ABSTRACT
Fucus vesiculosus and F. radicans (Phaeophyceae) are important habitat-formers on rocky shores in the Bothnian Sea. While both species occur sympatrically along the entire western Bothnian Sea coast, F. radicans has been found only in the northern part of the eastern coast. According to previous studies, the two species can be distinguished based on morphology, F. radicans having narrower thalli and a bushier appearance. However, marine mapping in the eastern Bothnian Sea has revealed that high morphological variation in Fucus, partly caused by gradients in salinity and exposure, makes differentiation between the two species difficult. We studied morphological and genetic variation to find out whether the two Fucus species can be differentiated in the south-eastern Bothnian Sea, and if F. radicans occurs in the area. The study was carried out in six subareas including 350 km of coast, with a salinity gradient of 3.5–6.5 PSU, and varying wave exposure. We found a gradual change towards smaller and narrower thalli and a higher number of holdfasts in Fucus populations when moving northwards to lower salinities. Distinct Fucus morphs were often found within the study sites but the morphs were genetically differentiated only at one study site in the Skaftung subarea, suggesting the occurrence of both species. However, in the Vasa subarea the sample size for analysing genetic differentiation was low due to high clonality. In the Luvia subarea south of Skaftung, Fucus morphology corresponded to that of F. radicans in earlier studies but the population was genetically more similar to F. vesiculosus in the southern subareas. We conclude that by using only morphological characteristics it is not possible to differentiate between the two species in central and northern parts of the eastern Bothnian Sea. Based on genetic analyses, the southernmost known occurrence of F. radicans in the eastern Bothnian Sea is in Skaftung.
Introduction
The brown algae of the genus Fucus are widely distributed on the temperate rocky shores of the northern hemisphere, where they mainly occur in the intertidal zone (Graham & Wilcox, Citation2003). In the brackish-water Baltic Sea Fucus is the only genus of large canopy-forming and habitat-structuring marine macroalgae, providing food and shelter for many other species (Wikström & Kautsky, Citation2007). In the Baltic Sea Fucus thalli are permanently submerged and subject to several stress factors, such as low salinity, ice-scour and high water turbidity (Wahl et al., Citation2011). Two species of Fucus occur in the northern Baltic Sea, Fucus vesiculosus L. and Fucus radicans L. Bergström & L. Kautsky (Bergström et al., Citation2005), the latter being endemic to the Baltic Sea (Pereyra et al., Citation2009). While F. vesiculosus is widely distributed in the Baltic Sea, extending to approximately 3.5 PSU salinity in the north (Nielsen et al., Citation1995; Bergström & Bergström, Citation1999) and to even lower salinities in the eastern Gulf of Finland (Lehvo & Bäck Citation2000; Schagerström, Citation2013), F. radicans is more limited in its distribution. The two species occur in sympatry along the Swedish Bothnian Sea coast (Bergström et al., Citation2005; Johannesson et al., Citation2011; Forslund et al., Citation2012), and in the north-eastern Bothnian Sea (Johannesson et al., Citation2011), suggesting a clear barrier to gene flow between the species (Bergström et al., Citation2005; Johannesson et al., Citation2011). Fucus radicans also occurs along the Estonian west coast (Johannesson et al., Citation2011; Pereyra et al., Citation2013; Schagerström, Citation2013) and in the eastern Gulf of Finland (Ardehed et al., Citation2016), although these populations are genetically different from the populations further north (Ardehed et al., Citation2016). So far, F. radicans has not been found in areas outside the currently described range (summarized in Schagerström, Citation2013), i.e. in the south-eastern Bothnian Sea, Åland Islands, Archipelago Sea or western Gulf of Finland.
According to Bergström et al. (Citation2005) the two species can be distinguished from each other based on morphological characteristics: F. radicans is smaller and has narrow thalli with a distinct midrib, a bushy appearance, elliptic receptacles and no vesicles. The two species have also shown ecological differences (e.g. differences in palatability and attractiveness to herbivores: Råberg & Kautsky, Citation2007; Forslund et al., Citation2012; Gunnarsson & Berglund, Citation2012). However, high morphological and physiological variation is typical of Fucus species (Kalvas & Kautsky, Citation1998; Ruuskanen & Bäck, Citation1999; Ferreira et al., Citation2015) and different forms of F. vesiculosus have been described in the Baltic Sea (Kjellman, Citation1890; Wærn, Citation1952) and also within small archipelagos (Häyrén, Citation1949, Citation1950). In contrast to the western Bothnian Sea where sympatric populations of F. radicans and F. vesiculosus show clear morphological differences (Bergström et al., Citation2005), it has become evident during the Finnish Inventory Programme for the Marine Environment (VELMU) that the morphology of Fucus is highly variable in the eastern Bothnian Sea on small geographic scales, but no clear morphological differences ensuring species identification exist.
The variation in Fucus morphology has traditionally been linked with phenotypic plasticity and environmental factors (Ruuskanen & Bäck, Citation1999, Citation2002; Kalvas & Kautsky, Citation1993; Scott et al., Citation2001), but physiological traits seem more genetically determined (Pearson et al., Citation2000; Johannesson et al., Citation2012). Although independently evolving Fucus populations may sometimes develop similar morphological characteristics in similar environments (Neiva et al., Citation2012), mosaics of different phenotypes at specific sites are more likely to be related to genotypic variation (Kalvas & Kautsky, Citation1998). In the Baltic Sea the main factors affecting Fucus morphology are salinity and exposure, both on large (Kalvas & Kautsky, Citation1993; Ruuskanen & Bäck, Citation1999) and more local scales (Bäck, Citation1993; Kalvas & Kautsky, Citation1993; Ruuskanen et al., Citation1999). The Gulf of Bothnia has a distinct salinity gradient with decreasing salinity towards the north, coupled with a gradual change towards smaller Fucus individuals with narrower fronds when moving north (Ruuskanen & Bäck, Citation1999). This phenomenon further complicates the differentiation between F. radicans and F. vesiculosus.
