ABSTRACT
Phytoplankton are present in a large variety of aquatic environments, ranging from small freshwater ponds to the oceans. Typically, freshwater and marine species are not closely related, indicating an ancient divergence and that salinity poses a strong dispersal barrier. Here we reveal a common recent origin of two dinoflagellates that are well adapted to different habitats. Gymnodinium baicalense inhabits the geologically old freshwater Lake Baikal, which is located in the middle of the Eurasian continent. Gymnodinium corollarium, on the other hand, is a brackish water species from the Baltic Sea. Both species form blooms under ice during the spring. We generated 10 DNA sequences from these species. The partial 28S rRNA gene from G. baicalense and the partial 18S rRNA gene and internal transcribed spacer-2 (ITS2) from G. corollarium were sequenced for the first time. A more detailed description of G. baicalense, which was previously known only from light microscopy observations, is also provided. In the laboratory we demonstrated that G. baicalense is strictly adapted to fresh water, while G. corollarium had a wide salinity tolerance. However, the two species have almost identical morphology, identical SSU rRNA gene sequences, and only small differences in the LSU rRNA gene and ITS2. We infer a common ancestor, which was a species from the Arctic region with a tolerance to a range of salinities. Our data support the scenario that the under-ice phytoplankton community in Lake Baikal has been formed recently, even though the lake is the oldest in the world.
Introduction
Protists (including microalgae) play a key role in many ecosystems, acting as primary producers, predators or parasites. Over the past few decades, their divergence into different ecological niches and their evolutionary relationships have been revealed with molecular tools. For instance, it has been shown for various groups of protists that marine and freshwater species were separated a long time ago and that the osmotic barrier prevents frequent transitions between marine and freshwater habitats (Logares et al., Citation2007, Citation2009). However, more recent studies suggest that this barrier is not as strict as the earlier works indicated, and groups of various protists that seemed to be exclusively marine can be found in fresh waters (e.g. Alverson et al., Citation2011; Simon et al., Citation2015). Kretschmann et al. (Citation2015) reported that the freshwater dinoflagellate Gymnodinium limneticum could survive for five weeks in 17 psu in the laboratory. Based on the uncorrelated molecular clock method, Žerdoner Čalasan et al. (Citation2019) suggested that marine-to-fresh water transitions within dinoflagellates have occurred several times independently at different points in time. Thus it appears that protists, in particular dinoflagellates, are able to adapt to new conditions faster and more frequently than expected. Because of the short reproduction time and large population sizes of free-living protists, the existence of a high level of genetic variability is likely (e.g. Rengefors et al., Citation2017). Consequently, some members of the population may be capable of thriving in the new conditions to which they are dispersed (e.g. Hairston et al., Citation1999; Logares et al., Citation2009). This hypothesis is corroborated by recent experimental work. For instance, several experimental studies on marine microalgae have demonstrated phenotypic adaptation to environmental stressors leading to higher fitness (e.g. Collins & Bell, Citation2004; Collins & de Meaux, Citation2009; Lohbeck et al., Citation2012). In addition, genetic adaptation has been identified in experimental evolution studies (e.g. López-Rodas et al., Citation2008; Lohbeck et al., Citation2012, Citation2013; Schlüter et al., Citation2016).
Despite the potential for rapid adaptation, there are only a handful of examples exploring recent speciation of microalgae in the wild. Annenkova et al. (Citation2018) showed a recent speciation event between the dinoflagellate species Apocalathium aciculiferum and Apocalathium malmogiense, based on transcriptomic data. These two species were found to be part of an Apocalathium species group, including several marine, brackish and freshwater species of dinoflagellates, which appears to have undergone a recent radiation event (Annenkova et al., Citation2015). All these species are a part of the under-ice spring algal blooms in their respective habitats (Baltic Sea, Arctic Ocean, Antarctic saline lakes and freshwater lakes in the temperate zone). Interestingly, in Lake Baikal three species from the group that co-occur include the morphospecies A. malmogiense, which is known from the Baltic Sea and Arctic Ocean. While the drivers for the divergence and speciation within this genus are unknown, salinity has been postulated as one of the selective agents (Annenkova et al., Citation2015, Citation2018).
Lake Baikal, located in the middle of the Eurasian continent, is one of the most interesting lakes in the world, due to its geological history (the most ancient lake, which has experienced various geological and climate changes during its history) and its biodiversity (it is a diversity hotspot with many endemic species; Kozhova & Izmest’eva, Citation1998). Usually, the lake is covered by ice from late January until early May. Intensive under-ice development of planktonic algae (mainly diatoms and dinoflagellates) plays an important role in the Baikal food-webs (Obolkina et al., Citation2000). Blooms are formed during the ice-covered season by dinoflagellates in the Apocalathium species complex and by Gymnodinium baicalense. The density of G. baicalense may reach 3 × 106 cells l–1 in the 0–5 m layer (Antipova, Citation1974). Annenkova (Citation2013) found that the 18S rRNA gene fragment (1553 bp) of G. baicalense has only two substitutions compared with an environmental DNA sequence from the glacial sample from Kongsfjorden, Svalbard (Arctic Ocean, Norway). There is evidence that the close relative of G. baicalense inhabits northern marine cold waters. We assumed that this relative should also form blooms under ice or in the early spring. The bloom-forming dinoflagellates known from the Arctic Ocean include Polarella glacialis (Montresor et al., Citation2003) and Apocalatium cf. malmogiense (Logares et al., Citation2008); in addition, Biecheleria baltica (Moestrup et al., Citation2009) and Gymnodinium corollarium (Sundström et al., Citation2009) are known as spring bloom-forming species in the Baltic Sea. Of these, we considered G. corollarium as the main candidate to be a close relative of G. baicalense, since both species belong to the same genus. The goal of the current study was to determine the evolutionary relation between G. corollarium and G. baicalense based on new and previously obtained genetic, morphological and physiological data. Their ability to grow at different salinities was tested in the light of the potential role of the salinity barrier in species differentiation. In addition more detailed morphological description of G. baicalense was provided.
