ABSTRACT
Four species of Gomphonema described by Hustedt with fusiform valve shape are studied for the first time using SEM. G. longissimum Hustedt, G. angustissimum Hustedt, G. woltereckii Hustedt and G. subtiliforme Hustedt were described, and are known only, from the ancient Malili lake system in Indonesia. Molecular investigations of two cultured strains of G. longissimum, using 18S rDNA and rbcL, are presented. In terms of morphology, these four species have unusually long valves and a fusiform shape otherwise unknown in Gomphonema, yet morphological similarities with other species include C-shaped or semi-circular areolae, uniseriate striae, pseudosepta, apical pore fields, a straight raphe with hooked internal proximal ends and a typical stigma in G. longissimum and G. angustissimum. Results of the molecular study support placement of G. longissimum with other ‘typical’ Gomphonema species. G. subtiliforme and G. woltereckii both lack a stigma. G. subtiliforme is similar in shape and morphology to members of the group G. subtile Ehrenberg, which all bear stigmata. G. woltereckii differs from G. longissimum and G. angustissimum by having areolae that are semi-circular, not C-shaped, and by the absence of a stigma. Therefore, based on morphology, we conclude these extraordinarily large Gomphonema taxa are similar to each other and generally resemble other species of the genus, yet there is enough variation in presence/absence of stigmata and structure of the areolae that they do not necessarily form a distinct group. Thus, despite their large size, and presence in the same lake system, we cannot conclude they form a species flock. Less than two dozen Gomphonema species are present in Malili lakes whereas some other ancient lakes, such as Lake Baikal, have many more species and exhibit species flocks.
Introduction
The genus Gomphonema Ehrenberg is one of the most widely distributed genera of pennate diatoms (Round et al., Citation1990). Species from this genus inhabit freshwater ecosystems with different ecological conditions, from acidic to alkaline, and from oligotrophic to eutrophic waters (Kulikovskiy et al., Citation2016a). Species of Gomphonema are known from all continents and all climatic zones in the world. Morphological diversity in the genus is great, and there are groups of species that can be recognized by robust size, differing types of areolar structure, width of the axial area, presence/absence and structure of stigmata, and position, extent and structure of the apical pore fields (Kociolek & Stoermer, Citation1993; Reichardt, Citation2005, Citation2015; Kociolek et al., Citation2016; Levkov et al., Citation2016). The monophyly of some of these groupings has been shown (Kociolek & Stoermer, Citation1993), but a broader analysis is lacking. In tropical zones, there are species groups that have wide axial areas (seen especially in the New World Tropics; Schmidt et al., Citation1902; Metzeltin & Lange-Bertalot, Citation2007), and others from Asia that have large, robust cells (Amossé, Citation1969; Kociolek et al., Citation2015), or are extremely narrow and elongated (species from Indonesia; Hustedt, Citation1942). Species with interesting shapes or morphologies are also known from the Holarctic region, especially from ancient lakes such as Lake Baikal or Lake Ohrid (Kulikovskiy et al., Citation2012a, Citation2012b, Citation2016b, Citation2016c; Levkov et al., Citation2007). In these ancient lakes, large, unusual species can be found co-occurring with widespread taxa inhabiting the lakeshore zones, especially in Lake Baikal (Kulikovskiy et al., Citation2012a; Kulikovskiy & Kuznetsova, Citation2016). Ancient lakes are very interesting ecosystems for understanding diatom diversity and biogeography, as well as places to conduct molecular investigations of taxa for understanding lineages and diversity of the diatom tree of life. Ancient lakes are rare and known from Eurasia (Ohrid, Baikal, Inle and Caspian), Africa (Malawi and Tanganyika), South America (Titicaca) and Indonesia (Poso Lake and Malili lake system including Matano, Mahalona, Towuti, Lontoa and Masapi lakes). Indonesia includes a large number of ancient lakes, but diatoms from these lakes are poorly studied in comparison with Lake Baikal (Kulikovskiy et al., Citation2011, Citation2012a, Citation2012b, Citation2013, Citation2014, Citation2015a, Citation2015b, Citation2015c, Citation2015d, Citation2015e, Citation2016b, Citation2016c, Citation2019; Genkal et al., Citation2013; Kociolek et al., Citation2013, Citation2018a, Citation2018b; Kulikovskiy & Kociolek, Citation2014; Vishnyakov et al., Citation2015; Kulikovskiy & Kuznetsova, Citation2016; Kapustin et al., Citation2017; Maltsev et al., Citation2017; Kapustin & Kulikovskiy, Citation2018).
Our investigation of diatoms from ancient lakes reveals some evolutionary peculiarities in species composition. In these ancient lakes there can be a large number of species in some genera not found in local areas surrounding the lake, such as streams or nearby lakes. Ancient lakes can also be hotspots of diatom species diversity including many endemic and relict species, flagship taxa, separation of diatom communities in near-shore and deeper part of lakes and the presence of species flocks (Kulikovskiy et al., Citation2015d).
Species flocks were defined by Ribbink (Citation1984) and Greenwood (Citation1974, Citation1984) as having several attributes; among these are that component species were closely related (later suggested to be monophyletic, see Eastman & McCune, Citation2000), and that flocks were geographically constrained, contain large numbers of species (at least in comparison to the proximate region) and evolved quickly. The concept might be thought of as analogous to adaptive radiation events resulting in numerous species (Herder et al., Citation2006; Glaubrecht & Rintelen, Citation2008; Schubart et al., Citation2008; Walter et al., Citation2009). Schluter (Citation1996) proposed that adaptive radiations could be diagnosed through recent common ancestry of component species, significant environment–phenotype correlations, trait utility in corresponding environments and rapid speciation.
Wallace (Citation1860) first commented on the striking uniqueness of the island of Sulawesi and its species diversity, and subsequent taxonomic investigations of the Malili lakes have revealed endemic species clusters in various aquatic organisms, such as fishes (Kottelat, Citation1991; Roy et al., Citation2007; Herder et al., Citation2008), molluscs (von Rintelen et al., Citation2014) and crustaceans (Pfaender et al., Citation2010). The relative isolation and age of the lakes (van Bemmelen, Citation1970), coupled with their unique limnological characteristics (Haffner et al., Citation2001; Bramburger et al., Citation2008; Katsev et al., Citation2010; Vaillant et al., Citation2011) make them important natural laboratories for understanding evolution of diatoms.
Hustedt (Citation1942) was the first to study diatoms from Indonesian ancient lakes. In his monograph, Hustedt prepared a floristic treatment of the diatoms from Indonesian ancient lakes, including the Malili lake system. Hustedt (Citation1942) mostly described new species on the basis of line drawings with some exceptions where he published light micrographs. Subsequently, Simonsen (Citation1987) studied the types of Hustedt and published light micrographs of them. Revisions of Indonesian diatom taxa, and Gomphonema in particular, using SEM are almost unknown with exception of investigations of Surirella Turpin, Tetralunata Hamsher et al. and Cymbella Agardh (Reichardt, Citation2005; Bramburger et al., Citation2006; Hamsher et al., Citation2014; Kapustin et al., Citation2017). Hustedt (Citation1942) described six new species of Gomphonema from lakes Towuti, Mahalona, Matano and Lontoa. The first three lakes form a connected system, and lakes Towuti and Matano combine to form Mahalona Lake. The six species described by Hustedt from this system include the following taxa: Gomphonema towutense Hustedt from Lake Towuti, G. malayense Hustedt from lakes Mahalona and Towuti, G. angustissimum Hustedt from lakes Mahalona and Towuti, G. woltereckii Hustedt from lakes Lontoa, Mahalona and Matano, G. longissimum Hustedt from lakes Towuti and Matano and G. subtiliforme Hustedt from Lake Matano. From this list of species, three are characterized by having very narrow and long, linear, fusiform valves with the presence of pseudosepta: G. angustissimum, G. woltereckii and G. longissimum. G. subtiliforme is morphologically similar to this group but lacking the extreme length.