Clonality is common in Fucus in the northern Baltic Sea (Johannesson et al., Citation2011; Ardehed et al., Citation2016). Asexual reproduction takes place mostly via adventitious branches that can produce rhizoids, re-attach and grow into adult clones of the parental plant (Tatarenkov et al., Citation2005). In the Gulf of Bothnia F. radicans shows particularly frequent clonality: up to 90% of the populations in the northern Baltic Sea may consist of only one clone, but asexual reproduction is also common in F. vesiculosus populations (Johannesson et al., Citation2011; Ardehed et al., Citation2016). Previously, high clonality in Fucus has been linked with the effects of low salinity causing polyspermy and reducing motility and longevity of the gametes (Serrão et al., Citation1996, Citation1999), thus favouring asexual reproduction in low salinity areas (Bergström et al., Citation2005; Johannesson et al., Citation2011). However, it was recently found that sexual reproduction can occur in very low salinities (2–3 PSU) in the eastern Gulf of Finland (Ardehed et al., Citation2016).
As species identification of F. radicans and F. vesiculosus relies on observed morphological differences and an underlying concordant genetic structuring between the morphs due to (assumed) reproductive isolation, both genetic and morphological data should be considered together for reliable identification. However, on a larger scale, e.g. during marine mapping efforts, genetic analysis of each sample is not feasible and thus an identification method based solely on morphology would greatly facilitate inventory and monitoring in the field. Prior to this, a clear correlation of local morphological variation and genetic structure should be established empirically. Information on genetic variation in Fucus (potentially small gene pools close to the distribution limits) and on the distribution of F. radicans in relation to F. vesiculosus is also important from a management perspective and should be considered in management plans and protected area designs.
In this study we investigated morphological variation in Fucus in relation to genetic variation in the eastern Bothnian Sea and eastern Åland islands, to determine if F. radicans occurs in the area. The specific aims of this study were: (i) to describe the patterns of morphological variation in Fucus from the eastern Bothnian Sea, especially in relation to exposure and geographic location/salinity gradient, (ii) to estimate genetic differentiation between studied populations in the eastern Bothnian Sea, and (iii) to estimate ‘within-site’ genetic differentiation between individuals visually identified as different morphs.
Materials and methods
Study area
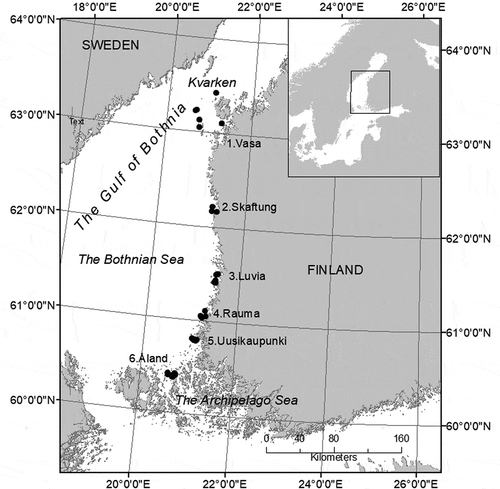
The study area is situated in the northern Baltic Sea, in the Gulf of Bothnia, along the west coast of Finland (). The area is characterized by a salinity gradient from about 6.5 PSU in the south to 3.5 PSU in the north. The steepest salinity gradient is in Kvarken, where water circulation is constrained by a shallow sill and a vast archipelago, resulting in the disappearance of many marine species in the area. The circulation of water in the Gulf of Bothnia is anti-clockwise (Voipio, Citation1981), with more saline waters moving northwards along the western coast of Finland and more limnic waters moving south along the east coast of Sweden. The west coast of Finland is relatively wave-exposed, but the rugged coastline and islands create more sheltered archipelago areas.
Data collection
The study was carried out during July–August 2011 in six subareas: Vasa (6 sites), Skaftung (3 sites), Luvia (6 sites), Rauma (6 sites), Uusikaupunki (6 sites) and Åland (9 sites). These areas are hereafter referred to as ‘subareas’, which contain ‘study sites’. As exposure is known to affect the morphology of Fucus, the study sites represented three different exposure classes (), based on the exposure model calculated using Wave Impact software (method described in Isæus Citation2004). The algal samples for morphological measurements were collected from depths of 1–4 m with maximum coverage of Fucus. Only fully grown individuals with receptacles or enough branching to indicate sexual maturity were analysed. Sampling was carried out by assuming that it is possible to distinguish between F. vesiculosus and F. radicans using morphological characteristics alone. If clearly distinct Fucus morphs were found at a study site (potentially representing separate species, narrow morph = potential F. radicans, wide morph = potential F. vesiculosus), 10 individuals of both morphs were collected. If Fucus individuals at a study site did not vary in appearance, only 10 individuals were collected. At site P5 in the Luvia subarea, only four individuals were sampled due to the low density of Fucus, but they were included in the study to increase the number of narrow morphs in the Luvia subarea. In order to make sure that samples represented different individuals, they were collected at least 10 m apart. When sampling, the whole alga was collected, including the holdfast which was scraped off the rock with a knife. Samples were individually marked and the growth depth of each individual was recorded.
Table 1. The abbreviations for study sites in each subarea and their division to exposure classes (sheltered, moderately exposed and exposed).
Morphological measurements
Five morphological variables previously used to characterize Fucus morphology in the Baltic Sea (Bäck, Citation1993; Ruuskanen & Bäck, Citation1999; Bergström et al., Citation2005) were measured on each individual: (1) frond length from holdfast to the longest tip, (2) frond width, measured midway between the youngest and the second youngest dichotomy, (3) stipe width, measured midway between the holdfast and the oldest dichotomy, (4) midrib width, measured midway between the youngest and second youngest dichotomy (average of three measurements) and (5) distance between dichotomies, measured from the oldest dichotomy onwards (average of five measurements). The number of fronds per holdfast was recorded. The sex of the individuals was determined when possible.