Methods
Collection sites
Gymnodinium baicalense dinoflagellate samples were collected during March–May 2011 from the ice-covered Lake Baikal, Russia near Listvyanka (51°51’07.2”N, 104°51’52.2”E) and Bolshie Koty villages (51°54’08.0”N, 105°06’10.8”E). The cells were collected by towing surface water using a plankton net (10 µm mesh size) after drilling holes in the ice. Additionally, samples were taken by a SCUBA diver, who collected visible dinoflagellate patches using a syringe. The strain SCCAP K-0983, from which the type of Gymnodinium corollarium was prepared, was provided by the Scandinavian Culture Collection of Algae and Protozoa (University of Copenhagen, Denmark). SCCAP K-0983 was isolated from the northern Baltic proper in the Baltic Sea, Sweden (Sundström et al., Citation2009). A monoclonal G. baicalense (Lake Baikal) was cultured in MWC+Se modified Woods Hole medium (Guillard & Lorenzen, Citation1972) based on Milli-Q with an addition of Na2SeO3.5H2O (0.008 μM). The monoculture of G. corollarium was cultured in f/2 medium (salinity 11) based on artificial seawater. Both algal cultures were grown non-axenically.
Microscopy and morphological measurements
Gymnodinium baicalense cell length and width were determined from measurements of 131 cells both from field samples and the culture, by using a Nikon Eclipse light microscope at 400× magnification (Tokyo, Japan). A histogram showing the distribution of cells based on their length was built using ggplot2 (Wickham, Citation2016). For SEM, two different fixation protocols were used.
Protocol 1: Cultured material was fixed in a mixture of OsO4 (1.2% final concentration) and HgCl2 (0.2% final concentration). After 20 min, the cells were placed on a poly-L-lysine-coated circular coverslip and washed in distilled water for 15 min. The coverslips were dehydrated in an ethanol series (30%, 50%, 70%, 90%, 96%, 99.9% and 100%), then critical-point dried in a BAL-TEC CPD 030 Critical Point Dryer (Bal-tec, Balzers, Liechtenstein). The coverslips were finally glued to SEM stubs with double-sided adhesive carbon tape, sputter-coated with palladium-platinum and examined in a JSM-6335F field emission scanning microscope (Jeol Ltd, Tokyo, Japan) at the University of Copenhagen, Denmark.
Protocol 2: A drop was taken from a field sample taken during a G. baicalense bloom, and was placed on a coverslip without any fixation. Further manipulations (dehydration, sputter coating) were as described above. The SEM stub was examined in a 525-M scanning microscope (Philips, the Netherlands) at LIN SB RAS, Russia.
Growth in different salinities
A salinity growth experiment was set up to compare salinity tolerance in the two species according to Sundström et al. (Citation2009). Cells of G. corollarium were originally maintained at a salinity of 11, and then 5 ml of the cells were successively transferred into salinities of 7, 4, 1.5 and 0.15, and also to 13 and 15.5. To obtain the lower salinities, medium with a salinity of 11 was diluted with freshwater MWC+Se medium. For higher salinities f/2 medium, prepared based on artificial marine water, was added to the initial medium. Cells of G. baicalense were transferred from MWC+Se medium (salinity 0.15) to salinities 1, 3 and 6 ppt, obtained by adding f/2 medium. When an inoculum culture had entered exponential growth phase at a given salinity, 1 ml of the culture was transferred to 20 ml of the next salinity level. Cultures were allowed to acclimate to the new salinity for 1 week before samples were collected for initial growth measurements. Cells were transferred to the new salinity when cells were in exponential growth after ~4 weeks. Cell counts were continued in the previous salinity until the cultures reached stationary phase. The cell density in each treatment was determined weekly by using a Fuchs-Rosenthal counting chamber and a light microscope at 100× magnification. Prior to sampling, the cultures were gently homogenized, and subsamples (1 ml) were taken and fixed with Lugol’s iodine (1.5%). Each count was based on at least 200 cells. Growth rates were measured as increase in cell numbers and were calculated assuming exponential growth: μ = (ln N1-ln N0)/t1-t0 where N0 and N1 are number of cells at time t0 and t1, and t is the difference in time between samples. Three sampling points were included in the calculation. All experiments were carried out in triplicate at 4°C, light:dark cycle of 12:12, and a light intensity of 25 µmol photons m–2 s–1.
Single-cell PCR and DNA sequencing
Individual cells were isolated from Lake Baikal field samples and from the culture of G. corollarium and photographed using a Nikon Eclipse TS100 microscope at 100× magnification. The selected cells of G. baicalense ranged in size from 34 to 57 µm in length. Each of these cells was prepared for PCR as described previously (Annenkova, Citation2018). Dinoflagellate-specific PCR primers (described in Annenkova et al., Citation2015) were used to amplify the partial 18S and 28S rRNA genes, and the internal transcribed spacer 2 (ITS2). 18S rRNA gene fragments (1179 bp, including the variable V4 domain) were amplified and sequenced for five different cells of G. baicalense. DNA fragments containing partial 5.8S rRNA gene and ITS2 (250 bp) and LSU rRNA gene (1045 bp, including the variable D1–D3 domains) were amplified and sequenced from eight different cells of G. baicalense and two cells of G. corollarium.
PCR reactions were performed using a 2xPCR Master Mix with high-fidelity DNA polymerase (Phusion, Finnzyme) and 0.4 mM of each primer. To amplify the SSU rRNA gene and ITS2-LSU rRNA gene fragments from one cell, multiplex PCR was performed with L1 and Dino1662R and 5L and R28Lo primers simultaneously (see details in Annenkova et al., Citation2015). The total volume of each PCR reaction was analysed by electrophoresis on a 1.5% agarose gel stained with GelRed Nucleic Acid Gel Stain in 0.5× TBE (Tris-Borate-EDTA) buffer. PCR products were excised from the gel and sequenced from both sides using the BigDye system (Perkin Elmer, Waltham, Massachusetts, USA), followed by electrophoresis using an Automated Sequencer (BaseStation, MJ Research).
New DNA sequences were deposited in GenBank under accession numbers MN416309–MN416313 and MN422065–MN422069.
