?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Macroalgal growth in temperate coastal ecosystems is primarily regulated by light and inorganic nitrogen availability. The effect of light (photon irradiance) on NO3− and NH4+ uptake, and NO3− assimilation, were studied in the red macroalga, Hemineura frondosa, which does not operate a carbon concentrating mechanism (non-CCM). Non-CCM macroalgae grow in low and high light environments but become increasingly dominant with depth, suggesting a mechanism for ‘preserving energy’ under low light levels. H. frondosa was acclimated to limiting (30 µmol photons m−2 s−1) and saturating (150 µmol photons m−2 s–1) irradiances for 8 days. Then, NO3− and NH4+ uptake rates were measured under limiting and saturating irradiances at six concentrations ranging from 2–64 μM. NO3− uptake did not follow saturating uptake kinetics at both irradiances suggesting multiple uptake mechanisms. NH4+ uptake saturated at concentrations <32 µM under limiting but not under saturating irradiance. Saturating irradiance resulted in greater maximum uptake rates of both NO3− and NH4+. There was no evidence that irradiance regulated NO3− reduction by nitrate reductase. Also illustrated is the importance of measuring nitrate reductase activity on fresh material, as freezing in liquid nitrogen and storage at −80°C for 7 days caused a 65% decline in activity. Photosynthetic pigments, soluble tissue nitrogen and % total tissue nitrogen were all higher in limiting irradiance. In this first study of the nitrogen physiology of a non-CCM seaweed, we show that light regulates NO3− and NH4+ uptake but not NO3− assimilation.
Introduction
Macroalgae are an intrinsic component of temperate coastal marine ecosystems and their growth is primarily regulated by the availability of light and nitrogen (Chapman & Craigie, Citation1977; Harrison & Hurd, Citation2001). Light regulates metabolic activities including photosynthesis and nutrient uptake (D’Elia & DeBoer, Citation1978; Udayakumar et al., Citation1981), while nitrogen is both an essential and limiting nutrient for growth (Phillips & Hurd, Citation2003; Hurd et al., Citation2014). The effects of light and nitrogen on macroalgal growth are tightly coupled and neither factor can facilitate primary production without the other (Lapointe et al., Citation1984; Turpin, Citation1991; Hurd et al., Citation2014). Both influence the biochemical composition of macroalgae (Figueroa et al., Citation1995, Citation1997; Marinho-Soriano, Citation2012), including light harvesting pigments – chlorophylls and red macroalgal accessory pigments (phycobilins) which are nitrogen rich – amino acids and inorganic soluble tissue nitrogen storage ‘pools’ (Figueroa et al., Citation1997; Barufi et al., Citation2011).
There are two main sources of dissolved inorganic nitrogen available for macroalgae: nitrate (NO3−) and ammonium (NH4+) (Smit, Citation2002; Ochoa-Izaguirre & Soto-Jiménez, Citation2015). NO3− is taken up across the plasma membrane via active transport with hydrogen ions (H+) (Syrett, Citation1981; Glass, Citation2003; Roleda & Hurd, Citation2019). In the cytoplasm it is reduced by the enzyme nitrate reductase (NR) to nitrite (NO2−) which uses NAD(P)H as an electron donor (Marquardt et al., Citation2010). NO2− is subsequently reduced to NH4+ by nitrite reductase (NiR) in the chloroplast before entering the glutamine synthetase-glutamine oxoglutarate aminotransferase (GS-GOGAT) pathway for assimilation into amino acids (Hipkin et al., Citation1983; Chow et al., Citation2007). In contrast, NH4+ is typically taken up by passive transport and directly assimilated into amino acids via GS-GOGAT, so has a much-reduced energetic cost compared with NO3− uptake; thus many macroalgae have a strong preference for NH4+ over NO3− as a nitrogen source (D’Elia & DeBoer, Citation1978; Raven et al., Citation1992; Nishihara et al., Citation2005; Abreu et al., Citation2011; Pritchard et al., Citation2015).
The mechanisms of NO3− and NH4+ uptake can be elucidated, in part, by examining the kinetic pattern of uptake at a range of concentrations. Active transport is indicated by a hyperbolic curve in which uptake rate reaches a maximum rate at a critical concentration i.e. uptake is ‘saturated’ indicating involvement of a saturable transmembrane transporter (Harrison & Druehl, Citation1982). Passive diffusion is indicated by an uptake rate which increases linearly with external nutrient concentration (Hurd et al., Citation2014). A curve that shows evidence of saturating uptake kinetics at low concentrations and linear components at higher concentrations indicates that both active and passive uptake mechanisms are operating (Hurd et al., Citation2014); or that a combination of two or more active transport systems with high- and low-affinities for the nutrient are operating (Martínez & Rico, Citation2004). The preference and acquisition mechanism(s) for each dissolved inorganic nitrogen source are species dependent (Smit, Citation2002; Ochoa-Izaguirre & Soto-Jiménez, Citation2015).
Macroalgae can store NO3− in the cell vacuole and cytoplasm when there is a surplus of NO3− in the marine environment or insufficient light energy in the environment for NO3− assimilation (Davison & Stewart, Citation1984; Raven et al., Citation1992; Young et al., Citation2007). A large store of NO3− can inhibit further NO3− uptake and result in increased NR activity, with the rate of depletion of the NO3− pool being proportional to an increase in growth rate (Thomas & Harrison, Citation1985; Hwang et al., Citation1987; McGlathery et al., Citation1996; Lartigue & Sherman, Citation2005). When sufficient light energy is available, NO3− may be assimilated to NH4+ which is subsequently assimilated into organic nitrogen such as pigments (chlorophyll, phycobilins), free amino acids (e.g. cirtulline, arginine) and proteins (Syrett, Citation1981; Harrison & Hurd, Citation2001). In red macroalgae, the synthesis of accessory pigments (phycobilins) has a relatively high requirement for nitrogen (Chopin et al., Citation1995). Their primary function is light harvesting, but they are sometimes considered nitrogen stores because when nitrogen supply is limiting, but light levels are optimal, the pigments are catabolized and used in growth (Lapointe, Citation1981; Marinho-Soriano, Citation2012). Under low light, phycobilin content can increase substantially to harvest more light energy but sufficient nitrogen supply is required for this process (Vergara & Niell, Citation1993; Barufi et al., Citation2011; Ak & Yücesan, Citation2012; Marinho-Soriano, Citation2012). In contrast to NO3−, NH4+ is not usually stored in large, unassimilated inorganic pools and is efficiently assimilated into amino acids, which in turn maintain the concentration gradient allowing NH4+ to continue to diffuse into the cell when external NH4+ is high (Fujita et al. Citation1988; Harrison & Druehl Citation1982; Raven et al. Citation1992).
NR activity is known to be up-regulated by the increased availability of NO3− in the surrounding medium (Davison & Stewart, Citation1984; Thomas & Harrison, Citation1987, Citation1988; Gordillo et al., Citation2006; Cabello-Pasini et al., Citation2011), and is also regulated by light levels (Corzo & Niell, Citation1992; Figueroa, Citation1996). Chow et al. (Citation2004) found that NR activity in Gracilaria chilensis (Rhodophyta) increased when light pulses were given during dark periods, and suggested that NR was regulated mainly by light and not by a biological clock. Additionally, Chow et al. (Citation2013) found a direct dependence of NR activity on PSII and PSI electron transport, but there is little information on the effect of light levels i.e. photon irradiance (µmol photons m−2 s−1) on the enzyme’s capacity to reduce NO3− in macroalgae. Saturating light can promote greater NO3− uptake rates compared with limiting light; this may be facilitated by an increase in NR enzyme activity due to the greater energy available (Wheeler, Citation1982; Hurd et al., Citation2014).