Statistical analysis of morphological data
As the morphological data did not fully meet the assumptions for parametric analysis of variance, 2-way permutational ANOVA was carried out using PERMANOVA+ (Anderson et al., Citation2008). The homogeneity of multivariate dispersion between groups (subareas and exposure classes) was tested prior to the analyses using PERMDISP. To test whether the two morphs differed significantly within each subarea, pairwise comparisons between the morphs were carried out using frond width and distance-between dichotomies as response variables. These variables (also used in Pereyra et al., Citation2013) were chosen based on Bergström et al. (Citation2005) as they contributed most to the morphological differences between F. radicans and F. vesiculosus. In other morphological analyses wide morphs and narrow morphs were treated separately, as the sampling was focused on two separate morphs. In order to avoid 3-way analyses (due to problems in interpretation of the results), the possible effects of depth on the morphology were tested prior to the main analyses by testing the interactions Area×Depth and Exposure×Depth together with the main effects, respectively. The effects of depth did not significantly differ between the study areas or between the exposure classes (non-significant interactions).
In the 2-way permutational MANOVA, the subareas (5 and 6 levels for narrow and wide morphs respectively) and exposure (2 and 3 levels for narrow and wide morphs respectively) were used as independent variables, both as fixed factors. All measured morphological variables were included, except for midrib width due to differences in measurement accuracy between the subareas. The data were log-transformed and standardized, and Euclidean distance was used when producing the similarity matrix used in the analysis. In addition, the effects of subarea and exposure were analysed separately for all morphological variables with log-transformed values. Pairwise comparisons of the exposure classes and subareas were carried out on the morphological variables. Permutations (n=4999) were run on residuals under the reduced model, following the recommendations in Anderson & ter Braak (Citation2003) and Anderson et al. (Citation2008).
Molecular analysis
The molecular analysis was carried out at the Centre of Evolutionary Applications, University of Turku. DNA was extracted from dried vegetative tips with the NucleoSpin Plant II kit according to the manufacturer’s instructions (Macherey-Nagel). Samples were genotyped using nine microsatellite loci L20, L38, L58, L85, L94, Fsp1, Fsp2, Fsp3, Fsp4 (Engel et al., Citation2003; Perrin et al., Citation2007). Eight bases were added to the 5’ end of the unlabelled primer in the locus Fsp2 to shift the allele range in order to avoid marker overlaps in multiplexing. To improve the microsatellite peak profiles, a GTTT-tail was added to the 5’ end of each unlabelled primer (Brownstein et al., Citation1996). PCR amplification was done in two multiplex reactions (MP1: Fsp1, Fsp-2, Fsp4, L38, L58, L94 MP2: Fsp3, L20) and one single reaction (S: L85). Each amplification was carried out in 8-µl reactions consisting of c. 50 ng of DNA, 0.07 to 0.6 µM of each primer (one of which was fluorescently labelled) and 1X Qiagen multiplex PCR master mix (Qiagen Inc. Valencia, CA, USA). The PCR conditions followed the manufacturer’s standard protocol with annealing temperatures of 61°C, 56°C and 57°C for MP1, MP2 and S, respectively. Amplifications were performed on PTC-100 (MJ Research) and AB 2720 (Applied Biosystems) thermal cyclers. For electrophoresis the PCR products were pooled by combining 1 µl, 1.2 µl and 0.8 µl of the reactions for MP1, MP2 and S, and diluted with 110 µl of sterile water. 2 µl of the pooled and diluted PCR product was combined with 0.1 µl GS600LIZ size standard (Applied Biosystems) and 10 µl HiDi-formamide (Applied Biosystems). Samples were denatured at 98°C for 3 min and the size of the fragments was determined by capillary electrophoresis on an ABI PrismTM 3130xl genetic analysis instrument. The genotypes were scored using GENEMAPPER version 4.0 (Applied Biosystems) with visual inspection, and exported to a spreadsheet program for downstream analyses.
Numerical analysis of microsatellite data
The data were checked for genotyping errors due to null alleles using Brookfield’s method (Brookfield, Citation1996). The number of individuals with unique genotypes was 399. Potential departure from Hardy–Weinberg equilibrium (HWE) was tested within populations (FIS) and for each locus separately according to Weir & Cockerham (Citation1984) following Bonferroni correction to adjust the level of significance (Rice, Citation1989). The presence of linkage disequilibrium was tested in Genepop version 4.2 (Raymond & Rousset, Citation1995), with 20 Markov chain Monte Carlo (MCMC) batches, 5000 iterations per batch, and 10000 as the dememorization number. A Bayesian framework implemented in the software Structure (Pritchard et al., Citation2000), version 2.3, was used to visualize the genetic structure. Validation of the number of genetically distinct groups (K) in the data set was conducted according to the Evanno method using Structure Harvester, version 0.6.94 (Dent & vonHoldt, Citation2012). In Structure, a model with admixture and correlated allele frequencies was run for 200 000 generations using a burn-in value of 50 000 with K values from 1 to 10. The analysis was conducted in five independent runs. The analyses were first conducted with ramets and genets included and then with only genets.
The amount of genetic differentiation among sampling locations was measured by calculating pairwise FST-values using Genepop. The statistical significance of pairwise FST was evaluated using Fisher’s exact probability test followed by Bonferroni correction. Potential isolation by distance (IBD) was tested in R (R Core Development Team) with a Mantel test (vegan) using FST and geographic distance matrices as input data.
Results
Morphological variation
Both narrow and wide morphs of Fucus were found in all subareas except for the Åland subarea, where only wide morphs were found. The cover of the two morphs at study sites where they occurred in sympatry varied, and there was no relationship with latitude or exposure (data not shown). The two morphs differed significantly in frond width and in distance between dichotomies within all subareas where both morphs were found (), confirming the existence of visually determined morphs.
