ABSTRACT
The dinoflagellate genus Bysmatrum encompasses five epibenthic or tide-pool species and has been characterized by separated anterior intercalary plates. In the present study, we obtained six strains of Bysmatrum from the South China Sea and French Atlantic coast by isolating single cells/cysts from plankton and sediment samples. All strains were examined with light microscopy and scanning electron microscopy. Based on morphological observations, three strains were identified as Bysmatrum subsalsum, characterized by the elongated and rectangular first and a hexagonal second anterior intercalary plate. They differ from each other in the number of sulcal lists and the configuration of the first anterior intercalary plate. One strain was identified as Bysmatrum gregarium and the other two as Bysmatrum granulosum. The cyst-theca relationship of B. subsalsum from the French Atlantic was established by incubation of the cyst, and the geochemical composition of the cyst wall was measured through micro-Fourier transform infrared spectroscopy. Bysmatrum subsalsum from Malaysia shows a bright red stigma in the sulcal area under light microscopy, which was confirmed with transmission electron microscopy: it was identified as a type B eyespot. Small subunit ribosomal DNA (SSU rDNA), partial large subunit ribosomal DNA (LSU rDNA) and internal transcribed spacer (ITS) sequences were obtained from all six strains. The maximum likelihood and Bayesian inference analysis based on concatenated SSU, ITS and LSU sequences revealed that Bysmatrum is monophyletic and nested within Peridiniales. Our strains of B. subsalsum form a new ribotype in the molecular phylogeny (designated as ribotype B). The genetic distance based on ITS sequences among Bysmatrum species ranged from 0.34 to 0.47 and those genetic distances at the intraspecific level of B. subsalsum could reach 0.41, supporting the possibility of hidden crypticity within B. subsalsum.
Introduction
The dinoflagellate genus Bysmatrum Faust & Steidinger was erected in Citation1998 (Faust & Steidinger, Citation1998). The plate formula of Bysmatrum is Po, X, 4′, 3a, 7′′, 6C, 4S, 5′′′, 2′′′′ with Bysmatrum subsalsum as type species. The type was initially assigned to Peridinium by Ostenfeld (Citation1908) and transferred later to Scrippsiella Balech ex Loeblich by Steidinger & Balech (Citation1977). However, Bysmatrum differs from Scrippsiella mainly in that the anterior intercalary plates 2a and 3a are separated by the third apical plate (Faust & Steidinger, Citation1998). Since then, two other Scrippsiella species were transferred to Bysmatrum: Bysmatrum arenicola Horiguchi & Pienaar (although Scrippsiella arenicola was invalidly published by Horiguchi & Pienaar, Citation1988a), and B. gregarium (Lombard & Capon) Horiguchi & Hoppenrath (= B. caponii, illegitimate). Three other species have been assigned to Bysmatrum: B. granulosum Ten-Hage, Quod, Turquet & Couté, B. teres Murray, Hoppenrath, Larsen & Patterson and B. austrafrum Dawut, Sym, Suda & Horiguchi (Ten-Hage et al., Citation2001; Murray et al., Citation2006; Dawut et al., Citation2018). Peridinium sociale (Henneguy) Biecheler also possesses three intercalary plates with 2a and 3a separated (Biecheler, Citation1952), and thus might belong to Bysmatrum as well (Murray et al., Citation2006). In fact, Biecheler (Citation1952, p. 59) regarded Peridinium sociale as a junior synonym of P. subsalsum. Useful characteristics to differentiate Bysmatrum species at the interspecific level include overall cell shape, plate ornamentation, cingulum displacement, the position of the nucleus and the shape of the apical pore complex (Horiguchi, Citation1983; Murray et al., Citation2006).
Bysmatrum species are considered photosynthetic, benthic dinoflagellates (Hoppenrath et al., Citation2014), although some specimens of B. subsalsum have been recovered from plankton, as well as floating detritus, sand and macroalgae (Faust, Citation1996). Bysmatrum teres was reported to be sand-dwelling (Murray et al., Citation2006) and B. granulosum has been recovered from surface sediment, coral rubble and macroalgal turf (Ten-Hage et al., Citation2001). Bysmatrum arenicola, B. gregarium and B. austrafrum are considered to inhabit tide pools (Horiguchi & Pienaar Citation1988a, Citation1988b; Dawut et al., Citation2018). Bysmatrum species are found in tropical to temperate waters (Hoppenrath et al., Citation2014) and may form blooms: B. subsalsum in mangrove detritus and salt marshes (Anglès et al., Citation2017), B. gregarium in tidal pools (Lombard & Capon, Citation1971) and B. arenicola on sandy sediments (Horiguchi & Pienaar, Citation1988b).
The molecular characterization of Bysmatrum is in its infancy: four out of the six described species have been sequenced: B. subsalsum, B. gregarium, B. arenicola and B. austrafrum have ribosomal DNA (rDNA) sequences available (Gottschling et al., Citation2012; Jeong et al., Citation2012; Anglès et al., Citation2017; Dawut et al., Citation2018). For B. subsalsum, only sequences from the Mediterranean Sea are available and two distinct clades in their small subunit (SSU), internal transcribed spacer (ITS) and large subunit (LSU) rDNA based phylogenies suggest hidden cryptic species (Anglès et al., Citation2017). Despite the peridinoid tabulation, the closest relative and higher rank of Bysmatrum remain unresolved (Gottschling et al., Citation2012; Anglès et al., Citation2017; Dawut et al., Citation2018), and the phylogenetic relationships among Bysmatrum species have not been elucidated. Bysmatrum subsalsum, B. gregarium, B. granulosum and B. austrafrum were described as possessing an eyespot (Faust & Steidinger, Citation1998; Ten-Hage et al., Citation2001; Jeong et al., Citation2012; Dawut et al., Citation2018), but detailed examination using transmission electron microscopy (TEM), which is necessary to characterize the eyespot, has only been carried out on B. austrafrum. The eyespot is considered of systematic importance at family level for other dinoflagellates (Lindberg et al., Citation2005; Moestrup & Daugbjerg, Citation2007), and its description may help better understand the systematic position of Bysmatrum within the Peridiniales. Information on plate overlap is helpful to identify possible plate homology, and will contribute to the understanding of the systematic relationship of armoured dinoflagellates (Netzel & Dürr, Citation1984).
Among the 2000 extant dinoflagellate species, only around 15% have been related to resting cysts (Head, Citation1996), although many recent studies have described new cyst-motile stage relationships (e.g. Gu et al., Citation2015a, Citationb). When describing Bysmatrum subsalsum (as Peridinium subsalsum Ostenfeld), Ostenfeld (Citation1908, p. 167) mentioned an ovoid cyst without illustration. Spherical to ovoid cysts were produced by B. subsalsum in culture (Gottschling et al., Citation2012; Anglès et al., Citation2017) while cysts with the typical plate pattern of B. subsalsum were identified in recent sediments, and survived palynological treatment (Limoges et al., Citation2015). In addition, unidentified Bysmatrum cells were germinated from ellipsoidal or ovoid cysts found in surface sediment from the Mediterranean lagoons and bays (Satta et al., Citation2013a, Citationb). The cyst–theca relationship of Bysmatrum has only been established through germination experiments for Mediterranean B. subsalsum (Anglès et al., Citation2017). Germination of more Bysmatrum cysts from elsewhere will be helpful to fully understand the diversity within the genus. The geochemical composition of the cyst wall can be studied with the aid of micro-Fourier transform infrared spectroscopy. Bogus et al. (Citation2014) concluded that cyst walls of autotrophic and heterotrophic species differ in their chemical composition, thus are useful indicators for infering nutrient strategies.
To increase knowledge on the morpho-molecular diversity of Bysmatrum species and to explore their phylogenetic relationships, five strains belonging to three species were established from the South China Sea (B. granulosum, B. gregarium, B. subsalsum), and one strain from the French Atlantic coast (B. subsalsum). Additionally, the morphology of their thecate stages was examined and the molecular phylogeny was inferred with concatenated SSU, LSU rDNA and ITS sequences. Finally, the cyst morphology, cyst wall chemistry and ultrastructure (with a focus on the type of eyespot) were analysed for B. subsalsum.
