ABSTRACT
The dinoflagellate order Peridiniales encompasses several well circumscribed families. However, the family level of some genera, such as Bysmatrum and Vulcanodinium, has remained elusive for many years. Four Peridinium-like strains were established from the Atlantic coast of France and North Sulawesi, Indonesia through cyst germination or isolation of single cells. The cyst-theca relationship was established on specimens from the French Atlantic. Their morphologies were examined using light, scanning and transmission electron microscopy. The cells were characterized by a much larger epitheca relative to the hypotheca, a large anterior sulcal (Sa) plate deeply intruding the epitheca and a small first anterior intercalary plate. The plate formula was identified as Po, cp, X, 4′, 3a, 7′′, 6C, 5S, 5′′′, 2′′′′, shared by Apocalathium, Chimonodinium, Fusiperidinium and Scrippsiella of the family Thoracosphaeraceae but the configuration of Sa plate and anterior intercalary plates is different. Transmission electron microscopy showed that the eyespot was located within a chloroplast comprising two rows of lipid globules and thus belongs to type A. All four strains were classified within a new genus Caladoa as C. arcachonensis gen. et sp. nov. Small subunit ribosomal DNA (SSU rDNA), partial large subunit ribosomal DNA (LSU rDNA) and internal transcribed spacer ribosomal DNA (ITS rDNA) sequences were obtained from all strains. Genetic distance based on ITS rDNA sequences between French and Indonesian strains reached 0.17, suggesting cryptic speciation in C. arcachonensis. The maximum likelihood and Bayesian inference analysis based on concatenated data from SSU and LSU rDNA sequences revealed that Caladoa is monophyletic and closest to Bysmatrum. Our results supported that Caladoa and Bysmatrum are members of the order Peridiniales but their family level remains to be determined. Our results also support that Vulcanodinium is closest to the family Peridiniaceae.
Introduction
The dinoflagellate order Peridiniales is characterized by a symmetrical first apical plate and two more or less symmetrical antapical plates (Fensome et al., Citation1993). Peridiniales currently encompasses several families including Blastodiniaceae, Heterocapsaceae, Kryptoperidiniaceae,Peridiniaceae, Peridiniopsidaceae, Protoperidiniaceae, Thoracosphaeraceae and Zooxanthellaceae (Gottschling et al., Citation2017). The morphological features in some of these families are well circumscribed, e.g. Peridiniopsidaceae has six cingular plates and less than two anterior intercalary plates (Gottschling et al., Citation2017); Protoperidiniaceae has three cingular plates and one transitional plate (Fensome et al., Citation1993). In other families, however, morphological features are not well defined. For instance, Kryptoperidiniaceae includes species with one or two anterior intercalary plates (Pienaar et al., Citation2007; Saburova et al., Citation2012; You et al., Citation2015), but some genera (e.g. Galeidinium M.Tamura & T. Horiguchi) do not show any thecal plate pattern (Tamura et al., Citation2005). The family Thoracosphaeraceae has unified the subfamily Calciodinelloideae and the order Thoracosphaerales, but its emended description was not provided by Elbrächter et al. (Citation2008).
Information on evolutionary history and cell ultrastructure are important for systematics, even at the family level. For instance, Kryptoperidiniaceae includes a small group of dinoflagellates hosting a tertiary endosymbiont derived from a diatom (Horiguchi & Takano, Citation2006; Kretschmann et al., Citation2018). Kryptoperidiniaceae currently includes Durinskia S. Carty & E.R. Cox, Blixaea M. Gottschling, Galeidinium, Kryptoperidinium Lindemann and Unruhdinium M. Gottschling. All these genera share a type D eyespot (Tamura et al., Citation2005; You et al., Citation2015; Yamada et al., Citation2017) that was probably derived from the original dinoflagellate chloroplast (Moestrup & Daugbjerg, 2007). The woloszynskioid dinoflagellate families Tovelliaceae and Borghiellaceae are characterized by a type C eyespot (composed of pigment globules not bound by membranes outside of the chloroplast) (Lindberg et al., Citation2005) and by a type B eyespot (intraplastidic with bricklike crystals overlying the chloroplast), respectively (Moestrup et al., Citation2009). In addition to the eyespot, peduncle microtubules were also suggested to be one of the diagnostic features to separate Peridiniopsidaceae from Peridiniaceae (Gottschling et al., Citation2017).
Among 2000 extant dinoflagellate species, only 13–16% of the motile stages have been related to resting cysts (Head, Citation1996). Cyst morphologies are often considered to be useful at interspecific level, e.g. Protoperidinium Bergh produces a number of cysts classified within different cyst genera (Gu et al., Citation2015), but also at the generic level, e.g. cysts of Biecheleria Ø. Moestrup, K. Lindberg & N. Daugbjerg are spherical and bear numerous short processes (Moestrup et al., Citation2009), or even at the subfamily level, e.g. Calciodinelloideae is characterized by possessing calcareous cysts (Elbrächter et al., Citation2008).
Molecularly, more than 90% of peridinialean species can be reliably placed into one of the families of Peridiniales (Gottschling et al., Citation2017), but some genera (e.g. Bysmatrum M.A. Faust & K.A. Steidinger, Vulcanodinium E. Nézan & N. Chomérat) have not been identified to the family level yet, although they display a peridinialean tabulation. Vulcanodinium was reported to be closely related to Peridinium cinctum (O.F. Müller) C.G. Ehrenberg based on LSU rDNA sequences (Nézan & Chomérat, Citation2011) and concatenated data from SSU, ITS and LSU rDNA sequences (Luo et al., Citation2018), but whether Vulcanodinium is also a member of Peridiniaceae is not clear. The phylogenetic position of Bysmatrum is elusive too: it is close to Peridinium C.G. Ehrenberg, with low support in the phylogeny based on concatenated data from SSU, ITS and LSU rDNA sequences (Anglès et al., Citation2017; Luo et al., Citation2018), but sometimes it is closer to Prorocentrum C.G. Ehrenberg (Gottschling et al., Citation2012; Dawut et al., Citation2018).
To clarify the phylogenetic positions of Vulcanodinium and Bysmatrum, sequences from closely related species of these two genera are essential. In the present study, four strains of Peridinium-like species were established from the Atlantic coast of France and North Sulawesi, Indonesia by incubating cysts or isolating single cells. The four cultured strains were examined morphologically and ultrastructurally, the latter with an emphasis on the eyespot. In addition, SSU rDNA, partial LSU rDNA and ITS rDNA sequences were determined for the cultured strains and molecular phylogeny was inferred using concatenated SSU and LSU rDNA sequences.
Materials and methods
Sample collection and treatment
Seagrass and sand samples were collected from the seabed by scuba divers near Manado and Bitung, North Sulawesi of Indonesia on 31 July and 1 August 2016 and placed into bottles containing seawater from the same location. The samples were stirred vigorously to detach the epibenthic cells and the suspension settled in a composite settling chamber. The settled material was rinsed with filtered seawater and transferred into a polycarbonate bottle. Single Peridinium-like cells were isolated from this material and the strains TIO339 and TIO340 were established ().
Table 1. Information on strains of Caladoa arcachonensis examined in the present study, including collection data, locations and sources.
