive success through the various seasons (Debusk et al., Citation1981; Battey, Citation2000; Fitter & Fitter, Citation2002; Dahlgreen et al., Citation2007).
Brown algae, such as kelps (Laminariales, Phaeophyceae, Ochrophyta), are the main habitat-forming primary producers in nearshore communities of the North Pacific (Steneck et al., Citation2002), and several species are harvested commercially as food and for their valuable carbohydrate extracts (e.g. fucoidan and alginate; Nyvall et al., Citation2003; Li et al., Citation2008). Many species of kelp have circannual growth patterns that are endogenous or triggered by photoperiod and may yield differences in tissue composition (Black, Citation1948, Citation1950; Parys et al., Citation2009; Adams et al., Citation2011; Kraan, Citation2012) and density (i.e. water content: Black, Citation1950; Bartsch et al., Citation2008). Few studies, however, have related functional traits to these changes in cell wall composition. Thus, it remains unclear whether changes in tissue composition have an impact on the properties of kelp tissue or whether they simply represent an accumulation of storage polysaccharides, as suggested by Black (Citation1950) and more recently Schiener et al. (Citation2015).
Laminaria is one of the most cosmopolitan kelp genera, found throughout the northern hemisphere (Bartsch et al., Citation2008), and shows widespread seasonal patterns of growth (e.g. Dieck, Citation1991; Schaffelke & Lüning, Citation1994; Toth & Pavia, Citation2002). Among these species is Laminaria setchellii, an important intertidal perennial that grows along exposed coasts in the north-east Pacific. Laminaria setchellii has a stiff, permanent stipe and a single, often dissected, blade with a basal meristem. Although some perennial kelp species are deciduous and lose their blade in the winter (e.g. Pleurophycus gardneri, Germann, Citation1986), L. setchellii tends to retain its blade year-round but goes through periods of punctuated rapid growth in the late winter and spring, followed by stunted or completely ceased growth in late summer to early winter (Dieck, Citation1991; Bartsch et al., Citation2008). The blades of this species therefore withstand winter storms without growing to replace old tissues, which may naturally weaken as they age (Krumhansl et al., Citation2015).
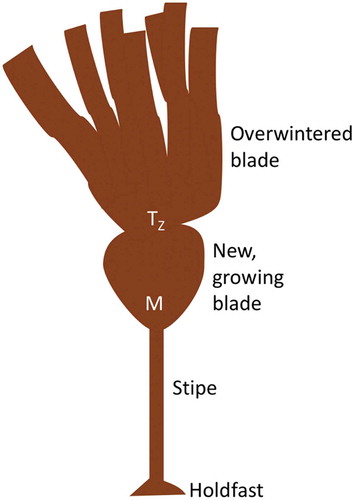
Although winter months are associated with increased storm prevalence (Supplementary fig. S1) and, thus, greater wave forces, the circannual growth rhythms of Laminaria species are believed to be an adaptive response to nutrient abundance (Chapman & Craigie, Citation1978; Dieck, Citation1991; Toth & Pavia, Citation2002); as nutrient availability decreases in late summer, growth rate slows until it ceases in the winter. Rapid growth then occurs following the shortest day of the year. At this time a new blade is actively synthesized, while, for a short time, the overwintering blade is retained. This pattern leads to a formation of ‘waists’, or transition zones (see ), between new and old blades (e.g. Black, Citation1948; Toth & Pavia, Citation2002), making it easy to distinguish between blades of different growing seasons and providing a unique opportunity to directly compare tissues produced during different seasons, with different longevities. Seasonal changes in tissue composition have been observed in several Laminaria species (see Black, Citation1948, Citation1950; Adams et al., Citation2011, Schiener et al., Citation2015); however, the function of these changes is not entirely clear. Tissues that ‘rest’ for several months may have material properties that are distinct from those prevalent during rapid growth. For example, increased material strength or extensibility could permit Laminaria to retain their blades during several months of autumn and winter storms. It is unknown, however, whether kelps can fine-tune their material properties in this way, or even what histological and chemical changes facilitate these physicomechanical attributes.
Fig. 1. Blade morphology of Laminaria setchellii, with meristem (M), transition zone (TZ) and both overwintered and newly formed blades labelled.
Despite their distinct evolutionary trajectories, the cell walls of brown algae are notably convergent with those of land plants, probably owing, in part, to the shared history of some metabolic pathways acquired by brown algae during secondary endosymbiosis with a red alga (see Michel et al., Citation2010). Cellulose is the main structural and load-bearing component of brown algal cell walls (Kloareg & Quatrano, Citation1988). However, other structural sugars are associated with cellulose fibres. For example, fucose-containing sulphated polysaccharides (FCSPs) are believed to strongly associate with cellulose (Doubet, Citation1983; Kloareg & Quatrano, Citation1988; Deniaud-Bouet et al., Citation2014). FCSPs are defined by the inclusion of sulphated α-L-fucose and are found in both the cell wall and in the intercellular space (Evans & Holligan, Citation1972; Kloareg & Quatrano, Citation1988; Stiger-Pouvreau et al., Citation2016). Some FCSPs have been suggested to perform hemicellulose-like functions in species from the Order Fucales (Doubet, Citation1983; Kloareg & Quatrano, Citation1988; Hackney et al., Citation1994; Deniaud-Bouet et al., Citation2014), though the functional significance of brown algal hemicellulose has never been demonstrated.
The purpose of this study was to compare the material properties, chemical composition, and histological characteristics of overwintered and newly synthesized blades from individuals of the perennial kelp, Laminaria setchellii to see if cell wall chemistry or tissue histology can be adjusted to fortify tissues against winter storms. We ask whether L. setchellii exhibits seasonal changes in material properties that are associated with changes in chemical composition of tissues. In doing so, we demonstrate that these kelps can adjust their material properties seasonally and quantify some changes in tissue composition that may be responsible for these adjustments.