Table 2. The results of pairwise comparisons (based on 2-way permutational ANOVA) of the two morphs (narrow, wide) within the subareas.
Fucus was found in sheltered areas only in the two southernmost subareas. The morphology varied significantly between the subareas and the different exposure classes (, ). Effects of area (resembling salinity differences) were stronger than effects of exposure. In general, Fucus individuals were larger with wider fronds, stipes and midribs in the southern areas. The change towards smaller and narrower individuals of both narrow and wide morphs was gradual going northwards (). In fact, what was considered narrow in the south would have been classified as wide in the north (see also for overlap between morphs).
Table 3. Results of 2-way permutational MANOVA describing the differences in Fucus sp. morphology between the subareas and exposure classes.
Fig. 2. Morphological variation of Fucus in subareas (1 =Vasa, 2=Skaftung, 3=Luvia, 4=Rauma, 5=Uusikaupunki, 6=Åland) and in different exposure classes (sheltered, moderately exposed and exposed, mean ±95% confidence intervals). Narrow forms were not found in Åland and Fucus was not found in the sheltered areas north of Uusikaupunki. The horizontal lines indicate the mean values for morphological variables of Fucus radicans in Bergström et al. (Citation2005; Population D1).
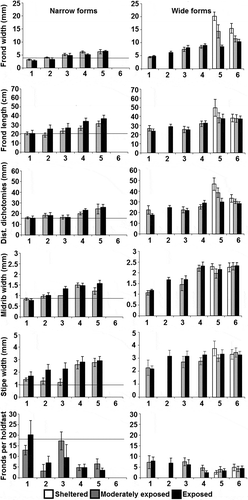
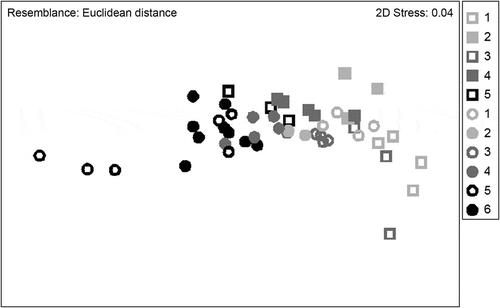
Fig. 3. NMDS ordination of morphological variation across the study area. All morphological variables were included in the analysis. Study areas are represented by different colours (study area numbering as in ) and different morphs by symbols: square = narrow, circle = wide.
The analyses carried out separately on different morphological variables showed that almost all variables differed significantly between the subareas and exposure classes (). However, there were also many significant interactions between the variables indicating that responses to exposure were not similar in all subareas. The effects of exposure on frond width were clear in the two southernmost subareas, Åland and Uusikaupunki: wider individuals occurred at sheltered sites than in other exposure classes (Åland: sheltered vs. moderately exposed sites: t=3.93, P<0.001, sheltered vs. exposed sites: t=5.78, P<0.001 Uusikaupunki: sheltered vs. moderately exposed sites: t=4.11, P<0.001, sheltered vs. exposed sites: t=16.84, P<0.001, ). However, in the more northern subareas where narrower forms occurred, the differences in frond width were not that clear between the exposure classes (). The effects of exposure on frond length were clear for wide morphs in the Uusikaupunki subarea, with longer fronds in the sheltered areas (sheltered vs. moderately exposed sites: t=2.30, P<0.05, sheltered vs. exposed sites: t=2.44, P<0.05, ). However, the fronds of the narrow morphs were often longer in more exposed areas, with significant differences between the moderately exposed and exposed sites in the Skaftung and Rauma subareas (Skaftung: t=3.00, P<0.01, Rauma: t=3.53, P<0.01). Furthermore, exposure had no significant effect on the distance between dichotomies on narrow morphs (). Stipe width generally increased southward but the number of fronds per holdfast varied more stochastically, especially among the narrow morphs ().
Table 4. Results from the 2-way permutational ANOVA describing differences in Fucus sp. morphology between subareas and exposure classes.
The NMDS ordination on the morphological variables revealed that the study areas formed no distinct groups. Instead the morphological variables formed a continuum (). Furthermore, the narrow forms of the southern sites and wide morphs of the northern sites overlapped with each other.
Vesicles were very abundant in Fucus thalli from sheltered shores of the two southernmost subareas but some individuals with small vesicles were present in more exposed sites. No vesicles were found north of Rauma. The number of adventitious branches was not calculated, but in general, they were more abundant in the northern subareas than in Åland and Uusikaupunki.
Molecular data quality control and clonality
The majority of loci showed negligible to moderate levels of null allele frequency (0.0–0.20) (). When analysing potential null allele frequencies per morph only one locus in the Vasa (wide morph) sample had a frequency above 0.20. Since we found no consistent pattern of null alleles across certain loci or locations, all loci were included in subsequent analyses. A global analysis including all loci and samples showed a significant deviation from HWE (heterozygote deficiency) in the Åland and Uusikaupunki samples (Bonferroni correction, P<0.05). More detailed analyses displayed significant heterozygote deficiency in locus Fsp-1 in the Åland population (Bonferroni correction, P<0.05). Locus Fsp-3 showed significant heterozygote deficiency in the narrow morphs in Rauma, in the wide morphs in Uusikaupunki and in the Åland population. Loci Fsp-4, L-20 and L-94 displayed an overwhelming presence of homozygotes in the Vasa samples. However, because of the low sample size (six wide morph genets/five narrow morph genets) no valid statistical analysis was possible.
Significant linkage disequilibrium was not found between loci across study sites (). Clonality became increasingly common towards the north. At one study site in Skaftung (‘Tis’, 62○10′N, 21○21′E), all samples consisted of the same clone (Clone A in ). The same clone was also found in Vasa, where most of the samples (76/80) represented three different clones, clone A constituting 35% of all samples (100% at one study site, all female), both wide and narrow morphs. Only four individuals in the entire subarea represented other genotypes ().