Sediment sampling was done using an Ekman grab on 15 and 19 April 2016 in a shallow natural reservoir used for oyster storage before commercialization (water depth 1.2–1.5 m). The small basin is located in Arcachon Bay in the Gironde region of south-western France. The top 2 cm of sediment were sliced off and stored in the dark at 4°C until further treatment. Approximately 5 g of wet sediment was mixed with 20 ml of filtered seawater and stirred vigorously to dislodge detrital particles. The settled material was subsequently sieved through 120 μm and 10 μm filters. Single cysts were isolated with a micropipette using an inverted Eclipse TS100 (Nikon, Tokyo, Japan) microscope, alternatively a small quantity of sediment was directly incubated in small containers with f/2-Si medium (Guillard & Ryther, Citation1962) at 20°C, 90 μmol photons m–2 s–1 under a 12:12 h light: dark cycle. The strains TIO278 and TIO282 were established using direct incubation ().
Morphological study of motile cells with microscopy
Live cells were examined and photographed using a Zeiss Axio Imager light microscope (Carl Zeiss, Göttingen, Germany) equipped with a Zeiss Axiocam HRc digital camera. The size of 30 cells was measured using Axiovision (4.8.2 version) software at ×1000 magnification. To observe the shape and location of the nucleus, cells were stained with 1:100 000 SYBR Green (Sigma Aldrich, St. Louis, USA) for 1 min, and photographed using the Zeiss fluorescence microscope with a Zeiss-38 filter set (excitation BP 470/40, beam splitter FT 495, emission BP 525/50). Chloroplast autofluorescence microscopy was carried out on live cells using the above-mentioned microscope equipped with a Chroma filter cube (emission filter ET480/20×, dichromatic mirror AT505dc, suppression filter AT515lp), and digitally photographed using a Zeiss Axiocam HRc digital camera.
For scanning electron microscopy (SEM), mid-exponential batch cultures of four strains were concentrated by a Universal 320 R centrifuge (Hettich-Zentrifugen, Tuttlingen, Germany) at 850 g for 10 min at room temperature. Cells were fixed with 2.5% glutaraldehyde for 3 h at 8°C, rinsed with Milli-Q water twice and post-fixed with 1% OsO4 overnight at 8°C in a tube. The supernatant was removed and the settled cells were transferred to a coverslip coated with poly-L-lysine (molecular weight 70 000–150 000). The cells attached to the cover slip were rinsed in Milli-Q water twice. The samples were then dehydrated in a graded ethanol series (10, 30, 50, 70, 90 and 3× in 100%, 10 min at each step), critical point dried (K850 Critical Point Dryer, Quorum/Emitech, West Sussex, UK), sputter-coated with gold and examined with a Zeiss Sigma FE (Carl Zeiss Inc., Oberkochen, Germany) scanning electron microscope. Labelling of tabulation follows a modified Kofoid system (Fensome et al., Citation1993), and the sulcal plate labelling is according to Balech (Citation1980).
Transmission electron microscopy (TEM)
Mid-exponential batch cultures of strain TIO278 (France) were fixed in 2.5% glutaraldehyde in phosphate buffered saline (PBS, 0.1 M at pH 7.4) for 1 h, concentrated by centrifugation and then washed three times with the same PBS for 10 min each. They were post-fixed in 1% OsO4 overnight at 4°C and washed three times with the same PBS for 10 min each. The cells were then dehydrated through a graded ethanol series (10, 30, 50, 70, 95, 3× in 100%, 10 min at each step). The pellet was embedded in Spurr’s resin (Spurr, Citation1969) and sectioned with a Reichert Ultracut E microtome (Leica, Vienna, Austria), mounted on Formvar-coated grids, stained with uranyl acetate and lead citrate, and observed in a JEOL JEM-100 transmission electron microscope (JEOL, Tokyo, Japan).
PCR amplifications and sequencing
The total algal DNA was extracted from 10 ml of exponentially growing cultures using a MiniBEST Universal DNA Extraction Kit (Takara, Tokyo, Japan) according to the manufacturer’s protocol. PCR amplifications were carried out using 1×PCR buffer, 50 µM dNTP mixture, 0.2 μM of each primer, 10 ng of template genomic DNA and 1 U of ExTaq DNA Polymerase (Takara, Tokyo, Japan) in 50 μl reactions. The SSU rDNA was amplified using the primers of PRIMER A/PRIMER B (Medlin et al., Citation1988). The LSU rDNA was amplified using the primers of D1R/28-1483R (Scholin et al., Citation1994; Daugbjerg et al., Citation2000). The total ITS1–5.8S–ITS2 was amplified using ITSA/ITSB primers (Adachi et al., Citation1996). The thermal cycle procedure was 4 min at 94°C, followed by 30 cycles of 1 min at 94°C, 1 min at 45°C, 1 min at 72°C and final extension of 7 min at 72°C with a Mastercycler (Eppendorf, Hamburg, Germany). The PCR product was purified using a SanPrep Column DNA Gel Extraction Kit (Sangon Biotech, Shanghai, China) and sequenced directly in both directions on an ABI PRISM 3730XL (Applied Biosystems, Foster City, California, USA) following the manufacturer’s instructions. Newly obtained sequences were deposited in GenBank with accession numbers MK012070–MK012084.
Sequence alignment and phylogenetic analysis
Newly-obtained sequences (SSU and partial LSU rDNA) were incorporated into a systematically representative set of dinoflagellates available in GenBank. Sequences were aligned using MAFFT v7.110 (Katoh & Standley, Citation2013) online program (http://mafft.cbrc.jp/alignment/server/) with default settings. Alignments were manually checked with BioEdit v. 7.0.5 (Hall, Citation1999). For Bayesian inference (BI), the program jModelTest (Posada, Citation2008) was used to select the most appropriate model of molecular evolution with Akaike Information Criterion (AIC). Bayesian reconstruction of the data matrix was performed using MrBayes 3.2 (Ronquist & Huelsenbeck, Citation2003) with the best-fitting substitution model (GTR+G). Four Markov chain Monte Carlo (MCMC) chains ran for 2 000 000 generations, sampling every 100 generations. The first 10% of burn-in trees were discarded. A majority rule consensus tree was created in order to examine the posterior probabilities of each clade. Maximum likelihood (ML) analyses were conducted with RaxML v7.2.6 (Stamatakis, Citation2006) on the T-REX web server (Boc et al., Citation2012) using the model GTR+G. Node support was assessed with 1000 bootstrap replicates.
Multiple ITS1-5.8S-ITS2 sequences of new strains were aligned using MAFFT v7.110 (Katoh & Standley, Citation2013) online program with default settings. Completed alignments were saved as NEXUS files and imported into PAUP*4b10 software (Swofford, Citation2002) to estimate divergence rates using simple uncorrected pairwise (p) distance matrices.
Results
Caladoa Z. Luo, K.N. Mertens & H.F. Gu, gen. nov
Diagnosis: Cells with a plate formula of Po, cp, X, 4′, 3a, 7′′, 6C, 5S, 5′′′, 2′′′′. A large anterior sulcal plate (Sa) deeply intrudes on the epitheca. Chloroplast with a stalked pyrenoid and a type A eyespot is present. Cells differ from Chimonodinium, Fusiperidinium, Apocalathium, Scrippsiella, Vulcanodinium and Bysmatrum in the size and location of Sa plate, and from Peridinium by the presence of an additional cingular plate.
Etymology: The epithet Caladoa is named after António José Calado, who carried out outstanding work on freshwater dinoflagellate taxonomy.
Type species: Caladoa arcachonensis Z. Luo, K.N. Mertens & H.F. Gu.