Materials and methods
Collection of specimens and material testing
Blades (n = 9) of Laminaria setchellii were collected in February 2014 from Brady’s Blowhole, a wave-exposed site in Barkley Sound, British Columbia (see Starko & Martone, Citation2016). Samples were kept in recirculating seawater for no more than 48 h prior to tensile testing. Both overwintered and newly formed blades were collected from the same individuals to allow for paired analysis of all mechanical and histological testing. Blades were cut directly above the meristem and material properties were compared by performing pull-to-break tests with an Instron (model 5500R). Standardized ‘dogbone’ specimens (see Demes et al., Citation2011) were cut between 3 and 6 cm from the meristem (or transition zone), clamped pneumatically and tested for tensile strength at a rate of 10 mm per minute. Samples were continually wetted prior to testing. Tensile strength (i.e. stress at break), stiffness (Young’s modulus) and extensibility (i.e. strain at break) were compared using paired t-tests of overwintered and new tissues from the same plants.
Histology of tissues
A subsample (n = 6 of both overwintered and newly formed) of blades were fixed in a 7% formalin seawater solution. Histological analyses of tissues were conducted using two methods: (1) In order to determine whether cell layer thickness differed between overwintered and newly formed tissues, medulla and cortex (including meristoderm) from the different blades were measured by making hand sections and measuring the thickness of each tissue type. Given that the meristoderm is a thin epidermal layer of tissue, any changes in combined thickness of cortex and meristoderm were assumed to be a consequence of cortical expansion, rather than changes in meristoderm thickness. Measurements of tissue-layer thickness were made by taking pictures under magnification, using a digital camera (DP21 Olympus Canada) mounted on a light microscope (model BX51wi, Olympus Canada). Measurements were then made using ImageJ (US National Institutes of Health, Bethesda, Maryland, USA). Tissue-layer thickness was taken as an average of three measurements per blade. (2) In order to measure cell-wall thickness, 10 μm thick cryosections (using freezing microtome: model CM1859, Leica Biosystems) were made of each blade and cortical cells (n = 5 per blade) were randomly selected (using a grid and random number generator) and cell wall thickness measured using the methods described above. Wall thickness measurements of each blade were then taken as a mean of three measurements per randomly selected cell. To determine how much water was present in each tissue, dry weight to wet weight ratios (dw:ww) were calculated by weighing hole-punches of tissues before and after drying (at 60°C until the sample weight equilibrated).
Pretreatment of tissues
Newly formed and overwintered blades were separated, cleaned of epiphytes, and dried in a 50°C oven overnight. They were then ground to a fine, homogenous powder (to pass a 60 mesh) using a Wiley Mill (Thomas Scientific, Swedesboro, New Jersey, USA). In order to have enough tissue to perform the necessary analyses, overwintered tissues and newly formed tissues were separately pooled. For this reason, confidence intervals and statistical analysis of chemical results were calculated from duplicates and triplicates of a pooled sample. Tissues were extracted for 24 h with hot acetone in a Soxhlet apparatus to remove extractives (e.g. soluble phenolics, lipids) that may interfere with further analysis of carbohydrates. The resulting residue shall hereafter be referred to as extractive-free tissue.
Hydrolysis and HPLC
Cell wall carbohydrates were analysed by secondary acid hydrolysis followed by quantification using high performance liquid chromatography (HPLC). Tissues were hydrolysed using the method described by Huntley et al. (Citation2003). In brief, approximately 100–200 mg of ground, extractive-free algal tissue was dried overnight at 50°C and weighed. Samples were then placed in a glass test tube and allowed to react with 3 ml of cold 72% H2SO4 for 2 h (with stirring every 10 min). The suspension was then transferred into a glass serum container and diluted with 112 ml of dH2O to a final concentration of ~3% H2SO4. Samples were heated in an autoclave at 120°C for 90 min, then filtered through a pre-weighed, medium coarseness sintered crucible. Solutions were analysed by HPLC using a Dionex AS50 high performance anion-exchange chromatography system, equipped with the CarboPac PA-1 column (Dionex) and an ED50 electrochemical detector (Dionex). Neutral sugars were eluted using an isocratic eluent of water at 1 ml per minute with a post-column addition of 250 mM sodium hydroxide at a rate of 1 ml per minute. Standards of neutral sugars were hydrolysed and analysed on the HPLC to account for breakdown of sugars during acid hydrolysis. All hydrolysis reactions were run as duplicates or triplicates to account for variability in the technique employed. Uronic acids were separated using an elution gradient of sodium acetate and sodium hydroxide, and post-column addition of 250 mM sodium hydroxide at a rate of 1 ml min–1. Although this method was effective for detecting uronic acids, their peaks could not be properly separated and thus this method was only successful to detect presence and absence of uronic acids.
Quantification of insoluble material
Extractive-free tissues were further washed with methanol, additional acetone, deionized water and diethyl ether and the remaining material (cell wall) was quantified. The procedure used was adapted from Koivikko et al. (Citation2004). The 13 washes, 15 min each, were performed as follows: (1) methanol, (2–4) deionized water, (5–9) methanol, (10–11) acetone, (12–13) diethyl ether. Following each wash (10 ml), samples were centrifuged for 5 min (2400 g) and the solute removed with a pipette. The objective was to wash the material exhaustively with both polar and non-polar solvents, such that only insoluble, wall-bound components remained in the tissue. The remaining insoluble residue was weighed to estimate the proportion of extractive-free tissue that was completely insoluble (wall-bound). After weighing, subsamples were secondarily hydrolyzed and neutral sugars were quantified as described above. This procedure was run using duplicates of each sample.