Tasmania has a globally unique macroalgal flora with an unusually high proportion (up to 90% of some sites) of macroalgae that solely use carbon dioxide (CO2) as a source of dissolved inorganic carbon, and not energetically expensive bicarbonate; they do not operate a CO2 concentrating mechanism (CCM) and are termed non-CCM seaweeds (Cornwall et al., Citation2015). These macroalgae can grow in both low and high light environments but become increasingly dominant with depth, suggesting a mechanism for ‘preserving energy’ at low light levels (Raven & Hurd, Citation2012; Raven et al., Citation2014; Cornwall et al., Citation2015). However, the nitrogen physiology of non-CCM species has not been examined to date, and we predict NH4+ to be preferred as it requires little energy for uptake and assimilation and could thus be another mechanism for preserving energy under low lightlevels (Raven et al., Citation1992). Pritchard et al. (Citation2015) found that low-light adapted Anotrichium crinitum had a strong preference for NH4+, however for non-CCM red macroalgae it is not understood how uptake and assimilation of NO3− and NH4+ is regulated by light. The objective of this study was to determine the effect of limiting and saturating irradiances on NO3− and NH4+ uptake, soluble tissue NO3− and NH4+ storage pools and NR activity of the non-CCM, red macroalga Hemineura frondosa (J.D.Hooker & Harvey) Harvey.
We hypothesized that for H. frondosa acclimated to limiting and saturating irradiance, (1) NO3− would exhibit saturating uptake kinetics indicative of active uptake, whilst (2) NH4+ would exhibit linear uptake with increasing concentration indicative of passive diffusion. (3) Pre-experimental acclimation to saturating irradiance would promote greater NO3− uptake rates and this increase in uptake would be facilitated by higher NR activity compared with limiting irradiance and (4) acclimation to limiting irradiance would promote larger internal storage pools of NO3− compared with saturating irradiance.
Materials and methods
Macroalgae collection and experimental conditions
For the main experiment, 20 individuals of H. frondosa (30–40 cm2 each) were collected by hand using SCUBA from Blackmans Bay (43°01′S, 147°33′E), south-eastern Tasmania, Australia, on 17 July 2017. Another 10 specimens were sampled for a subsequent NR experiment on 25 August 2017. Specimens were found growing in the subtidal on rock substratum at 2–6 m depth and were transported to the laboratory 20 min away in a cool box (25 litres, polypropylene/polyurethane). On this day, the irradiance at the collection depths, measured using a HOBO MX2202 (Mx temperature/light data logger), was between ~10–50 μmol photons m−2 s−1 at 10:00–11:00 AM. In the laboratory, all visible epiphytes were gently removed using tweezers and wiping with tissue paper. All experiments were conducted in a walk-in temperature-controlled room set at ambient temperature of collection, 12°C ± 0.4°C, with overhead irradiance provided by cool-white fluorescent tubes. A pulse-amplitude modulated (PAM) chlorophyll fluorescence meter (Walz Diving-PAM Underwater Fluorometer) was used to create rapid light curves (RLC), from which relative electron transport rate (rETR) were determined in sub-saturating and saturating irradiance of H. frondosa specimens. These irradiance levels were used as the ‘saturating irradiance’ and ‘limiting irradiance’ for experimental acclimation.
Seawater was available on tap from a 10 000 litre storage facility at the Institute for Marine and Antarctic Studies, University of Tasmania, and is collected bi-monthly from South Bruny Island, Tasmania (43°30′S, 147°13′E). Seawater was filtered to 1 μm and UV-sterilized prior to experiments (Emperor Aquatics Smart HO UV sterilizer, 025050-2, 50 W lamp, cartridge filter). All glassware was sterilized in 2% Decon 90TM and 10% v/v hydrochloric acid solution (Merck 37%) prior to use. Seawater samples for nutrient analyses from the experiments outlined below were filtered using glass microfibre filter paper (0.7 µM, Whatman, GF/F), stored in 12 ml polyethylene nutrient tubes (LabServ®) at −20°C until analysis. Concentrations of N(NO3− + NO2−) and NH4+ in seawater were analysed using a QuickChem® 8000 Automated Ion Analyser (LaChat Instruments) following methods outlined in Diamond (Citation2008) and Liao (Citation2008).
Pre-experimental acclimation to limiting and saturating irradiance
Each of 20 individual algae were cut into ten 4 cm2 pieces: pieces of each alga were placed into the same beaker with 500 ml of filtered seawater (0.7 uM, cartridge filter) and an air stone to maintain water motion. Ten of these beakers were placed under a limiting irradiance (30 µmol photons m−2 s−1) and the other 10 under a saturating irradiance (150 µmol photons m−2 s−1). The algae were acclimated for 8 days to daily seawater enrichment by adding 30 µM of NO3− as NaNO3 (EMSURE® ACS,ISO,Reag. Ph Eur) and seawater was changed every 3 days.
Experimental design
After the 8 days’ acclimation, the 10 seaweed pieces within each of the 20 beakers were removed and used in experiments and assays as follows. Six pieces of algae from one beaker were used immediately in the NO3− and NH4+ uptake experiments (see below). A seventh piece was used to determine pigment content and an eighth piece for NR activity: both these were immediately blotted dry, weighed (0.1 g ± 0.01) (± 0.01 mg, ME-T Analytical Balance), frozen with liquid nitrogen and stored at −80°C for 7 days. A ninth piece of alga was used to determine soluble tissue nitrogen and was immediately blotted dry, weighed (0.25 g ± 0.01), placed in a 50 ml boiling tube (borosilicate) with 20 ml of distilled water (ultrapure) and refrigerated overnight prior to boiling extraction. Finally, one piece of algae was randomly selected from 8 of the 20 flasks (i.e. four pieces of algae from each irradiance acclimation) for C:N analysis and were oven dried and weighed (5 mg ± 0.01) (see below).
NO3− and NH4+ uptake kinetics
Multiple flask uptake experiments were run to determine the maximum uptake rate (Vmax) and half saturation constant (Km) of both NH4+ and NO3− for H. frondosa which had been acclimated to either limiting or saturating irradiance (Harrison & Druehl, Citation1982; Hurd & Dring, Citation1990). Uptake experiments were conducted under the same irradiances as the 8 day acclimation period and consisted of the following treatments: 1. Limiting irradiance, NH4+ uptake; 2. Saturating irradiance, NH4+ uptake; 3. Limiting irradiance, NO3− uptake; 4. Saturating irradiance, NO3− uptake. There were five replicates for each of the four experimental treatments; each experimental replicate consisted of six seaweed pieces (see above) for which nutrient uptake was measured at each of six initial concentrations. Experiments were conducted in 250 ml conical flasks each containing 200 ml filtered seawater. Stock solutions of NO3− as NaNO3 (EMSURE® ACS,ISO,Reag. Ph Eur) and NH4+ as NH4Cl (EMSURE® ACS,ISO,Reag. Ph Eur) were added to seawater in flasks to give six initial experimental concentrations of 2, 4, 8, 16, 32 and 64 µM in addition to the background concentrations. Four flasks with no algae acted as controls, two containing filtered seawater and two containing seawater plus 64 µM of both nitrogen sources.