Phylogenetic analyses
The obtained sequences were edited manually and assembled using BioEdit v7.1.3 (Hall, Citation1999). A BLAST search was performed to find related sequences of other dinoflagellates in GenBank. Additionally, corresponding DNA fragments from well-known members of Gymnodiniaceae were included to the alignment for comparison. Separate alignments were constructed for 18S rRNA and 28S RNA genes fragments using Mafft V. 7 based on the L-INS-I model with default parameters (Katoh & Standley, Citation2013). The alignments were manually edited and gaps or ambiguous places were removed. The alignments were subsequently concatenated into one. A Maximum-likelihood (ML) tree was constructed using RAxML 8 (Stamatakis, Citation2014), and bootstrap values were estimated with 1000 replicates under the rapid bootstrapping algorithm. The ML tree was visualized in FigTree v1.4.0 (http://tree.bio.ed.ac.uk/). A Poisson Tree Processes (PTP) model was used to delimit species, because it allows inference of putative species boundaries on a given phylogenetic tree based on DNA sequences without time calibration (Zhang et al., Citation2013). The PTP model was performed on the obtained ML tree using the mPTP tool (Zhang et al., Citation2013). We used 1 000 000 MCMC generations and every 100th generation was collected. Bayesian support values to delimit species on the input tree were shown on the tree.
The ITS2 secondary structures of G. corollarium were predicted by homology modelling using the ITS2 Database (http://its2.bioapps.biozentrum.uni-wuerzburg.de/), with the secondary structure of G. baicalense ITS2 as a template (obtained from Annenkova et al., Citation2009). CBCAnalyzer was used to detect Compensatory Base Changes (CBC) (Wolf et al., Citation2005) based on the alignment of ITS-2 from G. baicalense, G. corollarium and G. aureolum.
Results
Microscopy
The vegetative cells of G. baicalense varied substantially in size, being 32–58 µm in length and 23–39 µm in width (–). The largest cell size corresponded to G. baicalense, and the smallest cell size to G. baicalense var. minor. However, the cells studied did not form separate groups based on their size: cell length was close to normally distributed (Supplementary fig. 1). Cells of different size could be found simultaneously in nature, as well as in culture. The large cells of G. baicalense (–) typically had a conical epicone, often with a pronounced truncated apex, while the hypocone was hemispherical, with a small subtle protrusion that was also apparent in SEM (, ). In small cells both epi- and hypocone were hemispherical, and the small protrusion was not apparent (). The cells were slightly dorsoventrally compressed. The cingulum was median and displaced c. one cingular width. The sulcus was narrow and shallow and had a rather indistinct extension into the epicone and the hypocone (, , ). A pronounced thickening bordered the right side of the sulcus, the so-called ventral ridge (). The amphiesma consisted of numerous polygonal vesicles, occasionally visible in cells prepared for SEM (). A shallow apical groove, lined by a rib with numerous small knobs, together termed an apical structure complex (ASC) sensu Moestrup et al. (Citation2014), ran counter-clockwise around the cell’s apex starting from a mid-ventral position (–, ). The nucleus was spherical to slightly elongated and situated in the right side of the cell at the same level as the cingulum (, ). The chloroplasts were elongated and radiated from the central part of the cell towards the cell margin (, , ). The cyst was thick-walled with a smooth surface and had small indentations indicating the presence of a paracingulum. It was considerably smaller than the large vegetative cells but comparable in size to the small vegetative cells (). Cells of G. corollarium (strain SCCAP K-0983), which were described in Sundström et al. (Citation2009), appeared very similar to the small cells of G. baicalense (–).
Figs 1–10. Gymnodinium species, light microscopy. Figs 1–6. Gymnodinium baicalense. Figs 1–3. Large cells. Note small protrusion (arrow) and the nucleus (n). Fig. 4. Small cell. Fig. 5. Epifluorescence microscopy displaying numerous chloroplasts Fig. 6. Cyst of G. baicalense; paracingulum is marked by arrowheads. Figs 7–9. Cells of G. corollarium from the monoculture. Fig. 10. Epifluorescence microscopy displaying numerous chloroplasts in G. corollarium.
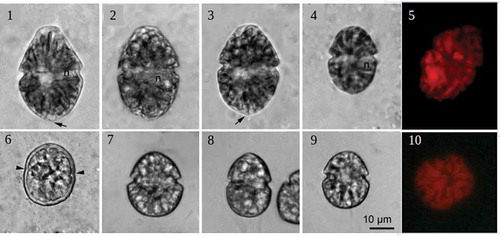
Figs 11–15. Gymnodinium baicalense, SEM. Fig. 11. Ventral view, ASC: apical structure complex, lf: longitudinal flagellum, tf: transverse flagellum, vr: ventral ridge; Fig. 12. left lateral view; Fig. 13. Dorsal view; Fig. 14. Polygonal amphiesmal vesicles (arrows), sulcal extension (arrowhead); Fig. 15. Details of the ASC with small knobs of the rib (arrow)
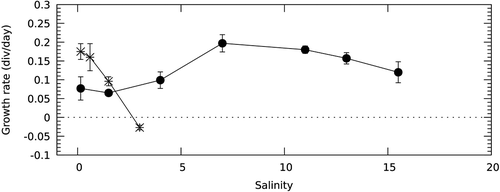
Salinity growth experiment
The salinity growth experiment demonstrated that G. corollarium and G. baicalense responded differently to salinity (). Growth of G. baicalense was reduced by 50% at salinity 1.5 and cells stopped growing and died at salinity 3. On the other hand, G. corollarium had a wide salinity tolerance. It was able to grow in both brackish water (salinity from 1.5–15.5) and freshwater medium. However, we observed that in freshwater medium, the population started to decrease after about two months of cultivation. The highest growth rates (up to μ = 0.21 divisions day–1) were attained at salinity 7. According to Sundström et al. (Citation2009), G. corollarium grows well up to a salinity of 25.
Molecular genetic analysis
The partial 18S rRNA gene sequences from five cells of G. baicalense were identical, as were ITS2 and 28S rRNA gene sequences from eight cells collected near the villages Bolshie Koty and Listvyanka, even though the studied cells varied substantially in size (from 34 to 57 µm in length). The partial 18S rRNA gene (1162 bp) sequenced from one cell of G. corollarium (strain K-0983) was identical to the corresponding fragment from G. baicalense. The ITS2 (340 bp) from the same cell of G. corollarium had 10 substitutions (97% similarity) compared with G. baicalense. The substitutions in the ITS2 fragment corresponded to 3 hemi-CBC substitutions in the ITS2 secondary structure, resulting in a difference in helix IV (Supplementary fig. 2). The partial 28S rRNA gene sequence of G. corollarium strain K-0983 in GenBank compared with the corresponding DNA from G. baicalense (1112 bp in length) showed 16 substitutions i.e. 99% similarity.