Cellulose and hemicellulose quantification
In embryophytes, structural polysaccharides can be isolated by performing a bleaching reaction with sodium chlorite to isolate holocellulose, followed by treatment with 17.5% NaOH to isolate α-cellulose, or true cellulose (Browning, Citation1967). Hemicellulose can then be calculated as the difference between holocellulose and alpha-cellulose. This method has been applied to brown algae (e.g. Rabemanolontsoa & Saka, Citation2013; Tamayo & Rosario, Citation2014) and surprisingly large amounts of hemicellulose have been reported. The composition of this hemicellulose fraction, however, has never been tested, and it has been assumed that bleaching will also remove any pectic material (i.e. alginates).
Cellulose was therefore analysed using a modified Browning (Citation1967) method described by Porth et al. (Citation2013) for isolating holocellulose and alpha-cellulose. This process involves the two extractions described above: (1) a bleaching reaction intended to remove any compounds not directly bonded to cellulose and (2) a saponification reaction with 17.5% NaOH intended to isolate alpha-cellulose (true cellulose) and remove hemicelluloses. 100–200 mg of tissue was added to a Pyrex tube with 3.5 ml of buffer solution (60 ml glacial acetic acid and 1.3 g NaOH) and 1.5 ml 80% (w/v) NaClO2. The tube was then sealed and shaken gently for 14–16 h at 50°C. Each sample was washed twice with 50 ml of 1% glacial acetic acid, followed by 10 ml acetone and filtered through a pre-weighed coarse sintered crucible. The samples were dried overnight at 50°C and weighed.
Next, 15–30 mg of bleached material was weighed and placed into a glass test tube and saponified by reacting with 2.5 ml of 17.5% sodium hydroxide for 30 min. 2.5 ml of dH2O was then added and the slurry was stirred for 1 min, and allowed to stand for 29 min. The remaining material was filtered through a pre-weighed coarse crucible and washed with 90 ml deionized water, soaked in 1.0 M glacial acetic acid for 5 min, washed again in 90 ml deionized water and then dried overnight in a 50°C drying oven. Subsamples of both residues (holocellulose and alpha-cellulose) were hydrolyzed to assess the composition of each isolate. Holocellulose was calculated as the total of all neutral sugars remaining in the bleached isolate. Hemicellulose was calculated, for each replicate, as the difference between holocellulose and alpha-cellulose.
Statistical analyses
All statistical analyses were conducted in R v. 3.2.3 (R Core Development Team). Paired t-tests were used to compare mechanical and histological properties for each blade type. Two sample t-tests were used to compare the means of chemical triplicates. In the cases where only duplicates were performed (as with ‘insoluble’ treatments), no formal statistical test was conducted.
Results
Mechanical properties
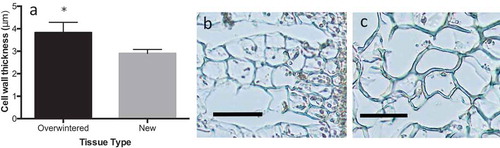
Overwintered blades were stiffer (t = 8.04, df = 8, p < 0.001, ) and stronger (t = 3.33, df = 8, p = 0.01, ) than newly formed tissues, but were less extensible (t = 5.83, df = 8, p < 0.01, ). Overwintered tissues were also notably darker in colour than new tissues and had substantially higher epiphyte abundance, with epiphytes covering the entire overwintered blades and lacking entirely on corresponding new blades. Overwintered blades had significantly thicker cortical cell layers than their new-blade counterparts (paired t-test: t = 4.6298; p < 0.01; df = 5, ) and significantly thicker cell walls (paired t-test: t = 4.5445; p < 0.01; df = 5, ). Overwintered tissues also had larger cells (paired t-test: t = –2.6661; p < 0.05; df = 5), but the proportion of cell radius occupied by the cell wall was not significantly different between tissue types (paired t-test: t = 0.9114; p > 0.05; df = 5). Medullary thickness (paired t-test: t = 0.9762; p > 0.05; df = 5, ) and total blade thickness (paired t-test: t = 0.0859; p > 0.05; df = 8, ) were not significantly different between overwintered and newly formed blades, but there was a strong trend towards increased medullary thickness in new tissues (). Overwintered blades also had higher dry weight to wet weight ratios (and thus lower water content) than new tissues (paired t-test: t = 8.2000; p < 0.001; df = 5, ), suggesting a difference in tissue composition.
Fig. 2. Material properties of overwintered (black bars) and newly formed (grey bars) blades. (A) Young’s modulus of stiffness (n = 9), (B) tensile strength (n = 9), (C) extensibility (n = 9) and (D) proportion dry weight of wet weight (n = 6) for newly formed and overwintered blades collected from the same plants of Laminaria setchellii. Error bars indicate 95% confidence intervals. Asterisks indicate a significant difference (p < 0.05) between treatments.
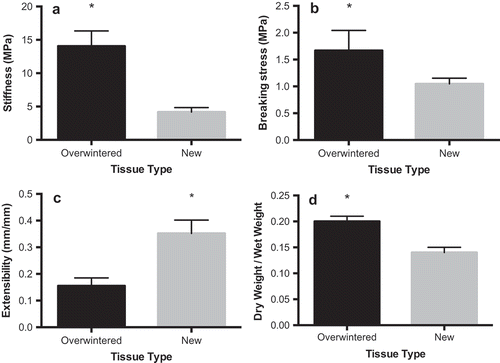
Fig. 3. (A) Cortical thickness (including meristoderm), (B) medullary thickness and (C) total tissue thickness for overwintering (black bars) and newly formed (grey bars) blades of Laminaria setchellii (n = 6). Error bars indicate 95% confidence intervals. Asterisks indicate a significant difference (p < 0.05) between treatments.