At the start of the experiment, one piece of algae was added to each of 120 flasks (five replicate algae × six cut pieces for each alga × four experimental treatments). Conical flasks were then randomly positioned on shaker tables set at 100 rpm under limiting and saturating irradiances corresponding to the prior acclimation. Initial seawater samples (10 ml) were taken 2 h after the algal pieces were added to each flask and final samples were taken at 3 h: this time period was chosen because our preliminary time-course experiment revealed a 2 h lag phase in NO3− uptake. At the end of the experiment, algae were removed, and a final seawater sample taken. Algae were photographed for surface area which was calculated using image processing program ImageJ, and nitrogen uptake rates were standardized to surface area.
Soluble tissue NO3− and NH4+ pools
Soluble tissue NO3− and NH4+ content were analysed to determine unassimilated inorganic NO3− and NH4+ within the alga cells using a boiling extraction method (Hurd et al., Citation1996). Boiling tubes (borosilicate, 50 ml) were filled with 20 ml of distilled water (ultrapure) and 0.25 ± 0.1 g pieces of alga tissue were added. Boiling tubes were removed after overnight refrigeration, capped in aluminium foil and placed in a boiling water bath (~100°C) for 40 min. Tubes were left to cool before the solution was filtered (Whatman, GF/F) and stored at −20°C. This process was repeated twice to ensure all soluble nitrogen was removed from the algal tissue. Each extract was analysed for concentrations of NO3− and NH4+. Results were standardized to wet weight using the following formula:
where N1, N2 are the NO3− or NH4+ concentrations (µM) in the supernatant after the first and second extractions, 0.02 is the volume of liquid in each boiling tube (l) and WW is the wet weight of the algae (g).
Photosynthetic pigment content and C:N ratio
Phycoerythrin (PE) and chlorophyll contents were analysed to study the acclimation of H. frondosa to limiting and saturating irradiances. Pigment contents were determined using preserved algal material (0.1 ± 0.01 g) following methods outlined in Pritchard et al. (Citation2013). Frozen tissue was ground using liquid nitrogen over ice in a mortar and pestle under dim light and phycobilins were extracted overnight in 15 ml centrifuge tubes (Greiner) using 5 ml of ice-cold 0.1 M phosphate buffer (pH 6.8, sodium phosphate dibasic and monobasic ≥ 99%, Sigma Life Sciences). The absorbance of the supernatant was measured at 564, 592, 455, 618 and 645 nm using a scanning spectrophotometer (Halo RB-10, Dynamica) and phycobilin contents were calculated using equations and coefficients by Beer & Eshel (Citation1985). Remaining pellets were re-suspended in 5 ml of 100% ethanol (99.8%, Merck) in 15 ml centrifuge tubes, vortexed and chlorophyll was extracted overnight at 4°C. The absorbance of the supernatant was measured at 629, 649 and 665 nm before chlorophyll contents were calculated using equations from Ritchie (Citation2006). Carbon and nitrogen content and C:N ratio were analysed to determine total nitrogen contents under saturating and limiting irradiances. Contents were analysed using a NA1500 elemental analyser coupled to a Thermo Scientific Delta V Plus via a Conflo IV. Tissue carbon and nitrogen content were expressed as a percentage of the alga’s dry weight and C:N ratios were indicated based on their atomic weights.
Nitrate reductase activity
During our preliminary experiments to optimize NR extraction, we achieved a NR activity of 36.55 (± 3.65) μmol NO3− reduced min−1 g−1 WW on fresh algal material. However, due to logistical limitations, for the experiment we froze samples in liquid nitrogen which were subsequently stored at −80°C for 7 days prior to measuring NR activity. The resulting activity was ~65% lower than that measured on fresh material. Therefore, to confirm our NR results (see later), we repeated the 1 week acclimation as described above between 27 August–4 September 2017, but this time measured the NR activity on fresh material immediately following the acclimation. In this second NR experiment, 10 individual algae were each cut into four pieces (each ~4 cm2 as in the first experiment). Of these four pieces, two were acclimated under saturating and two under limiting irradiance. For the pieces acclimated under saturating irradiance, one was used for NR assay and the second for inorganic NO3− and NH4+ pools to ensure nitrogen physiology of H. frondosa was comparable to the previous acclimation experiment. Similarly, under limiting irradiance, one piece of alga was used for NR assay and the second for inorganic NO3− and NH4+ pools.
NR assays were run to analyse the rate of nitrate assimilation and followed the protocol by Pritchard (Citation2013) who optimized the brown macroalgae methods of Hurd et al. (Citation1996) and Young et al. (Citation2005) for red macroalgae. Frozen algal pieces (0.1 g ± 0.01) were ground to a fine powder under liquid nitrogen in an ice-cold mortar and pestle. Algal powder was immediately transferred to a micro-centrifuge tube (1.5 ml, Eppendorf) and 1.5 ml of ice-cold NR buffer was added. NR buffer consisted of 200 mM phosphate extraction buffer (pH 7.9, sodium phosphate dibasic and monobasic ≥ 99%, Sigma Life Sciences), 5 mM EDTA (≥ 99.4%, chem-supply), 1% TritonTM × 100 (v/v, Sigma Life Sciences), 0.3% polyvinylpyrrolidone (PVP, ≥ 99%, Fluka Analytical), 3% bovine serum albumin (BSA, Bovogen Biologicals) and 2 mM dithiothreitol (DTT, ≥ 97%, BIO-RAD). Solution was homogenized and left on ice for no longer than 30 min before assays began. NR assays were conducted in microcentrifuge tubes in a total volume of 1 ml with 200 µl of homogenate. Reactions were started with the addition of 100 μl 10 mM stock KNO3 (≥ 98%, LabChem) solution and run for 40 min before being stopped using 500 µl of 50 mM zinc acetate (≥ 98%, Chem-supply) and suspension in a boiling water for 5 min. Homogenate was centrifuged (Centurion Scientific) at 13 000 g for 5 min and 1 ml of the supernatant was removed for colour reactions. Subsequent reactions indicated the presence of NO2− and included the addition of 20 µl 825 mM phenazine methosulphate (PMS, ≥ 99%, Sigma-Aldrich), 500 µl sulphanilamide (> 99%, Scharlau) and 500 µl N-(1-napthyl) ethylenediamine dihydrochloride (NEDD, > 98.0%, chem-supply). Concentrations of NO2− were determined spectrophotometrically at 540 nm and NR activity is reported as μmol NO3− reduced min−1 gWW−1. To account for any internal NO2− present in the algae a blank reaction was conducted by adding zinc acetate (< 98%, Chem-supply) prior to the addition of KNO3 for each replicate.
