ABSTRACT
Cyanobacteria synthesize many secondary metabolites of various chemical structures and biological activity and release them into the aquatic environment. Two new oligopeptides belonging to anabaenopeptins and cyanopeptolins were isolated from an enrichment culture dominated by Woronichinia naegeliana (Unger) Elenkin. The application of mass spectrometry and nuclear magnetic resonance spectroscopy allowed determination of their structure and identification of these compounds as cyanopeptolin 1081 and anabaenopeptin 899 with a molecular formula C52H75N9O16 and C48H65N7O10, respectively. The preliminary evaluation of their toxicity was conducted via tests based on crustacean Thamnocephalus platyurus. Although these peptides did not exert acute toxic effects at the concentration up to 40 μg ml–1, deterioration of the physiological state of the studied crustaceans, indicated by reduced mobility, was observed. Considering the limited knowledge of cyanopeptide presence in the aquatic environment, it is important to identify and characterize these compounds.
Introduction
Occurrences of massive blooms of cyanobacteria are undesirable for ecological, health and aesthetic reasons. Blooms adversely affect the organoleptic properties of water, give it an unpleasant smell, reduce the transparency of the water column, influencing the decrease in the number of phytoplankton and zooplankton, and consequently the fish and benthic fauna (Sukenik et al., Citation2015). Cyanobacteria adapting to the changing environment have evolved to synthesize and release into the aquatic environment many secondary metabolites of various chemical structures and mechanisms of biological effects. Most of the literature on cyanobacteria has focused on their ability to produce different types of toxins. Due to their harmful effects on vertebrates, these compounds are divided into four groups: hepatotoxins (which cause liver damage, e.g. microcystins), neurotoxins (which cause dysfunction of the nervous system, e.g. anatoxin-a), dermatotoxins (which cause irritation of the skin, e.g. aplysiatoxin) and cytotoxins (which exert negative effects on many organs, e.g. cylindrospermopsin) (Chorus & Bartram, Citation1999). Depending on the method of exposure to cyanotoxins (e.g. skin contact, ingestion, inhalation of water aerosol), symptoms may include skin rashes, diarrhoea, tremors, mild convulsion, paralysis, or even death (Svirčev et al., Citation2017). As a consequence monitoring of cyanobacteria and its metabolites are an important part of the water quality management.
In addition to toxins, cyanobacteria produce numerous bioactive compounds, some of which are of medical interest, while others are harmful to animals (Ali Shah et al., Citation2017; Singh et al., Citation2017). Taking into consideration the structural diversity, peptides represent more than 60% of the known biologically active cyanobacterial compounds and there are at least 500 oligopeptides ranging from 400 to 1900 Da described to date (Jansen, Citation2019). In terms of molecular structure cyanopeptides are divided into linear (e.g. aeruginosins, microginins), cyclic (e.g. anabaenopeptins, cyanopeptolins, cyclamides) and multicyclic (e.g. microviridins) (Welker & von Döhren, Citation2006). Most of the known cyanopeptides are synthesized outside the ribosomes through the multifunctional non-ribosomal peptide synthetase (NRPS) or the hybrid pathway non-ribosomal peptide synthetase/polyketide synthase (NRPS/PKS) which seems to be a very old evolutionary metabolic pathway of cyanobacteria (Welker & von Döhren, Citation2006). It seems natural selection did not minimize the available pool of peptides to only efficient compounds but favoured maintaining the synthesis of a wide range of compound efficacy (Czarnecki et al., Citation2006; Gademann et al., Citation2010). Cyanobacterial peptides belong to more than one class, each class containing more than 10 unique peptides but frequently only a few are dominant (Harada et al., Citation1993; Czarnecki et al., Citation2006). Cyanopeptides are often described as ‘non-toxic’, but there is the possibility of synergistic interactions between them and cyanotoxins. It has been discovered that crude cyanobacterial extracts exert stronger effects on vertebrates and invertebrates than exposure to purified toxins (Keil et al., Citation2002; Šuput et al., Citation2002). Therefore, the harmful effects of cyanobacteria cannot be attributed just to known cyanotoxins. Because cyanobacteria produce a mixture of potentially toxic metabolites, there is a need to identify and characterize these compounds.