Caladoa arcachonensis Z. Luo, K.N. Mertens & H.F. Gu, sp. nov. (–)
Diagnosis: Cells are 16.7–23.5 µm long and 13.1–19.4 µm wide. The cells have a rounded epitheca and hypotheca with an epitheca: hypotheca length ratio of 1.5–1.8. The thecae display a plate formula of Po, cp, X, 4′, 3a, 7′′, 6C, 5S, 5′′′, 2′′′′. A large anterior sulcal plate deeply intrudes on the epitheca. Plate 1a is about half the size of 3a and one quarter the size of 2a. Each cell has a single reticulated chloroplast with one stalked pyrenoid. An eyespot comprising two rows of lipid globules is situated within a chloroplast and belongs to type A. The nucleus is spherical and located posteriorly. Cysts are proximate with a chasmic archeopyle.
Figs 1–4. Light micrographs of cysts and cells of Caladoa arcachonensis from France. Fig. 1. Ventral view showing the outline and a post-equatorial cingulum of a living cyst with a pyrenoid (p) and eyespot (arrow). Fig. 2. The cyst in Fig. 1 upon germination showing the chasmic archeopyle (arrow). Fig. 3. The cyst in Fig. 1 upon germination showing granular surface. Fig. 4. Ventral view of the germinated cell from the cyst in Fig. 1, showing a red eyespot in the sulcal area (e). Scale bars = 5 μm.
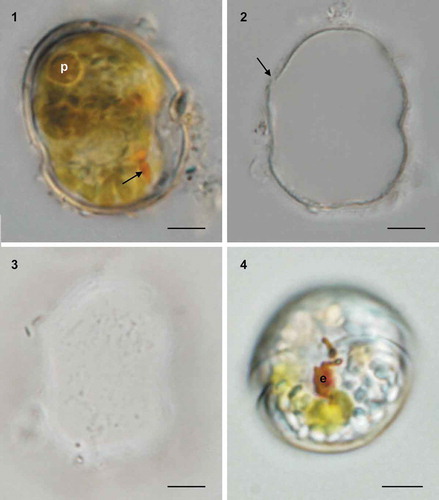
Figs 5–8. Light micrographs of cells of Caladoa arcachonensis strain TIO278 from France. Fig. 5. Ventral view showing a rounded epitheca and hypotheca with a post-equatorial cingulum. Fig. 6. Lateral view showing a ring-like pyrenoid (p) and a red eyespot (arrow). Fig.7. Epifluorescence image of a cell in ventral view showing the single reticulate chloroplast (c) in the periphery of the cell. Fig. 8. Epifluorescence image of a SYBR Green-stained cell showing a spherical nucleus (N). Scale bars = 5 μm.
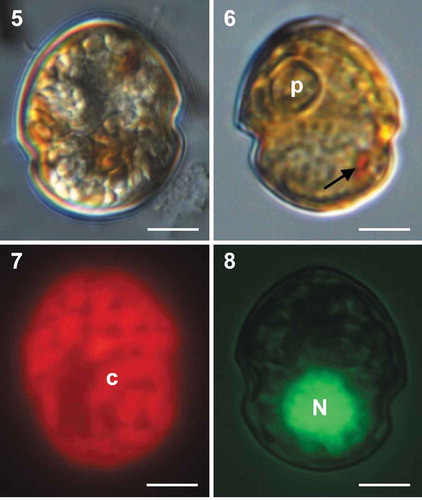
Figs 9–12. Scanning electron micrographs of vegetative cells of Caladoa arcachonensis strain TIO278 from France. Fig. 9. Ventral view showing the first apical plate (1′), anterior sulcal plate (Sa), right sulcal plate (Sd), posterior sulcal plate (Sp) and the first three cingular plates (C1–C3). Fig. 10. Dorsal view showing three anterior intercalary plates (1a–3a), three precingular plates (3′′–5′′), three cingular plates (C3–C5) and two postcingular plates (3′′′, 4′′′). Fig. 11. Ventral-lateral right view showing two precingular plates (6′′, 7′′), sixth cingular plate (C6) and fifth postcingular plate (5′′′). Fig. 12. Dorso-antapical view showing five postcingular plates (1′′′–5′′′) and two antapical plates (1′′′′, 2′′′′) of similar size. Scale bars = 5 μm.
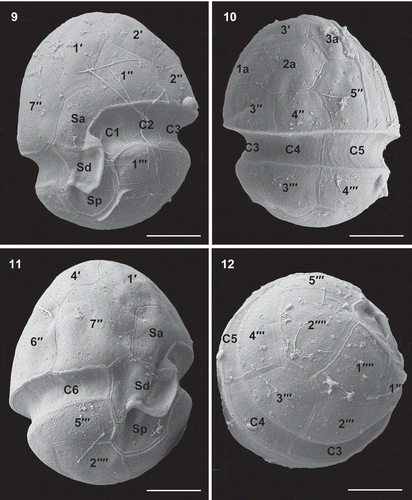
Figs 13–16. Scanning electron micrographs of vegetative cells of Caladoa arcachonensis strain TIO278 from France. Fig. 13. Ventro-apical view showing four apical plates (1′–4′), the anterior sulcal plate (Sa), the first anterior intercalary plate (1a) and three precingular plates (1′′, 2′′, 7′′). Fig. 14. Dorso-apical view showing three apical plates (2′–4′), three anterior intercalary plates (1a–3a) and five precingular plates (2′′–6′′). Fig. 15. Internal view of the hypotheca showing six cingular plates (C1–C6) and right sulcal plate (Sd), left sulcal plate (Ss), median sulcal plate (Sm) and posterior sulcal plate (Sp). Fig. 16. Sulcal area showing the first cingular plate (C1) and plates Sa, Sd, Sp and Ss (arrow). Scale bars = 5 μm.
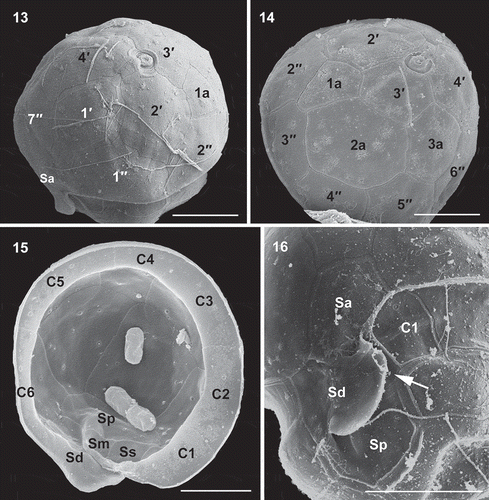
Figs 17–20. Schematic drawings of thecal plate patterns of Caladoa arcachonensis. Fig. 17. Ventral view. Fig. 18. Dorsal view. Fig. 19. Apical view showing plate overlap patterns. Fig. 20. Antapical view showing plate overlap patterns.
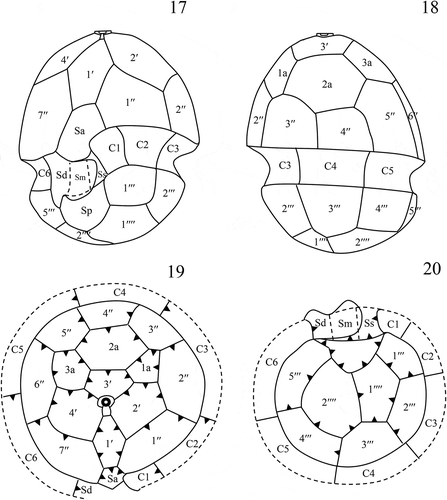
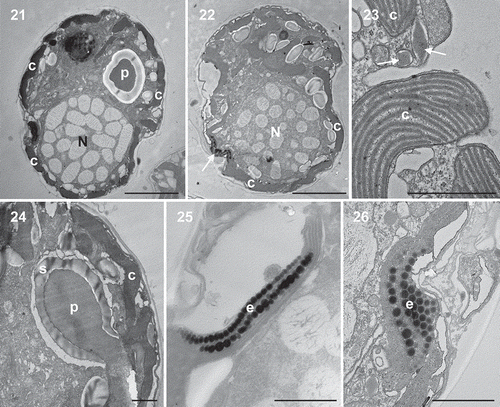
Figs 21–26. Transmission electron micrographs of vegetative cells of Caladoa arcachonensis strain TIO278 from France. Figs 21, 22. Transverse section through the cell showing a large nucleus (N), a stalked pyrenoid (p), a single chloroplast (c) in the periphery of the cell and an eyespot (arrow). Fig. 23. The chloroplast showing the thylakoids grouped in threes to form lamellae and several trichocysts (arrow). Fig. 24. Detail of the stalked pyrenoid (p) with dense matrices and surrounding starch (s). Figs 25, 26. The eyespot (e) located within a chloroplast (c) comprising several rows of globular lipids. Scale: Figs 21, 22 = 5 μm, Figs 23–26 = 1 μm.