Data analysis
Statistical analysis and curve fitting to nitrogen uptake data were conducted using R version 2.2 (R Development Core Team, Citation2012). A Shapiro–Wilk test was conducted to check the normality of the data and regressions of best fit were plotted using the graphing software package SigmaPlot (Systat Software Inc). Where possible, the Michaelis–Menten model was fitted to the uptake data to determine maximum uptake rate (Vmax) and half saturation constant (Km).
where V is the uptake rate, Vmax is the maximum uptake rate, S is the concentration of the limiting nutrient and Km is the half saturation constant. The function was used as a single model for all the data due to variability, excluding outliers. In cases where the data did not follow a rectangular hyperbola, the regression of best fit was plotted. For our data, the regression best fit was plotted using the following equation:
where V is the uptake rate, Vmax is the maximum uptake rate, S is the concentration of the limiting nutrient, Km is the half saturation constant and C is the linear trend. Again, the function was used as a single model for all the data due to variability, excluding outliers. To compare the uptake rates in the limiting- and saturating- irradiance acclimation, the relationship between uptake and concentration was determined by running an analysis of covariance (ANCOVA) on the following linear models:
Nitrate uptake rates:
Ammonium uptake rates:
Where α is the intercept of the linear model, β are the uptake regression coefficients, treat is a categorical variable that represents irradiance and ε is the model residuals. ANCOVA test assumptions were assessed using diagnostic plots of model residuals and data were transformed where necessary using values of λ from Box–Cox plots. A t-test or Mann–Whitney U test (depending on normality of the data) was used to compare responses of soluble tissue, pigment content and NR enzyme activity for saturated and limited irradiance acclimated thalli.
Results
NO3− and NH4+ uptake kinetics
For H. frondosa at both limiting and saturating irradiance, the regression of best fit for the uptake of NO3− was non-linear (saturating irradiance: adjusted R2 = 0.77, limiting irradiance: adjusted R2 = 0.53). At concentrations below 16 µM, the NO3− uptake rates appeared to saturate; however at higher concentrations, 16 µM and above, uptake rates further increased linearly (–). NH4+ uptake rates were best described by a Michaelis-Menten function (saturating irradiance: adjusted R2 = 0.47, Km = 152.93 µM and Vmax = 0.12 µmol cm−1 h−1 and limiting irradiance: adjusted R2 = 0.25, Km = 16.17 µM and Vmax = 0.01 µmol cm−1 whereby uptake rates saturate with increasin g NH4+ concentration (-). The conversion factor between wet weight and surface area was 1 cm2 = 10.67 g WW−1.
Fig. 1–4 Uptake rates (µmol cm−2 h−1) for Heminura frondosa as a function of increasing NO3– concentration (µM) for algae acclimated to either (1) saturating irradiance (150 µmol photons m−2 s−1) and (2) limiting irradiance (30 µmol photons m−2 s−1) and NH4+ under (3) saturating irradiance (150 µmol photons m–2 s–1) and (4) limiting irradiance (30 µmol photons m−2 s−1). Solid lines indicate regression of best fit. Note that the y-axis range for graphs 1 and 2 are different to those of 3 and 4. Each symbol represents an individual macroalgal replicate (n = 5)
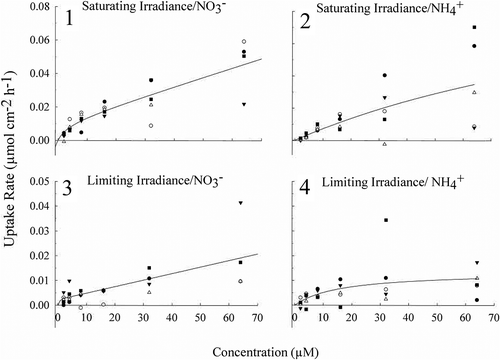
Irradiance acclimation had a significant effect on the uptake rates of NO3− (two-way ANCOVA, p < 0.001), with higher uptake rates at all concentrations of NO3− under saturating irradiance than limiting irradiance (–). Similarly, NH4+, uptake rates were higher in thalli under saturating irradiance when compared with the limiting irradiance acclimation (two-way ANCOVA, p = 0.0208) (–).
Soluble tissue NO3− and NH4+ pools
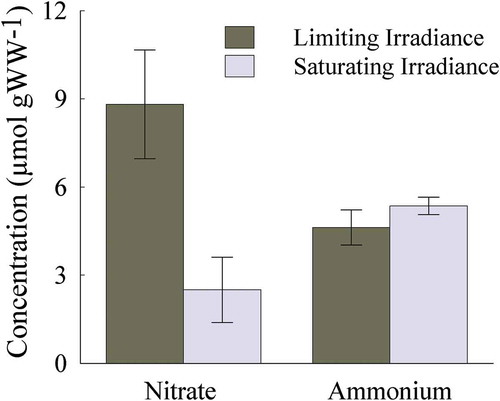
Soluble tissue NO3− pools were two-times higher in thalli acclimated to limiting than saturating irradiance (Wilcoxon rank sum test, p < 0.05, ), whereas there was no effect of irradiance on soluble tissue NH4+ pools (Wilcoxon rank sum test, p = 0.90).
Photosynthetic pigment contents
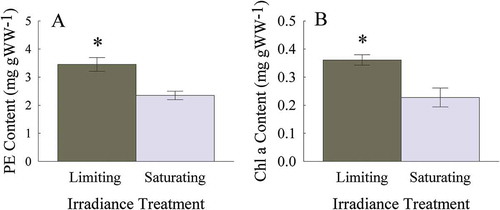
Hemineura frondosa acclimated in a limiting irradiance had 30% more PE than those acclimated under saturating irradiance (Wilcoxon rank sum test, p < 0.05; ). Similarly, Chl a content was 35% higher under limiting irradiance compared with saturating irradiance (Wilcoxon rank sum test, p < 0.05; ).
Nitrate reductase assay
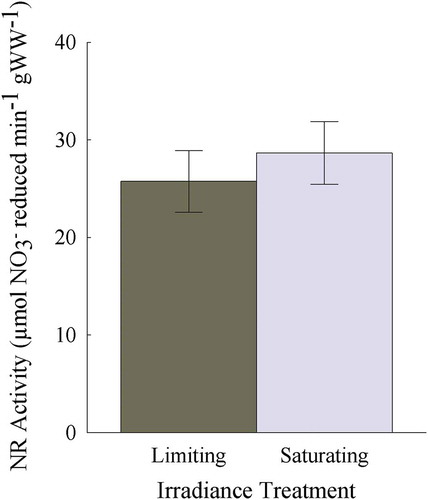
NR activity was similar (average of 27.2 ± 1.46 μmol NO3− reduced min−1) for individuals acclimated to saturating and limiting irradiance (two tailed t-test, p = 0.55; ).
Total tissue C, N and C:N ratio
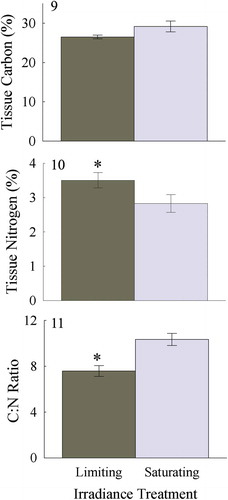
Total tissue carbon (%) was not significantly affected by irradiance acclimation (two tailed t-test, p = 0.84; ) whereas total tissue nitrogen (%) was 20% higher in thalli acclimated to limiting than saturating irradiance (two tailed t-test, p < 0.01; ). As a result, the C:N ratio was 25% lower for the limiting compared with saturating irradiance acclimation (two-tailed t-test, p < 0.05; ).