The aim of the present report is to offer a morphological investigation, with LM and SEM observations, of an interesting complex of large Gomphonema from the ancient Malili lake system, to compare them and to infer their possible interrelationships. We also present a molecular investigation, and typification, of Gomphonema longissimum for the first time on the basis of natural and cultured samples, to determine its position within the genus Gomphonema. It should be noted that there is still a tremendous amount of work to do in acquiring sequence data for diatoms in general, and especially for large genera such as Gomphonema. Kociolek et al. (Citation2019) list over 2000 described entities in the genus, yet there are only 27 named taxa in GenBank, and of these, only 23 have more than one gene sequenced. Making things more difficult, the large genera of diatoms are notoriously difficult to identify, and a limited number of the Gomphonema taxa listed in GenBank have any pictures presented (48%) or have specimens deposited into collections (67%). These data also allow us to discuss some aspects of diatom evolution in ancient lakes and offer some provisional remarks on species diversity in the genus Gomphonema.
Materials and methods
Benthic samples were collected by E.S. Gusev from Lake Matano, Island of Sulawesi, Indonesia (02°28.433′S, 121°15.710′E; temperature 28.5°C, pH 8.53 and conductivity 177 μS cm−1) on 14 November 2014. Two strains (Ind394, Ind395) were isolated from this sample. Isolated strains were deposited in the culture collection of the Institute of Plant Physiology, Russian Academy of Sciences (Moscow). A subsample of each collection was added to a WC liquid medium (Guillard & Lorenzen, Citation1972). Monoclonal strains were established by micropipetting single cells under the inverted microscope (Zeiss AxioVert A1). Non-axenic monocultures of the algae were cultivated in liquid WC medium in Petri dishes at 14°C with an alternating 12-hour light and dark photoperiod. Strains were analysed after one month of culturing.
Strains for LM and SEM investigations were processed by means of a standard procedure involving treatment with 10% HCl and concentrated hydrogen peroxide. The material was washed with distilled water. Permanent diatom preparations were mounted in Naphrax®. Light microscopic (LM) observations were performed using a Zeiss AxioScope A1 microscope (Germany) equipped with an oil immersion objective (×100/n.a.1.4, DIC). Ultrastructure of the valves was examined with a JSM-6510LV (Japan) scanning electron microscope and EMS FF200-Cu-50 (USA) transmission electron microscope.
Total DNA of monoclonal cultures was extracted using InstaGeneTM Matrix according to the manufacturer’s protocol. Fragments of 18S rDNA (430 bp, including V4 domain), and partial rbcL plastid genes (1332 bp) were amplified using primers from Zimmerman et al. (Citation2011) for 18S rDNA fragments and from Ruck & Theriot (Citation2011) for rbcL fragments.
Amplifications of the 18S rDNA fragments and partial rbcL gene fragment were carried out using the pre-made mix ScreenMix (Evrogen, Russia) for PCR. The conditions of amplification for 18S rDNA fragments were: an initial denaturation of 5 min at 95°C, followed by 35 cycles at 94°C for denaturation (30 s), 52°C for annealing (30 s) and 72°C for extension (50 s), and a final extension of 10 min at 72°C. The conditions of amplification for partial rbcL were: an initial denaturation of 5 min at 95°C, followed by 45 cycles at 94°C for denaturation (30 s), 60°C for annealing (30 s) and 72°C for extension (80 s), and a final extension of 10 min at 72°C.
The resulting amplicons were visualized by horizontal agarose gel electrophoresis (1.5%), coloured with SYBR Safe (Life Technologies, USA). Purification of DNA fragments was performed with the ExoSAP-IT kit (Affimetrix, USA) according to the manufacturer’s protocol. 18S rDNA fragments and partial rbcL gene were sequenced on both strands using forward and reverse PCR primers and the Big Dye system (Applied Biosystems, USA), followed by electrophoresis using a Genetic Analyzer 3500 sequencer (Applied Biosystems).
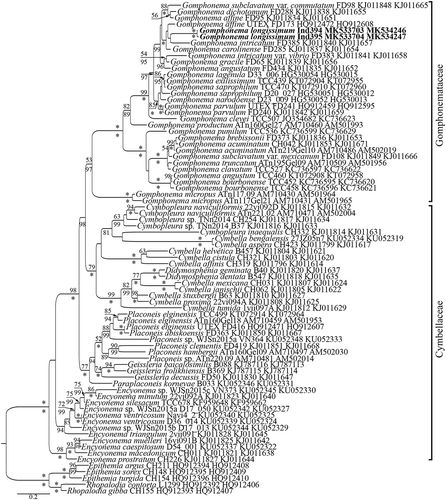
The obtained sequences were edited manually and assembled using BioEdit v7.1.3, Ridom TraceEdit (ver. 1.1.0) and Mega6 (Hall, Citation1999; Tamura et al., Citation2013). Newly determined sequences and DNA fragments from 76 other diatoms, which were downloaded from GenBank (taxa and accession numbers are given in the tree, ), were included in the alignments. Five species from the Rhopalodiaceae were chosen as the outgroups.
Fig. 1. Bayesian tree of Gomphonema longissimum (indicated in bold) constructed from a concatenated alignment of 78 partial rbcL and partial 18S rDNA sequences of 1771 characters. Values above the horizontal lines (slash) are bootstrap support from RAxML analyses (<50 are not shown); values below the horizontal lines (slash) are Bayesian posterior probabilities (<80 are not shown). All sequences have strain numbers (if available) and GenBank numbers. Species from Rhopalodiaceae were used as an outgroup. Families indicated according to Round et al. (Citation1990) with modification from Kulikovskiy et al. (Citation2016a). * is 100% statistical support.
The nucleotide sequences of the 18S rDNA and rbcL genes were aligned separately using the Mafft v7 software and the E-INS-i model (Katoh & Toh, Citation2010). A final alignment was then carried out: unpaired sites were visually determined and removed from the beginning and the end of the resulting matrices. For the protein-coding sequences of the rbcL gene, we checked that the beginning of the aligned matrix corresponded to the first position of the codon (triplet). The resulting alignments had lengths of 439 (18S rDNA) and 1332 (rbcL) characters.