Holotype: SEM stub of strain TIO278 designated as TIO201803 (illustrated in ) and deposited at Third Institute of Oceanography, State Oceanic Administration, Xiamen 361005, China.
Type locality: Arcachon Bay, France (1°4’0.0’’W, 44°38’11.0’’N); Collection date: 15 April 2016.
Etymology: The epithet arcachonensis is named after the type locality, Arcachon Bay.
Habitat: Benthic sand-dwelling or epiphytic on seagrass.
Distribution: Brackish and marine coastal water of French Atlantic (21.3–23 psu), North Sulawesi, Indonesia.
GenBank accession number sequences: MK012071 (SSU rDNA), MK012076 (LSU rDNA) and MK012081 (ITS rDNA) of strain TIO278.
Morphology
Cysts of Caladoa arcachonensis, isolated from Arcachon Bay, France surface sediment samples are spherical or ovoid in shape, proximate and with a well-defined posteriorly located cingulum (). They are 23.9–28.1 µm long (mean = 26.6 ± 2.3 μm, n = 3) and 19.9–24.4 µm wide (mean = 22.0 ± 2.3μm, n = 3). Each cyst contains an endospore, green to brown granules, a pyrenoid and the red eyespot. Two cysts were isolated, but only one germinated, displaying a chasmic archeopyle on the epitheca (). The cyst wall is transparent and it appears to be smooth (). Sometimes, small detrital particles can be attached to the cyst wall surface. The germinated cell had a pronounced eyespot in the sulcal area (). The cysts withstand palynological treatment.
Cells of the French strain TIO278 are 16.7–23.5 µm long (mean = 19.8 ± 2.2 μm, n = 30) and 13.1–19.4 µm wide (mean = 16.4 ± 1.8 μm, n = 30). The cells have a rounded epitheca and hypotheca with a much larger epitheca (). There is a ring-like pyrenoid located in the epicone and one pronounced orange eyespot in the sulcal area (). There is a single chloroplast forming a network in the periphery of the cell (‘c’ in ). The nucleus is rounded and located posteriorly (‘N’ in ).
The thecae have a plate formula of Po, cp, X, 4′, 3a, 7′′, 6C, 5S, 5′′′, 2′′′′ (–). The epitheca: hypotheca length ratio in the dorsal side is 1.5–1.8 (mean = 1.6 ± 0.1, n = 7). Thecal pores with a diameter of 0.1–0.2 μm are randomly scattered throughout the thecal plates (, , ). The epithecal plates are symmetrical in arrangement (bipesoid). There are four apical plates. Plates 1′ and 3′ are six-sided and are nearly symmetrical (, ). Plates 2′ and 4′ are seven-sided and similar in size, much larger than the other apical plates (, ). There are three anterior intercalary plates (1a, 2a and 3a), of which 1a and 3a are pentagonal and 2a is hexagonal. Plate 1a is about half the size of 3a and one quarter the size of 2a (). There are seven precingular plates among which the fourth is four-sided and smallest, whereas the others are five-sided (, , , ). The cingulum is 1.6–2.8 µm wide (mean = 2.3 ± 0.4 μm, n = 12), situated in the low part of the cell and descended c. half its width (). The cingulum comprises six plates. The cingular plates are similar in size except C1 is relatively smaller (, , ). The apical pore complex is tear-shaped comprising a round pore plate (Po), a round apical pore covered by a cover plate (cp) and an elongated canal plate (X) (, ). There are five postcingular plates, among them the third and fifth postcingular plates (3′′′, 5′′′) are five-sided whereas the other plates are four-sided (). The antapical plates (1′′′′, 2′′′′) are five-sided and similar in size ().
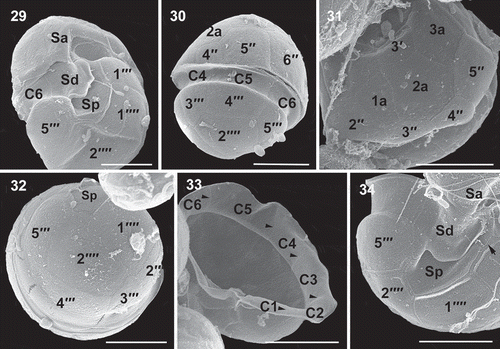
There are five sulcal plates. The large anterior sulcal plate (Sa) intrudes into the epitheca (). The left sulcal (Ss) is elongated and narrow. The median sulcal (Sm) is small and always masked by the sulcal list emerging from the right sulcal plate (Sd). The posterior sulcal plate (Sp) is seven-sided and has no contact with the cingulum (, ). A peduncle was not observed. Schematic drawings showing the plate patterns of Caladoa arcachonensis are provided in –. Plate overlap (, ) was identified individually for each suture by inspecting cells with visible growth bands or by internal theca views (–). The third precingular and postcingular plates and the fourth cingular plate were identified as the keystone plates (i.e. plates overlapping all their neighbours).
Longitudinal sections through the cell of TIO278 show a large spherical nucleus in the hypotheca, a stalked pyrenoid in the epitheca, a single chloroplast in the periphery and an eyespot in the sulcal region (, ). The nucleus consists of condensed chromosomes with a smooth nuclear envelope. The chloroplast is enveloped by three membranes and the thylakoids are grouped in threes to form lamellae (). The stalked pyrenoid is surrounded by a starch sheath without intrusion of lamellae (). Many diamond-shaped trichocysts were observed beneath the thecate wall (). The eyespot is located within a chloroplast comprising two rows of ~20 lipid globules in each row (, ). The lipid globules are ~100–150 nm in diameter. A microtubular strand of the peduncle and a pusular system were not observed but an intensive search was not carried out.
Cells of the Indonesian strain TIO339 are 13.2–18.9 µm long (mean = 16.5 ± 1.5 μm, n = 24) and 10.7–15.2 µm wide (mean = 12.9 ± 1.1 μm, n = 29). The cells show a spherical nucleus in the hypotheca, an orange eyespot in the sulcal region and a single reticulated chloroplast with a doughnut-shaped pyrenoid in the epitheca (, ). Cell morphology of strain TIO339 is indistinguishable from strain TIO278 (–).
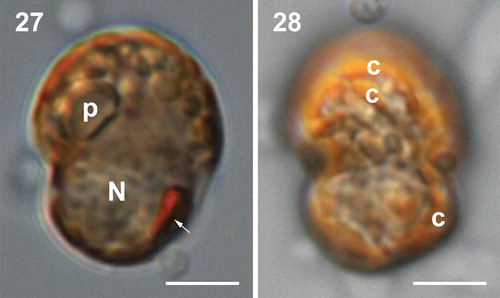
Figs 27–28. Light micrographs of vegetative cells of Caladoa arcachonensis strain TIO339 from Indonesia. Fig. 27. Lateral view of a living cell showing a pyrenoid (p), a nucleus (N) and an eyespot (arrow). Fig. 28. Dorsal view of a living cell showing a single chloroplast forming a network. Scale bars = 5 μm.