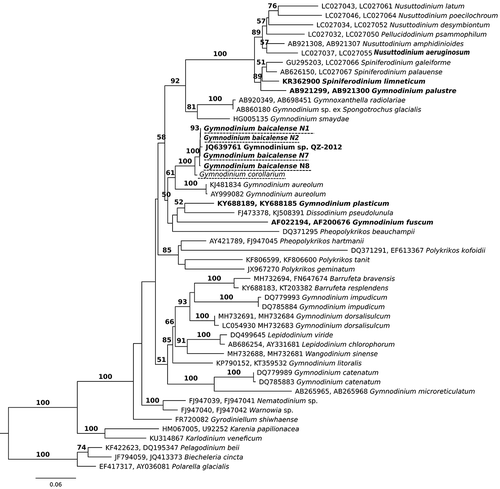
According to BLAST analysis and the phylogenetic tree (), Gymnodinium sp. QZ-2012 from the freshwater Lake Donghu (Wuhan city, China) had the greatest similarity to G. baicalense and G. corollarium. The marine species Gymnodinium aureolum was the most similar species to G. baicalense/Gymnodinium sp. QZ-2012/G. corollarium group (). shows the number of substitutions in the studied DNA markers between these species, based on available data.
Table 1. Nucleotide substitutions in the studied DNA markers of Gymnodinium baicalense in comparison with G. corollarium/Gymnodinium sp. QZ-2012/G. aureolum
Fig. 17. Maximum likelihood tree based on concatenated partial 18S and 28S rRNA genes. Samples sequenced in this study are underlined by a dotted line. Freshwater species are in bold. The numbers on the branches are ML bootstrap values (values <50 are not shown)
Phylogenetic analysis was performed based on concatenated 18S and 28S rRNA gene sequences. Three environmental 18S rRNA gene sequences were not included in the tree, because the corresponding 28S rRNA gene sequences were not available. Two of them belong to uncultured eukaryotes from Arctic Ward Hunt Lake and were identical to 18S rRNA gene sequences of G. baicalense and G. corollarium (1161 bp). The third environmental 18S rRNA gene sequence was from the North Pole sea ice and differed by two substitutions from the studied species.
The general tree topology shows that the genus Gymnodinium is polyphyletic (). Five species from the genus Gymnodinium clustered in a separate clade (ML 51, ) which also included the genera Barrufeta, Lepidodinium and Wangodinium. Another large clade (ML 58, ) contained the genera Nusuttodinium, Spiniferodinium, Pheopolykrikos and Dissodinium, as well as seven species of Gymnodinium (including G. fuscum, the type species of the genus). Within this clade, DNA sequences of G. baicalense, G. corollarium and Gymnodinium sp. QZ-2012 formed one clade with high statistical support (ML 100, ), while G. aureolum was the closest relative to the G. baicalense clade (ML 61, ).
We used the PTP method (Zhang et al., Citation2013) to check how many species could be delineated based on our DNA tree (). The estimated number of species was between 33 and 40 (Supplementary fig. 3). According to this analysis, DNA sequences of G. baicalense, Gymnodinium sp. QZ-2012 and G. corollarium may represent a single species, but without high statistical support (0.82 Bayesian posterior probabilities; the separation of G. baicalense and G. corollarium into two species had only 0.18 Bayesian PP support). Two other closely related species, Lepidodinium chlorophorum and L. viride, were also regarded as separate species with low support (0.55 Bayesian posterior probabilities) or as a single species depending on the program parameters.
Discussion
Salinity has been demonstrated to be a barrier for dispersal in many protists. However, salinity transitions among the Dinophyceae, most of which appear to have taken place during the Neogene (less than 25 million years ago), have been predicted solely based on molecular phylogenetic analyses (e.g. Žerdoner Čalasan et al., Citation2019). Here we present, based on combined data, a case of dinoflagellate transition across the salinity barrier, which took place very recently, probably in the Pleistocene (less than 1 million years ago). Our data provide evidence that Gymnodinium baicalense, a species from freshwater Lake Baikal with narrow salinity requirements, derives from a largely marine ancestor with greater salinity flexibility. Moreover, it is very closely related to the Baltic species Gymnodinium corollarium. Morphological and physiological evidence for species delimitation, as well as for evolutionary relationships to other gymnodinioid dinoflagellates, are discussed below.
Highly similar species from different environments
The species G. baicalense, isolated and described from Lake Baikal, had a very high genetic similarity to the freshwater strain Gymnodinium sp. QZ-2012 from a Chinese eutrophicated lake. Unfortunately, we did not find any published morphological observations of Gymnodinium sp. QZ-2012 and thus can only speculate that they represent the same or a highly similar species.
The next closest relative to G. baicalense proved to be G. corollarium, a cold-water species well adapted to brackish water conditions, which has been found in various parts of the Baltic Sea (Sundström et al., Citation2009). Moreover, we found identical or very similar 18S rRNA gene sequences from environmental studies of Arctic brackish water lakes (Canada) and from North Polar sea ice. This means that G. corollarium-like dinoflagellates may be found in other Arctic regions. In addition to genetic similarity (identical 18S sequence, few differences in ITS2 and 28S RNA sequences), G. baicalense and G. corollarium also have similar morphology. This includes the presence of a horseshoe-shaped apical groove, radiating chloroplasts, the location of the nucleus and similar cysts. In particular, the small cells of G. baicalense appear identical to G. corollarium.
Gymnodinium baicalense was also represented by a subpopulation of larger cells, which we did not observe in the G. corollarium culture. However, our DNA analysis did not detect any difference between the smaller and the larger G. baicalense cells. We propose that larger and smaller cells are different stages of the same population of G. baicalense, since these cells had identical ITS2 sequences and can be found simultaneously in both the monoculture and in nature. In fact, the different cell sizes constituted a continuum (Supplementary fig. 1). Larger cells of G. baicalense differed from the cells of G. corollarium by a subtle antapical protrusion (–; see also Kobanova, Citation2009). This feature may be regarded as a specific morphological feature of G. baicalense, which may be more visible in the ‘large-celled stage’. However, in nature G. corollarium is also variable in size (A. Kremp, pers.comm.), and the presence of the antapical protrusion should be rechecked. Moreover, larger cells of G. baicalense have a differently shaped epicone, often being a truncated cone. A truncated epicone was not included in the original description of G. baicalense by Antipova (Citation1955) and we suggest that this feature may be variable. It has also been observed in some cells of Gymnodinium catenatum and G. nolleri (e.g. cells depicted in Ellegaard & Oshima, Citation1998). Thus, the truncated epicone may be a common feature of gymnodinioids with a horseshoe-shaped apical groove, as the truncation corresponds to the position of the apical groove. The degree of truncation probably varies with the internal osmolarity of the cells and might be associated with specific Lake Baikal conditions (such as stratification, low mineralization, high oxygenation).