For each of the alignment partitions, the most appropriate substitution model was estimated using the Bayesian information criterion (BIC) as implemented in jModelTest 2.1.10 (Darriba et al., Citation2012). This BIC-based model selection procedure selected the following models, shape parameter α and a proportion of invariable sites (pinvar): TrN + I + G, α = 0.5830 and pinvar = 0.4870 for 18S rDNA; TPM1uf + I + G, α = 0.3520 and pinvar = 0.7030 for the first codon position of the rbcL gene; JC + I and pinvar = 0.8980 for the second codon position of the rbcL gene; GTR + I + G, α = 1.6380 and pinvar = 0.3310 for the third codon position of the rbcL gene. A phylogenetic tree was inferred by Bayesian inference (BI) using MrBayes version 3.2.7 (Ronquist et al., Citation2012). We used the GTR model of nucleotide substitution instead of TrN and the HKY model instead of TPM1uf, given that they were the best matching model available in MrBayes. All parameters were unlinked among partitions. Three ‘hot’ and one ‘cold’ Markov chains were run for 1×106 cycles in two repetitions with the selection of each 200th generated tree. The first 25% of the trees were discarded; the phylogenetic tree and posterior probabilities of its branching were obtained on the basis of the remaining trees, having stable estimates of the parameter models of nucleotide substitutions and likelihood. Maximum likelihood (ML) analysis was performed using the program RAxML (Stamatakis et al., Citation2008). The non-parametric bootstrap analysis with 1000 replicates was used. Statistical support values were visualized in FigTree v1.4.2 (http://tree.bio.ed.ac.uk/).
Sequence data were deposited as follows: Nuclear-encoded 18S rDNA partial sequences (GenBank accession MK534246 for strain Ind394, MK534247 for strain Ind395), plastid gene rbcL partial sequences (GenBank accession MK533703 for strain Ind394, MK533704 for strain Ind395).
Results
Gomphonema longissimum Hustedt Citation1942. Int. Rev. d. Hydrobiol. 42: 118, 119, figs 261, 262 (–, –)
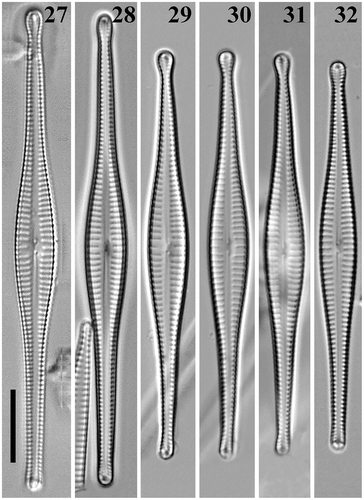
Figs 2–8. Gomphonema longissimum, Lake Matano, Indonesia, LM. Figs 2, 3. G. longissimum, natural population. Figs 4, 5. G. longissimum, strain Ind394. Figs 6–8. G. longissimum, strain Ind395. Scale bars: 10 µm.
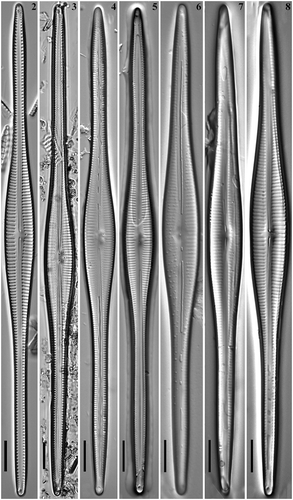
Figs 11–14. Gomphonema longissimum, Lake Matano, Indonesia, SEM, external views. Fig. 11. Whole valve. Fig. 12. Head pole. Fig. 13. Details of valve at centre. Fig. 14. Foot pole with pore field. Scale bars: Fig. 11, 20 µm; Figs 12–14, 1 µm.
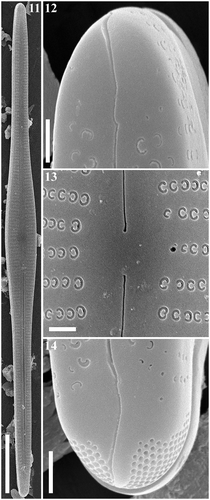
Figs 15–18. Gomphonema longissimum, Lake Matano, Indonesia, SEM, internal views. Fig. 15. Whole valve. Fig. 16. Head pole. Fig. 17. Details of valve at centre. Fig. 18. Foot pole with pore field. Scale bars: Fig. 15, 20 µm; Figs 16–18, 1 µm.
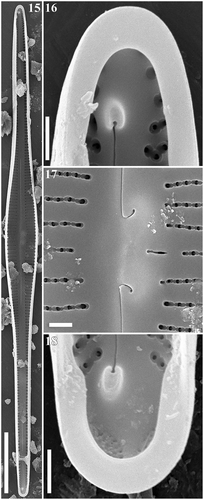
Figs 19–22. Gomphonema longissimum, strain Ind394, Lake Matano, Indonesia, SEM, external views. Fig. 19. Whole valve. Fig. 20. Head pole. Fig. 21. Details of valve at centre. Fig. 22. Foot pole with pore field. Scale bars: Fig. 19, 20 µm; Figs 20–22, 1 µm.
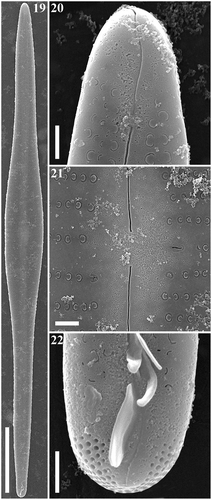
Figs 23–26. Gomphonema longissimum, strain Ind394, Lake Matano, Indonesia, SEM, internal views. Fig. 23. Whole valve. Fig. 24. Head pole. Fig. 25. Details of valve at centre. Fig. 26. Foot pole with pore field. Scale bars: Fig. 23, 20 µm; Figs 24–26, 1 µm.
Lectotype (designated by Simonsen, Citation1987, p. 288, pl. 427, figs 1–7): S2/84. Towoetisee. 60. c. Celebes.
LM: Valves are elongate, narrowly fusiform with cuneate-rounded headpole and slightly rostrate-rounded footpole. Cells are 160–192 µm long and 10–12 µm wide in natural populations and 149.6–199.0 µm long and 12.3–16.5 µm wide in culture. The axial area is narrow, linear; the central area is lanceolate. Striae are parallel becoming slightly radiate toward the poles, 9–10 (natural population) or 8–12 (cultures) in 10 µm at the centre and 10–11 (natural population) or 11–16 (cultures) per 10 µm near the poles. The raphe is straight, filiform. A single small stigma is present at the central area.
SEM: Externally (–, –), striae are uniseriate, composed of c-shaped areolae, occluded by siliceous flaps. In the central area, one isolated stigma with round or elongate opening is positioned close to the striae. The external proximal raphe slits are straight and terminate in very small, drop-like openings which are straight or very slightly deflected. The distal raphe ends are deflected to the secondary side of the valve and extend onto the mantle. At the footpole, an apical pore field is present. Porelli of the apical pore field are physically separate and structurally differentiated from the areolae. Internally (–, –), the proximal raphe ends terminate in small hooks curved towards the primary side of the valve and end in small helictoglossae near the poles. Helictoglossae appear offset to one side from the raphe. The central nodule bears a transversely elongated stigma opening. Pseudosepta are visible at the both poles. At the footpole, porelli of the APF are occluded.
Hustedt (Citation1942) mentioned the size range for this taxon to be 180–313 µm long and 13–15 µm wide.