Table 5. The percentage of different clones (A, B and C) at the study sites in the Vasa and Skaftung subareas.
Genetic structure
Probability assignment of individuals in Structure revealed two genetically divergent populations (K=2) (). The analysis including ramets also resulted in K=2. The samples from Vasa and parts of the Skaftung subarea constituted one population and the rest of the samples another genetically distinct population.
Fig. 4. Genetic structure in the microsatellite data as inferred by Structure analysis when assuming two genetic clusters (K=2). Each individual (n=399) is shown by a thin vertical bar on x-axis. This bar is divided into shaded segments representing each individual’s estimated assignment to assumed clusters. The areas in lighter grey indicate narrow morphs.
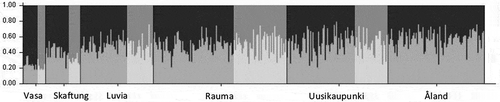
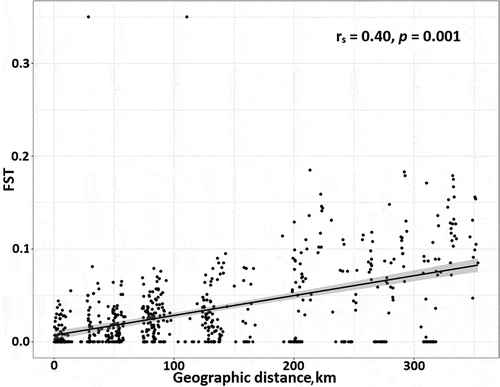
At study area level, FST values showed that the samples from Vasa were significantly differentiated from all other samples, except when comparing the wide morph samples from Vasa and Skaftung. In general, the narrow morphs in Vasa were the most genetically differentiated from other populations (), but there was no significant genetic differentiation between the morphs in Vasa. In general, the pairwise comparisons between morphs within the same study area showed no genetic differentiation (shaded grey in ). However, pairwise FST comparisons between individual study sites within the subareas showed that narrow and wide morphs were significantly genetically differentiated in Skaftung at study site ‘Sto’ (FST=0.090, P=0.001) (). This was also true for Vasa study sites ‘Syn’ and ‘Rev’ when ramets were included in the Fisher’s exact test (FST=0.229, P<0.0001 and FST=0.043, P<0.0001, respectively), but not when only genets were included. FST values showed that genetic differentiation between narrow and wide morphs, both within and between subareas south of Vasa and north of Åland (Skaftung, Luvia Rauma, Uusikaupunki) was generally low. However, the wide morphs in the Åland subarea were genetically different from Fucus in the other subareas. We found a weak, but significant IBD pattern () (R2=0.40, P=0.001) when FST values of both wide and narrow morphs were compared to geographic distance. Significant IBD was also detected for each morph separately (wide, R2=0.45, P=0.01, narrow, R2=0.29, P=0.04).
Table 6. Pairwise FST values describing genetic differentiation between the subareas and the two morphs (0 = no differentiation, 1= complete differentiation).
Discussion
Morphological variation
The results of this study show high variation in Fucus morphology along the Finnish west coast. The variation is, on a large scale, strongly linked to the environmental variability, especially to changing salinity. The gradual change towards shorter individuals with narrower thalli and shorter distances between dichotomies (also described by Ruuskanen & Bäck, Citation1999) was apparent when moving north towards the lower salinities. As far south as the Luvia subarea, the morphological features were comparable to those measured for F. radicans in Bergström et al. (Citation2005). Our findings on effects of salinity on Fucus morphology are fully in line with the current theory and empirical evidence (Kalvas & Kautsky, Citation1998; Ruuskanen & Bäck, Citation1999). However, since only empirical evidence exists today, additional experimental evidence could be useful to further confirm the causal relationship between salinity and Fucus morphology.
The effects of exposure on morphology were very clear in the two southernmost subareas, where Fucus was also found in sheltered conditions. As also noted in earlier studies (Wallentinus, Citation1979; Kalvas & Kautsky, Citation1993; Ruuskanen & Bäck, Citation1999), vesicles were abundant at sheltered sites and the individuals were clearly larger with wide thalli. North of Rauma, Fucus lacked vesicles even at moderately exposed sites. Generally, the effects of exposure on Fucus morphology were weaker and more complex in the north than in the southernmost subareas, and the different morphs responded differently to exposure. This weakening effect may be due to the overriding effects of decreasing salinity, which are to a large extent similar to those of increasing exposure (narrower and shorter fronds, with shorter distances between dichotomies; Bäck, Citation1993; Ruuskanen & Bäck, Citation1999). The common occurrence of Fucus in sheltered sites in the south, but its absence from such sites in the north is an interesting phenomenon that requires further study. The phenomenon may partly be due to the general decrease in salinity towards the north and the sheltered areas being close to the mainland, where salinity is even lower due to high freshwater input.
In addition to the morphological variation related to changes in the environment, individuals with narrow and wide thalli occurred in sympatry at study sites along the whole salinity gradient studied, except in the Åland subarea. As both narrow and wide morphs decreased in size towards low salinity, overlap between the morphs in some morphological characteristics was observed, e.g. frond width of the wide forms in the northern areas (Vasa and Skaftung) corresponded to the frond width of the narrow forms in Uusikaupunki.
Clonality
Clonality was very common in Vasa as most of the samples belonged to three clones only. Fucus radicans had been previously reported ~10 km north of the Skaftung subarea and the large female clone extending to the south-western Bothnian Sea was reported ~30 km north of the area (Johannesson et al., Citation2011; Ardehed et al., Citation2015). Thus, it is possible that the female clone found frequently in this study is the same clone reported in earlier studies. In addition, two extensive male clones were found in the area; clone B extending over 40 km and clone C covering 48 km within the Vasa subarea. Two widely distributed male clones in the area were also reported in Johannesson et al. (Citation2011) and Ardehed et al. (Citation2015).