The ecology of G. baicalense and G. corollarium is similar. Both proliferate in cold-water environments (between 0°C and 6°C) and their bloom-period occurs during the spring season (often under ice). There is no detailed observation of the G. corollarium life cycle in nature, but a five-month dormancy period has been confirmed for its cysts formed in culture (Parrow & Kremp, Citation2008). G. baicalense is known to be an abundant constituent of the under-ice bloom-forming phytoplankton community (), moreover it is able to develop in the ice interstitial water. Immediately after the ice disappears its bloom declines and cysts are formed.
The question thus arises whether G. baicalense and G. corollarium should be considered as different species, as they have very similar morphology, with the possible exception of the antapical protrusion in large cells of G. baicalense. The PTP model for delimiting species considered them as single species but without high certainty. Molecular genetic markers showed they were close, but not identical. According to Coleman (Citation2009), a single change at the 5′-side of helix III in the ITS2 secondary structure indicates sexual incompatibility between two organisms. In the G. baicalense/G. corollarium case, the only changes present in the secondary structure were observed in helix IV, which is not important for the species delineation (Coleman, Citation2009). This means that the two species have diverged recently and may even be regarded as one species although gene flow between them may be unlikely. Based on the 28S RNA gene, the two species differ by 1.4%. The average difference between other species in the tree () based only on 28S rRNA gene was 16%, with minimum species distance of 2% between Lepidodinium chlorophorum and L. viride. Gymnodinium strains within the same species (G. aureolum, G. dorsalicum, G. catenatum, G. impudicum) differed from each other by 0.2–0.4%. However, similar differences can be found between other closely related species. In particular, the brackish water species Biecheleria baltica and the freshwater species Biecheleria pseudopalustris differ from each other by only 0.7% in the 28S RNA gene (1221 bp) (Moestrup et al., Citation2009). Very close but distinct species of the Apocalathium complex have only 0.2% difference on average in the 28S rRNA partial gene sequence (Annenkova et al., Citation2015). Thus, we conclude that the difference in this marker between G. corollarium and G. baicalense is smaller than among other species of the family, but larger than between strains of marine gymnodinioids, and larger than between some known closely related dinoflagellates.
The separation of the two species is supported by the salinity experiment: the two species clearly differ in salinity tolerance. While G. corollarium grew at a wide interval of salinities in the laboratory and nature, G. baicalense appears to be strictly adapted to fresh water (). The salinity optimum for G. corollarium has earlier been shown to be between 1.5 ppt and 12 ppt (Sundström et al., Citation2009) and the current study supports this (). Investigations of other dinoflagellates from closely situated water reservoirs with different salinity status have shown that salinity may be an important driver of species divergence (Rengefors et al., Citation2008; Annenkova et al., Citation2018).
Overall, we conclude that the difference in the 28S rRNA gene and ITS2, in combination with the physiological and morphological difference, justifies separating G. baicalense and G. corollarium into two different, but very closely related species. Because of the recent divergence event, there has not been enough time for a large accumulation of substitutions in the ITS2. In the future, protein-coding genes, which are under selection in these species, should be investigated to search for more prominent genetic differences. In addition, it would be worthwhile to investigate the life cycles of G. corollarium and G. baicalense and check if there is sexual incompatibility, which is one of the main criteria for species delimitation.
Gymnodiniaceae phylogeny and distribution
Currently, the genus Gymnodinium is being actively revised and new genera have been separated from Gymnodinium sensu stricto as originally defined in Daugbjerg et al. (Citation2000). For example, the genera Barufeta, Nusuttodinium, Spiniferodinium and Wangodinium have been established (Hansen et al., Citation2007; Sampedro et al., Citation2011; Moestrup et al., Citation2014; Takano et al., Citation2014; Kretschmann et al., Citation2015; Luo et al., Citation2018), but the work is still far from finished. The present study contributes to this work, with G. corollarium and G. baicalense grouped in a well-supported clade within Gymnodinium sensu stricto ().
The closest relative to the G. corollarium/G. baicalense clade was the marine Gymnodinium aureolum (), as has also been shown in other studies (Sundström et al., Citation2009; Kretschmann et al., Citation2015; Luo et al., Citation2018). The three species have a very similar morphology. However, G. aureolum differs by a wider and more pronounced sulcal extension on the epicone. Its nucleus is generally more elongated and the irregularly branched chloroplasts are only occasionally in an indistinct radiating pattern. The cyst type of G. aureolum also differs, with no indication of a paracingulum (Hansen et al., Citation2000; Tang et al., Citation2008) as present in both G. baicalense and G. corollarium. The genetic difference between G. aureolum and G. corollarium is relatively large (8% and 13% differences in 28S rRNA gene and ITS2) and the position of G. aureolum in the phylogenetic tree had weak statistical support (, 61%). This makes it an unlikely direct ancestor of the species in the current study.
Other dinoflagellates from different genera, which clustered with the G. baicalense clade and G. aureolum, did not form a statistically well-supported clade (, ML 50), although this has been observed in various studies (e.g. Luo et al., Citation2018). Clearly, some representatives from this group are missing and more species must be described in future to understand the evolutionary relationships in this group. Based on previous morphological observations, freshwater Gymnodinium tylotum and G. baicalense were suggested to be very similar (Moestrup & Calado, Citation2018), however, this was not supported by our new data on the morphology of G. baicalense. In particular, these two species have different cyst morphology. Moreover, the freshwater G. baicalense did not group with the freshwater species G. fuscum and G. plasticum (). Instead it is nested within the marine clade of dinoflagellate species. This result should be regarded as evidence of the colonization of freshwater habitats by marine species (according to Logares et al., Citation2009). We conclude that G. baicalense is more closely related to brackish/marine species, than to any freshwater species from the family, including those which have similar adaptations (e.g. the freshwater member Spiniferodinium limneticum of the Gymnodiniaceae is evolutionarily distant from G. baicalense (), even though it inhabits cold freshwater mountain lakes (Kretschmann et al., Citation2015)).