The cyanobacterium Woronichinia naegeliana (Unger) Elenkin belonging to Chroococcales appears numerously in surface fresh water in Europe (including Sweden, Finland, Poland, the Czech Republic, Bulgaria, Belgium, Portugal, Spain), North America (USA, Mexico) and Australia (Sivonen et al., Citation1990; Wilk-Woźniak, Citation1998; Cronberg et al., Citation1999; Komárek & Anagnostidis, Citation1999; Rajaniemi-Wacklin et al., Citation2005; Willame et al., Citation2005; Oberholster et al., Citation2006; Quesada et al., Citation2006; Kopp et al., Citation2012; Santos et al., Citation2012; Dochin, Citation2019; Nandini et al., Citation2019). This cosmopolitan species prefers slightly eutrophic, mesotrophic and oligotrophic reservoirs in the temperate zones (Rajaniemi-Wacklin et al., Citation2005). Interestingly, it is speculated that the dominance of W. naegeliana might be classified as an indicator of lowering trophic status from eutrophic to meso-eutrophic (Pociecha & Wilk-Woźniak, Citation2005). W. naegeliana can form blooms at a lower temperature than other bloom-forming cyanobacteria; therefore, its outbreaks have usually been observed in late summer and autumn (Wilk-Woźniak & Mazurkiewicz-Boroń, Citation2003). Round, kidney-shaped, sometimes irregular colonies consist of an internal system of radially arranged mucilaginous stalks connected to the cells (Komárek & Anagnostidis, Citation1999). The presence of gas vesicles allows free movement of the colony in the water column. W. naegeliana multiplies through decay or throwing cells from the colony under stress. It is not capable of fixation of atmospheric nitrogen. In terms of the living strategy model, the cyanobacterium belongs to S-type ‘stress-tolerant’, able to grow in the condition of limited access to nutrients with the simultaneous availability of sufficient photosynthetically active radiation (Wilk-Woźniak & Mazurkiewicz-Boroń, Citation2003). Although many attempts to maintain its isolated strains in culture were performed (Rajaniemi-Wacklin et al. Citation2006; Lara et al. Citation2013) up to now there are no pure cultures of W. naegeliana in the world collection. Therefore, studies of this species have been based on natural populations and though this species frequently occurs worldwide in reservoirs that constitute drinking water, scarce information about its secondary metabolites production is available. The obtained results demonstrated that its cellular extract caused biological activity towards zooplankton (Bober & Bialczyk, Citation2017). Preliminary experiments show that W. naegeliana is a rich source of cyanopeptides (Bober et al., Citation2011), and most of these compounds belong to the microginins and cyanopeptolins whose role in the cyanobacteria metabolism and biological properties have not been clearly specified. Several unknown compounds whose structure has not been determined were also found. Therefore, the main aim of the present study was quantitative and qualitative identification of novel metabolites isolated from a natural population of W. naegeliana, which have not yet been described in the literature. In addition, their biological activity was determined by using the toxicity test on the freshwater crustacean Thamnocephalus platyurus.
Materials and methods
Cyanobacteria
Woronichinia naegeliana (Unger) Elenkin was collected from Dobczyce Reservoir (southern Poland) during a bloom in September 2017. Identification and isolation of the species were done according to Bober et al. (Citation2011). The field-collected samples dominated by W. naegeliana (over 95%) in association with Microcystis flos-aquae, determined by inverted microscopy (Eclipse TS-100F Nicon) were concentrated in test tubes under natural light. Due to a buoyancy mechanism colonies of W. naegeliana floated towards the surface and this layer was collected, passed through steel filters (30 μm diameter) and washed five times in Milli-Q water. Reexamination by microscopy showed that the samples contain over 99% W. naegeliana after this procedure. Lyophilized samples of an enrichment culture dominated by W. naegeliana were deposited in the Department of Plant Physiology and Development, Jagiellonian University, Krakow, Poland.
Sample preparation
The lyophilized cell material (18 g of dry weight (d.w.) divided into 4.5 g d.w. portions) was extracted three times with 100% methanol under constant shaking and then filtered through GF/C glass-fibre filters (Whatman, UK). The obtained extract was evaporated to dryness in a nitrogen atmosphere at 23°C and the residues were resolved in Milli-Q water. The sample was concentrated by solid-phase extraction (SPE) with a C18 silica cartridge (Baker Bond, USA). The cartridge was conditioned with 10 ml 100% methanol followed by 10 ml solution of methanol:10% acetic acid (4:1, v/v). The aqueous sample was passed through at 5 ml min–1 flow rate followed with 10 ml portions of 10%, 20% and 30% methanol. The eluate was obtained using 10 ml 80% methanol. After evaporation to dryness in a nitrogen atmosphere at 23°C, the residue was dissolved in Milli-Q water for further analysis.
Isolation and identification