Discussion
The patterns of NO3− and NH4+ uptake in H. frondosa were influenced by prior irradiance acclimation indicating that light plays a significant role in inorganic nitrogen uptake and utilization. The first hypothesis, that NO3− would exhibit saturating uptake kinetics, indicative of active uptake, was partly supported: uptake rates under both saturating and limiting irradiance appeared to saturate between 2 and 16 µM, but was linear at higher concentrations. This uptake pattern is similar to NO3− uptake observed in Palmaria palmata (Rhodophyta; Martínez & Rico, Citation2004), Gracilaria pacifica (Rhodophyta; Thomas et al., Citation1987), Ulva rigida (Chlorophyta; Lavery & McComb, Citation1991), Laminaria saccharina and Laminaria digitata (Phaeophyceae; Conolly & Drew, Citation1985) and has been explained by active uptake mechanism(s) at lower concentrations and passive diffusion at higher concentrations (see fig. 6.2d in Hurd et al., Citation2014). However NO3− uptake is typically via active transport rather than diffusive uptake (Hurd et al., Citation2014). NO3− transporters in algae and higher plants are encoded by multigene families which are differentially affected by external concentrations of NO3− (Runcie et al., Citation2003; Kakinuma et al., Citation2008). In terrestrial plants, high affinity transport systems are indicated by low Km values and saturable uptake rates, whilst low affinity systems have a relatively high Km, and at high nutrient concentrations they have a linear relationship with uptake rates (Glass, Citation2003). This pattern is similar to that observed for H. frondosa, and it is most likely that both high- and low-affinity NO3− transport systems are used for NO3− uptake, rather than a diffusive component. This would be advantageous under fluctuating ambient NO3− concentrations, dependent on the requirements of the algal tissue (Galván & Fernández, Citation2001). Our work highlights that for macroalgae, further research to identify the underpinning cellular and molecular regulatory mechanisms of NO3− uptake is needed.
The second hypothesis that NH4+ uptake would increase linearly with concentration, indicating uptake by passive diffusion, was partly supported at saturating, but not limiting, irradiance. Although NH4+ uptake rates under saturating irradiance appear to increase with NH4+ concentration, a rectangular hyperbola was the best fit curve suggesting that had we used higher concentrations, uptake rates would have saturated. This is reflected in Vmax which was very high (0.12 µmol cm−1 h−1), and the Km of 152.93 µM, which suggests saturation would occur at ~ 300 µM. Under limiting irradiance, NH4+ uptake saturated at 32.34 µM with a Km of 16.17 µM which was within the range of other marine Rhodophytes (range = 1.60–76.45 µM) although the Vmax of 0.01 µmol cm−1 h−1 was more than 10× lower than other subtidal Rhodophytes: Gracilaria foliifera, Neoagardhiella baileyi (D’Elia & DeBoer, Citation1978), Anotrichium crinitum (Pritchard et al., Citation2015) and Palmaria palmata (Martínez & Rico, Citation2004). We hypothesized that NH4+ uptake would exhibit non-saturating kinetics indicative of passive diffusion since H. frondosa has physiological characteristics of a species adapted to low-light environments (Raven et al., Citation1992; Cornwall et al., Citation2015). However, saturable uptake kinetics usually indicate active or mediated uptake mechanisms which are energy dependent (Harrison & Druehl, Citation1982; Harrison & Hurd, Citation2001) and assimilation rate may have exerted control over the pattern of uptake rate with concentration (Rees et al., Citation1998). Either one or both of these mechanisms are likely employed by H. frondosa as saturating irradiance promoted increased rates of NH4+ uptake and suggests an irradiance-regulated mechanism, however without mechanistic study using inhibitors, it is impossible to determine which uptake mechanim is used (Roleda & Hurd, Citation2019). To our knowledge, ours is the only study to examine the effect of light on NH4+ uptake rates by a non-CCM macroalga.
We hypothesized that acclimation to saturating irradiance would promote greater uptake of NO3− and that this would be facilitated by an increase in NR activity. This hypothesis was partially supported; NO3− uptake was greater under saturating irradiance, but NR activity was unaffected, suggesting that NO3− uptake and NR activity are uncoupled for this species. Furthermore, acclimation to limiting irradiance resulted in larger internal stores of NO3−, compared with saturating irradiance. This supported our hypothesis and may suggest that the differences in NO3− uptake rates of H. frondosa under limiting and saturating irradiances are instead facilitated by the preferential reduction of inorganic nitrogen pools under limiting irradiance before NO3− is actively taken out of the external medium. This method of nitrogen ‘recycling’ has been described for seagrass and was linked to a reduction of the plant’s dependence on external nitrogen sources (Borum et al., Citation1989). The preferential reduction of stored inorganic nitrogen contributed 40–69% of total nitrogen gained by Posidonia oceanica and 60% of that gained by Zostera marina (Borum et al., Citation1989; Alcoverro et al., Citation2000). To our knowledge, this reduced dependence on external nitrogen sources due to recycling of internal stores has not previously been described for macroalgae and clearly requires further work to test if it is the case.
Storage of unassimilated NH4+ in H. frondosa was unaffected by irradiance but content was high (5.36 μmol g WW−1 ± 0.93 SD, saturating irradiance, tissue nitrogen 2.8%) in comparison to other subtidal Rhodophytes, Gracilaria tikvahiae (3.80 μmol g WW−1, tissue nitrogen 2.0%; Bird et al., Citation1982) and Anotrichium crinitum (1.8 μmol g WW−1 ± 0.61 SD; Pritchard et al., Citation2015). The unassimilated stores of NH4+ may have decreased NH4+ uptake by H. frondosa as evidenced by a saturation of the uptake rates under limiting irradiance (McGlathery et al., Citation1996). High internal concentrations of unassimilated NO3− and NH4+ limit the uptake capacity of macroalgae and may have resulted in the relatively low uptake rates observed in this study for both NO3− and NH4+, as nutrient-limited algae generally show higher nutrient uptake rates than nutrient-replete algae (Kregting et al., Citation2008).
Phycobilins are the main light-harvesting accessory pigments of the Rhodophyta, and for some species can be a source of organic nitrogen which can be catabolized during periods of low nitrogen concentrations in ambient seawater (Martínez & Rico, Citation2002; Kim et al., Citation2007; Barufi et al., Citation2011). Phycobilins may be a major nitrogen store, but under these experimental conditions there is no evidence that the stores were used as the algae were nitrogen sufficient during the acclimation period. The algae responded to the limiting irradiance by increasing both PE and Chl a content, which would enhance the light harvesting capacity i.e. shade adaptation. Phycocyanin content was negligible (0.015 ± 0.005 mg g WW−1) indicating that it is not an important phycobilin for H. frondosa. PE and Chl a contents were similar to other Rhodophyta studies (Sampath-Wiley et al., Citation2008; Ribeiro et al., Citation2013; Wu, Citation2016).
An important finding of this study was that NR activity was strongly, negatively affected by freezing samples in liquid nitrogen and storing at −80°C for 2 weeks. Although we achieved the same result, i.e. no effect of light on NR activity, the activity in fresh material was ~65% greater than that of frozen material. This technique of freezing biological material for subsequent enzyme assays is common practice (Geiger et al., Citation1998; Chow et al., Citation2004, Citation2007; Chow & De Oliveira, Citation2008; Pritchard, Citation2013; Fernández et al., Citation2015), but our study illustrates the importance of testing the effect of freezing and storage prior to experiments.