Materials and methods
Sample collection and treatment
One litre surface water samples were collected from the South China Sea (China and Malaysia) using a plastic bottle and concentrated through a 20 μm filter, and the upper 5 cm of sand samples were collected from nearby Rawa Island (Malaysia) by divers. The sand samples were stirred vigorously to detach the epibenthic cells and the suspension settled in a composite settling chamber. The settled materials were rinsed with filtered seawater and transferred into a polycarbonate bottle. Single Bysmatrum cells were isolated by means of drawn-out Pasteur pipettes under an AE30 inverted microscope (Motic, Xiamen, China) and established into clonal cultures. Three strains of Bysmatrum were established by isolating a single cell from plankton samples, and two strains from the sand sample (). Sediment sampling was done using an Ekman grab on 15 and 19 April 2016 in a shallow natural reservoir used for oyster cleansing before commercialization (water depth 1.2–1.5 m). The small basin is located north of the community of Gujan-Mestras in the Gironde region of south-western France. The top 2 cm of sediment from France were sliced off and stored in the dark at 4°C until further treatment. Approximately 5 g of wet sediment was mixed with 20 ml of filtered seawater and stirred vigorously to dislodge detrital particles. The settled material was subsequently sieved through 120 μm and 10 μm filters. Single cysts were isolated with a micropipette under an inverted Eclipse TS100 (Nikon, Tokyo, Japan) microscope and incubated in small containers with f/2-Si medium (Guillard & Ryther, Citation1962) at 20ºC, 90 μm photons m–2s–1 under a 12:12 h light: dark cycle. Another strain (TIO406) was established in clonal cultures under the same culture conditions ().
Table 1. Bysmatrum strains examined in the present study, including the collection locality, coordinates and collection date.
Morphological study of thecate stages and cysts
Live cells of all strains were examined and photographed using a Zeiss Axio Imager light microscope (Carl Zeiss, Göttingen, Germany) equipped with a Zeiss Axiocam HRc digital camera. Cell size of 13–40 cells was measured using Axiovision (4.8.2 version) software at 1000× magnification. Fluorescence brightener Calcofluor white (Sigma Adrich, St. Louis, USA) was used to stain the plates following the method of Fritz & Triemer (Citation1985). To observe the shape and location of the nucleus, cells were stained with 1:100,000 Sybr Green (Sigma Aldrich, St. Louis, USA) for 1 min, and photographed under the Zeiss fluorescence microscope with a Zeiss-38 filter set (excitation BP 470/40, beam splitter FT 495, emission BP 525/50). Cysts of B. subsalsum from Gironde, France with cell contents were photographed and measured using an Olympus DP72 camera mounted on a BX41 microscope with 100× oil immersion objectives.
For scanning electron microscopy (SEM), mid-exponential batch cultures of all strains were concentrated by a Universal 320 R centrifuge (Hettich-Zentrifugen, Tuttlingen, Germany) at 850 g for 10 min at room temperature. The pellet was treated as described by Tillmann et al. (Citation2009) to strip off the outer cell membrane. Cells were fixed with 2.5% glutaraldehyde for 3 h at 8ºC, rinsed with Milli-Q water twice and post-fixed with 1% OsO4 overnight at 8ºC. The supernatant was removed and the settled cells were transferred to a coverslip coated with poly-L-lysine (molecular weight 70,000–150,000). The cells attached to the cover slip were rinsed in Milli-Q water twice. The samples were then dehydrated in a graded ethanol series (10%, 30%, 50%, 70%, 90% and 3× in 100%, 10 min at each step), critical point dried (K850 Critical Point Dryer, Quorum/Emitech, West Sussex, UK), sputter-coated with gold, and examined with a Zeiss Sigma FE (Carl Zeiss Inc., Oberkochen, Germany) scanning electron microscope at Xiamen University, China.
Single cysts of Bysmatrum subsalsum from France were picked from the residue filtered using polycarbonate membrane filters (Millipore, Billerica, MA, USA, GTTP Isopore, 0.22 μm pore size), sputter coated with gold, and examined using a FEI Quanta 200 SEM with an electron acceleration of 2.5 to 5 kV at IFREMER (Plouzané, France).
The plate overlap was derived for B. subsalsum from SEM micrographs. For Vulcanodinium rugosum, SEM micrographs previously obtained by Nézan & Chomérat (Citation2011) were used. For Parvodinium umbonatum, new SEM micrographs were made for thecae from a plankton sample from a lake to the north of Bergen, Norway (60.47°N, 4.95°E). Labelling of tabulation follows a modified Kofoid system that recognizes homologues (e.g. Fensome et al., Citation1993). The sulcal plate labelling is according to Balech (Citation1980).
Transmission electron microscopy (TEM) of B. subsalsum
Mid-exponential batch cultures of strains TBBYS02 and TBBYS03 were fixed in 2.5% glutaraldehyde in phosphate buffered saline (PBS, 0.1 M at pH 7.4) for 1 h, concentrated by centrifugation and then washed three times with the same PBS for 10 min each. They were post-fixed in 1% OsO4 overnight at 4°C and washed three times with the same PBS for 10 min each. The cells were then dehydrated through a graded ethanol series (10, 30, 50, 70, 95, 3× in 100%, 10 min at each step). The pellet was embedded in Spurr’s resin (Spurr, Citation1969) and sectioned with a Reichert Ultracut E microtome (Leica, Vienna, Austria), mounted on Formvar-coated grids, stained with uranyl acetate and lead citrate, and observed in a JEOL JEM-100 transmission electron microscope (JEOL, Tokyo, Japan).
Micro-Fourier infrared spectroscopy
For micro-FTIR analyses, cysts without cell contents of B. subsalsum were isolated from palynological residues, and were rinsed three times with organic solvents (methanol and dichloromethane) and MilliQ water to remove polar and apolar compounds. Cysts were placed on a gold-coated mirror, dried, and then analysed in reflective mode with 100 scans from 4000–600 cm–1 on a Hyperion 3000 FT-IR microscope (Bruker Optics, Elltingen, Allemagne) with a 15× objective, resulting in a consistent 50×50 µm window. The raw spectra (n=3) were subjected to background subtraction, atmospheric correction and rubberband baseline correction (5 iterations).
PCR amplifications and sequencing
The total algal DNA of six Bysmatrum strains () was extracted from 10 ml of exponentially growing cultures using a MiniBEST Universal DNA Extraction Kit (Takara, Tokyo, Japan) according to the manufacturer’s protocol. PCR amplifications were carried out using 1×PCR buffer, 50 µM dNTP mixture, 0.2 μM of each primer, 10 ng of template genomic DNA, and 1 U of ExTaq DNA Polymerase (Takara, Tokyo, Japan) in 50 μl reactions. The SSU rDNA was amplified using the primers PRIMERA/PRIMERB (Medlin et al., Citation1988). The LSU rDNA was amplified using the primers D1R/28-1483R (Scholin et al., Citation1994; Daugbjerg et al., Citation2000). The total ITS1–5.8S–ITS2 was amplified using ITSA/ITSB primers (Adachi et al., Citation1996). The thermal cycle procedure was 4 min at 94ºC, followed by 30 cycles of 1 min at 94ºC, 1 min at 45ºC, 1 min at 72ºC, and final extension of 7 min at 72ºC with a Mastercycler (Eppendorf, Hamburg, Germany). The PCR product was purified using a DNA purification kit (Shengong, Shanghai, China) and sequenced directly in both directions on an ABI PRISM 3730XL (Applied Biosystems, Foster City, California, USA) following the manufacturer’s instructions.
Cells of Vulcanodinium rugosum were isolated using a micropipette with the Olympus IX70 inverted light microscope, and deposited on a glass slide. Then, the cell was rinsed into two drops of double distilled water (ddH2O) before transfer to a 0.2 ml PCR tube containing 3 μl of ddH2O. PCR tubes were stored at −20ºC before the direct PCR amplifications. Each PCR tube was thawed before adding 25 pmol of each primer and 12.5 μl of PCR Master Mix 1X (Promega, Madison, Wisconsin, USA) containing the Taq DNA polymerase, dNTPs, MgCl2 and reaction buffers. Three nuclear markers: SSU rDNA, LSU rDNA, internal transcribed spacer region (ITS1-5.8S rDNA-ITS2) were amplified using different cells with several pairs of primers ( in Nézan et al., Citation2012). The PCRs were performed in a Mastercycler Personal (Eppendorf, Hamburg, Germany) as follows: one initial denaturation step at 94ºC for 2 min, followed by 45 cycles at 94ºC for 30 s, 52ºC for 30 s, and 72ºC for 4 min, and a final elongation at 72ºC for 5 min. The PCR products were purified using the Wizard SV Gel and PCR Clean-up system (Promega) according to the manufacturer’s recommendations. Then, they were sequenced directly using the ABI PRISM BigDye Terminator Cycle Sequencing Kit (Applied Biosystems, Carlsbad, CA, USA).
Newly obtained sequences were deposited in GenBank with accession numbers MG826100 to MG826115 and MG826360 to MG826367 (Supplementary table S1).