Except for the large female clone found at study site ‘Tis’ in Skaftung subarea, all samples in the subarea were unique genotypes. No clonality was found south of Skaftung. This is in line with Johannesson et al. (Citation2011), who found that clonality in F. radicans (F. vesiculosus not sampled) is relatively rare in the area (Sälskär 62○19′N, 21○10′E), but increases fast northward from this region. As sexual reproduction is common in very low salinities (Ardehed et al., Citation2016), low salinity is unlikely to be the only explanation for high clonality in the area. As F. vesiculosus colonized the Baltic Sea about 8000 years ago, high clonality may also be related to stochastic processes in a species colonizing new areas, where asexually recruited individuals dominate during the first phase of colonization, but are later replaced by sexually recruited individuals (Rafajlović et al., Citation2017).
Genetic differentiation
We could confirm genetic differentiation that clearly coincided with morphological characteristics only at one study site in Skaftung (‘Sto’). This indicated the existence of both F. vesiculosus and F. radicans in the Skaftung subarea. In Vasa where genetic differentiation between morphs was expected based on earlier studies (Johanneson et al., Citation2011), it was not confirmed. This may partly be because ramets of the same clone (clone A in ) were sometimes interpreted as wide, sometimes as narrow, depending on the morphology of the other individuals at the same site. Furthermore, due to high clonality, the number of genets in the area was low, reducing the power of statistical analyses. However, the clear genetic differentiation of narrow morphs in the Vasa subarea from other populations indicates the existence of F. radicans in the area (already confirmed in Johannesson et al., Citation2011 and Ardehed et al., Citation2015).
In subareas south of Skaftung and north of Åland (Luvia, Rauma, Uusikaupunki), no genetic differences between the morphs were found. There was also very little genetic differentiation between the subareas; only the wide morphs in the Rauma and Uusikaupunki subareas showed some degree of genetic differentiation from each other. However, wide morphs (i.e. F. vesiculosus) in Åland were genetically differentiated from all other Fucus thalli sampled. This result clearly shows that there is also substantial within-species variation in the genetic structure of F. vesiculosus.
The significant heterozygote deficiency in loci Fsp-1 and Fsp-3 in the Åland population, in locus Fsp-3 in the narrow morphs in Rauma and in the wide morphs in Uusikaupunki is probably not a result of inbreeding as only one to two loci displayed over-representation of homozygotes. In the Åland population these loci, Fsp-1 and Fsp-3, were the ones with the highest frequency of null alleles, and the observed heterozygote deficiency may be a result of inaccurate allele scoring.
Weak but significant IBD showed that, in general, genetic differentiation between the Fucus individuals increased with increasing distance. However, within-subarea genetic diversity was observed (FST>0 where distance between individuals is low). The weak IBD pattern across the entire study area is partly a result of clonality, observable as multiple zero FST values across the study area, despite increasing geographic distance. However, not all zero FST values should be interpreted as identical allele frequencies in two samples but merely as undetectable (weak) genetic differentiation. This is because the Weir & Cockerham (Citation1984) formula used requires large sample sizes if the real FST is low, and may result in negative FST values if the sample size is small or uneven between the groups compared. As these negative values were here considered as zeroes, they contribute to the number of zeroes in the analysis. As clonal lineages were detected only in Skaftung and Vasa (~150 km apart) FST values given as 0 and situated >150 km apart are not a sign of clonality but instead these values imply very small genetic differences.
Species identification
According to our results, the use of morphological characteristics alone as a basis for Fucus species identification in the eastern Bothnian Sea is problematic. The wide small-scale variation in morphological characteristics did not coincide with the genetic variation in nine microsatellite loci studied. This is contradictory to the findings in the western Bothnian Sea (Bergström et al., Citation2005; Pereyra et al., Citation2009), where morphological differences coincided better with genetic variation and formed the basis for describing the new species, F. radicans. Furthermore, the salinity gradient causes a northwards decrease in thallus width and length, as well as an increase in numbers of fronds per holdfast in F. vesiculosus, resulting in an appearance usually typical of F. radicans. As a result, in Luvia, where Fucus individuals were genetically similar to F. vesiculosus in the south (Rauma and Uusikaupunki), the morphological variables corresponded to values for F. radicans (Bergström et al., Citation2005). As no genetic differentiation between the morphs within the same subarea, or within the same study site, was found south of Skaftung, we may conclude that all Fucus sp. south of Skaftung (within our study area) are F. vesiculosus showing high variation in morphology, partly due to environmental conditions, but also due to high genotypic variation.
More studies are needed in order to clarify the relationship between the morphological characteristics and genetics within and between the two Fucus species in the eastern Bothnian Sea. This is especially true in the area north of Skaftung, where both species are found and where clonality becomes increasingly common. Further studies on, for example, within-clone variation in morphology as well as estimates of the clonality gradient, would offer interesting insight into the survival strategies of a species living close to its distribution limit.
Supplementary Information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2018.1453089
. Brookfield’s method showed that the majority of loci displayed negligible null allele frequencies. A few loci had moderate null allele frequencies. Only one locus, L-20 in wide morphs in Vasa (VasaW) had a high null allele frequency.
. The linkage disequilibrium between the microsatellite loci
. Pairwise FST values describing genetic differentiation between the narrow and wide morphs within the same study site.