Molecular investigation: We isolated two strains of G. longissimum, namely Ind394 and Ind395, from Matano Lake. These strains grouped in a unified branch in the molecular tree, closely related to G. intricatum and G. affine from GenBank (). The two strains were virtually identical genetically, but they differed slightly from one another morphologically and morphometrically. This clade was identical in the tree built on the basis of the concatenated 18S rDNA and rbcL gene sequence data, and in the individual trees for 18S DNA (Supplementary fig. 1) rDNA and the rbcL gene separately (Supplementary fig. 2). All highly supported clades in the rbcL–18S rDNA tree corresponded to similar clades in the individual 18S rDNA and rbcL trees. The only exception was the position of G. micropus Kützing strains ATn117.09 and ATn117Gel21 in the 18S rDNA tree; this was shown to be part of a monophyletic group with Gomphonema species in the rbcL–18S rDNA and rbcL trees. It should be noted that the positions of Encyonema triangulum (Ehrenberg) Kützing strain 2vii091, G. affine strain UTEX FD173, G. carolinense Hagelstein strain FD285 and Placoneis sp. strain ATn220.09 varied among the trees, with low statistical support in all trees.
Gomphonema angustissimum Hustedt Citation1942. Int. Rev. d. Hydrobiol. 42: 116, figs 248, 249 ()
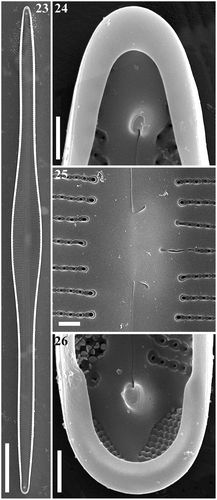
Figs 33–36. Gomphonema subtiliforme, Lake Matano, Indonesia, SEM, external views. Fig. 33. Whole valve. Fig. 34. Head pole. Fig. 35. Details of valve at centre. Fig. 36. Foot pole with pore field. Scale bars: Fig. 33, 20 µm; Figs 34–36, 1 µm.
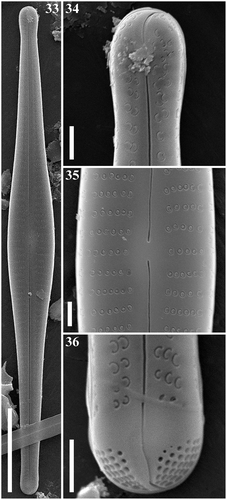
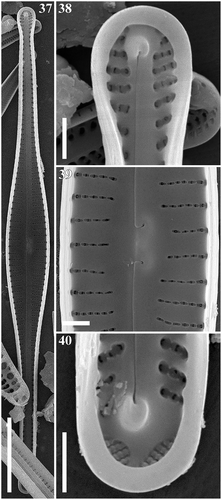
Figs 37–40. Gomphonema subtiliforme, Lake Matano, Indonesia, SEM, internal views. Fig. 37. Whole valve. Fig. 38. Head pole. Fig. 39. Details of valve at centre. Fig. 40. Foot pole with pore field. Scale bars: Fig. 37, µm; Figs 38–40, µm.
Lectotype (designated by Simonsen, Citation1987, p. 288, pl. 425, figs 5, 6): S2/83. Celebes. 68. Mahalone-See.
LM: Valves are elongate, narrowly lanceolate with cuneate-rounded headpole and footpole. Cells are 88–98 µm long and 6.0–6.5 µm wide. The axial area is narrow, linear; the central area is small circular. Striae are parallel becoming slightly radiate toward the poles, 12–14 in 10 µm at the centre and 17–18 in 10 µm near the poles. The raphe is straight, filiform. A single small stigma is present at the central area.
We observed only two valves under LM. According to Hustedt (Citation1942), the valves of this species are about 78–100 µm long and 6–7 µm wide; the striae have a density of 14–18 in 10 µm at the centre.
Gomphonema subtiliforme Hustedt Citation1942. Int. Rev. d. Hydrobiol. 42: 119, figs 250, 251 (–)
Lectotype (designated by Simonsen Citation1987, p. 288, 289, pl. 428, figs 8–10): S1/23. Celebes. 64. Matano-See.
LM: Valves are elongate, narrowly fusiform with capitate headpole and rounded to subcapitate footpole. Cells are 54.0–67.5 µm long and 5.0–5.8 µm wide. The axial area is narrow, linear; the central area is lanceolate. Striae are parallel becoming slightly radiate toward the poles, 11–14 in 10 µm at the centre and 15–17 in 10 µm near the poles. The raphe is straight, filiform. Stigma is absent.
SEM: Externally (–), striae are uniseriate, composed of c-shaped areolae, occluded by siliceous flaps (volae). The external raphe slits are straight and terminate proximally in very small, drop-like openings which are straight or very slightly deflected. The distal raphe ends are deflected to the secondary side of the valve and extend onto the mantle. At the footpole, the apical pore field is present. Porelli of the apical pore field are physically separate and structurally differentiated from the areolae. Internally (–), the proximal raphe ends terminating in small hooks curved towards the primary side of the valve and ending in small helictoglossae near the poles. The central nodule bears a transversely elongated stigma opening. Pseudosepta are visible at both poles. At the footpole, porelli of the APF are occluded.
According to Hustedt (Citation1942), the valves of this species are about 58 µm long and 5–6 µm wide; the striae have a density of 12–14 in 10 µm at the centre and about 16 in 10 µm near the poles.
Gomphonema woltereckii Hustedt Citation1942. Int. Rev. d. Hydrobiol. 42: 116, 118, figs 246, 247, 264, 265 (–)
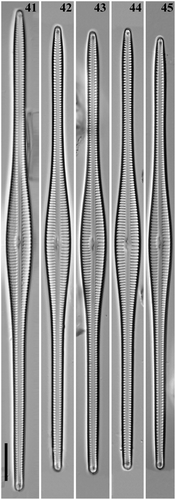
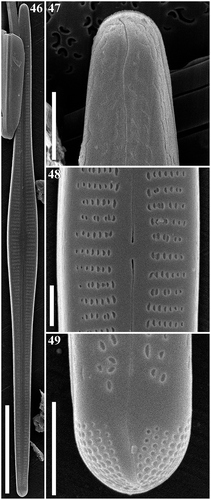
Figs 46–49. Gomphonema woltereckii, Lake Matano, Indonesia, SEM, external views. Fig. 46. Whole valve. Fig. 47. Head pole. Fig. 48. Details of valve at centre. Fig. 49. Foot pole with pore field. Scale bars: Fig. 46, 20 µm; Figs 47–49, 1 µm.
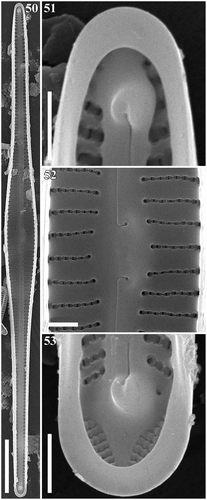
Figs 50–53. Gomphonema woltereckii, Lake Matano, Indonesia, SEM, internal views. Fig. 50. Whole valve. Fig. 51. Head pole. Fig. 52. Details of valve at centre. Fig. 53. Foot pole with pore field. Scale bars: Fig. 50, 20 µm; Figs 51–53, 1 µm.
Lectotype (designated by Simonsen, Citation1987, p. 288, pl. 426, figs 1–8): S1/24. Celebes. 64. Matano-See.