Sequence alignment and phylogenetic analysis
Newly obtained Bysmatrum sequences (nuclear-encoded SSU, partial LSU rDNA and ITS) were incorporated into a systematically representative set of dinoflagellates available in GenBank. Sequences were aligned using MAFFT v7.110 (Katoh & Standley, Citation2013) online program (http://mafft.cbrc.jp/alignment/server/) with default settings. Alignments were manually checked with BioEdit v. 7.0.5 (Hall Citation1999). For Bayesian inference (BI), the program jModelTest (Posada Citation2008) was used to select the most appropriate model of molecular evolution with Akaike Information Criterion (AIC). Bayesian reconstruction of the data matrix was performed using MrBayes 3.2 (Ronquist & Huelsenbeck, Citation2003) with the best-fitting substitution model (GTR+G). Four Markov chain Monte Carlo (MCMC) chains ran for 6 000 000 generations, sampling every 100 generations. The first 10% of burn-in trees were discarded. A majority rule consensus tree was created in order to examine the posterior probabilities of each clade. Maximum likelihood (ML) analyses were conducted with RaxML v7.2.6 (Stamatakis, Citation2006) on the T-REX web server (Boc et al., Citation2012) using the model GTR+G. Node support was assessed with 1000 bootstrap replicates.
Multiple ITS1-5.8S-ITS2 sequences of Bysmatrum species were aligned using MAFFT v7.110 (Katoh & Standley, Citation2013) online program (http://mafft.cbrc.jp/alignment/server/) with default settings. Completed alignments were saved as NEXUS files and imported into PAUP*4b10 software (Swofford, Citation2002) so that divergence rates could be estimated using simple uncorrected pairwise (p) distance matrices.
Results
Morphology
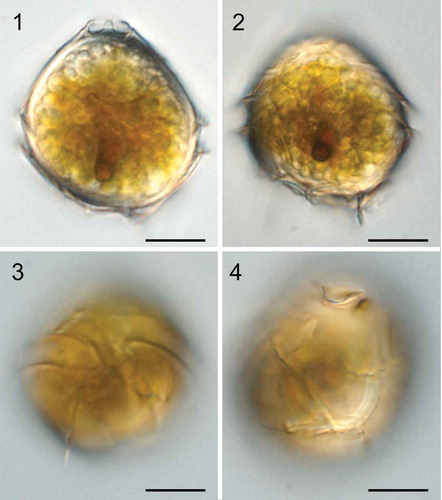
Bysmatrum subsalsum: Cysts of Bysmatrum subsalsum from Gironde, France were 30.0–40.9 µm long (mean = 36.0±3.4 μm, n=14) and 26.0–34.3 µm wide (mean =31.6±2.8 μm, n=14). They had a conical epitheca, and a round hypotheca (). The cysts possessed numerous golden granules and a red accumulation body in the sulcal area (, ). Plates were clearly visible and were identical to the thecal plates (, ).
Figs 1–4. Light micrographs of live cysts of Bysmatrum subsalsum from France. . Ventral view in mid-focus showing numerous granules, a red accumulation body and the apical stalk. . Dorsal view showing numerous granules and a red accumulation body. . Ventral view showing the cingulum displacement. . Dorsal view showing the plates. Scale = 10 μm.
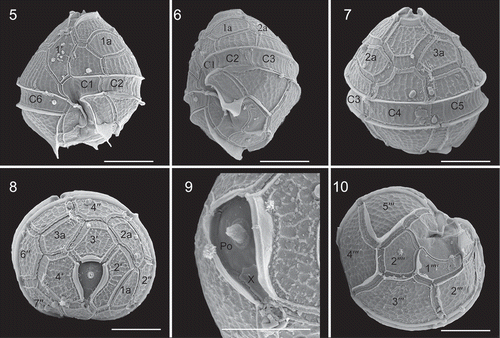
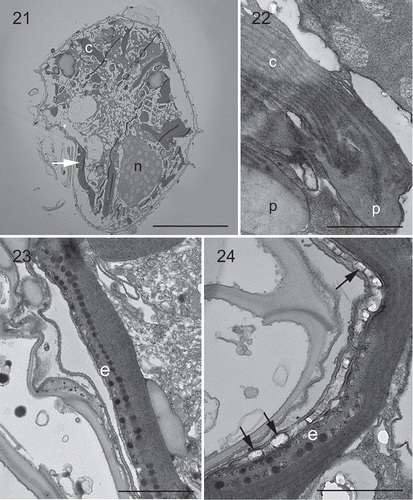
Cells of strain TIO406 from France were 22.7–31.7 µm long (mean =27.3±3.2 μm, n=15) and 22.0–29.4 µm wide (mean =23.9±2.3 μm, n=15). Under SEM, cysts and vegetative cells of Bysmatrum subsalsum from France showed a plate formula of Po, cp, X, 4′, 3a, 7′′, 6C, 4S, 5′′′, 2′′′′. The thecal plates were thick and covered with strong reticulations throughout (–). Thecal pores with a diameter of 0.14–0.28 μm were often observed along the plate margins. There were four apical plates. Plate 1′ was five-sided and asymmetrical with shorter anterior sutures than the posterior ones (). There were three anterior intercalary plates (1a, 2a and 3a), of which 3a is separated from 2a and 1a. Plate 1a was elongated rectangular whereas plates 2a and 3a were hexagonal and pentagonal, respectively (–). The length (of the upper side of 1a) and width (of the right side of 1a) ratio of the French strain TIO406 was 1.2–3.5 (mean =1.9±0.6, n=16). Occasionally, plate 1a was pentagonal (3 out of 37 cells). Plate 1′′ was five-sided and the upper and lower side length ratio was 0.63–0.84 (mean =0.74±0.07, n=14). The cingulum was deeply excavated, around 3.5–4.2 μm wide (mean = 3.8±0.3 μm, n=20) and descending to about its own width, comprising six plates. The first three cingular plates (C1, C2 and C3) were similar in size and much smaller than the other three plates (–). The apical pore complex (APC) was tear-shaped comprising a pore plate (Po) with a much smaller, round cover plate (cp) and an elongated pentagonal canal plate (X) with thick margins formed by the raised borders of the apical plates (, ). The APC ranged from 7.5 to 8.8 µm (mean =8.2±0.6, n=4) in length and from 3.2 to 3.5 µm (mean =3.4±0.1, n=4) in width. An apical stalk was sometimes observed protruding from the apical pore (). The first postcingular plate (1′′′) was much smaller than the other plates in the series plates and bore a sulcal list, as well as the 5′′′ (). The first antapical plate (1′′′′) was elongated and narrow, displaced to the left, whereas the second antapical plate (2′′′′) was pentagonal (). The anterior sulcal plate (Sa) and left sulcal (Ss) were elongated and narrow. The right sulcal (Sd) was triangular, with the internal sulcal list (i.s.l.) emerging from its left side. The posterior sulcal plate (Sp) was much wider than long. The left sulcal list (L.s.l.) emerged from the lower side of plate 1′′′ and the right sulcal list (R.s.l.) covered the entire left side of plate 5′′′ ().
Figs 5–10. Scanning electron micrographs of cysts of French Bysmatrum subsalsum. . Ventral view showing the first apical plate and cingulum displacement. . Left lateral view showing the first three cingular plates (C1, C2 and C3). . Dorsal view showing the separation of plates 2a and 3a. . Apical view showing four apical plates (1′–4′), three anterior intercalary (1a, 2a and 3a) plates and seven precingular plates (1′′–7′′). . Apical pore complex showing the apical stalk emerging from the apical pore, pore plate (Po) and the narrow canal plate (X). . Antapical view showing five postcingular plates (1′′′–5′′′) and two antapical plates (1′′′′, 2′′′′) of unequal size. Scale = 10 μm.
Figs 11–16. Scanning electron micrographs of vegetative cells of Bysmatrum subsalsum from French strain TIO406 (11) and Malaysian strain TBBYS02 (12–16). . The sulcus showing the anterior sulcal plate (Sa), left sulcal plate (Ss), right sulcal plate (Sd), posterior sulcal plate (Sp), the right sulcal list (R.s.l.), the left sulcal list (L.s.l.) and the internal sulcal list (i.s.l.). . Apical view showing four apical plates (1′–4′), three anterior intercalary (1a, 2a and 3a) plates and seven precingular plates (1′′–7′′). . Antapical view showing five postcingular plates (1′′′–5′′′) and left and internal sulcal lists. . Right lateral view showing the first apical plate. . Internal view showing six cingular plates (C1–C6). . Ventral view showing Sa, Ss, Sd, Sp plates, and L.s.l. (arrow), i.s.l. (arrowhead). Scale = 10 μm.
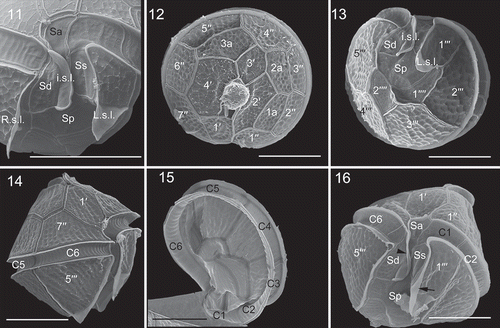
The morphologies of Malaysian strains TBBYS02 (–) and TBBYS03 (Supplementary figs S1–S6) were similar to the French strains (), although the Malaysian strains lacked a high sulcal list on 5′′′ (, , Supplementary figs S1, S5) and possessed a more elongated 1a plate. The length and width ratio of the 1a plate were 1.8–4.0 (mean =2.5±0.7, n=14) and 1.8–4.8 (mean =3.4±0.9, n=15) for strains TBBYS02 and TBBYS03. The length of the upper and lower side ratio of plate 1′′ was 0.69–0.90 (mean =0.83±0.06, n=13) and 0.60–0.82 (mean =0.74±0.06, n=14) for strains TBBYS02 and TBBYS03.