We have shown that irradiance plays a significant role in both NO3− and NH4+ uptake, as well as utilization for H. frondosa. Genomic characterization of NO3− and NH4+ transporters and their regulation by external factors would enhance our knowledge of inorganic nitrogen uptake in macroalgae in the future. The majority of studies on nitrogen uptake kinetics of red macroalgae are for species of commercial interest e.g. Porphyra/Pyropia spp. (Thomas & Harrison, Citation1985; Kraemer et al., Citation2004; Kang et al., Citation2014), Gracilaria spp. (Bird et al., Citation1982; Lapointe et al., Citation1984; Thomas et al., Citation1987; Naldi & Wheeler, Citation1999, Citation2002; Smit, Citation2002; Barufi et al., Citation2011), Palmaria palmata (Martínez & Rico, Citation2004; Corey et al., Citation2013; Grote, Citation2016) and Chondrus crispus (Amat & Braud, Citation1990; Corey et al., Citation2013). The limited knowledge of nitrogen uptake kinetics for non-commercial red macroalgae growing in natural systems highlights a key gap in the current phycological literature, along with the need for an improved understanding of the biochemical and molecular processes regulating inorganic nitrogen uptake and assimilation.
Author contributions
E. Paine: experimental design, conducted experiments and data analyses, drafting and editing of manuscript; M. Schmid: experimental design, laboratory assistance, editing manuscript; A. Revill: analysis of tissue carbon and nitrogen content; C. Hurd: original concept, experimental design, editing manuscript
Acknowledgements
We wish to thank Damon Britton, Dr Fanny Noisette, Harrison Vermont, Abbie Smith, Olivia Johnson and Joanna Smart for their help in the laboratory and field, Drs Erica Young and John Berges for critical feedback on the manuscript and Dr Daniel Pritchard for assistance with nitrate reductase assays.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Abreu, M.H., Pereira, R., Buschmann, A.H., Sousa-Pinto, I. & Yarish, C. (2011). Nitrogen uptake responses of Gracilaria vermiculophylla (Ohmi) Papenfuss under combined and single addition of nitrate and ammonium. Journal of Experimental Marine Biology and Ecology, 407: 190–199.
- Ak, I. & Yücesan, M. (2012). Effect of light intensity on the pigment composition of Gracilaria verrucosa (Rhodophyta). Fresenius Environmental Bulletin 21: 2126-2131.
- Alcoverro, T., Manzanera, M. & Romero, J. (2000). Nutrient mass balance of the seagrass Posidonia oceanica: the importance of nutrient retranslocation. Marine Ecology Progress Series, 194: 13–21.
- Amat, M.A. & Braud, J.P. (1990). Ammonium uptake by Chondrus crispus Stackhouse (Gigartinales, Rhodophyta) in culture. Hydrobiologia, 204–205: 467–471.
- Barufi, J.B., Korbee, N., Oliveira, M.C. & Figueroa, F.L. (2011). Effects of N supply on the accumulation of photosynthetic pigments and photoprotectors in Gracilaria tenuistipitata (Rhodophyta) cultured under UV radiation. Journal of Applied Phycology, 23: 457–466.
- Beer, S. & Eshel, A. (1985). Determining phycoerythrin and phycocyanin concentrations in aqueous crude extracts of red algae. Marine and Freshwater Research, 36: 785–792.
- Bird, K.T., Habig, C. & DeBusk, T. (1982). Nitrogen allocation and storage patterns in Gracilaria tikvahiae (Rhodophyta). Journal of Phycology, 18: 344–348.
- Borum, J., Murray, L. & Kemp, W.M. (1989). Aspects of nitrogen acquisition and conservation in eelgrass plants. Aquatic Botany, 35: 289–300.
- Cabello-Pasini, A., Macías-Carranza, V., Abdala, R., Korbee, N. & Figueroa, F.L. (2011). Effect of nitrate concentration and UVR on photosynthesis, respiration, nitrate reductase activity, and phenolic compounds in Ulva rigida (Chlorophyta). Journal of Applied Phycology, 23: 363–369.
- Chapman, A.R.O. & Craigie, J.S. (1977). Seasonal growth in Laminaria longicruris: relations with dissolved inorganic nutrients and internal reserves of nitrogen. Marine Biology, 40: 197–205.
- Chopin, T., Gallant, T. & Davison, I. (1995). Phophorus and nitrogen nutrition in Chondrus crispus (Rhodophyta): effects on total phosphorus and nitrogen content, carrageenan production, and photosynthetic pigments and metabolism. Journal of Phycology, 31: 283–293.
- Chow, F., Capociama, F.V., Faria, R. & De Oliveira, M.C. (2007). Characterization of nitrate reductase activity in vitro in Gracilaria caudata J. Agardh (Rhodophyta, Gracilariales). Brazilian Journal of Botany, 30: 123–129.
- Chow, F. & De Oliveira, M.C. (2008). Rapid and slow modulation of nitrate reductase activity in the red macroalga Gracilaria chilensis (Gracilariales, Rhodophyta): Influence of different nitrogen sources. Journal of Applied Phycology, 20: 775–782.
- Chow, F., De Oliveira, M.C. & Pedersén, M. (2004). In vitro assay and light regulation of nitrate reductase in red alga Gracilaria chilensis. Journal of Plant Physiology, 161: 769–776.
- Chow, F., Pedersén, M. & Oliveira, M.C. (2013). Modulation of nitrate reductase activity by photosynthetic electron transport chain and nitric oxide balance in the red macroalga Gracilaria chilensis (Gracilariales, Rhodophyta). Journal of Applied Phycology, 25: 1847–1853.
- Conolly, N.J. & Drew, E.A. (1985). Physiology of Laminaria: IV. Nutrient supply and daylength, major factors affecting growth of L. digitata and L. saccharina. Marine Ecology, 6: 299–320.
- Corey, P., Kim, J.K., Duston, J., Garbary, D.J. & Prithiviraj, B. (2013). Bioremediation potential of Palmaria palmata and Chondrus crispus (Basin Head): effect of nitrate and ammonium ratio as nitrogen source on nutrient removal. Journal of Applied Phycology, 25: 1349–1358.
- Cornwall, C.E., Revill, A.T. & Hurd, C.L. (2015). High prevalence of diffusive uptake of CO2 by macroalgae in a temperate subtidal ecosystem. Photosynthetic Research, 124: 181–190.
- Corzo, A. & Niell, X. (1992). Blue light induction of in situ nitrate reductase activity in the marine green alga Ulva rigida. Functional Plant Biology, 19: 625–635.
- D’Elia, C.F. & DeBoer, J.A. (1978). Nutritional studies of two red algae. II. Kinetics of ammonium and nitrate uptake. Journal of Phycology, 14: 266–272.
- Davison, I.R. & Stewart, W.D.P. (1984). Studies on nitrate reductase activity in Laminaria digitata (Huds.) Lamour. II. The role of nitrate availability in the regulation of enzyme activity. Journal of Experimental Marine Biology and Ecology, 79: 65–78.
- Diamond, D. (2008). Determination of nitrate/nitrite in brackish or seawater by flow injection analysis. 19. LaChat Instruments, Loveland, USA.
- Fernández, P.A., Roleda, M.Y. & Hurd, C.L. (2015). Effects of ocean acidification on the photosynthetic performance, carbonic anhydrase activity and growth of the giant kelp Macrocystis pyrifera. Photosynthetic Research, 124: 293–304.
- Figueroa, F.L. (1996). Effects of light quality on nitrate reductase and glutainine synthetase activities in the red alga Porphyra leucosticta Thur. in Le Jol. and other macroalgae. Scientia Marina, 60: 163–170.