LM: Valves are elongate, narrowly fusiform with cuneate rounded head pole and rounded foot pole. Cells are 98–137 µm long and 6.0–8.3 µm wide. The axial area is narrow, linear; the central area is not differentiated. Striae are parallel becoming slightly radiate toward the poles, 11–12 in 10 µm at the centre and 13–17 in 10 µm near the poles. The raphe is straight, filiform. A stigma is absent at the central area.
SEM: Externally (–), striae are uniseriate, composed of slit-like apically elongate areolae which are unoccluded. The striae extend onto the mantle. The external raphe slits are straight and terminate proximally in very small, drop-like openings which are straight or very slightly deflected. The distal raphe ends are deflected to the secondary side of the valve and extend onto the mantle. At the footpole, the apical pore field (APF) is present. Porelli of the apical pore field are present on the valve face and mantle, and are physically separate and structurally differentiated from the areolae. Internally (–), the proximal raphe ends terminating in small hooks curved towards the primary side of the valve and ending in small helictoglossae near the poles. Pseudosepta are visible at the both poles. At the footpole, porelli of the APF are occluded.
Discussion
Based on our observations presented here, we attempt to differentiate four species of Gomphonema from one another on the basis of morphological data, and to understand the relationship of G. longissimum within the genus on the basis of molecular data. The taxon most dissimilar to the others is G. subtiliforme. Unlike the other three species considered, G. subtiliforme has medium-sized valves characterized by the distinct capitate headpole sitting on a narrow ‘neck’. This diatom is morphologically similar to a group of species akin to G. subtile Ehrenberg and its allies. This species complex was studied by Reichardt (Citation2015) who made observations on G. subtile and closely related species such as G. pseudosubtile Reichardt, G. sagitta Schumann, G. cathedrale Lange-Bertalot & Reichardt, G. maclaughlinii Reichardt, G. subsagitta Reichardt and G. pantropicum Reichardt. After this investigation, Reichardt (Citation2015) concluded that ‘G. subtiliforme Hust. is a much more slender diatom and in spite of its name unmistakable compared to forms described in this paper’. The first SEM investigation of G. subtiliforme is provided herein. This species is 5.0 to 5.8 µm wide and is not noticeably more slender than others in the G. subtile group, which vary from 5 to 10 µm (Reichardt, Citation2015). G. subtiliforme shares morphological features with other species of the G. subtile group, such as C-shaped areolae, raphe morphology with internal raphe endings that are long and curved, and a bilobed apical pore field. More precisely, the striae of G. subtiliforme are small inside, round with reniform stalked flaps located in shallow foraminal rows. However, G. subtiliforme lacks a stigma, and that feature distinguishes it from other members of the G. subtile species complex (Glushchenko et al., Citation2017).
Glushchenko et al. (Citation2017) studied the morphology of G. pantropicum from Southeast Asia and offered the description of two new species from the G. subtile group, G. dalatica Glushchenko, Kulikovskiy & Kociolek and G. prowsei Glushchenko, Kulikovskiy & Kociolek. These species fully correspond to the G. subtile group in terms of having similar morphology, and differ from G. subtiliforme by each having a stigma.
G. longissimum is a unique large species about 200 µm in length and has obvious narrow valves with cuneate ends. Here, we offer the first SEM observations of this species. G. longissimum is characterized by features that are typical for Gomphonema, including the presence of C-shaped areolae that are occluded by large reniform semi-lunar siliceous flaps connected to the valve surface by a narrow strut, filiform raphe with straight ends externally and hooked ends internally. The raphe divides the pore field into two groups of porelli. This species differs from the previously discussed G. subtiliforme by the presence of a stigma. The stigma opening externally is small and rounded, while internally the stigma is an elongated slit located in a long groove.
A stigma is present in both G. longissimum and G. angustissimum. Since G. angustissimum is a rather rare species (we observed just two valves), it is still unknown whether G. longissimum and G. angustissimum are conspecific or not. We have not ruled out that G. angustissimum represents nothing more than smaller valves of G. longissimum. In our investigation of cultured specimens of G. longissimum, the strain Ind394 corresponds well with Hustedt’s original description and our observations of specimens from a natural population of this species. Its valves are 197–199 µm long and 12.4–13.3 µm wide; the striae density is 9–10 in 10 µm at the centre and about 11–13 in 10 µm near the poles. The strain Ind395 has transapically asymmetrical, narrowly lanceolate valves rather than the fusiform ones of strain Ind394. Valves of the strain Ind395 are shorter and wider than the dimensions mentioned in Hustedt’s original description and our measurements from natural populations (see ); stria density is 8–12 in 10 µm at the centre and about 11–16 in 10 µm near the poles. Strain Ind395 is more similar to G. angustissimum than to G. longissimum. In strain Ind395 we found valves intermediate between G. longissimum and G. angustissimum. However, no molecular difference was observed between strains Ind395 and Ind394, leading us to conclude that specimens of G. longissimum and G. angutissimum may represent a single species.
Table 1. Morphometric comparison of Gomphonema longissimum and related taxa
G. woltereckii is almost identical in shape to G. angustissimum and G. longissimum but shorter than G. longissimum and is distinguished from both by the absence of a stigma. Hustedt (Citation1942) noted the absence of a stigma for this species in his original description, and this was confirmed here in the first published SEM images of this taxon. G. woltereckii differs from G. longissimum and G. subtiliforme in the structure of the external areolae. Uniseriate striae of G. woltereckii are formed by areolae partially occluded to a variable degree. At the headpole, areolae are almost slit-like, while in the central part of the valve and at the footpole they are semi-circular in shape.
Comparison of the four species with more or less fusiform valves described by Hustedt (Citation1942) from the ancient Malili lake system in Indonesia shows that these species differ with respect to some aspects of their morphology. It is possible to divide these species into two groups on the basis of presence or absence of a stigma, with G. subtiliforme and G. woltereckii having no stigma and G. angustissimum and G. longissimum possessing a stigma. On the other hand, G. woltereckii differs from the other three species by having both slit-like and semi-circular areolae. Presence or absence of stigmata, and the number of stigmata, are possibly constant features for taxa at the species level in the genera Gomphonema, Gomphoneis Cleve and Gomphosinica Kociolek, You, Wang & Liu; some members of the family Gomphonemataceae Kützing and the genus Reimeria Kociolek & Stoermer; and all members of the order Cymbellales D.G. Mann (Kociolek et al., Citation2013, Citation2015; Kulikovskiy et al., Citation2016a; Levkov et al., Citation2016). In the closely related family Cymbellaceae Greville, species are known to both possess and lack stigmata (Krammer, Citation2002; Kulikovskiy et al., Citation2016a). In these large Gomphonema species from Indonesia, it is possible that differences in areolar structure and the presence of a stigma may be better indicators of relationships than fusiform valve shape. If so, then these large, fusiform species may belong to different evolutionary lines, having evolved from different ancestors in this ancient ecosystem.