Separation of sample on fractions containing secondary metabolites was performed according to the procedure described in Bober & Bialczyk (Citation2017). Briefly, a high-performance liquid chromatography (HPLC) Waters system containing a 600E gradient pump, 717 plus autosampler, a Jetstream 2 plus column thermostat, 996 photodiode array detector, and Millennium32 SS Software with PDA option was used. The extract separation was achieved using a Symmetry C18 column (4.6 × 250 mm; 5 μm; Waters) maintained at 23°C and gradient mobile phases consisting of: (A) water/trifluoroacetic acid (0.05%, v/v) and (B) acetonitrile/trifluoroacetic acid (0.05%, v/v). The elution gradient was changed from 70% to 35% of eluent A over 35 min at a flow rate of 0.7 ml min–1. Chromatograms were monitored at 220 nm. A detailed qualitative identification was carried out for the two fractions isolated manually at retention time 14.2 min (A) and 28.5 min (B), which were quantitatively dominated in the extract. These fractions were collected from a series of consecutive injections, evaporated to dryness in a nitrogen atmosphere at 23°C to yield 11.6 mg of A and 1.9 mg of B, and then were stored at −20°C to prevent possible modification. The content and the purity of the collected fractions were analysed by ultra-performance liquid chromatography – mass spectrometry (UPLC-MS/MS) using Waters ACQUITY system coupled to a Waters TQD Electrospray Ionization – tandem quadrupole mass spectrometer. Chromatographic separations were performed on an Acquity UPLC BEH (bridged ethyl hybrid) C18 column (2.1 × 100 mm; 1.7 μm; Waters) equipped with an Acquity UPLC BEH C18 VanGuard pre-column (2.1 × 5 mm; 1.7 μm; Waters) maintained at 40°C. The gradient mobile phases consisting of: (A) water/formic acid (0.1%, v/v) and (B) acetonitrile/formic acid (0.1%, v/v) changed from 5% to 100% of eluent B over 10 min at a flow rate 0.3 ml min–1. Operating conditions of Waters TQD mass spectrometer were as follows: source temperature 150°C, desolvation temperature 350°C, desolvation gas flow rate 600 l h–1 and cone gas flow 100 l h–1. The capillary and cone potential were set at 3.00 kV and 20 V, respectively. Nitrogen was used for both nebulizing and drying gas. The scan mode ranging from 50 to 2000 m/z in 1.0 s intervals was applied; 8 scans were summed up to get the final spectrum. Collision activated dissociation (CAD) analyses were carried out with the energy of 40 eV, and all the fragmentations were observed in the source. Consequently, the ion spectra were obtained by scanning from 50 to 1100 m/z range. The MassLynx V 4.1 (Waters) software was used for data evaluation. Structures of peptides present in the two isolated fractions were further determined by nuclear magnetic resonance (NMR) analyses. The NMR spectra were collected using a 700 MHz Bruker Avance III HD spectrometer equipped with a QCI CryoProbe. All the data were acquired in either CD3CN/H2O (4:1) or CD3CN/D2O (4:1) mixtures at 25°C. The recorded spectra were then processed using the TopSpin 3.6 software (Bruker) and analysed with NMRFAM-SPARKY (Lee et al., Citation2015). Proton chemical shifts were referenced to sodium 3-trimethylsilyl-[2,2,3,3-D4]-propionate (TSP) and then used to indirectly reference the carbon shifts. The full set of spectra recorded for each sample comprised: COSY, TOCSY, multiplicity-edited 1H-13C HSQC, broad spectral window 1H-13C HMBC (200 ppm window in the carbon dimension), narrow spectral window 1H-13C HMBC centred at the carbonyl resonances (around 40 ppm spectral window; the exact width of the window was selected such as to minimize the folding of other resonances into the 170–178 ppm carbonyl region) and ROESY (400 ms mixing time). For the peptide A an additional 1H-15N HMBC spectrum was also acquired.
Toxicity assay
The evaluation of biological activity towards zooplankton of isolated and structurally determined peptides was performed through the application of commercially available acute toxicity assay based on the freshwater crustacean Thamnocephalus platyurus (Thamnotoxkit F™) following the respective Standard Operational Procedures (Microbiotests Inc., Belgium). The dilution series of the peptides were prepared with appropriate exposure media in the range from 2.5 to 40.0 μg ml–1. The quantitative importance of the toxic effects was calculated as a 50% effective concentration. Mortality (LC50) of T. platyurus larvae was recorded after 24 h incubation in dark condition at 25°C. The exposure medium was used as a control. The bioassays were considered as valid if the mortality of tested organisms in the controls did not exceed 10%.
Chemicals
All reagents were analytical, HPLC or MS grade and were purchased from Sigma-Aldrich (USA) or delivered within Thamnotoxkit F test (Microbiotests Inc., Belgium). NMR solvents were bought from Across Organics (USA) and ultrapure grade water (Milli-Q water) was obtained from Millipore (USA).
Results
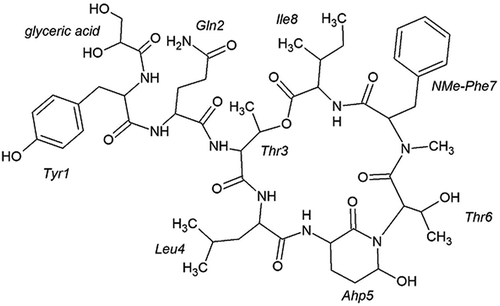
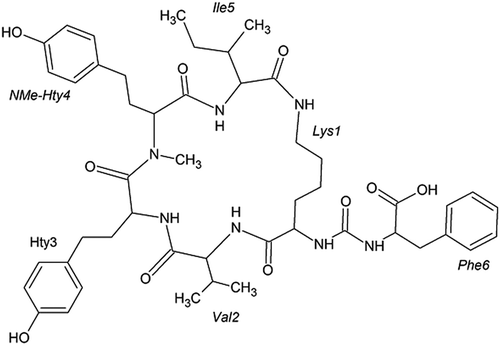
HPLC separation of the extract from an enrichment culture dominated by W. naegeliana led to the isolation of a highly homogeneous analyte A at a retention time of 14.2 min with an absorption maximum at 276 nm and analyte B at a retention time of 28.5 min with an absorption maximum at 278 nm. These compounds have not been described in the literature yet. Their structural elucidation was performed based mainly on NMR spectra, while analyses of additional data such as MS/MS spectra were also helpful. Thus, compound A (cyanopeptolin 1081, ) was identified as a member of the family of cyanopeptolins, while compound B (anabaenopeptin 899, ) was recognized as a member of the anabaenopeptins class.