Figs 29–34. Scanning electron micrographs of vegetative cells of Caladoa arcachonensis strain TIO339 from Indonesia. Fig. 29. Ventro-antapical view showing the anterior sulcal plate (Sa), the right sulcal plate (Sd), posterior sulcal plate (Sp), two postcingular plates (1′′′, 5′′′) and two antapical plates (1′′′′, 2′′′′). Fig. 30. Dorsal view showing three precingular plates (4′′–6′′), three cingular plates (C4–C6) and three postcingular plates (3′′′–5′′′). Fig. 31. Dorso-apical view showing four precingular plates (2′′–5′′), three anterior intercalary plates (1a–3a). Fig. 32. Antapical view showing four postcingular plates (2′′′–5′′′), plate Sp and two antapical plates (1′′′′, 2′′′′) of similar size. Fig. 33. Internal view of the hypotheca showing six cingular plates (C1–C6). Fig. 34. The sulcus showing Sa, Sd, Sp and left sulcal plate (arrow). Scale bars = 5 μm.
Molecular analysis and phylogeny
The French strains TIO278 and TIO282 shared identical SSU, LSU and ITS rDNA sequences, as did the Indonesian strains TIO339 and TIO340. Strain TIO278 differed from TIO339 at 13 positions (SSU rDNA, 99.2% similarity out of 1724 bp), 61 positions (LSU rDNA, 95.5% similarity out of 1331 bp) and 131 positions (ITS rDNA, 79.8% similarity out of 648 bp). The genetic distance based on ITS rDNA sequences was 0.17 between French and Indonesian strains.
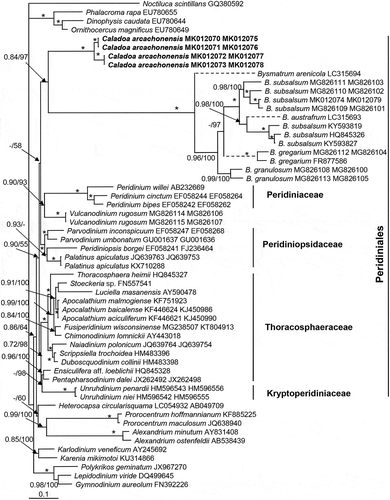
The maximum likelihood (ML) and Bayesian inference (BI) analysis based on combined SSU rDNA and partial LSU rDNA sequences yielded similar phylogenetic trees. The BI tree is illustrated in . The order Peridiniales is well resolved (0.9 BPP/55 BS) encompassing the families Peridiniaceae, Peridiniopsidaceae, Thoracosphaeraceae, Kryptoperidiniaceae, and the genera Caladoa, Bysmatrum and Vulcanodinium. The genus Caladoa was monophyletic with maximal support (1.0 BPP/100 BS), which formed a sister clade of Bysmatrum with maximal support. They were closest to a well resolved clade (0.90 BPP/93 BS) formed by Peridiniaceae and Vulcanodinium rugosum E. Nézan & N. Chomérat with a low Bayesian posterior probability (<0.7 BPP) and moderate ML bootstrap support (58 BS).
Fig. 35. Phylogeny of Caladoa inferred from concatenated SSU and partial LSU rDNA sequences using Maximum likelihood (ML). New sequences are indicated in bold. Branch lengths are drawn to scale, with the scale bar indicating the number of nucleotide substitutions per site. Numbers on branches are statistical support values to clusters on the right of them (left: ML bootstrap support (BS) values; right: Bayesian posterior probabilities (BPP)). Bootstrap support values >50% and Bayesian posterior probabilities above 0.7 are shown. Asterisk indicates maximal support (ML BS = 100% and BPP = 1.00).
Discussion
Morphology
To determine plate homologies, the plate overlap pattern was investigated. Plate overlap pattern of strain TIO278 (, ) is generally consistent with other dinoflagellates, following a trend from dorsal to ventral and from equatorial to poles (Netzel & Dürr, Citation1984). The third precingular plate is identified as the keystone plate in our strain, also previously reported for Parvodinium marciniakii J. Kretschmann, P. Owsianny, A.Z. Zerdoner & M. Gottschling by Kretschmann et al. (Citation2018) and Parvodinium umbonatum (F. Stein) S. Carty by Luo et al. (Citation2018). In contrast, the fourth precingular plate is the keystone plate in many other species of Peridiniales including Bysmatrum subsalsum (C.H. Ostenfeld) M.A. Faust & K.A. Steidinger and Vulcanodinium rugosum (Luo et al., Citation2018), Scrippsiella acuminata (C.G. Ehrenberg) J. Kretschmann, M. Elbrächter, C. Zinssmeister, S. Soehner, M. Kirsch, W.-H. Kusber & M. Gottschling (Kretschmann et al., Citation2015), Peridinium cinctum (Dürr, Citation1979), Fusiperidinium wisconsinense (S. Eddy) F.M.G. McCarthy, H. Gu, K.N. Mertens & C. Carbonell-Moore (McCarthy et al., Citation2018), Heterocapsa triquetra (C.G. Ehrenberg) F. Stein (Tillmann et al., Citation2017), Parvodinium travinskii J. Kretschmann, P. Owsianny, A.Z. Zerdoner & M. Gottschling, Parvodinium mixtum Wołoszyńska ex J. Kretschmann, P. Owsianny, A.Z. Zerdoner & M. Gottschling (Kretschmann et al., Citation2018) and Parvodinium umbonatum (Elbrächter & Meyer, Citation2001). In addition, Caladoa has a primtegulate series that touches the terttegulate series, which can also be found in Scrippsiella Balech ex A.R. Loeblich III, Fusiperidinium F.M.G. McCarthy, H. Gu, K.N. Mertens & C. Carbonell-Moore, Parvodinium, and Heterocapsa F. Stein, but is different from Bysmatrum, Vulcanodinium, Peridinium and Protoperidinium, where both series are separated (partly) by the sectegulate series.
The central ventral plate of strain TIO278 (denoted here by Sa) is overlapped by all adjacent epithecal plates including the first apical plate 1′. This ventral plate is identified as homologous with the anterior sulcal plate (Sa) instead of a precingular plate because plate 1′ is generally overlapped by precingular plates (Dürr & Netzel, Citation1974; Fensome et al., Citation1993). The plate pattern of strain TIO278 was thus determined as Po, cp, X, 4′, 3a, 7′′, 6C, 5S, 5′′′, 2′′′′. This plate formula is reminiscent of several previously described genera, including the freshwater genera Chimonodinium S.C. Craveiro, A.J. Calado, N. Daugbjerg, Gert Hansen & Ø. Moestrup and Fusiperidinium (Craveiro et al., Citation2011; McCarthy et al., Citation2018), the freshwater-brackish genus Apocalathium S.C. Craveiro, N. Daugbjerg, Ø. Moestrup & A.J. Calado (Craveiro et al., Citation2016), and the brackish-marine genera Bysmatrum, Scrippsiella and Vulcanodinium (Nézan & Chomérat, Citation2011; Luo et al., Citation2016, Citation2018). The strain TIO278 can be separated from these genera based primarily on the size and location of Sa plate, which is large and intrudes deeply into the epitheca. This kind of Sa plate has only been reported in marine Heterocapsa (Tillmann et al., Citation2017) and Laciniporus Saburova & N. Chomérat (Saburova & Chomérat Citation2018), freshwater Parvodinium (Carty, Citation2008) and Palatinus S. Craveiro, A. Calado, N. Daugbjerg & Ø. Moestrup (Craveiro et al., Citation2009). A similar plate was observed in Sphaerodinium cracoviense J. Wołoszyńska (Craveiro et al., Citation2010), but the authors expressed some doubts about whether it should be considered as a Sa plate. Interestingly, this type of deeply intruding Sa, with similar overlap with the 1′, has also been recorded for the fossil cyst Susadinium? pinna (Below) Lentin & Williams by Below (Citation1987, as Dodekovia pinna Below) described from the Lower Jurassic (Toarcian), a species assigned to the Heterocapsaceae (Fensome et al., Citation1993); suggesting that this type of Sa is a primitive evolutionary trait not present in genera that have presumably evolved later such as Protoperidinium.