- Figueroa, F.L., Aguilera, J. & Niell, F.X. (1995). Red and blue light regulation of growth and photosynthetic metabolism in Porphyra umbilicalis (Bangiales, Rhodophyta). European Journal of Phycology, 30: 11–18.
- Figueroa, F.L., Salles, S., Aguilera, J., Jiménez, C., Mercado, J., Viñegla, B., Flores-Moya, A. & Altamirano, M. (1997). Effects of solar radiation on photoinhibition and pigmentation in the red alga Porphyra leucosticta. Marine Ecology Progress Series, 151: 81–90.
- Fujita, R.M., Wheeler, P.A., & Edwards, R.L. (1988). Metabloic regulation of ammonium uptake by Ulva rigida (Chlorophyta): A compartmental analysis of the rate-limiting step for uptake. Journal of Phycology, 24: 560-566.
- Galván, A. & Fernández, E. (2001). Eukaryotic nitrate and nitrite transporters. Cellular and Molecular Life Sciences, 58: 225–233.
- Geiger, M., Walch-Liu, P., Engels, C., Harnecker, J., Schulze, E.D., Ludewig, F., Sonnewald, U., Scheible, W.R. & Stitt, M. (1998). Enhanced carbon dioxide leads to a modified diurnal rhythm of nitrate reductase activity in older plants, and a large stimulation of nitrate reductase activity and higher levels of amino acids in young tobacco plants. Plant, Cell & Environment, 21: 253–268.
- Glass, A.D.M. (2003). Nitrogen use efficiency of crop plants: physiological constraints upon nitrogen absorption. Critical Reviews in Plant Sciences, 22: 453–470.
- Gordillo, F.J.L., Aguilera, J. & Jiménez, C. (2006). The response of nutrient assimilation and biochemical composition of Arctic seaweeds to a nutrient input in summer. Journal of Experimental Botany, 57: 2661–2671.
- Grote, B. (2016). Bioremediation of aquaculture wastewater: evaluating the prospects of the red alga Palmaria palmata (Rhodophyta) for nitrogen uptake. Journal of Applied Phycology, 28: 3075–3082.
- Harrison, P.J. & Druehl, L.D. (1982). Nutrient uptake and growth in the Laminariales and other macrophytes: a consideration of methods In Synthetic and Degradative Processes in Marine Macrophytes (Srivastava, L.M., editor), 99–120. Walter de Gruyter, Berlin.
- Harrison, P.J. & Hurd, C.L. (2001). Nutrient physiology of seaweeds: application of concepts to aquaculture. Cahiers de Biologie Marine, 42: 71–82.
- Hipkin, C.R., Thomas, R.J. & Syrett, P.J. (1983). Effects of nitrogen deficiency on nitrate reductase, nitrate assimilation and photosynthesis in unicellular marine algae. Marine Biology, 77: 101–105.
- Hurd, C.L., Berges, J.A., Osborne, J. & Harrison, P.J. (1996). Erratum: An in vitro reductase assay for marine microalgae: optimization and characterization of the enzyme for Fucus gardneri (Phaeophyta) (Journal of Phycology 31 (835–431)). Journal of Phycology, 32: 1094.
- Hurd, C.L. & Dring, M.J. (1990). Phosphate uptake by intertidal algae in relation to zonation and season. Marine Biology, 107: 281–289.
- Hurd, C.L., Harrison, P.J., Bischof, K. & Lobban, C.S. (2014). Seaweed Ecology and Physiology. 2nd ed. Cambridge University Press, Cambridge.
- Hwang, S.P.L., Williams, S.L. & Brinkhuis, B.H. (1987). Changes in internal dissolved nitrogen pools as related to nitrate uptake and assimilation in Gracilaria tikvahiae McLachlan (Rhodophyta). Botanica Marina, 30: 11–20.
- Kakinuma, M., Coury, D.A., Nakamoto, C., Sakaguchi, K. & Amano, H. (2008). Molecular analysis of physiological responses to changes in nitrogen in a marine macroalga, Porphyra yezoensis (Rhodophyta). Cell Biology and Toxicology, 24: 629–639.
- Kang, Y.H., Kim, S., Lee, J.B., Chung, I.K. & Park, S.R. (2014). Nitrogen biofiltration capacities and photosynthetic activity of Pyropia yezoensis Ueda (Bangiales, Rhodophyta): groundwork to validate its potential in integrated multi-trophic aquaculture (IMTA). Journal of Applied Phycology, 26: 947–955.
- Kim, J.K., Kraemer, G.P., Neefus, C.D., Chung, I.K. & Yarish, C. (2007). Effects of temperature and ammonium on growth, pigment production and nitrogen uptake by four species of Porphyra (Bangiales, Rhodophyta) native to the New England coast. Journal of Applied Phycology, 19: 431–440.
- Kraemer, G.P., Carmona, R., Chopin, T., Neefus, C., Tang, X. & Yarish, C. (2004). Evaluation of the bioremediatory potential of several species of the red alga Porphyra using short-term measurements of nitrogen uptake as a rapid bioassay. Journal of Applied Phycology, 16: 489–497.
- Kregting, L.T., Hurd, C.L., Pilditch, C.A. & Stevens, C.L. (2008). The relative importance of water motion on nitrogen uptake by the subtidal macroalga Adamsiella chauvinii (Rhodophyta) in winter and summer. Journal of Phycology, 44: 320–330.
- Lapointe, B.E. (1981). The effects of light and nitrogen on growth, pigment content, and biochemical composition on Gracilaria folifera v. Angustissima (Gigartinales, Rhodophyta). Journal of Phycology, 17: 90–95.
- Lapointe, B.E., Dawes, C.J. & Tenore, K.R. (1984). Interactions between light and temperature on the physiological ecology of Gracilaria tikvahiae (Gigartinales: Rhodophyta) – II. Nitrate uptake and levels of pigments and chemical constituents. Marine Biology, 80: 171–178.
- Lartigue, J. & Sherman, T.D. (2005). Response of Enteromorpha sp. (Chlorophyceae) to a nitrate pulse: nitrate uptake, inorganic nitrogen storage and nitrate reductase activity. Marine Ecology Progress Series, 292: 147–157.
- Lavery, P.S. & McComb, A.J. (1991). The nutritional eco-physiology of Chaetomorpha linum and Ulva rigida in Peel Inlet, Western Australia. Botanica Marina, 34: 251–260.
- Liao, N. (2008). Determination of ammonia in brackish or seawater by flow injection analysis. 20. LaChat Instruments Loveland, USA.
- Marinho-Soriano, E. (2012). Effect of depth on growth and pigment contents of the macroalgae Gracilaria bursapastoris. Brazilian Journal of Botany, 22: 730–735.
- Marquardt, R., Schubert, H., Varela, D.A., Huovinen, P., Henríquez, L. & Buschmann, A.H. (2010). Light acclimation strategies of three commercially important red algal species. Aquaculture, 299: 140–148.
- Martínez, B. & Rico, J.M. (2002). Seasonal variation of P content and major N pools in Palmaria palmata (Rhodophyta). Journal of Phycology, 38: 1082–1089.
- Martínez, B. & Rico, J.M. (2004). Inorganic nitrogen and phosphorus uptake kinetics in Palmaria palmata (Rhodophyta). Journal of Phycology, 40: 642–650.