Table 2. Morphological comparisons of Bysmatrum strains examined in the present study with closely related species.
Under LM, Malaysian strains showed a lot of banded chloroplasts in the periphery of the cells (‘c’ in ). One pronounced orange eyespot was seen in the sulcal area (). The nucleus was large, elongated and located posteriorly (‘n’ in , ). TEM confirmed the presence of an eyespot in the sulcal area, and a posterior nucleus as well (). The chloroplasts had terminal pyrenoids with lamellae intruding the pyrenoid (). The thylakoids were grouped in twos or threes to form lamellae. The eyespot was located within a chloroplast comprising two rows of lipid globules (around 50 in each row) with a diameter of 70–110 nm (). There was one row of brick-like crystals enveloped by a vesicle overlying the eyespot, varying in size from 110 nm long and 80 nm wide to 220 nm long and 140 nm wide ().
Figs 17–20. Light micrographs of live cells of Bysmatrum subsalsum from Malaysian strains TBBYS02 (17–19) and TBBYS03 (20). . Ventral view showing the gross morphology and cingulum displacement. . Ventral view showing the banded chloroplasts (c) and a red eyespot in the sulcal area (arrow). . Dorsal view showing an elongated nucleus (n) and numerous chloroplasts (c). . Dorsal view showing an elongated nucleus (n) (Sybr Green staining). Scale = 10 μm.
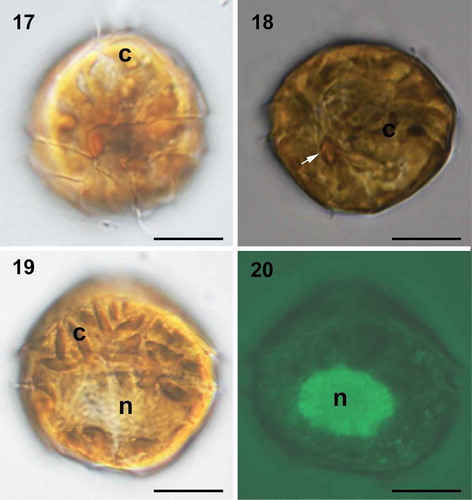
Figs 21–24. Transmission electron micrographs of vegetative cells of Bysmatrum subsalsum strain TBBYS02. . A longitudinal section through the cell showing a nucleus (n), several chloroplasts (c) and an eyespot (arrow). . Several chloroplasts (c) with terminal pyrenoids (p). . An eyespot (e) comprising two rows of lipid globules located within a chloroplast nearby sulcus. . Detail of the eyespot showing one row of overlying brick-like crystals (arrows). Scale: Fig. 21 = 10 μm; Figs 22–24 = 1 μm.
Bysmatrum gregarium: The cells of strain TIO316 from the South China Sea were 27.2–37.9 µm long (mean =31.0±3.1 μm, n=20) and 25.5–35.9 µm wide (mean =31.1±2.7 μm, n=20). The cells had a conical epitheca and a rounded hypotheca (, ). Bysmatrum gregarium showed a plate formula of Po, cp, X, 4′, 3a, 7′′, 6C, 4S, 5′′′, 2′′′′. The thecal plates were thick and covered with strong reticulations. Thecal pores were often observed on the plates.
Figs 25–30. Scanning electron micrographs of vegetative cells of Bysmatrum gregarium strain TIO316. . Ventral view showing the first apical plate. . Dorsal view showing the cingulum plates. . Apical view showing four apical plates (1′–4′), three anterior intercalary (1a, 2a and 3a) plates and seven precingular plates (1′′–7′′). . Antapical view showing five postcingular plates (1′′′–5′′′), two antapical plates (1′′′′, 2′′′′) of unequal size and the left sulcal list (L.s.l.). . Apical pore complex showing the oval pore plate (Po), elongated canal plate (X) and round apical pore. . The sulcus showing an anterior sulcal plate (Sa), a right sulcal plate (Sd), a left sulcal plate (Ss), and a posterior sulcal plate (Sp). Scale = 10 μm.
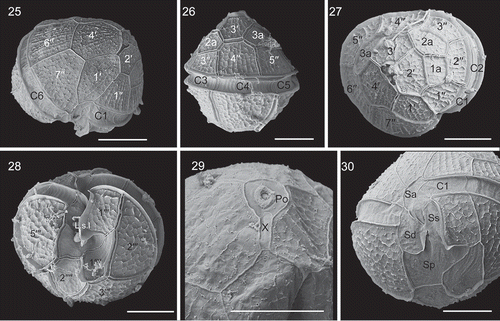
The first apical plate (1′) was five-sided and asymmetrical with longer anterior sutures than the posterior ones (). There were three anterior intercalary plates (1a, 2a and 3a). Plate 1a was nearly square and 2a and 3a were hexagonal and pentagonal respectively (, ). The length (of the upper side of 1a) and width (of the right side of 1a) ratio was 0.9–4.0 (mean =1.9 ± 1.1, n=9). Plate 1′′ was five-sided and the length of the upper and lower side ratio was 0.45–0.57 (mean =0.50±0.05, n=7). The cingulum was deeply excavated and descending to about 1.5 times its own width, comprising six plates of unequal size (–). The first three cingular plates (C1, C2 and C3) were similar in size and much smaller than the three other plates. The first postcingular plate (1′′′) was much smaller than other plates in this series (). The first antapical plate (1′′′′) was elongated and narrow, displaced to the left, whereas the second antapical plate (2′′′′) was pentagonal (). The apical pore complex comprised a round pore plate (Po), a round cover plate (cp) and an elongated canal plate (X) with a round apical pore located in the middle of the pore plate ().
There were four sulcal plates and two sulcal lists. The anterior sulcal plate (Sa) was elongated and narrow. The left sulcal (Ss) was elongated and narrow too. The right sulcal (Sd) was fan-shaped, with a small internal sulcal list (i.s.l.) at its left side. The posterior sulcal plate (Sp) was wider than long (). The left sulcal list (L.s.l.) emerged from the right of plate 1′′′ ().
Bysmatrum granulosum: Cells of the strain SP004 from the South China Sea were 34.0–42.8 µm long (mean =38.8±2.5 μm, n=13) and 30.2–42.6 µm wide (mean =35.5±3.8 μm, n=18). The cells had a conical epitheca and a round hypotheca (). Bysmatrum granulosum showed a plate formula of Po, cp, X, 4′, 3a, 7′′, 6C, 4S, 5′′′, 2′′′′. The thecal plates were thick and covered with small wart-like projections except on the sulcal and cingular plates (–). The apical pore complex was elongated comprising a pore plate (Po) and a narrow canal plate (X) with thickened margins formed by the raised borders of the apical plates ().
Figs 31–36. Scanning electron micrographs of vegetative cells of Bysmatrum granulosum strain SP004. . Ventral view showing the first apical plate (1′) and cingulum displacement. . Left lateral view showing the two anterior intercalary plates (1a, 2a). . Dorsal view showing the cingulum plates (C4, C5) and the third anterior intercalary plate (3a). . Apical view showing the apical pore complex, four apical plates (1′–4′), three anterior intercalary (1a, 2a and 3a) plates and seven precingular plates (1′′–7′′). . Antapical view showing five postcingular plates (1′′′–5′′′), two antapical plates (1′′′′, 2′′′′) of unequal size and the left sulcal list (L.s.l.). . The sulcus showing an anterior sulcal plate (Sa), a right sulcal plate (Sd), a left sulcal plate (Ss), a posterior sulcal plate (Sp), and the internal sulcal list (i.s.l.). Scale = 10 μm.