Strain TIO278 differs from Chimonodinium in the presence of a pyrenoid and lack of a peduncle (Craveiro et al., Citation2011), from Scrippsiella in the size of the first cingular plate (Luo et al., Citation2016) and it differs from Fusiperidinium, Apocalathium and Vulcanodinium in the presence of an eyespot (Nézan & Chomérat, Citation2011; Craveiro et al., Citation2016; McCarthy et al., Citation2018), as well as differing from Bysmatrum in the configuration of anterior intercalary plates. Plates 2a and 3a are separated in Bysmatrum, but they touch in strain TIO278. Strain TIO278 shares a similar Sa plate with Heterocapsa and Laciniporus, but the plate pattern differs. Only two anterior intercalary plates are present in Heterocapsa and Laciniporus and an eyespot is not observed (Tillmann et al., Citation2017; Saburova & Chomérat, Citation2018). Strain TIO278 shares a type A eyespot with Peridinium but differs in the number of cingular plates (six versus five), therefore, a new genus Caladoa is established to incorporate TIO278 and related strains.
Caladoa arcachonensis is morphologically close to the freshwater ‘Peridinium subtatranum’ (invalidly described), Apocalathium aciculiferum var. inerme (J. Wołoszyńska) Ø. Moestrup & A.J. Calado and Chimonodinium lomnickii var. minus (J. Wołoszyńska) Ø. Moestrup based on the plate pattern and a large Sa plate, but it differs in the relative size of anterior intercalary plates and the size of the apical pore (Wołoszyńska, Citation1916, Citation1937, Citation1952; Moestrup & Calado, Citation2018). Caladoa arcachonensis is also morphologically close to Chimonodinium lomnickii (J. Wołoszyńska) S.C. Craveiro, A.J. Calado, N. Daugbjerg, Gert Hansen & Ø. Moestrup and Chimonodinium lomnickii var. wierzejskii (J. Wołoszyńska) S.C. Craveiro, A.J. Calado, N. Daugbjerg, Gert Hansen & Ø. Moestrup but differs in the size and location of the Sa and relative size of anterior intercalary plates (Craveiro et al., Citation2011; Moestrup & Calado, Citation2018). Caladoa arcachonensis differs from Peridinium gutwinskii J.Wołoszyńska and Peridinium bipes F.Stein in the number of cingular plates and relative size of anterior intercalary plates (Boltovskoy, Citation1989; Lee et al., Citation2006), and differs from Peridinium allorgei M.Lefèvre in the cell shape and relative size of anterior intercalary plates (Moestrup & Calado, Citation2018). Caladoa arcachonensis differs from Apocalathium malmogiense (G. Sjöstedt) S.C. Craveiro, N. Daugbjerg, Ø. Moestrup & A.J. Calado in a much larger epitheca and relative size of anterior intercalary plates (Craveiro et al., Citation2016).
Phylogeny and genetic differentiation
The only morphological difference between French strain TIO278 and Indonesian strain TIO339 is the cell size, therefore, they are considered to be the same morphospecies. However, these strains inhabit temperate and tropical waters, respectively, and are likely to differ in their eco-physiological traits. The two strains also have a genetic distance, based on ITS sequences, of as much as 0.17, which is much larger than the threshold value (0.04) for differentiating dinoflagellate species at interspecific level (Litaker et al., Citation2007), therefore suggesting the existence of cryptic species. The French strain has a cyst stage, but whether the Indonesian strain produces cysts is yet to be determined.
Our molecular phylogeny supports the classification of Caladoa within the order Peridiniales, consistent with the morphological features characteristic of Peridiniales, such as a symmetrical 1′ plate and two symmetrical antapical plates. However, the close relationship between Caladoa and Bysmatrum is surprising. Bysmatrum shows an asymmetrical 1′ plate and two somewhat asymmetrical antapical plates that do not fit with the definition of Peridiniales (Fensome et al., Citation1993). However, Bysmatrum and Caladoa share identical plate pattern and, most importantly, they share a type A eyespot (located within the chloroplast and consisting of two rows of opaque globules) (Moestrup & Daugbjerg, Citation2007; Dawut et al., Citation2018). A type A eyespot appears to be a common feature of Peridiniales, reported also in Scrippsiella acuminata and Chimonodinium lomnickii (Craveiro et al., Citation2011), Palatinus apiculatus (C.G. Ehrenberg) S. Craveiro, A. Calado, N. Daugbjerg & Ø. Moestrup (Craveiro et al., Citation2009), Naiadinium polonicum (J. Wołoszyńska) S. Carty (Craveiro et al., Citation2015) and Peridinium willei H. Huitfeldt-Kaas (Moestrup & Daugbjerg, 2007). Bysmatrum can also have a type B eyespot, but this is a modified type A eyespot with the only difference being the presence of overlying brick like crystals (Luo et al., Citation2018). Caladoa and Bysmatrum both have proximate cysts that are similar to the motile cells (Anglès et al., Citation2017; Luo et al., Citation2018). A new family is possible for Caladoa and Bysmatrum in view of their morphological similarity, but evidence from more closely related species is needed.
The close relationship between Vulcanodinium and Peridinium sensu stricto has previously been reported based on the LSU rDNA sequences (Nézan & Chomérat, Citation2011), concatenated data from SSU, ITS and LSU rDNA sequences (Luo et al., Citation2018), and is supported here again based on concatenated data from SSU and LSU rDNA sequences. It is worth noting that the closest relative of Peridinium is a brackish species, in disagreement with the suggestion that marine and freshwater species are usually not closely related (Logares et al., Citation2007). However, Vulcanodinium has six cingular plates compared with the five in Peridinium sensu stricto (Boltovskoy, Citation1975; Nézan & Chomérat, Citation2011), and Vulcanodinium does not have a type A eyespot whereas Peridinium cinctum and P. willei do (Spector & Triemer, Citation1979; Moestrup & Daugbjerg, 2007). The assignment of Vulcanodinium to Peridiniaceae is possible depending on the emendation of Peridiniaceae. Multi-gene phylogenies or phylotranscriptomics should provide more evidence for a definite assignment.
Author contributions
Z. Luo: light microscopy, electron microscopy and drafting manuscript; K.N. Mertens: original concept, drafting and editing manuscript, incubation experiment, light microscopy; E. Nézan: drafting and editing manuscript; Li Gu: electron microscopy; Vera Pospelova: palynological treatment, drafting and editing manuscript; H. Thoha: sampling and editing manuscript; H. Gu: original concept and editing of manuscript.