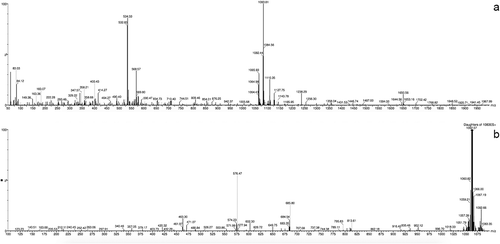
Cyanopeptolin 1081 was isolated as a colourless amorphous powder (11.6 mg, 0.064% yield). The analysis of the proton-proton and proton-carbon connectivities peptide allowed identification of the following residues in its structure: leucine (Leu), isoleucine (Ile), N-methylated phenylalanine (NMe-Phe), tyrosine (Tyr), glutamine (Gln), two instances of threonine (Thr), one highly unusual amino acid (later identified as 3-amino-6-hydroxy-2-piperidone amino acid (Ahp)) and one non-amino acid residue (glyceric acid (GA)) (Supplementary figs 1–8). The chemical shifts of the assigned proton and carbon atoms, as well as the full set of HMBC correlations, are presented in . The HMBC connectivities involving the carbonyl groups (C1) allowed to unambiguously establish the amino acid sequence as: GA-Tyr1-Gln2-Thr3-Leu4-Ahp5-Thr6-NMePhe7-Ile8. In addition, the HMBC cross-peak between the Thr3-H2 and Ile8-C1 reports on the cyclization of the depsipeptide, achieved by ester formation between the C-terminal carboxylic group of Ile8 and the side chain OH group of Thr3. The following data support the identification of the non-standard amino acid as Ahp: (a) the scalar connectivities identify the side chain of this residue as constructed by two consecutive CH2 groups (C3 and C4) followed by another carbon atom with only a single proton attached (C5). The C5 atom thus has to have two additional bonding partners; (b) two 1H-13C HMBC peaks such as Ahp5-H5 to Thr6-C2 and Thr6-H2 to Ahp5-C5 report on the direct covalent bonding between the side chain C5 of the non-standard residue and the following Thr6 residue while strongly supporting the idea of Ahp5-C5 to Thr6-N bonding. This observation was also supported by analysis of 1H-15N HMBC spectrum (Supplementary fig. 8); (c) the last bonding partner of Ahp5-C5 was established to be an OH group, based on the presence of a hydroxyl proton resonance at 5.91 ppm, giving rise to a COSY cross-peak with Ahp5-H5 (theoretically it might be a thiol SH group instead, but such an interpretation would not fit the known mass of the compound – ). Furthermore, it was observed that three out of six backbone amide protons, namely those belonging to Leu4, Ahp5 and Ile8, appear to be protected from an exchange with the solvent. The most likely reason is the involvement of these protons in hydrogen-bonding interactions. Similarly, the crystal structure of another cyanopeptoline-like peptide scyptolin A bound to a protein partner (PDB_ID: 1OKX deposited by Matern et al., Citation2003) shows hydrogen-bonding interactions between the equivalent of Ile8 amide proton and the OH oxygen of Ahp, as well as between the amide proton of Ahp and (the equivalent of) Ile8 carbonyl group. The exchange protection of these two amide protons in cyanopeptolin 1081 thus suggests that this peptide may contain the same hydrogen bonding pattern and thus assume a very similar three-dimensional structure. In addition, in the recorded NMR spectra the expected COSY cross-peak between H2 and H3 of Thr3 was missing, likely due to the dihedral angle H2-C2-C3-H3 being fixed to a value for which the J H2-H3 is very small. Interestingly, the same observation was made by Okumura et al. (Citation2009) for another cyanopeptoline-like peptide. This observation is a further hint that the ring portion of cyanopeptolin 1081 assumes a single, well-defined, rigid three-dimensional structure probably similar with that found in PDB_ID: 1OKX (Matern et al., Citation2003). The MS spectrum obtained for cyanopeptolin 1081 in positive mode showed [M+H]+ and [M+H – H2O]+ peaks at m/z 1082 and 1064, respectively, indicating a molecular weight of 1081 (). Cyanopeptolins are generally detected as intact molecules or water abstracted derivatives, thus the [M – H2O + H]+ can have the highest intensity (Welker & von Döhren, Citation2006). In the product ion spectrum several ions were prominently observed, indicating the partial sequences of [Tyr-GA-Gln-Thr-Leu – H2O + H]+ at m/z 576, [Tyr-GA-Gln-Thr – H2O + H]+ at m/z 463 and [Thr-Leu-Ahp-Thr-NMePhe-Ile – H2O + H]+ at m/z 685. The mass signals following fragmentation series were also assigned to: [Ahp-Thr-NMePhe-Ile – H2O + H]+ at m/z 471, [Leu-Ahp-Thr-NMePhe-Ile + H]+ at m/z 602 and [Tyr-GA-Gln + H]+ at m/z 379. The molecular formula of cyanopeptolin 1081 was deduced as C52H75N9O16 by MS and NMR spectral data. Summing up all the above presented information the proposed structure of this peptide is presented in .