- McGlathery, K.J., Pedersen, M.F. & Borum, J. (1996). Changes in intracellular nitrogen pools and feedback controls on nitrogen uptake in Chaetomorpha linum (Chlorophyta). Journal of Phycology, 32: 393–401.
- Naldi, M. & Wheeler, P.A. (1999). Changes in nitrogen pools in Ulva fenestrata (Chlorophyta) and Gracilaria pacifica (Rhodophyta) under nitrate and ammonium enrichment. Journal of Phycology, 35: 70–77.
- Naldi, M. & Wheeler, P.A. (2002). 15N measurements of ammonium and nitrate uptake by Ulva fenestrata (Chlorophyta) and Gracilaria pacifica (Rhodophyta): comparison of net nutrient disappearance, release of ammonium and nitrate, and 15N accumulation in algal tissue. Journal of Phycology, 38: 135–144.
- Nishihara, G.N., Terada, R. & Noro, T. (2005). Effect of temperature and irradiance on the uptake of ammonium and nitrate by Laurencia brongniartii (Rhodophyta, Ceramiales). Journal of Applied Phycology, 17: 371–377.
- Ochoa-Izaguirre, M.J. & Soto-Jiménez, M.F. (2015). Variability in nitrogen stable isotope ratios of macroalgae: consequences for the identification of nitrogen sources. Journal of Phycology, 51: 46–65.
- Phillips, J.C. & Hurd, C.L. (2003). Nitrogen ecophysiology of intertidal seaweeds from New Zealand: N uptake, storage and utilisation in relation to shore position and season. Marine Ecology Progress Series, 264: 31–48.
- Pritchard, D.W. (2013). Ecophysiology of the deep-water macroalga Anotrichium crinitum (Kutzing) Baldock. In Aquatic Botany. University of Otago, Dunedin, New Zealand.
- Pritchard, D.W. (2013). Ecophysiology of the deep-water macroalga Anotrichium crinitum (Kutzing) Baldock. In Aquatic Botany University of Otago, Dundedin, New Zealand.
- Pritchard, D.W., Hurd, C.L., Beardall, J. & Hepburn, C.D. (2015). Restricted use of nitrate and a strong preference for ammonium reflects the nitrogen ecophysiology of a light-limited red alga. Journal of Phycology, 51: 277–287.
- R Development Core Team (2012). R: A Language and Environment for Statistical Computing. R foundation for Statistical Computing, Vienna.
- Raven, J.A., Beardall, J. & Giordano, M. (2014). Energy costs of carbon dioxide concentrating mechanisms in aquatic organisms. Photosynthesis Research, 121: 111–124.
- Raven, J.A. & Hurd, C.L. (2012). Ecophysiology of photosynthesis in macroalgae. Photosynthesis Research, 113: 105–125.
- Raven, J.A., Wollenweber, B. & Handley, L.L. (1992). A comparison of ammonium and nitrate as nitrogen sources for photolithotrophs. New Phytologist, 121: 19–32.
- Rees, A.V.T., Grant, C.M., Harmens, H.E. & Taylor, R.B. (1998). Measuring rates of ammonium assimilation in marine algae: use of the protonophore carbonyl cyanide m-chlorophenylhydrazone to distinguish between uptake and assimilation. Journal of Phycology, 34: 264–272.
- Ribeiro, A.L.N.L., Tesima, K.E., Souza, J.M.C. & Yokoya, N.S. (2013). Effects of nitrogen and phosphorus availabilities on growth, pigment, and protein contents in Hypnea cervicornis J. Agardh (Gigartinales, Rhodophyta). Journal of Applied Phycology, 25: 1151–1157.
- Ritchie, R.J. (2006). Consistent sets of spectrophotometric chlorophyll equations for acetone, methanol and ethanol solvents. Photosynthetic Research, 89: 27–41.
- Roleda, M.Y. & Hurd, C.L. (2019). Seaweed nutrient physiology: application of concepts to aquaculture and bioremediation. Phycologia, 58: 552–562.
- Runcie, J.W., Ritchie, R.J. & Larkum, A.W.D. (2003). Uptake kinetics and assimilation of inorganic nitrogen by Catenella nipae and Ulva lactuca. Aquatic Botany, 76: 155–174.
- Sampath-Wiley, P., Neefus, C.D. & Jahnke, L.S. (2008). Seasonal effects of sun exposure and emersion on intertidal seaweed physiology: fluctuations in antioxidant contents, photosynthetic pigments and photosynthetic efficiency in the red alga Porphyra umbilicalis Kützing (Rhodophyta, Bangiales). Journal of Experimental Marine Biology and Ecology, 361: 83–91.
- Smit, A.J. (2002). Nitrogen uptake by Gracilaria gracilis (Rhodophyta): adaptations to a temporally variable nitrogen environment. Botanica Marina, 45: 196–209.
- Syrett, P.J. (1981). Uptake and utilisation of nitrogenous compounds. Canadian Bulletin of Fisheries and Aquatic Sciences, 210: 182–210.
- Systat Software, Inc. San Jose, California. www.systatsoftware.com.
- Thomas, T.E. & Harrison, P.J. (1985). Effect of nitrogen supply on nitrogen uptake, accumulation and assimilation in Porphyra perforata (Rhodophyta). Marine Biology, 85: 269–278.
- Thomas, T.E. & Harrison, P.J. (1987). Rapid ammonium uptake and nitrogen interactions in five intertidal seaweeds grown under field conditions. Journal of Experimental Marine Biology and Ecology, 107: 1–8.
- Thomas, T.E. & Harrison, P.J. (1988). A comparison of in vitro and in vivo nitrate reductase assays in three intertidal seaweeds. Botanica Marina, 31: 101–108.
- Thomas, T.E., Harrison, P.J. & Turpin, D.H. (1987). Adaptations of Gracilaria pacifica (Rhodophyta) to nitrogen procurement at different intertidal locations. Marine Biology 93: 569–580.
- Turpin, D.H. (1991). Effects of inorganic nitrogen availability on algal photosynthesis and carbon metabolism. Journal of Phycology, 27: 14–20.
- Udayakumar, M., Devendra, R., Reddy, V.S. & Sastry, K.S.K. (1981). Nitrate availability under low irradiance and its effect on nitrate reductase activity. New Phytologist, 88: 289–297.
- Vergara, J.J. & Niell, F.X. (1993). Effects of nitrate avaliabilty and irradiance on internal nitrogen constituents in Corallina elongata (Rhodophyta). Journal of Phycology, 29: 285-293.
- Wheeler, W.N. (1982). Nitrogen nutrition of Macrocystis. In Synthetic and Degradative Processes in Marine Macrophytes (Srivastivia, L.M., editor), 121–137. Walter de Gruyter, Berlin.
- Wu, H. (2016). Effect of different light qualities on growth, pigment content, chlorophyll fluorescence, and antioxidant enzyme activity in the red alga Pyropia haitanensis (Bangiales, Rhodophyta). BioMed Research International 2016.
- Young, E.B., Dring, M.J. & Berges, J.A. (2007). Distinct patterns of nitrate reductase activity in brown algae: light and ammonium sensitivity in Laminaria digitata is absent in Fucus species. Journal of Phycology, 43: 1200–1208.
- Young, E.B., Lavery, P.S., Van Elven, B., Dring, M.J. & Berges, J.A. (2005). Nitrate reductase activity in macroalgae and its vertical distribution in macroalgal epiphytes of seagrasses. Marine Ecology Progress Series, 288: 103–114.