Acknowledgements
We thank two anonymous reviewers for constructive suggestions that improved the manuscript greatly. Claire Meteigner and Myriam Rumèbe are acknowledged for collecting samples from Arcachon Bay.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Adachi, M., Sako, Y. & Ishida, Y. (1996). Analysis of Alexandrium (Dinophyceae) species using sequences of the 5.8S ribosomal DNA and internal transcribed spacer regions. Journal of Phycology, 32: 424–432.
- Anglès, S., Reñé, A., Garcés, E., Lugliè, A., Sechi, N., Camp, J. & Satta, C.T. (2017). Morphological and molecular characterization of Bysmatrum subsalsum (Dinophyceae) from the western Mediterranean Sea reveals the existence of cryptic species. Journal of Phycology, 53: 833–847.
- Balech, E. (1980). On the thecal morphology of dinoflagellates with special emphasis on circular and sulcal plates. Anales del Centro de Ciencias del Mar y Limnologia, Universidad Nacional Autonomia de Mexico, 7: 57–68.
- Below, R. (1987). Evolution und Systematik von Dinoflagellaten-Zysten aus der Ordnung Peridiniales. I. Allgemeine Grundlagen und Subfamilie Rhaetogonyaulacoideae (Familie Peridiniaceae). Palaeontographica Abteilung B, 205: 1–164.
- Boc, A., Diallo, A.B. & Makarenkov, V. (2012). T-REX: a web server for inferring, validating and visualizing phylogenetic trees and networks. Nucleic Acids Research, 40: W573–W579.
- Boltovskoy, A. (1975). Peridinium cinctum (Müller) Ehrenberg. Physis, secciones B, 34: 73–84.
- Boltovskoy, A. (1989). Thecal morphology of the dinoflagellate Peridinium gutwinskii. Nova Hedwigia, 49: 369–380.
- Carty, S. (2008). Parvodinium gen. nov. for the Umbonatum group of Peridinium (Dinophyceae). Ohio Journal of Science, 108: 103–107.
- Craveiro, S., Calado, A.J., Daugbjerg, N. & Moestrup, Ø. (2009). Ultrastructure and LSU rDNA-based revision of Peridinium group palatinum (Dinophyceae) with the description of Palatinus gen. nov. Journal of Phycology, 45: 1175–1194.
- Craveiro, S.C., Moestrup, Ø., Daugbjerg, N. & Calado, A.J. (2010). Ultrastructure and large subunit rDNA-based phylogeny of Sphaerodinium cracoviense, an unusual freshwater dinoflagellate with a novel type of eyespot. Journal of Eukaryotic Microbiology, 57: 568–585.
- Craveiro, S.C., Calado, A.J., Daugbjerg, N., Hansen, G. & Moestrup, Ø. (2011). Ultrastructure and LSU rDNA-based phylogeny of Peridinium lomnickii and description of Chimonodinium gen. nov. (Dinophyceae). Protist, 162: 590–615.
- Craveiro, S.C., Daugbjerg, N., Moestrup, Ø. & Calado, A.J. (2015). Fine-structural characterization and phylogeny of Peridinium polonicum, type species of the recently described genus Naiadinium (Dinophyceae). European Journal of Protistology, 51: 259–279.
- Craveiro, S.C., Daugbjerg, N., Moestrup, Ø. & Calado, A.J. (2016). Studies on Peridinium aciculiferum and Peridinium malmogiense (= Scrippsiella hangoei): comparison with Chimonodinium lomnickii and description of Apocalathium gen. nov. (Dinophyceae). Phycologia, 56: 21–35.
- Daugbjerg, N., Hansen, G., Larsen, J. & Moestrup, Ø. (2000). Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia, 39: 302–317.
- Dawut, M., Sym, S.D., Suda, S. & Horiguchi, T. (2018). Bysmatrum austrafrum sp. nov. (Dinophyceae), a novel tidal pool dinoflagellate from South Africa. Phycologia, 57: 169–178.
- Dürr, G. (1979). Elektronenmikroskopische Untersuchungen am Panzer von Dinoflagellaten: II. Peridinium cinctum. Archiv für Protistenkunde, 122: 88–120.
- Dürr, G. & Netzel, H. (1974). The fine structure of the cell surface in Gonyaulax polyedra (Dinoflagellata). Cell and Tissue Research, 150: 21–41.
- Elbrächter, M. & Meyer, B. (2001). Plate pattern variability and plate overlap in a clonal culture of the freshwater dinoflagellate Peridinium umbonatum Stein species complex (Dinophyceae). Neues Jahrbuch für Geologie und Paläontologie/Abhandlungen, 219: 221–227.
- Elbrächter, M., Gottschling, M., Hildebrand-Habel, T., Keupp, H., Kohring, R., Lewis, J., Meier, S.K.J., Montresor, M., Streng, M. & Versteegh, G.J.M. (2008). Establishing an agenda for calcareous dinoflagellate research (Thoracosphaeraceae, Dinophyceae) including a nomenclatural synopsis of generic names. Taxon, 57: 1289–1303.
- Fensome, R.A., Taylor, F.J.R., Norris, G., Sarjeant, W.A.S., Wharton, D.I. & Williams, G.L. (1993). A classification of fossil and living dinoflagellates. Micropaleontology Special Publication, 7: 1–245.
- Gottschling, M., Soehner, S., Zinssmeister, C., John, U., Plötner, J., Schweikert, M., Aligizaki, K. & Elbrächter, M. (2012). Delimitation of the Thoracosphaeraceae (Dinophyceae), including the calcareous dinoflagellates, based on large amounts of Ribosomal RNA sequence data. Protist, 163: 15–24.
- Gottschling, M., Kretschmann, J. & Čalasan, A.Ž. (2017). Description of Peridiniopsidaceae, fam. nov. (Peridiniales, Dinophyceae). Phytotaxa, 299: 293–296.
- Gu, H., Liu, T. & Mertens, K. (2015). Cyst—theca relationship and phylogenetic positions of Protoperidinium (Peridiniales, Dinophyceae) species of the sections Conica and Tabulata, with description of Protoperidinium shanghaiense sp. nov. Phycologia, 54: 49–66.
- Guillard, R.R.L. & Ryther, J.H. (1962). Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea Cleve. Canadian Journal of Microbiology, 8: 229–239.
- Hall, T.A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. pp. 95–98.
- Head, M.J. (1996). Modern dinoflagellate cysts and their biological affinities. In Palynology: Principles and Applications (Jansonius, J. & McGregor, D.C., editors), 1197–1248. American Association of Stratigraphic Palynologists Foundation, Dallas.
- Horiguchi, T. & Takano, Y. (2006). Serial replacement of a diatom endosymbiont in the marine dinoflagellate Peridinium quinquecorne (Peridiniales, Dinophyceae). Phycological Research, 54: 193–200.
- Katoh, K. & Standley, D.M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution, 30: 772–780.
- Kretschmann, J., Elbrächter, M., Zinssmeister, C., Soehner, S., Kirsch, M., Kusber, W.-H. & Gottschling, M. (2015). Taxonomic clarification of the dinophyte Peridinium acuminatum Ehrenb.,≡ Scrippsiella acuminata, comb. nov. (Thoracosphaeraceae, Peridiniales). Phytotaxa, 220: 239–256.
- Kretschmann, J., Žerdoner Čalasan, A. & Gottschling, M. (2018). Molecular phylogenetics of dinophytes harboring diatoms as endosymbionts (Kryptoperidiniaceae, Peridiniales), with evolutionary interpretations and a focus on the identity of Durinskia oculata from Prague. Molecular Phylogenetics and Evolution, 118: 392–402.