Table 1. The NMR assignments for cyanopeptolin 1081
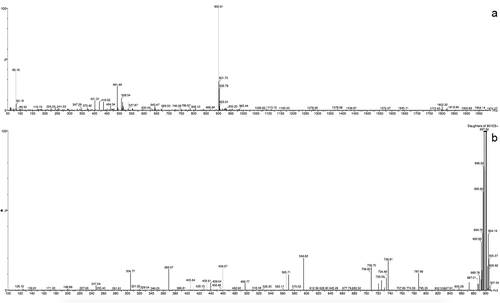
Anabaenopeptin 899 was isolated as a colourless amorphous powder (1.9 mg, 0.011% yield). The analysis of proton spin systems, as well as multiple bond13C-1H connectivities allowed identification of the following six residues within this peptide: isoleucine (Ile), valine (Val), lysine (Lys), phenylalanine (Phe) and two instances of homotyrosine (Hty), one of which was N-methylated (NMe-Hty) (Supplementary figs 9–15). The NMR data are summarized in . The lysine residue was missing one of its side chain NH2 protons, while the remaining one was showing a clear 1H–13C HMBC correlation to a carbonyl carbon atom. These observations strongly suggested the side chain NH2 group of lysine was involved in amide formation. Another peculiar spectral feature observed for the studied peptide was the presence of an additional carbonyl resonance at 160.6 ppm (over 10 ppm upfield from all the other CO resonances in the molecule). This CO resonance was also showing clear 1H–13C HMBC connectivity to two backbone amide protons and two H2 protons. The unusual chemical shift and the observed connectivity pattern taken together strongly suggested that this carbonyl group was involved in the formation of two amide bonds (as is the case for CO in urea). Following the 1H–13C HMBC connectivities demonstrated that the two residues directly flanking this ureido-group were lysine and phenylalanine. On the side of the phenylalanine, no connectivity to further residues were observed. On the lysine side, on the other hand, the 1H–13C HMBC connectivities involving the carbonyl groups allowed to map a typical peptide chain of the following sequence: Lys1-Val2-Hty3-NMeHty4-Ile5. Moreover, the CO group of the C-terminal residue Ile5 turned out to be the carbonyl forming the amide bond with the side chain NH2 group of Lys1. This last interaction leads to the cyclization of the peptide. The MS spectrum of anabaenopeptin 899 in positive mode displayed protonated molecule [M+H]+ at m/z 900, indicating a molecular weight of 899 (). The most abundant ions observed in the product ion spectrum of this compound revealed the following fragmentation series, indicating the partial sequences of [Phe-CO-Lys-Val-Hty-NMeHty + H]+ at m/z 787, [CO-Lys-Val-Hty-NMeHty-Ile + H]+ at m/z 736, [CO-Lys-Val-Hty + H]+ at m/z 432, [Lys-Val-Hty-NMeHty-Ile + H]+ at m/z 708, [Lys-Val-Hty-NMeHty + H]+ at m/z 594 and [Lys-Ile-NMeHty + H]+ at m/z 432. The molecular formula of anabaenopeptin 899 was established to be C48H65N7O10 based on MS and NMR spectral data. With all the conclusions presented above, the structure of anabaenopeptin 899 best corresponding to the obtained data is presented in .
Table 2. The NMR assignments for anabaenopeptin 899
In the toxicity assessment of isolated and structurally determined cyanopeptides neither cyanopeptolin 1081 nor anabaenopeptin 899 exerted acute toxic effects on T. platyurus at concentrations up to 40 μg ml–1. However, deterioration of the physiological state of the examined crustaceans manifested in reduced mobility, as compared with the control condition, was observed at the highest tested concentrations.
Discussion
Two new oligopeptides, so far not described in the literature, belonging to cyanopeptolins and anabaenopeptins were isolated from an enrichment culture dominated by W. naegeliana. Cyanopeptolins are cyclic hexapeptides with a side chain of variable length and composition. Their common feature is the presence of the conserved 3-amino-6-hydroxy-2-piperidone amino acid and an ester linkage between the hydroxy group of threonine and the carboxyl group of the terminal amino acid. However, the occurrence of O-methylated Ahp (Amp) and the replacement of threonine by 3-hydroxy-4-methylproline have also been reported (Chlipala et al., Citation2011). Generally, the amino acids sequence in the cyclic part is usually composed with: Thr – (Arg/MeLys/Leu/Trp/Tyr/Gln) – Ahp – (Leu/Thr/Ile/Phe/Val) – N-Me(Phe/Tyr) – (Val/Ile/Leu) (Demay et al., Citation2019; Jansen, Citation2019). Variability of some amino acids is shown in brackets. One of the residues is N-methyl aromatic amino acid, typically tyrosine or phenylalanine, while next to this position is commonly occupied by a small non-polar amino acid, i.e. isoleucine/leucine or valine. In the case of studied cyanopeptolin 1081 the presence of N-methylated-phenylalanine and leucine were confirmed. The side chain of cyanopeptolins may contain an aliphatic fatty acid or glyceric acid that is linked either directly to the threonine or through one or two amino acids (Welker & von Döhren, Citation2006). The glyceric acid located between tyrosine and glutamine formed the side chain of cyanopeptolin 1081. The high structural variability of cyanopeptolins representing over 170 variants is also reflected by the wide range of masses between 590 Da and 1200 Da (Le Manach et al., Citation2019). To this class of cyclic depsipeptides, belong also: aeruginopeptins, anabaenopeptolides, hoffmanolins, microcystilides, micropeptins, nostocyclins, nostopeptins, oscillapeptillides, oscillapeptins, planktopeptins, scyptolins, somamides, symplostatins and tasipeptins (Welker & von Döhren, Citation2006). They are synthesized mainly by cyanobacteria of the genera Anabaena, Lyngbya, Microcystis, Nostoc, Planktothrix, Scytonema, Symploca and as is shown in our preliminary study in W. naegeliana (Le Manach et al., Citation2019).