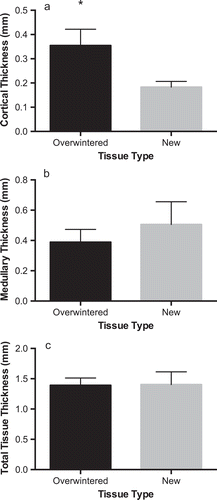
Fig. 4. (A) Cell wall thickness of overwintered and newly formed tissues of Laminaria setchellii (n = 6). Also shown, cross-sections of cortical cells from (B) new tissue and (C) overwintered tissues. Note the increase in both cell wall thickness and cell size in the cortex of overwintered tissues. Error bars in (A) represent 95% confidence intervals and scale bars in (B) and (C) represent 50 μm. Asterisks indicate a significant difference (p < 0.05) between treatments.
Neutral cell wall carbohydrates
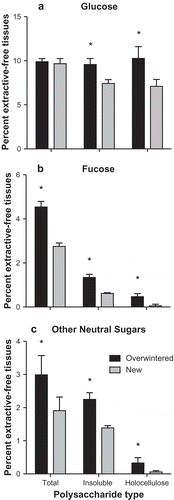
Following acetone-extraction, higher proportions of material were retained from overwintering blades (93.98 ± 0.01%; n = 3) than from newly synthesized blades (87.21 ± 0.02%; n = 3). Acetone-extracted residues from overwintered tissues had higher percentages of neutral cell wall carbohydrates than residues of newly formed tissues. Substantial amounts of glucose, fucose, xylose, galactose, rhamnose and mannose were found in both overwintered and new tissues (), and arabinose was also present in trace amounts (0.01–0.02%). Overwintered tissues had significantly higher percentages of total neutral carbohydrates than new tissues (t = 5.76, p < 0.01), and significantly higher percentages of all sugars other than glucose (glucose: t = 2.43, p = 0.08; fucose: t = 11.01, p < 0.01; mannose t = 4.44, p < 0.05; galactose t = 5.81, p < 0.05; rhamnose t = 4.48, p < 0.05; xylose: t = 9.48, p < 0.01).
Table 1. Neutral carbohydrate composition of extractive-free tissues, measured as percentage dry weight.
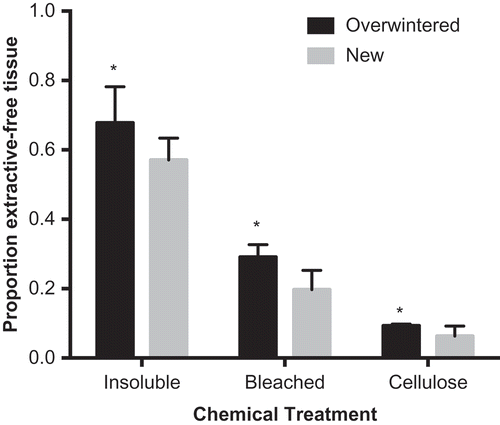
Insoluble residue of overwintered tissues also had higher percentages of neutral carbohydrates than the insoluble residue of newly formed tissue. Following total extraction of soluble material, higher yields were obtained () and more total carbohydrates remained in the overwintered tissues (). Furthermore, higher percentages of glucose, fucose and xylose were present in the overwintered tissues (Supplementary table S1). Though statistical tests were not performed on the monosaccharide composition of insoluble residue, 95% confidence intervals for these sugars do not overlap between treatments (). Large proportions of glucose, which are likely storage polysaccharides (e.g. laminarin), were removed during the wash of newly formed tissue (). Conversely, very little, if any, glucose was removed from the overwintered tissues ().
Table 2. Composition of insoluble residue measured as percentage dry weight.
Cellulose and holocellulose
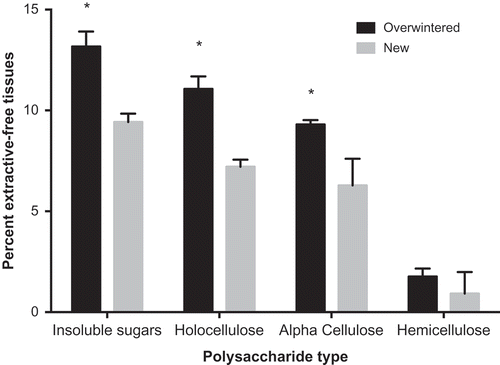
Following cellulose isolation, significantly higher yields were obtained from overwintered tissues than from newly formed tissues, indicating that overwintered tissues had a higher proportion of cellulose-associated material (). The material remaining after sodium chlorite treatment contained detectable amounts of uronic acids. HPLC analysis showed signatures of mannuronate, guluronate and possibly glucuronate. For this reason, true holocellulose was calculated as the sum of all neutral carbohydrates in the isolate. Overwintered tissues had more holocellulose (t = 5.73, p < 0.01, df = 5) and alpha-cellulose (t = 4.69, p < 0.01, df = 5) than newly formed tissues (), but the composition of holocelluloses from each tissue type were similar (). Only fucose and rhamnose concentrations were significantly higher in residues isolated from overwintered tissues than new tissues (fucose: t = 5.734707, p < 0.01, df = 5; rhamnose: t = 4.77497, p < 0.05, df = 5; Supplementary table S2). Following hydrolysis of the alpha-cellulose isolate, glucose was the only carbohydrate detected, as expected. Sodium chlorite treatment allowed for alpha-cellulose isolation by the saponification reaction; on its own, this was not enough to isolate cellulose (data not shown here) and suggests that cross-linking compounds had been successfully removed by the isolation reaction. Thus, the two-step method used here is an effective way to estimate alpha-cellulose from kelps.
Table 3. Holocellulose composition, measured as percentage dry weight of isolated residue.