The most likely evolutionary scenario for the divergence of G. baicalense and G. corollarium is that brackish-water G. corollarium, freshwater G. baicalense and Gymnodinium sp. QZ-2012 (if it is another species) share a single Arctic ancestor. This ancestor seems to be tolerant of a range of salinities (like G. corollarium), including very low ones, which can be found in some sites of the northern seas, near estuaries of great Siberian rivers (e.g. Spielhagen et al., Citation2005). The reverse pathway, i.e. from freshwater to marine habitats, is less likely, because in that case, a G. baicalense-like dinoflagellate would have had to acquire additional physiological means to tolerate higher salinities (such as changing the membrane structure and lipid metabolism, acquiring various osmolytes (Kirst, Citation1990)).
Evolutionary relationships between Baltic, Arctic and Baikal organisms have been observed in other studies. In particular, based on metagenome-assembled genomes it was shown that some Baikal bacteria (e.g. the one truly freshwater Pelagibacter representative, actinobacteria, flavobacterium and Cyanobium sp.) were most closely affiliated to Baltic Sea bacteria (Cabello-Yeves et al., Citation2018). The dinoflagellate morphospecies Apocalatium malmogiense inhabits Lake Baikal, the Baltic Sea, and the Arctic Ocean (Annenkova et al., Citation2015). Moreover, one of the organisms that originated from Arctic waters and adapted to the Lake Baikal habitat about 1 million years ago was the ancestor of the freshwater seal Pusa sibirica (Fulton & Strobeck, Citation2010). Thus, even for this large multicellular organism the distance was not a barrier. Likely, the resting cysts of G. corollarium-like dinoflagellate have served as dispersal propagules, as cysts are more resistant to desiccation feeding by invertebrates (Kremp et al., Citation2003) and survival in bird guts (Tesson et al., Citation2018). The expansion of the G. baicalense ancestor could be similar to the scenario suggested for Apocalathium, in which the species likely adapted to the Lake Baikal habitat during the last cooling period (1.8–0.15 million years ago), when the pelagic zone of the lake was less inhabited after geological shifts leading to the formation of modern ultra-deep lake (Annenkova et al., Citation2015). Žerdoner Čalasan et al. (Citation2019) corroborate a recent divergence of G. baicalense (and Apocalathium), and their indirect counts show that the divergence occurred less than 5 million years ago.
Overall, we conclude that both diatoms from the under-ice phytoplankton (Khursevich et al., Citation2001) and spring-blooming dinoflagellates from Lake Baikal have a modern origin. Here we show this for G. baicalense as previously demonstrated for other spring-blooming dinoflagellates from Lake Baikal: A. baicalense, A. euryceps and A. aff. malmogiense (Annenkova et al., Citation2015). There are a few observations based on light microscopy of allegedly endemic Baikal diatoms and of G. baicalense in several mountain lakes in the Baikal region, but whether these were colonized before or after their appearance in Lake Baikal is unknown (Popovskaya & Genkal, Citation2008; Bondarenko, Citation2009). Considering Gymnodinium sp. QZ-2012, which is genetically very similar to G. baicalense, we hypothesize that G. baicalense-like dinoflagellates are distributed in various cold-water lakes across eastern Asia.
Author contributions
N. Annenkova: analysing molecular-genetic data, salinity experiment, light and partly SEM microscopy, drafting and editing of the manuscript; G. Hansen: scanning electron microscopy, analysing morphological data, editing manuscript; K. Rengefors: original concept, drafting and editing of the manuscript.
Supplementary information
Supplementary fig. 1. The distribution of Gymnodinium baicalense cells based on their length. Mean value is 42 μm (standard deviation = 7 μm).
Supplementary fig. 2. Secondary structure of ITS2: A. Gymnodinium baicalense, B. Gymnodinium corollarium, C. Gymnodinium aureolum. Arrows indicate changes in the secondary structure due to mutations compared with G. baicalense.
Supplementary fig. 3. Species delimitation using the Poisson Tree Processes (PTP) model based on the ML tree from . Bayesian support values shown on the tree correspond to the posterior probabilities of those taxa that form one species under the PTP model.
TEJP-2019-0129-File009.pdf
Download PDF (181.1 KB)TEJP-2019-0129-File008.jpg
Download JPEG Image (613.1 KB)TEJP-2019-0129-File007.jpg
Download JPEG Image (165.9 KB)Acknowledgements
Salinity experiments and analysis of the molecular genetic data were carried out under the project 16-04-01704 of the Russian Foundation for Basic Research, and microscopic observations were supported by project 18-74-00054 of the Russian Science Foundation. We thank the Experimental Freshwater Aquarium Complex for Baikal Hydrobionts LIN SB RAS that provided us the resources to cultivate the algal cultures. We are grateful to I. Khanaev, who photographed the dinoflagellates blooming in Lake Baikal.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplemental Material
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2020.1750057
References
- Alverson, A.J., Beszteri, B., Julius, M.L. & Theriot, E.C. (2011). The model marine diatom Thalassiosira pseudonana likely descended from a freshwater ancestor in the genus Cyclotella. BMC Evolutionary Biology, 11: 125.
- Annenkova, N.V. (2013). Phylogenetic relations of the dinoflagellate Gymnodinium baicalense from Lake Baikal. Central European Journal of Biology, 8: 366–373.
- Annenkova, N.V. (2018). Identification of Lake Baikal plankton dinoflagellates from the genera Gyrodinium and Gymnodinium using single-cell PCR. Russian Journal of Genetics, 54: 1302–1313.
- Annenkova, N.V., Belykh, O.I., Denikina, N.N. & Belikov, S.I. (2009). Identification of dinoflagellates from the Lake Baikal on the basis of molecular genetic data. Doklady Biological Sciences, 426: 253–256.
- Annenkova, N.V., Hansen, G., Moestrup, Ø. & Rengefors, K. (2015). Recent adaptive radiation in a marine and freshwater dinoflagellate species flock. ISME Journal, 9: 1821–1834.