TEJP-2017-0059-File007.docx
Download MS Word (17.2 KB)Acknowledgements
We are grateful to Ellen Schagerström for sharing Swedish expertise in Fucus radicans identification and assistance in the field, Jon Ögård and Suvi Kiviluoto with her field team for assistance in the fieldwork, Hans-Göran Lax and Tapio Suominen for co-operation in making fieldwork possible and Juha Hyvärinen for valuable information on Fucus in the Bothnian Sea. Meri Lindqvist at the Centre of Evolutionary Applications, University of Turku carried out the molecular analysis. We are also grateful to Kerstin Johannesson for valuable comments that improved the manuscript.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
Notes on contributors
Henna Rinne
H. Rinne: original idea, field work, statistical analysis, manuscript drafting and editing; U. Björkman: most of the fieldwork, manuscript editing; C. Sjöqvist: numerical analyses related to population genetics, manuscript drafting and editing; S. Salovius-Laurén: field work, manuscript editing; J. Mattila: supervision of the project and manuscript editing. All authors approved the final version for publication.
References
- Anderson, M., Gorley, R. & Clarke, K. (2008). PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods. PRIMER-E, Plymouth.
- Anderson, M. & Ter Braak, C. J. F. (2003). Permutation test for multi-factorial analysis of variance. Journal of Statistical Computation and Simulation, 73: 85–113.
- Ardehed, A., Johansson, D., Schagerström, E., Kautsky, L., Johannesson, K. & Pereyra, R.T. (2015). Complex spatial clonal structure in the macroalgae Fucus radicans with both sexual and asexual recruitment. Ecology and Evolution, 5: 4233–4245.
- Ardehed, A., Johansson, D., Sundqvist, L., Schagerström, E., Zagrodzka, Z., Kovalchouck, N.A., Bergström, L., Kautsky, L., Rafajlovic, M., Pereyra, R.T. & Johannesson, K. (2016). Divergence within and among seaweed siblings (Fucus vesiculosus and F. radicans) in the Baltic Sea. PLoS ONE, 11: e0161266. doi:10.1371/journal.pone.0161266.
- Bäck, S. (1993). Morphological variation of northern Baltic Fucus vesiculosus along the exposure gradient. Annales Botanici Fennici, 30: 275–283.
- Bergström, L. & Bergström, U. (1999). Species diversity and distribution of aquatic macrophytes in the Northern Quark, Baltic Sea. Nordic Journal of Botany, 19: 375–383.
- Bergström, L., Tatarenkov, A., Johannesson, K., Jönsson, R. & Kautsky, L. (2005). Genetic and morphological identification of Fucus radicans sp. nov. (Fucales Phaeophyceae) in the brackish Baltic Sea. Journal of Phycology, 41: 1025–1038.
- Brookfield, J. (1996). A simple new method for estimating null allele frequency from heterozygote deficiency. Molecular Ecology, 5: 453–455.
- Brownstein, M.J., Carpten, J.D. & Smith, J.R. (1996). Modulation of non-templated nucleotide addition by Taq DNA polymerase: primer modifications that facilitate genotyping. BioTechniques, 20: 1004–1010.
- Dent, E.A. & vonHoldt, B.M. (2012). STRUCTURE HARVESTER: a website and program for visualizing STRUCTURE output and implementing the Evanno method. Conservation Genetics Resources, 4: 359–361.
- Engel, C.R., Brawley S.H., Edwards K.J. & Serrão E. (2003). Isolation and cross-species amplification of microsatellite loci from the fucoid seaweeds Fucus vesiculosus, F. serratus and Ascophyllum nodosum (Heterokontophyta, Fucaceae). Molecular Ecology Notes, 3: 180–182.
- Ferreira, J.G., Hawkins, S.J. & Jenkins, S.R. (2015). Patterns of reproductive traits of fucoid species in core and marginal populations. European Journal of Phycology, 50: 457–468.
- Forslund, H., Eriksson, O. & Kautsky, L. (2012). Grazing and geographic range of the Baltic seaweed Fucus radicans (Phaeophyceae). Marine Biology Research, 8: 322–330.
- Graham, L.E. & Wilcox, L.W. (2003). Algae. Prentice-Hall, Englewood Cliffs, New Jersey.
- Gunnarsson, K. & Berglund, A. (2012). The brown alga Fucus radicans suffers heavy grazing by the isopod Idotea baltica. Marine Biology Research, 8: 87–89.
- Häyrén, E. (1949). Botaniska anteckningar från Raumo skärgård. Bidrag till kännedom af Finlands natur och folk, 6: 3–19.
- Häyrén, E. (1950). Botaniska anteckningar från Nystads skärgård. Bidrag till kännedom af Finlands natur och folk, 7: 3–23.
- Isæus, M. (2004). Factors structuring Fucus communities at open and complex coastlines in the Baltic Sea: PhD Thesis, Stockholm University.
- Johannesson, K., Johansson, D., Larsson, K.H., Huenchuñir, C.J., Perus, J., Forslund, H., Kautsky, L. & Pereyra, R.T. (2011). Frequent clonality in fucoids (Fucus radicans and Fucus vesiculosus; Fucales, Phaeophyceae) in the Baltic Sea. Journal of Phycology, 47: 990–998.
- Johannesson, K., Forslund, H., Åstrand Capetillo, N., Kautsky, L., Johansson, D., Pereyra, R.T. & Råberg, S. (2012). Phenotypic variation in sexually and asexually recruited individuals of the Baltic Sea endemic macroalga Fucus radicans: in field and after growth in a common-garden. BMC Ecology, 12: 2.
- Kalvas, A. & Kautsky, L. (1993). Geographical variation in Fucus vesiculosus morphology in the Baltic and North Seas. European Journal of Phycology, 28: 85–91.
- Kalvas, A. & Kautsky, L. (1998). Morphological variation in Fucus vesiculosus populations along temperature and salinity gradients in Iceland. Journal of the Marine Biology Association of the United Kingdom, 78: 985–1001.
- Kjellman, F.R. (1890). Handbok i Skandinaviens hafsalgflora. Oscar L. Lamms Förlag, Stockholm.
- Lehvo, A. & Bäck, S. (2000). Notes on the phytobenthos, northeastern Gulf of Finland. Memoranda Societatis pro Fauna et Flora Fennica, 76: 7–13.