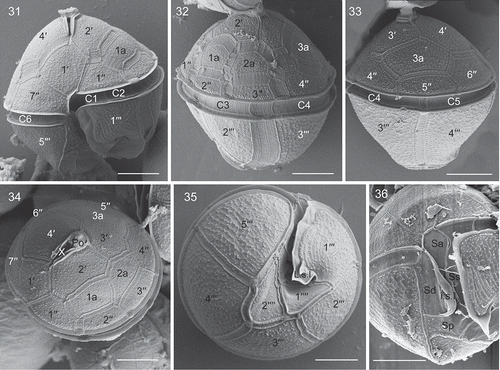
The first apical plate (1′) was five-sided and asymmetrical with anterior sutures equal in length to the posterior ones (). There were three anterior intercalary plates (1a, 2a and 3a). Plates 1a, 2a and 3a were rectangular, hexagonal and pentagonal respectively (, ). The length (of the upper side of 1a) and width (of the right side of 1a) ratio was 1.1–2.3 (mean =1.5±0.4, n=12). Plate 1′′ was five-sided and the length of the upper and lower side ratio was 0.65–0.85 (mean =0.74±0.05, n=12). The cingulum was deeply excavated and descended about 1.5 times of own width, comprising six plates of unequal size (, ). The first three cingular plates (C1, C2 and C3) were similar in size and much smaller than the three other plates. The first postcingular plate (1′′′) was smaller than other plates in this series (). The first antapical plate (1′′′′) was wider than long, displaced to the left, whereas the second antapical plate (2′′′′) was five-sided and slightly larger ().
There were four sulcal plates and two sulcal lists. The anterior sulcal plate (Sa) was triangular and the left sulcal (Ss) and right sulcal (Sd) were elongated and narrow. A small internal sulcal list (i.s.l.) was located at the left side of Sd. The posterior sulcal plate (Sp) was wider than long (). The left sulcal list (L.s.l.) emerged from the right side of plate 1′′′ ().
Cells of strain A10-P14-R2 from the South China Sea were 37.7–55.4 µm long (mean =46.2±4.2 μm, n=40) and 34.8–50.3 µm wide (mean =43.3±4.1 μm, n=40). The cells shared similar morphology with strain SP004 (Supplementary figs S7–S12, ).
Epithecal plate overlap in Bysmatrum, Parvodinium and Vulcanodinium
The epithecal plate overlap of all studied Bysmatrum species is identical and is exemplified in B. subsalsum (). The epithecal plate overlap of Vulcanodinium Nézan & Chomérat, Parvodinium Carty and Bysmatrum is characteristic for each genus (, ). These morphological differences are concordant with the genetic distances between the genera ().
Figs 37–39. Plate overlaps of epitheca in Bysmatrum, Vulcanodinium and Parvodinium. . Plate overlaps of Bysmatrum subsalsum. . Plate overlaps of Vulcanodinium rugosum. . Plate overlaps of Parvodinium umbonatum.
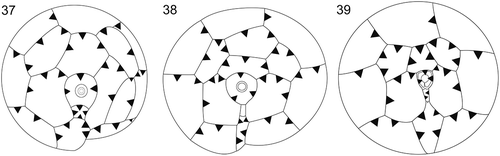
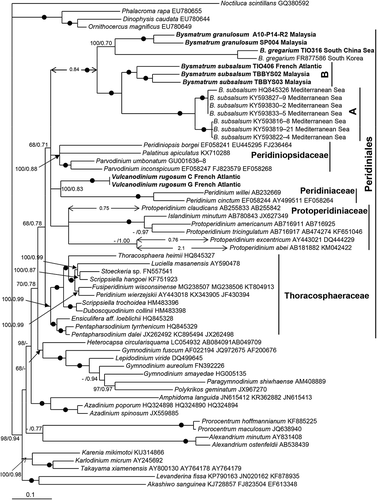
Fig. 40. Phylogeny of Bysmatrum inferred from concatenated SSU, partial LSU rDNA and ITS sequences using Bayesian inference . New sequences are indicated in bold. Branch lengths are drawn to scale, with the scale bar indicating the number of nucleotide substitutions per site. The long branches are reduced in size with their actual size indicated. Numbers on branches are statistical support values to clusters to the right of them (left: maximum likelihood bootstrap support values; right: Bayesian posterior probabilities). Black circles indicate maximal support (bootstrap = 100% in ML and pp = 1.00 in BI respectively).
MicroFTIR analysis of cysts of Bysmatrum subsalsum from French Atlantic
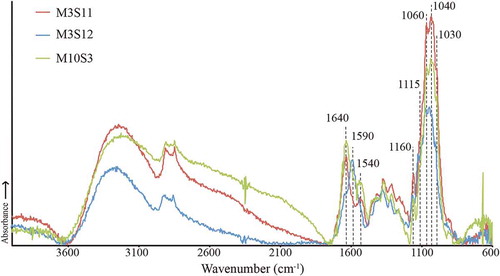
The geochemical analysis of the cyst walls of three specimens (M3S11, M3S12, M10S3) is illustrated in . There were absorptions for OH stretching centred at ~3300 cm–1 as well as aliphatic CH stretching (2725 & 2850 cm–1) and bending (1420 & 1370 cm–1) (not delineated in ). There were also absorptions that are characteristic for polysaccharides (Pandey Citation1999; Kačuráková & Wilson Citation2001), including 1640 cm–1 (C=O stretching), 1160 cm–1 (C-O-C asymmetric vibration), 1115 cm–1 (glucose ring stretching) and C-O stretching absorptions at 1060 cm–1, 1040 cm–1 and 1030 cm–1.
Overall, M3S11 and M10S3 showed the greatest similarity, including a shoulder at 1540 cm–1 that could suggest either extracellular contamination or amide groups within the cyst wall; however, there were no other clear absorptions for proteinaceous material (see contamination discussion in Mertens et al., Citation2015). The M3S12 spectrum is different in that it exhibits a shoulder at 1640 cm–1 and a band at 1590 cm–1 that reflect C=O and C=C stretching, respectively.
Molecular analysis and phylogeny
The genetic distance based on ITS1-5.8S-ITS2 sequences among Bysmatrum morphospecies ranged from 0.34 to 0.47 and those genetic distances at intraspecific level were 0.15 (B. gregarium and B. granulosum) and 0.09 to 0.41 (B. subsalsum) ().
Table 3. Uncorrected pairwise distances among Bysmatrum species based on ITS sequences.
The maximum likelihood (ML) and Bayesian inference (BI) analysis based on combined SSU rDNA, partial LSU rDNA and ITS sequences yielded similar phylogenetic trees. The BI tree is illustrated in . The genus Bysmatrum was monophyletic with maximal support. It was clearly nested within the Peridiniales and formed a clade with Parvodinium, Peridiniopsis borgei, Palatinus apiculatus, Vulcanodinium rugosum, Peridinium cintum and Protoperidinium with low support (68/0.71). Bysmatrum subsalsum consisted of two well-resolved subclades. One subclade comprised strains from the Mediterranean Sea (referred to ribotype A) and the other included strains from Malaysia and the French Atlantic (referred to ribotype B). Bysmatrum gregarium and B. granulosum grouped together with strong ML support (100), but low posterior probability (0.70).
Discussion
Morphology and biogeography
Bysmatrum subsalsum: The strains of B. subsalsum from the French Atlantic were characterized by the separation of plates 2a and 3a and an elongated rectangular 1a plate, thus fitting the original descriptions, especially the presence of three sulcal lists (Ostenfeld, Citation1908, fig. 50). Sulcal lists were also reported in specimens from Argentina (Balech, Citation1964, as Peridinium subsalsum), USA (Faust, Citation1996) and Mediterranean Sea (Gottschling et al., Citation2012; Anglès et al., Citation2017), but the right sulcal list was not present in the latter two reports or in the Malaysian strains TBBYS02 and TBBYS03 reported here. The shape of plate 1a is often rectangular in B. subsalsum, although a pentagonal 1a was occasionally observed (Faust & Steidinger, Citation1998; present study). The ratio of the length and width of 1a plate might be a useful indicator to differentiate B. subsalsum strains, but the ratio of the length of the upper and lower side of 2′′ plate appears rather conservative among these strains (). A significant correlation between APC length and total cell length was reported in B. subsalsum from the Mediterranean Sea (Anglès et al., Citation2017), and also confirmed in the Malaysian and French strains. Because of the morphological similarity between our strains and strains from elsewhere, we identified them as B. subsalsum.
Bysmatrum subsalsum has a worldwide distribution, being recorded in the Aral Sea (Ostenfeld, Citation1908), the Caribbean (Faust & Steidinger, Citation1998), the Gulf of Mexico (Limoges et al., Citation2015), the Mediterranean Sea (Gottschling et al., Citation2012; Anglès et al., Citation2017), Argentina (Balech, Citation1964, as P. subsalsum), Japan (Horiguchi, Citation1983) and in the South China Sea and French Atlantic as reported here.
Bysmatrum subsalsum is morphologically close to Peridinium sociale (Henneguy) Biecheler as described by Biecheler (Citation1952), but our strains are much smaller and have less cingulum displacement (1.0 versus 1.5 cingular widths). However, the Mediterranean B. subsalsum strains can have a cingulum displacement from 1.0 to 1.5 and have a large variability in cell size (Anglès et al., Citation2017). Therefore, the species reported by Biecheler (Citation1952) might be conspecific with B. subsalsum. However, whether B. subsalsum is a junior synonym of Peridinium sociale remains to determined since Henneguy (Citation1890) described Glenodinium sociale without giving information about the tabulation.