Anabaenopeptins are cyclic hexapeptides consisting of a ring formed by five amino acids, including a conserved lysine, which both closes the ring and establishes a ureido bond with one amino acid of the side chain. Usually the sequence of the amino acids in the cyclic part is composed with: Lys–(Phe/Ile/Leu/Tyr/Acetyl-Ser/Br-Trp/Tyr)–(Me-Ala/Me-Ile/Hty/Me-Hty/Hph/Gly/MeTrp)–(Hty/MeHty/Hph/Leu)–(Val/Me-Ile/Ala/Leu/Me-Met) (Welker & von Döhren, Citation2006; Jansen, Citation2019). Except for the conserved lysine, all amino acids are variable (in brackets) and one of them in the ring is always N-methylated. In the structure of anabaenopeptin 899 the presence of N-methylated-homotyrosine, as well as homotyrosine was confirmed. Homo-amino acids have extra methylene (CH2) group in the carbon side chain and are frequently found in cyanobacterial natural products (Shishido et al., Citation2019). In a cyclic part position next to lysine has been reported to be occupied primarily by valine or isoleucine/leucine and less frequently by methionine (Welker & von Döhren, Citation2006). The presence of valine was found in anabaenopeptin 899. More than 75 variants of anabaenopeptins with masses ranging from 750 to 960 Da have been described to date (Le Manach et al., Citation2019). These compounds have been detected in cyanobacteria of the genera Anabaena, Microcystis, Planktothrix, Lyngbya, Nodularia, Schizotrix and Nostoc (Miller et al., Citation2019). The isolation of anabaenopeptin 899 from an enrichment culture dominated by W. naegeliana confirms its ability to produce a wide range of cyanopeptides.
Considering the limited knowledge of the presence of cyanopeptides in the aquatic environment, their identification and characterization, particularly those extracted from the cyanobacterial material collected from drinking water reservoirs, are crucial. The detected novel cyanopeptides were most likely produced by W. naegeliana since it was the most abundant species in the analysed sample, but it cannot be excluded that the origin could also have been M. flos-aquae. Most of the currently identified metabolites synthesized by W. naegeliana belong to several classes of oligopeptides including microginins and cyanopeptolins, in which non-ribosomal synthesis is a significant part of cell metabolism (Welker & von Döhren Citation2006; Bober et al., Citation2011, Citation2014). The obtained result indicating its ability to also produce anabaenopeptin is another example of the cyanobacterial capability to synthesize peptides belonging to more than one class (Harada et al., Citation1993; Kodani et al., Citation1999). However, amongst the number of compounds discovered in a given class of peptides only a few are dominant, e.g. anabaenopeptins in Planktothrix rubescens, cyanopeptolins and microcystins in Microcystis sp., and microginins and cyanopeptolins in W. naegeliana (Bober et al., Citation2011). The highest number of oligopeptides have been identified in species belonging to Oscillatories and Nostocales, fewer in Chroococcales and Stigonematales, and only a few from Pleurocapsales (Welker & von Döhren, Citation2006). A large variety of secondary metabolites produced by cyanobacteria is an interesting issue because its synthesis requires high material and energy cost. Thus, it indicates that these compounds have a potentially important function. The characteristics and potential of most cyanobacterial peptides are still poorly understood. Many of them have shown protease inhibitory activities, but their biological significance and role in nature remain unknown. The protease inhibition may be connected with an anti-grazing strategy, however, additional research is required to justify this theory (Gademann & Portmann, Citation2008). The preliminary assessment of biological activity of newly discovered cyanopeptolin 1081 and anabaenopeptin 899 towards zooplankton T. platyurus did not allow clear assessment of the importance of their biological significance. However, the observed deterioration of the physiological state of crustacean larvae caused by studied peptides indicates their possible negative effects on the aquatic organism. Members of the cyanopeptolin and anabaenopeptin classes of compounds are known protease inhibitors. Many structural variants of cyanopeptolins inhibit serine protease, e.g. chymotrypsin, trypsin and elastase, by preventing hydrolytic attack on substrates by covering the active centre with a rigid ring structure (Matern et al., Citation2003; Chlipala et al., Citation2011). Considering the possible similarity of the three-dimensional structure of the cyanopeptolin 1081 ring portion to the well-known elastase inhibitor scyptolin A, indicated by NMR analyses, their similar inhibitory activity might be assumed. On the other hand, anabaenopeptins have been reported to inhibit carboxypeptidase A as well as protein phosphatases PP1 and PP2A (Sano et al., Citation2001). Although they show various inhibitory activities a recent study discovered that both cyanopeptolins and anabaenopeptins induced significant toxicity effects in Caenorhabditis elegans including reduction of reproduction, decreasing of growth rate, shortening lifespan and some severe ageing-related vulval integrity defects (Lenz et al., Citation2019). Thus, further biological tests of these compounds are warranted.
Supplementary fig. 1. 1H NMR spectrum of cyanopeptolin 1081.
Supplementary fig. 2. COSY spectrum of cyanopeptolin 1081.
Supplementary fig. 3. TOCSY spectrum of cyanopeptolin 1081.
Supplementary fig. 4. 1H-13C HSQC spectrum of cyanopeptolin 1081.
Supplementary fig. 5. 1H-13C HMBC spectrum of cyanopeptolin 1081.
Supplementary fig. 6. 1H-13C HMBC narrow spectral window of cyanopeptolin 1081.
Supplementary fig. 7. ROESY spectrum of cyanopeptolin 1081.
Supplementary fig. 8. 1H-15N HMBC spectrum of cyanopeptolin 1081.
Supplementary fig. 9. 1H NMR spectrum of anabaenopeptin 899.
Supplementary fig. 10. COSY spectrum of anabaenopeptin 899.
Supplementary fig. 11. TOCSY spectrum of anabaenopeptin 899.
Supplementary fig. 12. 1H-13C HSQC spectrum of anabaenopeptin 899.
Supplementary fig. 13. 1H-13C HMBC spectrum of anabaenopeptin 899.
Supplementary fig. 14. 1H-13C HMBC narrow spectral window of anabaenopeptin 899.