- Annenkova, N.V., Ahrén, D., Logares, R., Kremp, A. & Rengefors, K. (2018). Delineating closely related dinoflagellate lineages using phylotranscriptomics. Journal of Phycology, 54: 571–576.
- Antipova, N.L. (1955). New species of the genera Gymnodinium Stein (Gymnodiniaceae) in the Lake Baikal. Proceedings of the Academy of Sciences of the USSR, 103: 325–328. (in Russian)
- Antipova, N.L. (1974). Interannual changes in phytoplankton of Lake Baikal near Bolshii Koty, 1960–1970 years. In Baikal Productivity and Anthropogenic Changes of its Nature (Kozhova, O.M., editor), 75–94. ISU, Irkutsk. (in Russian)
- Bondarenko, N.A. (2009). Ecology and taxonomy diversity of planktonic algae in mountain lakes from the Eastern Siberia. Dr. Sci. thesis, Institute for Biology of Inland Waters RAS, Borok, Russia. (in Russian)
- Cabello-Yeves, P.J., Zemskaya, T.I., Rosselli, R., Coutinho, F.H., Zakharenko, A.S., Blinov, V.V. & Rodriguez-Valera, F. (2018). Genomes of novel microbial lineages assembled from the sub-ice waters of Lake Baikal. Applied and Environmental Microbiology, 84: e02132–17.
- Coleman, A.W. (2009). Is there a molecular key to the level of “biological species” in eukaryotes? A DNA guide. Molecular Phylogenetics and Evolution, 50: 197–203.
- Collins, S. & Bell, G. (2004). Phenotypic consequences of 1,000 generations of selection at elevated CO2 in a green alga. Nature, 431: 566–569.
- Collins, S. & De Meaux, J. (2009). Adaptation to different rates of environmental change in Chlamydomonas. Evolution, 63: 2952–2965.
- Daugbjerg, N., Hansen, G., Larsen, J. & Moestrup, Ø. (2000). Phylogeny of some of the major genera of dinoflagellates based on ultrastructure and partial LSU rDNA sequence data, including the erection of three new genera of unarmoured dinoflagellates. Phycologia, 39: 302–317.
- Ellegaard, M. & Oshima, Y. (1998). Gymnodinium nolleri Ellegaard et Moestrup sp. ined. (Dinophyceae) from Danish waters, a new species producing Gymnodinium catenatum-like cysts: molecular and toxicological comparisons with Australian and Spanish strains of Gymnodinium catenatum. Phycologia, 37: 369–378.
- Fulton, T.L. & Strobeck, C. (2010). Multiple fossil calibrations, nuclear loci and mitochondrial genomes provide new insight into biogeography and divergence timing for true seals (Phocidae, Pinnipedia). Journal of Biogeography, 37: 814–829.
- Guillard, R.R.L. & Lorenzen, C.J. (1972). Yellow-green algae with chlorophyllide C. Journal of Phycology, 8: 10–14.
- Hairston, Jr. N.G., Lampert, W., Cáceres, C.E., Holtmeier, C.L., Weider, L.J., Gaedke, U., Fischer, J.M., Fox, J.A. & Post, D.M. (1999). Lake ecosystems: rapid evolution revealed by dormant eggs. Nature, 401: 446.
- Hall, T.A. (1999). BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41: 95–98.
- Hansen, G., Daugbjerg, N. & Henriksen, P. (2000). Comparative study of Gymnodinium mikimotoi and Gymnodinium aureolum, comb. nov (= Gyrodinium aureolum) based on morphology, pigment composition, and molecular data. Journal of Phycology, 36: 394–410.
- Hansen, G., Botes, L. & De Salas, M. (2007). Ultrastructure and large subunit rDNA sequences of Lepidodinium viride reveal a close relationship to Lepidodinium chlorophorum comb. nov. (= Gymnodinium chlorophorum). Phycological Research, 55: 25–41.
- Katoh, S. & Standley, D.M. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution, 30: 772–780.
- Khursevich, G.K., Karabanov, E.B., Prokopenko, A.A., Williams, D.F., Kuzmin, M.I., Fedenya, S.A., Gvozdkov, A.N. & Kerber, E.V. (2001). Detailed diatom biostratigraphy of Baikal sediment during the Brunhes Chron and climatic factors of species formation. Russian Geology and Geophysics, 42: 108–129. (in Russian)
- Kirst, G.O. (1990). Salinity tolerance of eukaryotic marine algae. Annual Review of Plant Biology, 41: 21–53.
- Kobanova, G.I. (2009). Morphology and life cycle of Gymnodinium baicalense Ant. (Dinophyceae) from Lake Baikal. Contemporary Problems of Ecology, 2: 581.
- Kozhova, O.M. & Izmest’eva, L.R. (1998). Lake Baikal: Evolution and Biodiversity. Backhuys Publ., Leiden.
- Kremp, A., Shull, D.H. & Anderson, D.M. (2003). Effects of deposit-feeder gut passage and fecal pellet encapsulation on germination of dinoflagellate resting cysts. Marine Ecology Progress Series, 263: 65–73.
- Kretschmann, J., Filipowicz, N.H., Owsianny, P.M., Zinßmeister, C. & Gottschling, M. (2015). Taxonomic clarification of the unusual dinophyte Gymnodinium limneticum Wołosz. (Gymnodiniaceae) from the Tatra Mountains. Protist, 166: 621–637.
- Logares, R., Shalchian-Tabrizi, K., Boltovskoy, A. & Rengefors, K. (2007). Extensive dinoflagellate phylogenies indicate infrequent marine-freshwater transitions. Molecular Phylogenetics and Evolution, 45: 887–903.
- Logares, R., Daugbjerg, N., Boltovskoy, A., Kremp, A., Laybourn-Parry, J. & Rengefors, K. (2008). Recent evolutionary diversification of a protist lineage. Environmental Microbiology, 10: 1231–1243.
- Logares, R., Bråte, J., Bertilsson, S., Clasen, J.L., Shalchian-Tabrizi, K. & Rengefors, K. (2009). Infrequent marine-freshwater transitions in the microbial world. Trends in Microbiology, 17: 414–22.
- Lohbeck, K.T., Riebesell, U. & Reusch, T.B. (2012). Adaptive evolution of a key phytoplankton species to ocean acidification. Nature Geoscience, 5: 346.