An apical stalk emerged from the apical pore of B. subsalsum (), and has been reported for B. arenicola and B. gregarium as well, suggesting that this is a common feature for the genus, possibly related to their epibenthic life stage. Mucus excreted from the apical stalk was observed in B. subsalsum and B. granulosum, which could help them to attach to a substrate, such as macroalgal surfaces, as illustrated for B. arenicola by Horiguchi & Pienaar (Citation1988b).
Cysts of Bysmatrum subsalsum from the French Atlantic are covered by thecal plates, as was reported for the Gulf of Mexico and Mediterranean Sea (Limoges et al., Citation2015; Anglès et al., Citation2017). The geochemical analysis of the cyst walls of three specimens (M3S11, M3S12, M10S3; ) showed overall consistency with other extant dinoflagellate cysts analysed from surface sediments (e.g. Bogus et al., Citation2014; Mertens et al., Citation2015). The M3S12 spectrum could indicate that aromatic bonds are present, similar to Pyrodinium bahamense (Mertens et al., Citation2015). The variability among the three specimens could reflect intraspecific differences even in cysts from the same location. The spectra of B. subsalsum cysts did not resemble those from heterotrophic dinoflagellates (Bogus et al., Citation2014), supporting that they have an autotrophic nutritional strategy.
Bysmatrum gregarium: Lombard & Capon (Citation1971) described a new thecate dinoflagellate isolated from tidal pools in southern California and named it Peridinium gregarium Lombard & Capon. After the illegitimate renaming of P. gregarium as Scrippsiella caponii by Horiguchi & Pienaar (Citation1988a), and its therefore illegitimate transfer to Bysmatrum caponii by Faust & Steidinger (Citation1998), the correct name for this species is now considered to be Bysmatrum gregarium (see Hoppenrath et al., Citation2014, for further details). The Chinese strain TIO316 generally fits the original description of B. gregarium but differs in the relative size of C2 and C3 (similar size of C2 and C3 in TIO316 versus half length of C3 in the type material) (Horiguchi & Pienaar, Citation1988a). Strain TIO316 is morphologically indistinguishable from the Korean strains described by Jeong et al. (Citation2012) which also have plates C2 and C3 of similar size. Bysmatrum gregarium is morphologically very close to B. subsalsum (Anglès et al., Citation2017), however, the ratio of length of upper and lower sides of 2′′ plate appears useful to differentiate them (0.5 vs 0.7, see ). Additionally, the ratio of total cell length and APC length can reach 5.0 in B. gregarium strain TIO316, much larger than those of B. subsalsum strains (less than 4.0).
Bysmatrum gregarium has been reported in southern California, western Korea (Jeong et al., Citation2012), the Mexican Caribbean (Almazán-Becerril et al., Citation2015), Hawai’i (Parsons & Preskitt, Citation2007) and in the South China Sea (this study). This is also the first report of Bysmatrum in Chinese waters.
Bysmatrum granulosum: The Malaysian strains SP004 and A10-P14-R2 fit the original description having wart-like projections on the thecal surface. Bysmatrum granulosum was described from sediment and coral samples collected from Reunion Island, South West Indian Ocean (Ten-Hage et al., Citation2001) and later found in Sabah, Malaysia (Mohammad-Noor et al., Citation2007) and the Mexican Caribbean (Almazán-Becerril et al., Citation2015). In this study, it was found in Rawa Island and Perhentian Island, extending its distribution to the Malay Peninsula. Our strains were also isolated from coral reef debris, supporting the idea that this species is always associated with corals (Ten-Hage et al., Citation2001).
Eyespot structure of Bysmatrum subsalsum from Malaysia
Moestrup & Daugbjerg (Citation2007) reviewed five different types of eyespot in dinoflagellates, excluding the complex ocelloid of the Warnowiaceae (Greuet, Citation1987): Type A, characterized by one to several layers of opaque globules inside a chloroplast; Type B, a vesicle containing crystal-like units overlying an eyespot type A-like chloroplast; Type C, layers of opaque lipid globules not bounded by a membrane; Type D, layers of opaque globules inside a reduced chloroplast; and Type E, several layers of crystal-like units contained in a vesicle. Craveiro et al. (Citation2010) reported a new type of eyespot (as Type F), characterized by a single layer of vesicle-contained crystal-like units overlying layers of more or less fused globules not bounded by membranes. The eyespot of B. subsalsum is located within the chloroplast and consists of two rows of lipid globules with overlying brick-like crystals, thus was identified as a Type B eyespot, as also reported in Borghiella dodgei (Moestrup et al., Citation2008) and Baldinia anauniensis (Hansen et al., Citation2007).
A prominent eyespot has been reported in B. subsalsum (Faust & Steidinger, Citation1998; Anglès et al., Citation2017), B. granulosum (Ten-Hage et al., Citation2001) and B. gregarium (Jeong et al., Citation2012) and is confirmed here in B. subsalsum. However, it was not described for B. arenicola (Horiguchi & Pienaar, Citation1988b) or B. teres (Murray et al., Citation2006). A Type A eyespot was reported in B. austrafrum (Dawut et al., Citation2018), and in species of Peridiniales such as Scrippsiella trochoidea (Craveiro et al., Citation2011), Palatinus apiculatus (Craveiro et al., Citation2009), Naiadinium polonicum (Craveiro et al., Citation2015) and Peridinium willei (Moestrup & Daugbjerg, Citation2007), suggesting that Bysmatrum is systematically distant from Thoracosphaeraceae but close to Peridiniaceae and Peridiniopsidaceae ().
Phylogeny and genetic differentiation
The phylogenetic position of Bysmatrum was first reported by Gottschling et al. (Citation2012) using concatenated SSU, ITS and LSU sequences. In their trees, Bysmatrum subsalsum appears distant from other Peridiniales, and this led Gottschling et al. (Citation2012) to suggest that Bysmatrum is closer to Gonyaucales. Later however, Jeong et al. (Citation2012) reported that Bysmatrum gregarium is close to Protoperidinium and Peridinium based on SSU and LSU rDNA sequences. The phylogenetic tree based on SSU, partial LSU rDNA and ITS () supports the nesting of Bysmatrum within the Peridiniales (Anglès et al., Citation2017) and a close relationship between Bysmatrum and the freshwater planktonic genus Parvodinium. These two genera share the separation of anterior intercalary plates (Carty, Citation2008), although Parvodinium only has two anterior intercalary plates in contrast to the three found in Bysmatrum (Hansen & Flaim, Citation2007; Carty, Citation2008). Parvodinium was classified in the family Peridiniopsidaceae together with Peridiniopsis Bourrelly and Palatinus Craveiro, Calado, Daugbjerg & Moestrup (Gottschling et al., Citation2017). An eyespot is probably present in Parvodinium inconspicuum (Lemmermann) Carty (Carty, Citation2008), but detailed study has not been carried out and should be the focus of future research.
The close relationship between Bysmatrum and Vulcanodinium is not surprising because of their similarity in shape and plate pattern and thick, reticulate plates. The major difference is the separation of plates 2a and 3a in Bysmatrum, which does not occur in Vulcanodinium (Nézan & Chomérat, Citation2011). In addition, Bysmatrum subsalsum strain TBBYS02 is not able to produce pinnatoxin (Krock, B. personal communication) while Vulcanodinium rugosum is always toxic (Selwood et al., Citation2014). The ITS-based genetic distances at intraspecific level in B. subsalsum reached 0.4, similar to those between the different sequenced Bysmatrum species () and much greater than the threshold of 0.04 used to delimit species based on ITS based genetic distances (Litaker et al., Citation2007), supporting the idea that there is cryptic diversity within B. subsalsum (Anglès et al., Citation2017). It is worth noting that two Malaysian strains of B. subsalsum were recovered from a single water sample and showed large genetic differentiation, as also reported in the two B. granulosum strains collected in neighbouring locations. Whether such genetic differentiations also occurs in other Bysmatrum species remains to be confirmed.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at http://10.1080/09670262.2018.1449014
Supplementary table S1. GenBank accession numbers of strains or isolates examined in the present study.
Supplementary figs S1–S6. Scanning electron micrographs of vegetative cells of Bysmatrum subsalsum from Malaysian strain TBBYS03. Fig. S1. Left lateral view showing the elongated 1a plate and three cingular plates (C1–C3). Fig. S2. Apical view showing four apical plates (1′–4′), three anterior intercalary (1a, 2a and 3a) plates and seven precingular plates (1′′–7′′). Fig. S3. Dorsal view showing three anterior intercalary (1a, 2a and 3a) plates. Fig. S4. Ventral view showing five postcingular plates (1′′′–5′′′) and two antapical plates (1′′′′, 2′′′′) of unequal size. Fig. S5. The sulcus showing the anterior sulcal plate (Sa), left sulcal plate (Ss), right sulcal plate (Sd), posterior sulcal plate (Sp), the left sulcal list (L.s.l.) and the internal sulcal list (i.s.l.). Fig. S6. Antapical view showing five postcingular plates (1′′′–5′′′) and two antapical plates (1′′′′, 2′′′′) of unequal size. Scale bars = 10 μm.