Supplementary fig. 15. ROESY spectrum of anabaenopeptin 899.
Author contributions
B. Bober: original concept, analyses, analysis of data and drafting manuscript; E. Chrapusta-Srebrny: analysis of data and editing manuscript; J. Bialczyk: review and editing manuscript.
TEJP-2019-0147-File020.tif
Download TIFF Image (46.4 KB)TEJP-2019-0147-File019.tif
Download TIFF Image (26.9 KB)TEJP-2019-0147-File018.tif
Download TIFF Image (24.5 KB)TEJP-2019-0147-File017.tif
Download TIFF Image (25.7 KB)TEJP-2019-0147-File016.tif
Download TIFF Image (26.5 KB)TEJP-2019-0147-File015.tif
Download TIFF Image (18 KB)TEJP-2019-0147-File014.tif
Download TIFF Image (26.7 KB)TEJP-2019-0147-File013.tif
Download TIFF Image (26.9 KB)TEJP-2019-0147-File012.tif
Download TIFF Image (43.1 KB)TEJP-2019-0147-File011.tif
Download TIFF Image (29.1 KB)TEJP-2019-0147-File010.tif
Download TIFF Image (34.6 KB)TEJP-2019-0147-File009.tif
Download TIFF Image (30.1 KB)TEJP-2019-0147-File008.tif
Download TIFF Image (35.2 KB)TEJP-2019-0147-File007.tif
Download TIFF Image (30.4 KB)TEJP-2019-0147-File006.tif
Download TIFF Image (34.4 KB)Acknowledgements
The authors are grateful to Professor Zbigniew Lechowski for his thoughtful comments and valuable advice. The authors thank Dr Witold Andrałojć and Dr Karol Pasternak (Institute of Bioorganic Chemistry, Polish Academy of Sciences) for technical assistance and useful comments on NMR spectra.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2020.1813809
Additional information
Funding
References
- Ali Shah, S.A., Akhter, N., Auckloo, B.N., Khan, I., Lu, Y., Wang, K., Wu, B. & Guo, Y.-W. (2017). Structural diversity, biological properties and applications of natural products from cyanobacteria. A review. Marine Drugs, 15: 354.
- Bober, B. & Bialczyk, J. (2017). Determination of the toxicity of the freshwater cyanobacterium Woronichinia naegeliana (Unger) Elenkin. Journal of Applied Phycology, 29: 1355–1362.
- Bober, B., Lechowski, Z. & Bialczyk, J. (2011). Determination of some cyanopeptides synthesized by Woronichinia naegeliana (Chroococcales, Cyanophyceae). Phycological Research, 59: 286–294.
- Bober, B., Kaminski, A., Chrapusta, E.& Bialczyk, J. (2014). Stability of some microginins synthesized by the cyanobacterium Woronichinia naegeliana (Unger) Elenkin. Phycological Research, 62: 228–231.
- Chlipala, G.E., Mo, S.& Orjala, J. (2011). Chemodiversity in freshwater and terrestrial cyanobacteria – a source for drug discovery. Current Drug Targets, 12: 1654–1673.
- Chorus, I. & Bartram, J., editors (1999). Toxic Cyanobacteria in Water: A Guide to their Public Health Consequences. Monitoring and Management. World Health Organization, Taylor & Francis Group, London and New York.
- Cronberg, G., Annadotter, H.& Lawton, L. (1999). The occurrence of toxic blue-green algae in Lake Ringsjön, southern Sweden, despite nutrient reduction and fish biomanipulation. Hydrobiologia, 404: 123–129.
- Czarnecki, O., Henning, M., Lippert, I.& Welker, M. (2006). Identification of peptide metabolites of Microcystis (Cyanobacteria) that inhibit trypsin-like activity in planktonic herbivorous Daphnia (Cladocera). Environmental Microbiology, 8: 77–87.
- Demay, J., Bernard, C., Reinhardt, A. & Marie, B. (2019). Natural products from cyanobacteria: focus on beneficial activities. Marine Drugs, 17: 320.
- Dochin, K. (2019). Functional and morphological groups in the phytoplankton of large reservoirs used for aquaculture in Bulgaria. Bulgarian Journal of Agricultural Science, 25: 166–176.
- Gademann, K. & Portmann, C. (2008). Secondary metabolites from Cyanobacteria: complex structures and powerful bioactivities. Current Organic Chemistry, 12: 326–341.
- Gademann, K., Portmann, C., Blom, J.F., Zeder, M. & Juttner, F. (2010). Multiple toxin production in the cyanobacterium Microcystis: isolation of the toxic protease inhibitor cyanopeptolin 1020. Journal of Natural Products, 73: 980–984.
- Harada, K.-I., Mayumi, T., Shimada, T., Suzuki, M., Kondo, F. & Watanabe, M.F. (1993). Occurrence of four depsipeptides, aeruginopeptins, together with microcystins from toxic cyanobacteria. Tetrahedron Letters, 34: 6091–6094.
- Jansen, E.M.-L. (2019). Cyanobacterial peptides beyond microcystins – a review on co-occurrence, toxicity, and challenges for risk assessment. Water Research, 151: 488–499.
- Keil, C., Forchert, A., Fastner, J., Szewczyk, U., Rotard, W., Chorus, I. & Kraetke, R. (2002). Toxicity and microcystin content of extracts from a Planktothrix bloom and two laboratory strains. Water Research, 36: 2133–2139.