Discussion
Overwintered tissues were significantly stiffer and stronger than newly formed tissues, but were less extensible. Because previous work has demonstrated that the materials of some kelp blades become weaker as they age (Krumhansl et al., Citation2015), results demonstrating increased strength in older tissues suggest a difference in tissue composition or structure. The biomechanical characteristics of the different tissues correlate with notable differences in water content, cortical layer thickness, cell wall thickness and insoluble carbohydrate abundance, including differences in cellulose, holocellulose and fucan quantity. Together this suggests that seasonal changes in carbohydrate abundance and composition are associated with cell wall thickening and mechanical reinforcement rather than with energetic metabolism (e.g. laminarin production), as previously suggested (Black, Citation1948, Citation1950; Schiener et al., Citation2015).
Biochemically, extractive-free overwintered tissues had approximately 30% more cellulose, about 25% more acetone-insoluble carbohydrates and more than 30% greater completely insoluble carbohydrates than newly formed tissues (). Among these carbohydrates were fractions that were not degraded by the holocellulose isolation reaction and, thus, constitute hemicellulosic polysaccharides. Differences in cellulose quantity have been shown to cause changes in both strength and rigidity of plant tissues (e.g. Turner & Somerville, Citation1997; Salmén, Citation2002; Burgert & Fratzl, Citation2009) and material properties of fibres have been shown to depend largely on hemicellulose abundance and composition (e.g. pine trees; Spiegelberg, Citation1966). Although kelps do not possess secondary cell walls like some other wave-swept seaweeds (Martone et al., Citation2009; Janot & Martone, Citation2016), thicker walls with increased holocellulose in older, overwintering tissues suggest that cells add to their primary walls by producing more structural polymers. While previous work suggested that cell wall elaboration was restricted to the red algae (Martone, Citation2007), this work demonstrates a similar strategy in a kelp. In addition, previous work has shown that cortical thickening occurs seasonally in the stipes of L. setchellii (Klinger & DeWreede, Citation1988) and our study suggests that seasonal cortical thickening also occurs in blades, likely providing mechanical support (Harder et al., Citation2006). Along this coast, the largest storms tend to begin in October and last until April (Supplementary fig. S1). Thus, mechanically reinforced tissues are unlikely to be adapted to winter storms, specifically. Instead, reinforcement probably allows for longer-lived tissues that might otherwise degrade and weaken with age (Krumhansl et al., Citation2015).
Fig. 6. Per cent extractive-free material attributed to (A) glucose, (B) fucose and (C) all other neutral carbohydrates (mannose, galactose, xylose, arabinose) for each chemical treatment of Laminaria setchellii blades. Error bars indicate 95% confidence intervals. Asterisks indicate a significant difference (p < 0.05) between treatments.
There were also greater amounts of fucose, both soluble and insoluble (including the holocellulose fraction), in the overwintered tissues than in the newly formed tissues. FCSPs are likely to be involved in cross-linking cell wall components, such as cellulose and alginate (Kloareg & Quatrano, Citation1988; Deniaud-Bouet et al., Citation2014). Kloareg & Quatrano (Citation1988) suggest that some FCSPs, specifically xylofucoglycans, may act as hemicelluloses in brown algae. Hemicelluloses work to cross-link cellulose microfibrils and can therefore play a key role in determining the mechanical properties of such tissues (Niklas, Citation1992; Whitney et al., Citation1999). In this study, fucose, mannose, xylose, galactose, and a fraction of glucose, were retained following isolation and therefore form part of the hemicelluloses or hemicellulose-like polymers. Collectively, cortical growth and cell wall deposition, through increased cellulose and hemicellulose production, may be important mechanisms by which kelps can adjust material properties.
Schiener et al. (Citation2015) found little seasonal variation in the cellulose content of Laminaria digitata, L. hyperborea, Saccharina latissima and Alaria esculenta. Although it is possible that these species do not share the ability of L. setchellii to accumulate cellulose, the presence of non-cellulosic glucose in insoluble and holocellulosic fractions ( and ) draws into question the indirect measures of cellulose used in their study. Recently, Salmean et al. (Citation2017) confirmed that a mixed (1-3), (1-4)-β-D-glucan is a common component of brown algal cell walls. The presence of glucose-containing hemicelluloses, like this mixed glucan, and complex FCSPs, coupled with incomplete extraction of soluble glucose-containing molecules, could inflate estimates of cellulose. This is especially apparent when considering that, in our study, large proportions of xylose and fucose were associated with an increase in non-cellulosic glucose that were retained through the holocellulose isolation procedure, suggesting that xylofucoglucans (as suggested by Kloareg & Quatrano, Citation1988) may be an important component of holocellulose in Laminaria (). Nonetheless, it remains unknown whether seasonal changes in material properties and cell-wall composition are widespread across the kelps.
In our study, hemicellulose content was found to be substantially lower (1–3% w/w; ) than in some previous studies on brown algae (e.g. 19.6%; Tamayo & Rosario, Citation2014). These studies have quantified holocellulose from brown algae using a sodium chlorite bleaching reaction, similar to that shown here, but defined hemicellulose as the difference between holocellulose and alpha-cellulose isolates (Rabemanolontsoa & Saka, Citation2013; Tamayo & Rosario, Citation2014). In our study, other components, such as alginates, were found in this bleached residue. Thus, HPLC was necessary to quantify hemicellulose quantity with this method, calling into question previously published results.
Fig. 7. Per cent dry weight of insoluble neutral carbohydrates, holocellulose, alpha-cellulose and hemicellulose of Laminaria setchellii blades. Asterisks indicate a significant difference between newly formed and overwintered tissues. Error bars indicate 95% confidence intervals.