Supplementary figs S7–S12. Scanning electron micrographs of vegetative cells of Bysmatrum granulosum strain A10-P14-R2. Fig. S7. Ventral view showing the first apical plate and cingulum displacement. Fig. S8. Left lateral view showing the two anterior intercalary plates (1a, 2a). Fig. S9. Dorsal view showing the cingulum plates and the third anterior intercalary plate (3a). Fig. S10. Apical view showing the apical pore complex, four apical plates (1′–4′), three anterior intercalary (1a, 2a and 3a) plates and seven precingular plates (1′′–7′′). Fig. S11. Antapical view showing five postcingular plates (1′′′–5′′′), two antapical plates (1′′′′, 2′′′′) of unequal size and the left sulcal list (L.s.l.). Fig. S12. The sulcus showing an anterior sulcal plate (Sa), a right sulcal plate (Sd), a left sulcal plate (Ss) and a posterior sulcal plate (Sp). Scale bars = 10 μm.
Supplementary_material.zip
Download Zip (18.2 MB)Acknowledgements
We thank two anonymous reviewers for constructive suggestions that greatly improved the manuscript. N. Gayet is acknowledged for critical-point drying of the samples for SEM. Claire Meteigner and Myriam Rumebe are thanked for sediment sampling in France. Data on Malaysian strains was part of research dissertation of ZFL.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
Notes on contributors
Zhaohe Luo
Z. Luo: electron microscopy and drafting manuscript; Z.F. Lim: culture experiments; K.N. Mertens: original concept, drafting and editing manuscript, light microscopy, scanning electron microscopy; P. Gurdebeke:drafting and editing manuscript, interpretation of FTIR spectra; K. Bogus: drafting and editing manuscript, interpretation of FTIR spectra; M. C. Carbonell-moore: drafting and editing manuscript, interpretation of SEM images, illustration; H. Vrielinck: drafting and editing manuscript, interpretation of FTIR spectra; C.P. Leaw: analysis of molecular data; P.T. Lim: analysis of molecular data; N. Chomérat: drafting and editing manuscript, scanning electron microscopy; X. Li: culture experiment; H. Gu: original concept and editing of manuscript.
References
- Adachi, M., Sako, Y. & Ishida Y. (1996). Analysis of Alexandrium (Dinophyceae) species using sequences of the 5.8S ribosomal DNA and internal transcribed spacer regions. Journal of Phycology, 32: 424–432.
- Almazán-Becerril, A., Escobar-Morales, S., Rosiles-González, G. & Valadez, F. (2015). Benthic-epiphytic dinoflagellates from the northern portion of the Mesoamerican Reef System. Botanica Marina, 58: 115–128.
- Anglès, S., Reñé, A., Garcés, E., Lugliè, A., Sechi, N., Camp, J. & Satta, C.T. (2017). Morphological and molecular characterization of Bysmatrum subsalsum (Dinophyceae) from the western Mediterranean Sea reveals the existence of cryptic species. Journal of Phycology, 53: 833–847.
- Balech, E. (1964). Tercera contribucion al conocimiento del genero Peridinium. Museo Argentino de ciencias naturales ‘Bernadino Rivadavia’e Instituto nacional de investigacion de las ciencias naturales, Revista, Hydrobiologia, 4: 179–195.
- Balech, E. (1980). On the thecal morphology of dinoflagellates with special emphasis on circular and sulcal plates. Anales del Centro de Ciencias del Mar y Limnologia, Universidad Nacional Autonomia de Mexico, 7: 57–68.
- Biecheler, B. (1952). Recherches sur les Peridiniens. Bulletin biologique de la France et de la Belgique, 36: 1–147.
- Boc, A., Diallo, A.B. & Makarenkov, V. (2012). T-REX: a web server for inferring, validating and visualizing phylogenetic trees and networks. Nucleic Acids Research, 40: W573–W579.
- Bogus, K., Mertens, K.N., Lauwaert, J., Harding, I.C., Vrielinck, H., Zonneveld, K.A. & Versteegh, G.J. (2014). Differences in the chemical composition of organic-walled dinoflagellate resting cysts from phototrophic and heterotrophic dinoflagellates. Journal of Phycology, 50: 254–266.
- Carty, S. (2008). Parvodinium gen. nov. for the Umbonatum group of Peridinium (Dinophyceae). Ohio Journal of Science, 108: 103–107.
- Craveiro, S., Calado, A.J., Daugbjerg, N. & Moestrup, Ø. (2009). Ultrastructure and LSU rDNA-based revision of Peridinium group palatinum (Dinophyceae) with the description of Palatinus gen. nov. Journal of Phycology, 45: 1175–1194.
- Craveiro, S.C., Moestrup, Ø., Daugbjerg, N. & Calado, A.J. (2010). Ultrastructure and large subunit rDNA‐based phylogeny of Sphaerodinium cracoviense, an unusual freshwater dinoflagellate with a novel type of eyespot. Journal of Eukaryotic Microbiology, 57: 568–585.
- Craveiro, S.C., Calado, A.J., Daugbjerg, N., Hansen, G. & Moestrup, Ø. (2011). Ultrastructure and LSU rDNA-based phylogeny of Peridinium lomnickii and description of Chimonodinium gen. nov. (Dinophyceae). Protist, 162: 590–615.
- Craveiro, S.C., Daugbjerg, N., Moestrup, Ø. & Calado, A.J. (2015). Fine-structural characterization and phylogeny of Peridinium polonicum, type species of the recently described genus Naiadinium (Dinophyceae). European Journal of Protistology, 51: 259–279.
- Daugbjerg, N., Hansen, G., Larsen, J. & Moestrup, Ø. (2000). Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia, 39: 302–317.
- Dawut, M., Sym, S.D., Suda, S. & Horiguchi, T. (2018). Bysmatrum austrafrum sp. nov. (Dinophyceae), a novel tidal pool dinoflagellate from South Africa. Phycologia, 57: 169–178.
- Faust, M.A. (1996). Morphology and ecology of the marine benthic dinoflagellate Scrippsiella subsalsa (Dinophyceae). Journal of Phycology, 32: 669–675.
- Faust, M.A. & Steidinger, K.A. (1998). Bysmatrum gen. nov. (Dinophyceae) and three new combinations for benthic scrippsielloid species. Phycologia, 37: 47–52.
- Fensome, R.A., Taylor, F.J.R., Norris, G., Sarjeant, W.A.S., Wharton, D.I. & Williams, G.L. (1993). A classification of fossil and living dinoflagellates. Micropaleontology Special Publication, 7: 1–245.
- Fritz, L. & Triemer, R. (1985). A rapid simple technique utilizing calcofluor white M2R for the visualization of dinoflagellate thecal plates. Journal of Phycology, 21: 662–664.
- Greuet, C. (1987). Complex organelles. In The Biology of Dinoflagellates (Taylor, F.J.R., editor), 117–142. Blackwell, Oxford.
- Gottschling, M., Soehner, S., Zinssmeister, C., John, U., Plötner, J., Schweikert, M., Aligizaki, K. & Elbrächter, M. (2012). Delimitation of the Thoracosphaeraceae (Dinophyceae), including the calcareous dinoflagellates, based on large amounts of ribosomal RNA sequence data. Protist, 163: 15–24.
- Gottschling, M., Kretschmann, J. & Čalasan, A.Ž. (2017). Description of Peridiniopsidaceae, fam. nov. (Peridiniales, Dinophyceae). Phytotaxa, 299: 293–296.
- Gu, H., Luo, Z., Mertens, K.N., Price, A.M., Turner, R.E. & Rabalais, N.N. (2015a). Cyst-motile stage relationship, morphology, ultrastructure, and molecular phylogeny of the gymnodinioid dinoflagellate Barrufeta resplendens comb. nov., formerly known as Gyrodinium resplendens, isolated from the Gulf of Mexico. Journal of Phycology, 51: 990–999.
- Gu, H., Liu, T. & Mertens, K. (2015b). cyst-theca relationship and phylogenetic positions of Protoperidinium (Peridiniales, Dinophyceae) species of the sections Conica and Tabulata, with description of Protoperidinium shanghaiense sp. nov. Phycologia, 54: 49–66.
- Guillard, R.R.L. & Ryther, J.H. (1962). Studies of marine planktonic diatoms. I. Cyclotella Nana Hustedt and Detonula confervacea Cleve. Canadian Journal of Microbiology, 8: 229–239.
- Hall, T.A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT, pp. 95–98.
- Hansen, G., Daugbjerg, N. & Henriksen, P. (2007). Baldinia anauniensis gen. et sp. nov.: a “new” dinoflagellate from Lake Tovel, N. Italy. Phycologia, 46: 86–108.