- Lohbeck, K.T., Riebesell, U., Collins, S. & Reusch, T.B. (2013). Functional genetic divergence in high CO2 adapted Emiliania huxleyi populations. Evolution, 67: 1892–1900.
- López-Rodas, V., Marvá, F., Rouco, M., Costas, E. & Flores-Moya, A. (2008). Adaptation of the chlorophycean Dictyosphaerium chlorelloides to stressful acidic, mine metal-rich waters as result of pre-selective mutations. Chemosphere, 72: 703–707.
- Luo, Z., Hu, Z., Tang, Y., Mertens, K.N., Leaw, C.P., Lim, P.T., Teng, S.T., Wang, L. & Gu, H.F. (2018). Morphology, ultrastructure, and molecular phylogeny of Wangodinium sinense gen. et sp. nov. (Gymnodiniales, Dinophyceae) and revisiting of Gymnodinium dorsalisulcum and Gymnodinium impudicum. Journal of Phycology, 54: 744–761.
- Moestrup, Ø. & Calado, A.J. (2018). Süßwasserflora von Mitteleuropa, Bd. 6 – Freshwater Flora of Central Europe, Vol. 6: Dinophyceae. Springer-Verlag, Berlin.
- Moestrup, Ø., Lindberg, K. & Daugbjerg, N. (2009). Studies on woloszynskioid dinoflagellates IV: the genus Biecheleria gen. nov. Phycological Research, 57: 203–220.
- Moestrup, Ø., Hakanen, P., Hansen, G., Daugbjerg, N. & Ellegaard, M. (2014). On Levanderina fissa gen. and comb. nov. (Dinophyceae) (syn. Gymnodinium fissum, Gyrodinium instriatum, Gyr. uncatenum), a dinoflagellate with a very unusual sulcus. Phycologia, 53: 265–92.
- Montresor, M., Lovejoy, C., Orsini, L., Procaccini, G. & Roy, S. (2003). Bipolar distribution of the cyst-forming dinoflagellate Polarella glacialis. Polar Biology, 26: 186–194.
- Obolkina, L.A., Bondarenko, N.A., Doroshchenko, L.F., Gorbunova, L.A. & Molozhavaya, O.A. (2000). The find of a cryophilic association in Lake Baikal. Doklady Earth Sciences, 371: 592–594.
- Parrow, M.W. & Kremp, A. (2008). Asexual resting cysts: a common dinoflagellate survival strategy? In Book of Abstracts Eighth International Conference on Modern and Fossil Dinoflagellates, 42–43.
- Popovskaya, G.I. & Genkal, S.I. (2008). Materials to flora of diatom algae (Centrophyceae, Bacillariophyta) in some lakes of Pribaikal and Zabaikal regions. Inland Water Biology, 4: 3–11.
- Rengefors, K., Laybourn‐Parry, J., Logares, R., Marshall, W.A. & Hansen, G. (2008). Marine‐derived dinoflagellates in antarctic saline lakes: community composition and annual dynamics. Journal of Phycology, 44: 592–604.
- Rengefors, K., Kremp, A., Rheusch, T. & Wood, M. (2017). Genetic diversity and evolution of eukaryotic phytoplankton: revelations from population genetic studies. Journal of Plankton Research, 39: 165–179.
- Sampedro, N., Fraga, S., Penna, A., Casaianca, S., Zapata, M., Grünewald, C.F., Riobó, P. & Camp, J. (2011). Barrufeta bravensis gen. nov. sp. nov. (Dinophyceae): a new bloom-forming species from the northwest Mediterranean Sea. Journal of Phycology, 47: 375–392.
- Schlüter, L., Lohbeck, K.T., Gröger, J.P., Riebesell, U. & Reusch, T.B. (2016). Long-term dynamics of adaptive evolution in a globally important phytoplankton species to ocean acidification. Science Advances, 2: e1501660.
- Simon, M., Jardillier, L., Deschamps, Ph., Moreira, D., Restoux, G., Bertolino, P. & López García, P. (2015). Complex communities of small protists and unexpected occurrence of typical marine lineages in shallow freshwater systems. Environmental Microbiology, 17: 3610–3627.
- Spielhagen, R.F., Erlenkeuser, H. & Siegert, Ch. (2005). History of freshwater runoff across the Laptev Sea (Arctic) during the last deglaciation. Global and Planetary Change, 48: 187–207.
- Stamatakis, A. (2014). RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics, 30: 1312–1313.
- Sundström, A.M., Kremp, A., Daugbjerg, N., Moestrup, Ø., Ellegaard, M., Hansen, R. & Hajdu, S. (2009). Gymnodinium corollarium sp. nov. (Dinophyceae) – a new cold-water dinoflagellate responsible for cyst sedimentation events in the Baltic Sea. Journal of Phycology, 45: 938–952.
- Takano, Y., Yamaguchi, H., Inouye, I., Moestrup, Ø. & Horiguchi, T. (2014). Phylogeny of five species of Nusuttodinium gen. nov. (Dinophyceae), a genus of unarmoured kleptoplastidic dinoflagellates. Protist, 165: 759–778.
- Tang, Y.Z., Egerton, T.A., Kong, L.S. & Marshall, H.G. (2008). Morphological variation and phylogenetic analysis of the dinoflagellate Gymnodinium aureolum from a tributary of Chesapeake Bay. Journal of Eukaryotic Microbiology, 55: 91–99.
- Tesson, S.V., Weißbach, A., Kremp, A., Lindström, Å. & Rengefors, K. (2018). The potential for dispersal of microalgal resting cysts by migratory birds. Journal of Phycology, 54: 518–528.
- Wickham, H. (2016). ggplot2: elegant graphics for data analysis. Springer-Verlag, New York.
- Wolf, M., Friedrich, J., Dandekar, T. & Müller, T. (2005). CBCAnalyzer: inferring phylogenies based on compensatory base changes in RNA secondary structures. In Silico Biology, 5: 291–294.
- Žerdoner Čalasan, A., Kretschmann, J. & Gottschling, M. (2019). They are young and they are many: dating freshwater lineages in unicellular dinophytes. Environmental Microbiology, 21: 4125–4135.
- Zhang, J., Kapli, P., Pavlidis, P. & Stamatakis, A.A. (2013). General species delimitation method with applications to phylogenetic placements. Bioinformatics, 29: 2869–2876.