Chapman & Kraemer (Citation1991) noted an increase in cell wall investment by the kelp species, Egregia menziesii, both when tension was imposed on the tissue and when ambient nutrient levels were reduced. Moreover, Stephens & Hepburn (Citation2016) found that blades of Macrocystis pyrifera were maintained, rather than elongated, during periods of low environmental nitrogen and this was associated with an increase in per cent total carbon of tissues. Although cued by photoperiod, seasonal growth patterns of Laminaria species, including L. setchellii, strongly correlate with environmental nutrient availability (Dieck, Citation1991; Bartsch et al., Citation2008). One possible explanation for the increased cell wall incorporation reported here is that kelps may invest in cell wall polysaccharides when nutrients are too low for tissue elongation. This could be driven by a lack of environmental nitrogen that limits cell division through restricting DNA replication or protein formation. These circannual rhythms have been demonstrated in distantly related kelp species (e.g. Pterygophora californica; Lüning & Kadel, Citation1993) and therefore may be important adaptations shared throughout the order Laminariales. Although we report notable differences in the abundance of structural polysaccharides, it remains unclear whether this seasonal cell wall expansion is also associated with changes in the interactions between compounds. Changes in the nature of covalent or non-covalent interactions between polymers could have important impacts on the material properties of tissues (Kloareg & Quatrano, Citation1988; Deniaud-Bouet et al., Citation2014). Investigating how linkages and interactions between cell wall constituents may influence brown algal materials and how kelps might adjust these interactions seasonally would be an enlightening next step. Specifically, alginates are an abundant component of brown algal cell walls that were not investigated in this study. Changes in the composition of alginate could contribute to the rigidity of tissues (Kraemer & Chapman, Citation1991) and should be investigated in future work. Recently, carbohydrate binding modules (CBMs) have been developed for various brown algal polysaccharides (e.g. Torode et al., Citation2016; Salmeán et al., Citation2017), and combining the analytical techniques shown here with CBMs to localize and characterize the different polymers within tissues of differing material characteristics would be another powerful way to move forward.
In conclusion, overwintering blades of L. setchellii have thicker cortex, thicker cell walls and higher concentrations of holocellulose than young, fast-growing blades from the same individuals, helping explain differences in material properties observed between these two tissues. Holocellulose included a notable portion of fucose, suggesting that at least some hemicellulose polymers incorporate sulphated fucose into their structure, likely in the form xylofucoglucans and xylofucogalactans (Kloareg & Quatrano, Citation1988). Hemicelluloses in brown algae are still poorly understood and probably include a large number of different polymers, incorporating many different neutral carbohydrates. Investment in cell wall polysaccharides and thickening of cortical layers during the winter months could help individuals maintain longer-lived blades, allowing for increased carbon fixation year-round. This may allow Laminaria spp. to maintain metabolic processes throughout the year.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://doi.org/10.1080/09670262.2018.1449013
Supplementary table. S1. Statistics for extractive-free tissues
Supplementary table. S2. Statistics for holocellulose
Supplementary fig. S1. Significant wave height data from La Perouse Buoy off the west coast of Barkley Sound
Supplementary_material.docx
Download MS Word (257.5 KB)Acknowledgements
The authors would like to thank F. Hart and F. Unda for helping perform chemical analyses and providing important insight into the results of each analysis. We would also like to thank K. Mittl for collecting much of the histological data. Thanks to A. Fortune and N. Weiwel for assistance with collections during late-night low tides in February. Thanks also to E. Clelland, S. Gray and other staff at BMSC for making these collections possible.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Samuel Starko
S.Starko conceived the study, collected tissues in the field, analysed the data and wrote the manuscript, with contributions from S.Mansfield and P.Martone. S.Starko, S.Mansfield and P.Martone designed the experiment. S.Mansfield and P.Martone provided laboratory equipment and funding.
References
- Adams, J.M.M., Toop, T.A., Donnison, I.S. & Gallagher, J.A. (2011). Seasonal variation in Laminaria digitata and its impact on biochemical conversion routes to biofuels. Bioresource Technology, 102: 9976–9984.
- Bartsch, I., Wiencke, C., Bischof, K., Buchholz, C.M., Buck, B.H., Eggertm, A., Feuerpfeil, P., Hanelt, D., Jacobsen, S., Karez, R., Karsten, U., Molis, M., Roleda, M.Y., Schubert, H., Schumann, R., Valentin, K., Weinberger, F. & Wiese, J. (2008). The genus Laminaria sensu lato: recent insights and developments. European Journal of Phycology, 43: 1–86.
- Battey, N.H. (2000). Aspects of seasonality. Journal of Experimental Botany, 51:1769–1780.
- Black, W.A.P. (1948). Seasonal variation in the chemical constitution of some of the Laminariaceae common to Scotland. Journal of the Society of Chemical Industry, 67: 165–72.
- Black, W.A.P. (1950). The seasonal variation in weight and chemical composition of the common British Laminariaceae. Journal of the Marine Biological Association of the United Kingdom, 29: 45–72.
- Blue, M.P. & Jensen, R.J. (1988). Positional and seasonal variabilityin oak (Quercus; Fagaceae) leaf morphology. American Journal of Botany, 75: 939–947.
- Briffa, K.R., Schweingruber, F.H., Jones, P.D., Osborn, T.J., Harris, I.C., Shiyatov, S.G., Vaganov, E.A. & Grudd, H. (1998). Trees tell of past climates: but are they speaking less clearly today? Philosophical Transactions of the Royal Society B, 353: 65–73.
- Briffa, K.R., Osborn, T.J. & Schweingruber, F.H. (2004). Large-scale temperature inferences from tree rings: a review. Global and Planetary Change, 40: 11–26.
- Browning, B.L. (1967). The Chemistry of Wood. 1st ed. Interscience, USA.
- Burgert, I. & Fratzl, P. (2009). Plants control the properties and actuation of their organs through the orientation of cellulose fibrils in their cell walls. Integrative and Comparative Biology, 49: 69–79.