Ancient lakes are refugia for many organisms and have high numbers of endemic species and high species diversity (von Rintelen et al., Citation2014). The presence of fewer than 10 known Gomphonema species in Malili lakes is interesting (Hustedt, Citation1942; Bramburger et al., Citation2004, Citation2008). The low numbers of Gomphonema species from Malili lakes are correlated with the small number of diatom species in the lakes in general. Bramburger et al. (Citation2004) published a list of diatom species from Malili lakes that included just 256 taxa. This number is much lower than Lake Baikal, as we estimate diversity at about 1500 diatom species in that ancient lake. Of course, we cannot exclude the possibility that more Gomphonema species will be found in Lake Malili in the future. For example, our investigation of samples from Malili lakes allowed us to describe a new, large species, Gomphonema matanensis Kapustin, Kociolek & Kulikovskiy (Kociolek et al., Citation2018a). The same situation occurred with a more comprehensive investigation of Surirella Turpin species, when 11 additional species new to science were described (Bramburger et al., Citation2006).
A possible explanation for the small number of Gomphonema in Malili lakes in comparison with, for example, Lake Baikal, is that there is a very smooth, clayey bottom (inorganic particulate matter sensu Bramburger et al., Citation2006) in the Indonesian lakes, whilst the shore of Lake Baikal is characterized by stones. In Baikal, species of Gomphonema occur attached to stones along with Gomphoneis and Cymbella, two genera that are also very abundant and common in Lake Baikal (Kulikovskiy et al., Citation2012a; Kociolek et al., Citation2013). This surface structure may also explain why some genera appear to have radiated in the Malili lake system while others have not. A large number of species of Surirella in Malili lakes was shown by Bramburger et al. (Citation2006), who connected the morphological evolution of Surirella with the substratum and life in inorganic particulate matter. Some species of Surirella in Malili lakes have siliceous tendrils that they use for attachment to substrata, and the evolutionary development of attachment features contributes to the high diversity of Surirella (Bramburger et al., Citation2006). The large fusiform Gomphonema species do not appear to have unique combinations of morphological characteristics to help them to live in the inorganic particulate matter that covers the shore of the lakes. We can speculate that there was an absence of special morphological features in Gomphonema species that colonized this lake system not so long ago from surrounding rivers, a phenomenon shown for other organisms too (Poettinger & Schubart, Citation2014).
From a biogeographic point of view, any trends related to Gomphonema species may be masked owing to the large size of the genus, the taxonomic lumping that is common in hard-to-identify groups and the lack of phylogenetic analyses of species groupings. G. longissimum and related taxa discussed here are rare species which may result from absence of appropriate ecological niches in the Malili lake system or from the increase in anthropogenic influences, with elevated metal concentrations and a paucity of nutrients in Lake Matano’s water column (Bramburger et al., Citation2008). Bramburger et al. (Citation2014) studied the ecology of a littoral epilithic diatom community of the ancient Lake Matano and showed that species such as Achnanthidium minutissumum var. gracillima (Meister) Bukhtiyarova, Brachysira longirostris (Hustedt) Mann in Round, Crawford & Mann and Gomphonema angustissimum Hustedt are relatively weak competitors regardless of environmental conditions in comparison with some other taxa.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2019.1664771
Supplementary fig. 1. Bayesian tree of Gomphonema longissimum (indicated in bold) constructed from a concatenated alignment of 78 partial 18S rDNA sequences of 439 characters. Values above the horizontal lines are bootstrap support from RAxML analyses (<50 are not shown); values below the horizontal lines are Bayesian posterior probabilities (<80 are not shown). All sequences have strain numbers (if available) and GenBank numbers. Species from Rhopalodiaceae were used as an outgroup. Families indicated according to Round et al. (Citation1990) with modification from Kulikovskiy et al. (Citation2016a). * is 100% statistical support.
Supplementary fig. 2. Bayesian tree of Gomphonema longissimum (indicated in bold) constructed from a concatenated alignment of 78 partial rbcL sequences of 1332 characters. Values above the horizontal lines are bootstrap support from RAxML analyses (<50 are not shown); values below the horizontal lines are Bayesian posterior probabilities (<80 are not shown). All sequences have strain numbers (if available) and GenBank numbers. Species from Rhopalodiaceae were used as an outgroup. Families indicated according to Round et al. (Citation1990) with modification from Kulikovskiy et al. (Citation2016a). * is 100% statistical support.
Author contributions
M. Kulikovskiy: original concept, drafting manuscript; D. Kapustin: wrote manuscript and performed LM and SEM observations; A. Glushchenko: editing of manuscript, creation of figures; S. Sidelev and E. Gusev: molecular investigation of strains; Y. Maltsev: molecular investigation of strains and creating of phylogenetic tree; E. Kezlya, N. Shkurina and I. Kuznetsova: morphological investigation of samples; P. Kociolek: wrote discussion, critical reading, revision and editing of manuscript.
Additional information
Funding
References
- Amossé, A. (1969). Note sur des Diatomées récoltées en Indochine. Revue Algologique, Nouvelle Série, 9: 326‒344.
- Bramburger, A.J., Haffner, G.D. & Hamilton, P.B. (2004). Examining the distributional patterns of the diatom flora of the Malili Lakes, Sulawesi, Indonesia. In Proceedings of the 17th International Diatom Symposium (Poulin, M., editor), 11–25. Biopress, Bristol.
- Bramburger, A.J., Haffner, G.D., Hamilton, P.B., Hinz, F. & Hehanussa, P.E. (2006). An examination of species within the genus Surirella from the Malili Lakes, Sulawesi Island, Indonesia, with descriptions of 11 new taxa. Diatom Research, 21: 1–56.
- Bramburger, A.J., Hamilton, P.B., Hehanussa, P.E. & Haffner, G.D. (2008). Processes regulating the community composition and relative abundance of taxa in the diatom communities of the Malili Lakes, Sulawesi Island, Indonesia. Hydrobiologia, 615: 215–224.
- Bramburger, A.J., Hamilton, P.B. & Haffner, G.D. (2014). Effects of a simulated upwelling event on the littoral epilithic diatom community of an ancient tropical lake (Lake Matano, Sulawesi Island, Indonesia). Hydrobiologia, 739: 133–143.
- Darriba, D., Taboada, G.L., Doallo, R. & Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9: 772.
- Eastman, J.T. & McCune, A.R. (2000). Fishes on the Antarctic continental shelf: evolution of a marine species flock? Journal of Fish Biology, 57: 84–102.
- Genkal, S.I., Kulikovskiy, M.S. & Kuznetsova, I.V. (2013). New data on Centrophyceae (Bacillariophyta) of Lake Baikal, Russia. International Journal on Algae, 15: 50–64.
- Glaubrecht, M. & Rintelen, T.v. (2008). The species flocks of lacustrine gastropods: Tylomelania on Sulawesi as models in speciation and adaptive radiation. Hydrobiologia, 615: 181–199.
- Glushchenko, A.M., Kulikovskiy, M.S. & Kociolek, J.P. (2017). New diatom species from Gomphonema subtile-group in Southeast Asia. Phytotaxa, 329: 223‒232.
- Greenwood, P.H. (1974). The haplochromine fishes of Lake Victoria, East Africa: the biology and evolution of a species flock. Bulletin of the British Museum of Natural History Zoology, 6: 1‒134.
- Greenwood, P.H. (1984). African cichlids and evolutionary theories. In Evolution of Fish Species Flocks (Echelle, A.A. & Kornfield, I., editors), 93‒110. University of Maine, Orono.
- Guillard, R.R.L. & Lorenzen, C.J. (1972). Yellow-green algae with chlorophyllide C. Journal of Phycology, 8: 10‒14.