- Neiva, J., Hansen, G.I., Pearson, G.A., Van de Vliet, M.S., Maggs, C.A. & Serrão, E.A. (2012). Fucus cottonii (Fucales, Phaeopyceae) is not a single genetic entity but a convergent salt-marsh morphotype with multiple independent origins. European Journal of Phycology, 47: 461–468.
- Nielsen, R. Kristiansen, A., Mathiesen, L. & Mathiesen, H. (1995). Distributional index of the benthic macroalgae of the Baltic Sea area. Acta Botanica Fennica, 155.
- Pearson, G., Kautsky, L. & Serrão, E. (2000). Recent evolution in Baltic Fucus vesiculosus: reduced tolerance to emersion stress compared to intertidal (North Sea) populations. Marine Ecology Progress Series, 202: 67–79.
- Pereyra, R., Bergström, L., Kautsky, L. & Johannesson, K. (2009). Rapid speciation in a newly opened postglacial marine environment, the Baltic Sea. BMC Evolutionary Biology, 9: 1–9.
- Pereyra, R.T., Huenchuñir, C., Johansson, D., Forslund, H., Kautsky, L., Jonsson, P.R. & Johannesson, K. (2013). Parallel speciation or long‐distance dispersal? Lessons from seaweeds (Fucus) in the Baltic Sea. Journal of Evolutionary Biology, 26: 1727–1737.
- Perrin, C., Daguin, C., Vliet, M. Van De Engel, C.R., Pearson, G.A. & Serrão, E.A. (2007). Implications of mating system for genetic diversity of sister algal species: Fucus spiralis and Fucus vesiculosus (Heterokontophyta, Phaeophyceae). European Journal of Phycology, 42: 219–230.
- Pritchard, J.K., Stephens, M. & Donnelly, P. (2000). Inference of population structure using multilocus genotype data. Genetics, 155: 945–959.
- Råberg, S. & Kautsky, L. (2007). A comparative biodiversity study of the associated fauna of perennial fucoids and filamentous algae. Estuarine Coastal and Shelf Science, 73: 249–258.
- Rafajlović, M., Kleinhans, D., Gulliksson, C., Fries, J., Johansson, D., Ardehed, A., Sundqvist, L., Pereyra, R.T., Mehlig, B., Johnsson, P.R. & Johannesson, K. (2017). Neutral processes forming large clones during colonization of new areas. Journal of Evolutionary Biology, 30: 1544–1560.
- Raymond, M. & Rousset, F. (1995). GENEPOP (version 1.2): population genetics software for exact tests and ecumenicism. Journal of Heredity, 86: 248–249.
- Rice, W.R. (1989). Analyzing tables of statistical tests. Evolution, 43: 223–225.
- Ruuskanen, A. & Bäck, S. (1999). Morphological variation of northern Baltic Sea Fucus vesiculosus L. Ophelia, 50: 43–59.
- Ruuskanen, A. & Bäck, S. (2002). Morphological changes in submerged Fucus vesiculosus (L.) (Phaeophyta) along the salinity gradient of the River Keret estuary, Russia. Sarsia, 87: 185–188.
- Ruuskanen, A., Bäck, S. & Reitalu, T. (1999). A comparison of two exposure methods using Fucus vesiculosus as an indicator. Marine Biology, 134: 139–145.
- Schagerström, E. (2013). On the endemic Fucus radicans in the Baltic Sea. PhD Thesis, Stockholm University.
- Scott, G., Hull, S., Hornby, S., Hardy, F.G. & Owens, N. (2001). Phenotypic variation in Fucus spiralis (Pheophyceae): morphology, chemical phenotype and their relationship to the environment. European Journal of Phycology, 36: 43–50.
- Serrão, E., Kautsky, L. & Brawley, S. (1996). Distributional success of the marine seaweed Fucus vesiculosus L. in the brackish Baltic Sea correlates with osmotic capabilities of Baltic gamets. Oecologia, 107: 1–12.
- Serrão, E., Brawley, S.H., Hedman, J., Kautsky, L. & Samuelsson, G. (1999). Reproductive success of Fucus vesiculosus (Phaeophyceae) in the Baltic Sea. Journal of Phycology, 35: 254–269.
- Tatarenkov, A., Bergström, L., Jönsson, R.B., Serrão, L., Kautsky, L. & Johannesson, K. (2005). Intriguing asexual life in the marginal populations of the brown seaweed Fucus vesiculosus. Molecular Ecology, 14: 647–651.
- Voipio, A. (ed.) (1981). The Baltic Sea. Elsevier Oceanography Series 30. Elsevier, Amsterdam.
- Wærn, M. (1952). Rocky-shore algae in the Öregrund archipelago. Acta Phytogeographica Suecica, 30: 1–298.
- Wahl, M., Jormalainen, V., Eriksson, B.K., Coyer, J.A., Molis, M., Schubert, H., Dethier, M., Karez, R., Kruse, I., Lenz, M., Pearson, G., Rohde, S., Wikström, S.A. & Olsen, J.L. (2011). Stress ecology in Fucus: abiotic, biotic and genetic interactions. Advances in Marine Biology, 59: 37–106.
- Wallentinus, I. (1979). Environmental influence on benthic macrovegetation in the Trosa-Askö area, northern Baltic Proper. II. The ecology of macroalgae and submersed phanerogams. Contributions from the Askö Laboratory, University of Stockholm, 25. 210 pp.
- Weir, B.S. & Cockerham C.C. (1984). Estimating F-statistics for the analysis of population structure. Evolution, 38: 1358–1370.
- Wikström, S. & Kautsky, L. (2007). Structure and diversity of invertebrate communities in the presence and absence of canopy-forming Fucus vesiculosus in the Baltic Sea. Estuarine, Coastal and Shelf Science, 72: 168–176.