- Caritat, A., Gutierrez, E. & Molinas, M. (2000). Influence of weather on cork-ring width. Tree Physiology, 20: 893–900.
- Chapman, A.R.O. & Craigie, J.S. (1978). Seasonal growth in Laminaria longicuris: relations with reserve carbohydrate storage and production. Marine Biology, 46: 209–213.
- Chapman, D.J. & Kraemer, G.P. (1991). Effects of tensile force and nutrient availability on carbon uptake and cell wall synthesis in blades of juvenile Egregia menziesii (Turn.) Aresch. (Phaeophyta). Journal of Experimental Marine Biology and Ecology, 149: 267–277.
- Dahlgreen, J.P, von Zeipel, H. & Ehrlen, J. (2007). Variation in vegetative and flowering phenology in a forest herb caused by environmental heterogeneity. American Journal of Botany, 94: 1570–1576.
- Debusk, T.A., Ryther, J.H., Hanisak, M.D. & Williams, L.D. (1981). Effects of seasonality and plant density on the productivity of some freshwater macrophytes. Aquatic Biology, 10: 133–142.
- Demes, K.W., Carrington, E., Gosline, J. & Martone, P.T. (2011). Variation in anatomical and material properties explains differences in hydrodynamic performances of foliose red macroalgae (Rhodophyta). Journal of Phycology, 47: 1360–1367.
- Deniaud-Bouet, E., Kervarec, N., Michel, G., Tonon, T., Kloareg, B. & Herve, C. (2014). Chemical and enzymatic fractionation of cell walls from Fucales: insight into the structure of the extracellular matrix of brown algae. Annals of Botany, 114: 1203–1216.
- Dieck, I. (1991). Circannual growth rhythm and photoperiodic sorus induction in the kelp Laminaria setchellii (Phaeophyta). Journal of Phycology, 27: 341–350.
- Doubet, R.S. (1983). PhD Thesis, Oregon State University, Corvallis, Oregon, 107 pp.
- Evans, L.V. & Holligan, S. (1972). Correlated light and electron microscope studies on brown algae. I. Localization of alginic acid and sulphated polysaccharides in Dictyota. New Phytologist, 71: 1161–1172.
- Ferreira, A., Lopes, F. & Pereira, H. (2000). Characterization of cork growth and quality in one region of production. Annals of Forest Science, 57: 187–193.
- Fitter A.H. & Fitter R.S.R. (2002). Rapid changes in flowering time in British plants. Science, 296: 1689–1691.
- Germann, I. (1986). Growth phenology of Pleurophycus gardneri (Phaeophyceae, Laminariales), a deciduous kelp of the northeast Pacific. Canadian Journal of Botany, 64: 2538–2547.
- Hackney, J., Kraemer, G.P., Atalla, R., Vanderhart, D. & Chapman, D.J. (1994). Influence of hydrodynamic environment on composition and macromolecular organization of structural polysaccharides in Egregia menziesii cell walls. Planta, 192: 461–472.
- Harder, D.L., Hurd, C.L. & Speck, T. (2006). Comparison of mechanical properties of four large, wave-exposed seaweeds. American Journal of Botany, 93: 1426–1432.
- Huntley, S.K., Ellis, D., Gilbert, M., Chapple, C. & Mansfield, S.D. (2003). Significant increases in pulping efficiency in C4H:F5H-transformed poplars: improved chemical savings and reduced environmental toxins. Journal of Agricultural and Food Chemistry, 51: 6178–6183.
- Janot, K. & Martone, P.T. (2016). Convergence of joint mechanics in independently evolving, articulated coralline algae. Journal of Experimental Biology, 219: 383–391.
- Klinger, T. & DeWreede, R.E. (1988). Stipe rings, age, and size in populations of Laminaria setchellii Silva (Laminariales, Phaeophyta) in British Columbia, Canada. Phycologia, 27: 234–240.
- Kloareg, B. & Quatrano, R.S. (1988). Structure of cell walls of marine algae and ecophysiological functions of the matrix polysaccharides. Oceanography and Marine Biology, An Annual Review, 26: 259–315.
- Koivikko, R., Loponen, J., Honkanen, P. & Jormalainen, V. (2004). Contents of soluble, cell-wall-bound and exuded phlorotannins in the brown alga Fucus vesiculosus, with implications on their ecological functions. Journal of Chemical Ecology, 31: 195–211.
- Kraan, S. (2012). Algal polysaccharides, novel applications and outlook. In Carbohydrates – Comprehensive Studies on Glycobiology and Glycotechnology (Chuan-Fa Chang, editor). INTECH Open Access Publisher.
- Kraemer, G.P. & Chapman, D.J. (1991). Biomechanics and alginic acid composition during hydrodynamic adaptation by Egregia menziesii (Phaeophyta) juveniles. Journal of Phycology, 27: 47–53.
- Krumhansl, K., Demes, K., Carrington, E. & Harley, C. (2015). Divergent growth strategies in red algae vs. kelps influence biomechanical properties. American Journal of Botany, 102: 1938–1944.
- Li, B., Lu, F., Wei, X. & Zhao, R. (2008). Fucoidan: structure and bioactivity. Molecules, 13: 1671–1695.
- Lüning, K. & Kadel, P. (1993). Daylength range for circannual rhythmicity in Pterygophora californica (Alariaceae, Phaeophyta) and synchronization of seasonal growth by daylength cycles in several other brown algae. Phycologia, 32: 379–387.
- Martone, P.T. (2007). Kelp versus coralline: cellular basis for mechanical strength in the wave-swept alga Calliarthron (Corallinaceae, Rhodophyta). Journal of Phycology, 43: 882–891.
- Martone, P.T., Estevez, J., Lu, F., Ruel, K., Denny, M., Somerville, C. & Ralph, J. (2009). Discovery of lignin in seaweed reveals convergent evolution of cell-wall architecture. Current Biology, 19: 169–175.