- Hansen, G. & Flaim, G. (2007). Dinoflagellates of the Trentino Province, Italy. Journal of Limnology, 66: 107–141.
- Head, M.J. (1996). Modern dinoflagellate cysts and their biological affinities. In Palynology: principles and applications (Jansonius, J. & McGregor, D.C., editors), 1197–1248. American Association of Stratigraphic Palynologists Foundation, Dallas.
- Henneguy, M.F. (1890). Contributions a l’étude de la faune des marais salants. Compte Rendu Societé Biologique, 9: 625–627.
- Hoppenrath, M., Murray, S.A., Chomérat, N. & Horiguchi, T. (2014). Marine benthic dinoflagellates – unveiling their worldwide biodiversity. Kleine Senckenberg-Reihe 54, Schweizerbart Science, Stuttgart.
- Horiguchi, T. (1983). Life history and taxonomy of benthic dinoflagellates (Pyrrophyta). PhD thesis. University of Tsukuba, Tsukuba, Japan. 142 pp.
- Horiguchi, T. & Pienaar, R.N. (1988a). A redescription of the tidal pool dinoflagellate Peridinium gregarium based on re-examination of the type material. European Journal of Phycology, 23: 33–39.
- Horiguchi, T. & Pienaar, R. (1988b). Ultrastructure of a new sand-dwelling dinoflagellate, Scrippsiella arenicola sp. nov. Journal of Phycology, 24: 426–438.
- Jeong, H.J., Jang, S.H., Kang, N.S., Yoo, Y.D., Kim, M.J., Lee, K.H., Yoon, E.Y., Potvin, É., Hwang, Y.J. & Kim, J.I. (2012). Molecular characterization and morphology of the photosynthetic dinoflagellate Bysmatrum caponii from two solar saltons in western Korea. Ocean Science Journal, 47: 1–18.
- Kačuráková, M. & Wilson, R. H. (2001). Developments in mid-infrared FT-IR spectroscopy of selected carbohydrates. Carbohydrate Polymers, 44: 291–303.
- Katoh, K. & Standley, D.M. (2013). MAFFT Multiple Sequence Alignment Software Version 7: improvements in performance and usability. Molecular Biology and Evolution, 30: 772–780.
- Limoges, A., Mertens, K.N., Ruíz-Fernández, A.C. & Vernal, A. (2015). First report of fossilized cysts produced by the benthic Bysmatrum subsalsum (Dinophyceae) from a shallow Mexican lagoon in the Gulf of Mexico. Journal of Phycology, 51: 211–215.
- Lindberg, K., Moestrup, Ø. & Daugbjerg, N. (2005). Studies on woloszynskioid dinoflagellates I: Woloszynskia coronata re-examined using light and electron microscopy and partial LSU rDNA sequences, with description of Tovellia gen. nov. and Jadwigia gen. nov. (Tovelliaceae fam. nov.). Phycologia, 44: 416–440.
- Litaker, W.R., Vandersea, M.W., Kibler, S.R., Reece, K.S., Stokes, N.A., Lutzoni, F.M., Yonish, B.A., West, M.A., Black, M.N.D. & Tester, P.A. (2007). Recognizing dinoflagellate species using ITS rDNA sequences. Journal of Phycology, 43: 344–355.
- Lombard, E.H. & Capon, B. (1971). Peridinium gregarium, a new species of dinoflagellate. Journal of Phycology, 7: 184–187.
- Medlin, L., Elwood, H.J., Stickel, S. & Sogin, M.L. (1988). The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene, 71: 491–499.
- Mertens, K.N., Wolny, J., Carbonell-Moore, C., Bogus, K., Ellegaard, M., Limoges, A., de Vernal, A., Gurdebeke, P., Omura, T. & Al-Muftah, A. (2015). Taxonomic re-examination of the toxic armored dinoflagellate Pyrodinium bahamense Plate 1906: Can morphology or LSU sequencing separate P. bahamense var. compressum from var. bahamense? Harmful Algae, 41: 1–24.
- Moestrup, Ø. & Daugbjerg, N. (2007). On dinoflagellate phylogeny and classification. In Unravelling the Algae: The Past, Present, and Future of Algal Systematics (Brodie, J. & Lewis J., editors), 215–230. The Systematics Association Special Volume Series, CRC Press, Boca Raton, FL.
- Moestrup, Ø., Hansen, G. & Daugbjerg, N. (2008). Studies on woloszynskioid dinoflagellates III: on the ultrastructure and phylogeny of Borghiella dodgei gen. et sp. nov., a cold-water species from Lake Tovel, N. Italy, and on B. tenuissima comb. nov. (syn. Woloszynskia tenuissima). Phycologia, 47: 54–78.
- Mohammad-Noor, N., Daugbjerg, N., Moestrup, Ø. & Anton, A. (2007). Marine epibenthic dinoflagellates from Malaysia – a study of live cultures and preserved samples based on light and scanning electron microscopy. Nordic Journal of Botany, 24: 629–690.
- Murray, S., Hoppenrath, M., Larsen, J. & Patterson, D.J. (2006). Bysmatrum teres sp. nov., a new sand-dwelling dinoflagellate from north-western Australia. Phycologia, 45: 161–167.
- Nézan, E. & Chomérat, N. (2011). Vulcanodinium rugosum gen. et sp. nov. (Dinophyceae), un nouveau dinoflagellé marin de la côte méditerranéenne française. Cryptogamie-Algologie, 32: 3–18.
- Nézan, E., Tillmann, U., Bilien, G., Boulben, S., Chèze, K., Zentz, F., Salas, R. & Chomérat, N. (2012). Taxonomic revision of the dinoflagellate Amphidoma caudata: transfer to the genus Azadinium (Dinophyceae) and proposal of two varieties, based on morphological and molecular phylogenetic analyses. Journal of Phycology, 48: 925–939.
- Netzel, H. & Dürr, G. (1984). Dinoflagellate cell cortex. In Dinoflagellates (Spector, D.L., editor), 43–105. Academic Press, Orlando, FL.
- Ostenfeld, C.H. (1908). The phytoplankton of the Aral Sea and its affluents, with an enumeration of the algae observed. Wissenschaftliche Ergebnisse der Aralsee- Expedition, 8: 123–225.
- Pandey, K K. (1999). A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. Journal of Applied Polymer Science, 71: 1969–1975.
- Parsons, M.L. & Preskitt, L.B. (2007). A survey of epiphytic dinoflagellates from the coastal waters of the island of Hawai’i. Harmful Algae, 6: 658–669.
- Posada, D. (2008). jModelTest: phylogenetic model averaging. Molecular Biology and Evolution, 25: 1253–1256.
- Ronquist, F. & Huelsenbeck, J.P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19: 1572–1574.
- Satta, C.T., Anglès, S., Garcés, E., Sechi, N., Pulina, S., Padedda, B.M., Stacca, D. & Lugliè, A. (2013a). Dinoflagellate cyst assemblages in surface sediments from three shallow Mediterranean lagoons (Sardinia, North Western Mediterranean Sea). Estuaries and Coasts, 37: 1–18.
- Satta, C. T., Anglès, S., Lugliè, A., Guillén, J., Sechi, N., Camp, J. & Garcés, E. (2013b). Studies on dinoflagellate cyst assemblages in two estuarine Mediterranean bays: a useful tool for the discovery and mapping of harmful algal species. Harmful Algae, 24: 65–79.
- Scholin, C.A., Herzog, M., Sogin, M. & Anderson, D.M. (1994). Identification of group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. Journal of Phycology, 30: 999–1011.
- Selwood, A.I., Wilkins, A.L., Munday, R., Gu, H., Smith, K.F., Rhodes, L.L. & Rise, F. (2014). Pinnatoxin H: a new pinnatoxin analogue from a South China Sea Vulcanodinium rugosum isolate. Tetrahedron Letters, 55: 5508–5510.
- Spurr, A.R. (1969). A low-viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructure Research, 26: 31–43.
- Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22: 2688–2690.
- Steidinger, K. A. & Balech, E. (1977). Scrippsiella subsalsa (Ostenfeld) comb nov. (Dinophyceae) with a discussion on Scrippsiella. Phycologia, 16: 69–73.
- Swofford, D.L. (2002). PAUP*. Phylogenetic analysis using parsimony (* and other methods), version 4. Sinauer Associates, Sunderland, MA.
- Ten-Hage, L., Quod, J., Turquet, J. & Couté, A. (2001). Bysmatrum granulosum sp. nov. a new benthic dinoflagellate from the southwestern India sea. European Journal of Phycology, 36: 129–135.
- Tillmann, U., Elbrächter, M., Krock, B., John, U. & Cembella, A. (2009). Azadinium spinosum gen. et sp. nov. (Dinophyceae) identified as a primary producer of azaspiracid toxins. European Journal of Phycology, 44: 63–79.