- Lee, J.-J., Jang, S.-H., Lee, J.-H. & Lee, J.-H. (2006). Morphology and ecology of Peridinium bipes var. occultatum Lindem. (Dinophyceae) forming freshwater red tides in Korean dam reservoirs. Algae, 21: 433–443.
- Lindberg, K., Moestrup, Ø. & Daugbjerg, N. (2005). Studies on woloszynskioid dinoflagellates I: Woloszynskia coronata re-examined using light and electron microscopy and partial LSU rDNA sequences, with description of Tovellia gen. nov. and Jadwigia gen. nov. (Tovelliaceae fam. nov.). Phycologia, 44: 416–440.
- Litaker, W.R., Vandersea, M.W., Kibler, S.R., Reece, K.S., Stokes, N.A., Lutzoni, F.M., Yonish, B.A., West, M.A., Black, M.N.D. & Tester, P.A. (2007). Recognizing dinoflagellate species using ITS rDNA sequences. Journal of Phycology, 43: 344–355.
- Logares, R., Shalchian-Tabrizi, K., Boltovskoy, A. & Rengefors, K. (2007). Extensive dinoflagellate phylogenies indicate infrequent marine-freshwater transitions. Molecular Phylogenetics and Evolution, 45: 887–903.
- Luo, Z., Mertens, K.N., Bagheri, S., Aydin, H., Takano, Y., Matsuoka, K., McCarthy, F. M.G. & Gu, H. (2016). Cyst-theca relationship and phylogenetic positions of Scrippsiella plana sp. nov. and S. spinifera (Peridiniales, Dinophyceae). European Journal of Phycology, 51: 188–202.
- Luo, Z., Lim, Z.F., Mertens, K.N., Gurdebeke, P., Bogus, K., Carbonell-Moore, C., Vrielinck, H., Leaw, C.P., Lim, P.T., Chomérat, N., Li, X. & Gu, H. (2018). Morpho-molecular diversity and phylogenetic relationship of Bysmatrum species (Dinophyceae) from the South China Sea and France. European Journal of Phycology, 53: 318–335.
- McCarthy, F.M., Gu, H., Mertens, K.N., Carbonell-Moore, C., Krueger, A.M., Takano, Y. & Matsuoka, K. (2018). Transferring the freshwater dinoflagellate Peridinium wisconsinense (Dinophyceae) to the family Thoracosphaeraceae, with the description of Fusiperidinium gen. nov. Phycological Research, 66: 137–168.
- Medlin, L., Elwood, H.J., Stickel, S. & Sogin, M.L. (1988). The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene, 71: 491–499.
- Moestrup, Ø. & Calado, A.J. (2018). Dinophyceae. In Freshwater Flora of Central Europe (Büdel, B., Gärtner, G., Krienitz, L. & Schagerl, M., editors), 6: 1–560. Springer, Berlin.
- Moestrup, Ø. & Daugbjerg, N. (2007). On dinoflagellate phylogeny and classification. In Unravelling the Algae: The Past, Present, and Future of Algal Systematics (Brodie, J. & Lewis, J., editors). The Systematics Association Special Volume Series, 75: 215–230.
- Moestrup, Ø., Lindberg, K. & Daugbjerg, N. (2009). Studies on woloszynskioid dinoflagellates IV: The genus Biecheleria gen. nov. Phycological Research, 57: 203–220.
- Netzel, H. & Dürr, G. (1984). Dinoflagellate cell cortex. In Dinoflagellates (Spector, D.L., editor), 43–105. Academic Press, Orlando.
- Nézan, E. & Chomérat, N. (2011). Vulcanodinium rugosum gen. et sp. nov. (Dinophyceae), un nouveau dinoflagellé marin de la côte méditerranéenne française. Cryptogamie-Algologie, 32: 3–18.
- Pienaar, R.N., Sakai, H. & Horiguchi, T. (2007). Description of a new dinoflagellate with a diatom endosymbiont, Durinskia capensis sp. nov. (Peridiniales, Dinophyceae) from South Africa. Journal of Plant Research, 120: 247–258.
- Posada, D. (2008). jModelTest: phylogenetic model averaging. Molecular Biology and Evolution, 25: 1253–1256.
- Ronquist, F. & Huelsenbeck, J.P. (2003). MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19: 1572–1574.
- Saburova, M. & Chomérat, N. (2018). Laciniporus arabicus gen. et sp. nov. (Dinophyceae, Peridiniales), a new thecate, marine, sand‐dwelling dinoflagellate from the northern Indian Ocean (Arabian Sea). Journal of Phycology, doi: 10.1111/jpy.12783.
- Saburova, M., Chomérat, N. & Hoppenrath, M. (2012). Morphology and SSU rDNA phylogeny of Durinskia agilis (Kofoid & Swezy) comb. nov. (Peridiniales, Dinophyceae), a thecate, marine, sand-dwelling dinoflagellate formerly classified within Gymnodinium. Phycologia, 51: 287–302.
- Scholin, C.A., Herzog, M., Sogin, M. & Anderson, D.M. (1994). Identification of group- and strain-specific genetic markers for globally distributed Alexandrium (Dinophyceae). II. Sequence analysis of a fragment of the LSU rRNA gene. Journal of Phycology, 30: 999–1011.
- Spector, D. & Triemer, R. (1979). Ultrastructure of the dinoflagellate Peridinium cinctum f. ovoplanum. I. Vegetative cell ultrastructure. American Journal of Botany, 66: 845–850.
- Spurr, A.R. (1969). A low-viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructure Research, 26: 31–43.
- Stamatakis, A. (2006). RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics, 22: 2688–2690.
- Swofford, D. (2002). 2002PAUP*. Phylogenetic analysis using parsimony (* and other methods), version 4 b10. Sinauer Associates, Sunderland, MA.
- Tamura, M., Shimwia, S. & Horigucki, T. (2005). Galeidinium rugatum gen. et sp. nov. (Dinophyceae), a new coccoid dinoflagellate with a diatom endosymbiont. Journal of Phycology, 41: 658–671.
- Tillmann, U., Hoppenrath, M., Gottschling, M., Kusber, W.-H. & Elbrächter, M. (2017). Plate pattern clarification of the marine dinophyte Heterocapsa triquetra sensu Stein (Dinophyceae) collected at the Kiel Fjord (Germany). Journal of Phycology, 53: 1305–1324.
- Wołoszyńska, J. (1916). Polskie Peridineae słodkowodne. – Polnische Süsswasser -Peridineen. Bulletin International de l’Academie des Sciences de Cracovie, Classe des Sciences Mathématiques et Naturelles, série B: Sciences Naturelles, 1915: 260–285.
- Wołoszyńska, J. (1937). Die Algen der Tatraseen und Tümpel. III. Peridineen im Winterplankton einiger Tatraseen. Archiwum Hydrobiologji i Rybactwa, 10: 188–196.
- Wołoszyńska, J. (1952). Bruzdnice Tatr i Karpat Wschodnich [Peridineae montium Tatrensium et Carpathorum Orientalium]. Acta Societatis Botanicorum Poloniae, 21: 311–316.
- Yamada, N., Sym, S.D. & Horiguchi, T. (2017). Identification of highly divergent diatom-derived chloroplasts in dinoflagellates, including a description of Durinskia kwazulunatalensis sp. nov. (Peridiniales, Dinophyceae). Molecular Biology and Evolution, 34: 1335–1351.
- You, X., Luo, Z., Su, Y., Gu, L. & Gu, H. (2015). Peridiniopsis jiulongensis, a new freshwater dinoflagellate with a diatom endosymbiont from China. Nova Hedwigia, 100: 313–326.