- Michel, G., Tonon, T., Scornet, D., Cock, J.M. & Kloareg, B. (2010). The cell wall polysaccharide metabolism of the brown alga Ectocarpus siliculosus. Insights into the evolution of extracellular matrix polysaccharides in Eukaryotes. New Phytologist, 188: 82–97.
- Niklas, K.J. (1992). Plant Biomechanics: An Engineering Approach to Plant Form and Function. University of Chicago Press. Chicago, IL.
- Nyvall, P., Corre, E., Boisset, C., Barbeyron, T., Rousvoal, S., Scornet, D., Kloareg, B. & Boyen, C. (2003). Characterization of mannuronan C-5-epimerase genes from the brown alga Laminaria digitata. Plant Physiology, 133: 726–735.
- Parys, S., Kehraus, S., Pete, R., Küpper, F.C., Glombitza, K.-W. & König, G.M. (2009). Seasonal variation of polyphenolics in Ascophyllum nodosum (Phaeophyceae). European Journal of Phycology, 44: 331–338.
- Porth, I., Klápště, J., Skyba, O., Lai, B.S., Geraides, A., Muchero, W., Tuskan, G.A., Douglas, C.J., El-Kassaby, Y.A. & Mansfield, S.D. (2013). Populus trichocarpa cell wall chemistry and ultrastructure trait variation, genetic control and genetic correlations. New Phytologist, 197: 777–790.
- Rabemanolontsoa, H. & Saka, S. (2013). Comparative study on chemical composition of various biomass species. RSC Advances, 3: 3946–3956.
- Salmeán, A.A., Duffieux, D., Harholt, J., Qin, F, Michel, G., Czjzek, M., Willats, W.G.T. & Hervé, C. (2017). Insoluble (1 → 3), (1 → 4)-β-D-glucan is a component of cell walls in brown algae (Phaeophyceae) and is masked by alginates in tissues. Scientific Reports, 7: 2880. doi: 10.1038/s41598-017-03081-5.
- Salmén, L. (2002). Micromechanics of wood cell walls – a tool for a better understanding of its structure. In Proceedings of 1st International Conference of the European Society of Wood Mechanics (Navi P, editor), 385–398. EPFL, Lausanne, Switzerland.
- Schaffelke, B. & Lüning, K. (1994). A circannual rhythm controls seasonal growth in the kelps Laminaria hyperborea and L. digitata from Helgoland (North Sea). European Journal of Phycology, 29: 49–56.
- Schiener, P., Black, K.D., Stanley, M.S. & Green, D.H. (2015). The seasonal variation in the chemical composition of the kelp species Laminaria digitata, Laminaria hyperborea, Saccharina latissima and Alaria esculenta. Journal of Applied Phycology, 27: 363–373.
- Schweingruber, F.H. & Briffa, K.R. (1996). Tree-ring density networks for climate reconstruction. In Climatic Variations and Forcing Mechanisms of the Last 2000 Years (Jones, P.D., Bradley, R.S. & Jouzel, J., editors), 44–66. NATO ASI Series 141.
- Skormarkova, M.V., Vaganov, E.A., Mund, M., Knohl, A., Linke, P., Boerner, A. & Schulze, E.D. (2006). Inter-annual and seasonal variability of radial growth, wood density and carbon isotope ratios in tree rings of beech (Fagus sylvatica) growing in Germany and Italy. Trees, 20: 571–586.
- Spiegelberg, H.L. (1966). PhD Thesis. The Institute of Wood Chemistry, Lawrence University, Appleton, Wisconsin.
- Starko, S. & Martone, P.T. (2016). Evidence of an evolutionary-developmental trade-off between drag avoidance and tolerance strategies in wave-swept intertidal kelps (Laminariales, Phaeophyceae). Journal of Phycology, 52: 54–63.
- Steneck, R.S., Graham, M.H., Bourque, B.J., Corbett, D., Erlandson, J.M., Estes, J.A. & Tegner, M.J. (2002). Kelp forest ecosystems: biodiversity, stability, resilience and future. Environmental Conservation, 29: 436–459.
- Stephens, T.A. & Hepburn, C.D. (2016). A kelp with integrity: Macrocystis pyrifera prioritises tissue maintenance in response to nitrogen fertilisation. Oecologia, 182: 71–84.
- Stiger-Pouvreau, V., Bourgougnon, N., & Deslandes, E. (2016). Carbohydrates from seaweeds. In Seaweed in Health and Disease Prevention (Fleurence, J. & Levine, I. editors), 223–274. Elsevier.
- Tamayo, J.P. & Rosario, E.J.D. (2014). Chemical analysis and utilization of Sargassum sp. as substrate for ethanol production. Iranica Journal of Energy and Environment, 5: 202–208.
- Torode, T.A., Siméon, A., Marcus, S.E., Jam, M., Le Moigne, M.A., Duffieux, D., Knox, J.P. & Hervé, C. (2016). Dynamics of cell wall assembly during early embryogenesis in the brown alga Fucus. Journal of Experimental Botany, 67: 6089–6100.
- Toth, G. & Pavia, H. (2002). Lack of phlorotannin induction in the kelp Laminaria hyperborea in response to grazing by two gastropod herbivores. Marine Biology, 140: 403–409.
- Turner, S.R. & Somerville, C.R. (1997). Collapsed xylem phenotype of Arabidopsis mutants deficient in cellulose deposition in the secondary cell wall. The Plant Cell, 9: 689–701.
- Whitney, S.E.C., Gothard, M.G.E., Mitchell, J.T. & Gidley, M.J. (1999). Roles of cellulose and xyloglucan in determining the mechanical properties of primary plant cell walls. Plant Physiology, 21: 657–665.