- Haffner, G., Hehanussa, P.E. & Hartoto, D. (2001). The biology and physical processes of large lakes of Indonesia: Lakes Matano and Towuti. In The Great Lakes of the World. Food-web, Health and Integrity (Munawar, M. & Hecky, R.E., editors), 183‒192. Backhuys Publishers, Leiden.
- Hall, T.A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41: 95–98.
- Hamsher, S.E., Graeff, C.L., Stepanek, J.G. & Kociolek, J.P. (2014). Frustular morphology and polyphyly in freshwater Denticula (Bacillariophyceae) species, and the description of Tetralunata gen. nov. (Epithemiaceae, Rhopalodiales). Plant Ecology and Evolution, 147: 346‒365.
- Herder, F., Nolte, A.W., Pfaender, J., Schwarzer, J., Hadiaty, R.K. & Schliewen, U.K. (2006). Adaptive radiation and hybridization in Wallace’s Dreamponds: evidence from sailfin silversides in the Malili Lakes of Sulawesi. Proceedings of the Royal Society B: Biological Sciences, 273: 2209‒2217.
- Herder, F., Pfaender, J. & Schliewen, U.K. (2008). Adaptive sympatric speciation of polychromatic “roundfin” sailfin silverside fish in Lake Matano (Sulawesi). Evolution, 62: 2178‒2195.
- Hustedt, F. (1942). Süßwasser-Diatomeen des indomalayischen Archipels und der Hawaii-Inseln. Int. Rev. Gesamten Hydrobiol. Hydrogr., 42: 1–252.
- Kapustin, D.A. & Kulikovskiy, M.S. (2018). Transfer of Stenopterobia and Surirella taxa (Bacillariophyceae) described from the insular Southeast Asia to the genus Iconella. Nova Hedwigia, Beiheft, 147: 237–245.
- Kapustin, D.A., Kulikovskiy, M.S. & Kociolek, J.P. (2017). Celebesia gen. nov., a new cymbelloid diatom genus from the ancient Lake Matano (Sulawesi Island, Indonesia). Nova Hedwigia, Beiheft, 146: 147‒155.
- Katoh, K. & Toh, H. (2010). Parallelization of the MAFFT multiple sequence alignment program. Bioinformatics, 26: 1899–1900.
- Katsev, S., Crowe, S.A., Mucci, A., Sundby, B., Nomosatryo, S., Haffner, G.D. & Fowle, D.A. (2010). Mixing and its effects on biogeochemistry in the persistently stratified, deep, tropical Lake Matano, Indonesia. Limnology and Oceanography, 55: 763–776.
- Kociolek, J.P. & Stoermer, E.F. (1993). Freshwater gomphonemoid diatom phylogeny: preliminary results. Hydrobiologia, 269/270: 31–38.
- Kociolek, J.P., Balasubramanian, K., Blanco, S., Coste, M., Ector, L., Liu, Y., Kulikovskiy, M., Lundholm, N., Ludwig, T., Potapova, M., Rimet, F., Sabbe, K., Sala, S., Sar, E., Taylor, J., Van de Vijver, B., Wetzel, C.E., Williams, D.M., Witkowski, A. & Witkowski, J. (2019). DiatomBase. http://www.diatombase.org on 2019- 06-15.
- Kociolek, J.P., Kulikovskiy, M.S. & Solak, C.N. (2013). The diatom genus Gomphoneis Cleve (Bacillariophyceae) from Lake Baikal, Russia. Phytotaxa, 154: 1‒37.
- Kociolek, J.P., Glushchenko, A.M. & Kulikovskiy, M.S. (2015). Typification, valve ultrastructure, and systematic position of Gomphonema gomphopleuroides Amossé ex Kociolek, Glushchenko & Kulikovskiy, an endemic diatom from Southeast Asia. Diatom Research, 30: 247‒255.
- Kociolek, J.P., Kapustin, D.A. & Kulikovskiy, M.S. (2018a). A new, large species of Gomphonema Ehrenberg from ancient Lake Matano, Indonesia. Diatom Research, 33: 241–250.
- Kociolek, J.P., Kulikovskiy M.S., Kuznetsova, I.V., Glushchenko, A.M. & Solak, C.N. (2018b). A putative species flock in the diatom genus Gomphonema Ehrenberg (Bacillariophyta: Gomphonemataceae) from Lake Baikal, Russia: description of six new species similar to G. ventricosum W. Gregory. Cryptogamie, Algologie, 39: 365–388.
- Kociolek, J.P., Woodward, J. & Graeff, C. (2016). New and endemic Gomphonema C.G. Ehrenberg (Bacillariophyceae) species from Hawaii. Nova Hedwigia, 102: 141‒171.
- Kottelat, M. (1991). Sailfin silversides (Pisces: Telmatherinidae) of Lake Matano, Sulawesi, Indonesia, with descriptions of six new species. Ichthyological Exploration of Freshwaters, 1: 321‒344.
- Krammer, K. (2002). Cymbella. Diatoms of Europe, 3: 1‒584.
- Kulikovskiy, M.S. & Kociolek, J.P. (2014). The diatom genus Gomphonema Ehrenberg in Lake Baikal. I. Morphology and taxonomic history of two endemic species. Nova Hedwigia, Beiheft, 143: 507‒518.
- Kulikovskiy, M.S. & Kuznetsova, I.V. (2016). Morphology, taxonomical position, and distribution of the genera of diatoms Ochigma and Khursevichia from Lake Baikal. Inland Water Biology, 9: 226–233.
- Kulikovskiy, M.S., Lange-Bertalot, H., Witkowski, A. & Khursevich, G.K. (2011). Achnanthidium sibiricum (Bacillariophyta), a new species from bottom sediments in Lake Baikal. Algological Studies, 136/137: 77‒87.
- Kulikovskiy, M.S., Lange-Bertalot, H., Metzeltin, D. & Witkowski, A. (2012a). Lake Baikal: hotspot of endemic diatoms I. Iconographia Diatomologica, 23: 7‒608.
- Kulikovskiy, M.S., Witkowski, A. & Khursevich, G.K. (2012b). Encyonema horstii sp. nov., a species of unusual valve outline from Pleistocene deposits of Lake Baikal. Nova Hedwigia, Beiheft, 141: 365–374.
- Kulikovskiy, M.S., Lange-Bertalot, H. & Witkowski, A. (2013). Gliwiczia gen. nov. a new monoraphid diatom genus from Lake Baikal with a description of four species new for science. Phytotaxa, 109: 1‒16.
- Kulikovskiy, M.S., Lange-Bertalot, H., Witkowski, A. & Kuznetsova, I.V. (2014). Description of four species belonging in Cavinula D.G. Mann & Stickle from Lake Baikal with notes on family Cavinulaceae D.G. Mann in Round et al. 1990. Nova Hedwigia, 99: 487–499.
- Kulikovskiy, M.S., Kociolek, J.P., Solak, C.N. & Kuznetsova, I.V. (2015a). The diatom genus Gomphonema Ehrenberg in Lake Baikal. II. Revision of taxa from Gomphonema acuminatum and Gomphonema truncatum-capitatum complexes. Phytotaxa, 233: 251‒272.
- Kulikovskiy, M.S., Lange-Bertalot, H. & Kuznetsova, I.V. (2015b). Lake Baikal: hotspot of endemic diatoms II. Iconographia Diatomologica, 26: 1‒657.
- Kulikovskiy, M.S., Lange-Bertalot, H., Kuznetsova, I.V. & Khursevich, G.K. (2015c). Three new species of Eolimna Lange-Bertalot & Schiller (Bacillariophyta) from Lake Baikal. Nova Hedwigia, Beiheft, 144: 199‒209.
- Kulikovskiy, M.S., Lange-Bertalot, H., Witkowski, A., Khursevich, G.K. & Kociolek, J.P. (2015d). New species of Eunotia (Bacillariophyta) from Lake Baikal with comments on morphology and biogeography of the genus. Phycologia, 54: 248‒260.
- Kulikovskiy, M.S., Lange-Bertalot, H., Witkowski, A., Khursevich, G.K. & Kuznetsova, I.V. (2015e). Lectotypification of diatoms from Lake Baikal. I. Some species described by A.P. Skabitschewsky. Nova Hedwigia, 100: 215–223.
- Kulikovskiy, M.S., Glushchenko, A.M., Genkal, S.I. & Kuznetsova, I.V. (2016a). Identification Book of Diatoms from Russia. Yaroslavl, Filigran, 1‒804.
- Kulikovskiy, M.S, Lange-Bertalot, H., Annenkova, N.V., Gusev, E.S. & Kociolek, J.P. (2016b). Morphological and molecular evidence support description of two new diatom species from the genus Ulnaria in Lake Baikal. Fottea, 16: 34‒42.
- Kulikovskiy, M.S., Lange-Bertalot, H. & Kuznetsova, I.V. (2016c). Cocconeis nanoburyatica sp. nov. – a new monoraphid diatom species from Lake Baikal. Inland Water Biology, 9: 112‒115.
- Kulikovskiy, M.S., Maltsev, Y., Andreeva, S.A., Glushchenko, A.M., Gusev, E.S., Podunay, Y., Ludwig, T.V., Tusset, E. & Kociolek, J.P. (2019). Description of a new diatom genus Dorofeyukea gen. nov. with remarks on phylogeny of the family Stauroneidaceae. Journal of Phycology, 55: 173–185.
- Levkov, Z., Krstic, S., Metzeltin, D. & Nakov, T. (2007). Diatoms from Lakes Prespa and Ohrid. Iconographia Diatomologica, 16: 1–603.
- Levkov, Z., Mitic-Kopanja, D. & Reichardt, E. (2016). The diatom genus Gomphonema from the Republic of Macedonia. Diatoms of Europe, 8: 1‒552.
- Maltsev, Y.I., Andreeva, S.A., Kulikovskiy, M.S. & Kociolek, J.P. (2017). Molecular phylogeny of the diatom genus Envekadea (Bacillariophyceae, Naviculales). Nova Hedwigia, Beiheft, 146: 241–252.
- Metzeltin, D. & Lange-Bertalot, H. (2007). Tropical diatoms of South America II. Iconographia Diatomologica, 18: 1‒876.
- Poettinger, T. & Schubart, C.D. (2014). Molecular diversity of freshwater crabs from Sulawesi and the sequential colonization of ancient lakes. Hydrobiologia, 739: 73–84.
- Pfaender, J., Schliewen, U.K. & Herder, F. (2010). Phenotypic traits meet patterns of resource use in the radiation of “sharpfin” sailfin silverside fish in Lake Matano. Evolutionary Ecology, 24: 957–974.
- Reichardt, E. (2005). Die Identität von Gomphonema entolejum Østrup (Bacillariophyceae) sowie. Revision ähnlicher Arten mit weiter Axialarea. Nova Hedwigia, 81: 115‒144.
- Reichardt, E. (2015). Taxonomy and distribution of Gomphonema subtile Ehrenberg (Bacillariophyceae) and six related taxa. Fottea, 15: 27‒38.
- Ribbink, A.J. (1984). Is the species flock concept tenable? In Evolution of Fish Species Flocks (Echelle, A.A. & Kornfield, I., editors), 21‒25. University of Maine, Orono.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012). MRBAYES 3.2: Efficient Bayesian phylogenetic inference and model selection across a large model space. Systematic Biology, 61: 539–542.
- Round, F.E., Crawford, R.M. & Mann, D.G. (1990). The Diatoms. Biology and Morphology of the Genera. Cambridge: Cambridge University Press, 1–747.
- Roy, D., Docker, M.F., Haffner, G.D. & Heath, D.D., 2007. Body shape vs. colour associated initial divergence in the Telmatherina radiation in Lake Matano, Sulawesi, Indonesia. Journal of Evolutionary Biology, 20: 1126‒1137.
- Ruck, E.C. & Theriot, E.C. (2011). Origin and evolution of the canal raphe system in diatoms. Protist, 162: 723–737.
- Schluter, D. (1996). Adaptive radiation along genetic lines of least resistance. Evolution, 50: 1766‒1774.
- Schmidt, A. et al. (1874–1959). Atlas der Diatomaceenkunde. Aschersleben, Leipzig, Heft 1–120, Tafeln 1–460.
- Schubart, C.D., Santl, T. & Koller, P. (2008). Mitochondrial patterns of intra- and interspecific differentiation among endemic freshwater crabs of ancient lakes in Sulawesi. Contributions to Zoology, 77: 83‒90.
- Simonsen, R. (1987). Atlas and Catalogue of the Diatom Types of Friedrich Hustedt. Volume 3. J. Cramer, Berlin-Stuttgart, pp. 1‒620.
- Stamatakis, A., Hoover, P. & Rougemont, J. (2008). A rapid bootstrap algorithm for the RAxML web-servers. Systems Biology, 75: 758–771.
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Molecular Biology and Evolution, 30: 2725–2729.
- Vaillant, J., Haffner, G.D. & Cristescu, M.E. (2011). The ancient lakes of Indonesia: towards integrated research on speciation. Integrative and Comparative Biology, 51: 634–643.
- van Bemmelen, R.W. (1970). The Geology of Indonesia. 2nd Edition. Martinus Nijhoff, The Hague.
- Vishnyakov, V.S., Kulikovskiy, M.S., Genkal, S.I. & Kuznetsova, I.V. (2015). Comparative morphological characteristic of diatoms of genus Hannaea Patrick of the two largest lakes of the Baikal rift zone with a description of the new species. Inland Water Biology, 8: 222–231.
- von Rintelen, T., Marwoto, R.M., Haffner, G.D. & Herder, F. (2014). Preface: species research in ancient lakes – classic concepts and new approaches. Hydrobiologia, 739: 1–6.
- Wallace, A.R. (1860). On the zoological geography of the Malay Archipelago. Zoological Journal of the Linnean Society, 4: 172–184.
- Walter, R.P., Haffner, G.D. & Heath, D.D. (2009). Dispersal and population genetic structure of Telmatherina antoniae, an endemic freshwater Sailfin silverside from Sulawesi, Indonesia. Journal of Evolutionary Biology, 22: 314–323.
- Zimmermann, J., Jahn, R. & Gemeinholzer, B. (2011). Barcoding diatoms: evaluation of the V4 subregion on the 18S rRNA gene, including new primers and protocols. Organisms Diversity & Evolution, 11: 173–192.