- Kodani, S., Suzuki, S., Ishida, K. & Murakami, M. (1999). Five new cyanobacterial peptides from water bloom materials of Lake Teganuma (Japan). FEMS Microbiology Letters, 178: 343–348.
- Komárek, J. & Anagnostidis, K. (1999). Cyanoprokaryota. 1 Teil: Chroococcales. Süßwasserflora von Mitelleuropa 19/1: 1–548. Gustav Fischer Verlag, Stuttgart.
- Kopp, R., Vítek, T., Štastny, J., Sukop, I., Brabec, T., Zivkova, A., Spurny, P. & Mares, J. (2012). Water quality and biotic community of a highland stream under the influence of a eutrophic fishpond. International Review of Hydrobiology, 97: 26–40.
- Lara, Y., Lambion, A., Menzel, D., Codd, G.A. & Wilmotte, A. (2013). A cultivation-independent approach for the genetic and cyanotoxin characterization of colonial cyanobacteria. Aquatic Microbial Ecology, 69: 135–143.
- Le Manach, S., Duval, C., Marie, A., Djediat, C., Catherine, A., Edery, M., Bernard, C. & Marie, B. (2019). Global metabolomic characterizations of Microcystis spp. highlights clonal diversity in natural bloom-forming populations and expands metabolite structural diversity. Frontiers in Microbiology, 10: 791.
- Lee, W., Tonelli, M. & Markley, J.L. (2015). NFRAM-SPARKY: enhanced software for biomolecular NMR spectroscopy. Bioinformatics, 31: 1325–1327.
- Lenz, K.A., Miller, T.R. & Ma, H. (2019). Anabaenopeptins and cyanopeptolins induce systemic toxicity effects in a model organism the nematode Caenorhabditis elegans. Chemosphere, 214: 60–69.
- Matern, U., Schleberger, C., Jelakovic, S., Weckesser, J.& Schulz, G.E. (2003). Binding structure of elastase inhibitor scyptolin A. Chemistry & Biology, 10: 997–1001.
- Miller, T.R., Barlett, S.L., Weirich, C.A. & Hernandez, J. (2019). Automated subdaily sampling of cyanobacterial toxins on a buoy reveals new temporal patterns in toxin dynamics. Environmental Science & Technology, 53: 5661–5670.
- Nandini, S., Sanchez-Zamora, C. & Sarma, S.S.S. (2019). Toxicity cyanobacterial blooms from the reservoir Valle de Bravo (Mexico): a case study on the rotifer Brachionus calyciflorus. Science of the Total Environment, 688: 1348–1358.
- Oberholster, P.J., Botha, A.-M. & Cloete, T.E. (2006). Toxic cyanobacterial blooms in a shallow, artificially mixed urban lake in Colorado, USA. Lakes & Reservoirs: Research and Management, 11: 111–123.
- Okumura, H.S., Philmus, B., Portmann, C., Hemscheidt, T.K. (2009). Homotyrosine-containing cyanopeptolins 880 and 960 and anabaenopeptins 908 and 915 from Planktothrix agardhii CYA 126/8. Journal of Natural Products, 72: 172–176.
- Quesada, A., Moreno, E., Carrasco, D., Paniagua, T., Wormer, L., De Hoyos, C. & Sukenik, A. (2006). European Journal of Phycology, 41: 39–45.
- Pociecha, A. & Wilk-Woźniak, E. (2005). Dynamics of phyto- and zooplankton in the submountane dam reservoirs with different trophic status. Limnological Review, 5: 215–221.
- Rajaniemi-Wacklin, P., Rantala, A., Mugnai, M.A., Turicchia, S., Ventura, S., Komárková, J., Lepistö, L. & Sivonen, K. (2005). Correspondence between phylogeny and morphology of Snowella spp. and Woronichinia naegeliana, cyanobacteria commonly occurring in lakes. Journal of Phycology, 42: 226–232.
- Sano, T., Usui, T., Ueda, K., Osada, H. & Kaya, K. (2001). Isolation of new protein phosphatase inhibitors from two cyanobacteria species, Planktothrix spp. Journal of Natural Products, 64: 1052–1055.
- Santos, M.C.R., Muelle, H. & Pacheco, D.M.D. (2012). Cyanobacteria and microcystins in Lake Furnas (S. Miguel island-Azores). Limnetica, 31: 107–118.
- Shishido, T.K., Jokela, J., Humisto, A., Suurnakki, S., Wahlsten, M., Alvarenga, D.O., Sivonen, K. & Fewer, D.P. (2019). The biosynthesis of rare homo-amino acid containing variants of microcystin by a benthic cyanobacterium. Marine Drugs, 17: 271.
- Singh, R., Parihar, P., Singh, M., Bajguz, A., Kumar, J., Singh, S., Singh, V.P. & Prasad, S.M. (2017). Uncovering potential applications of cyanobacteria and algal metabolites in biology, agriculture and medicine: current status and future prospects. Frontiers in Microbiology, 8: 515.
- Sivonen, K., Niemelä, S.I., Niemi, R.M., Lepistö, L., Luoma, T.H. & Räsänen, L.A. (1990). Toxic cyanobacteria (blue-green algae) in Finnish fresh and coastal waters. Hydrobiologia, 3: 256–275.
- Sukenik, A., Quesada, A. & Salmaso, N. (2015). Global expansion of toxic and non-toxic cyanobacteria: effect on ecosystem functioning. Biodiversity and Conservation, 24: 889–908.
- Šuput, D., Milutinovič, A., Serša, I.& Sedmak, B. (2002). Chronic exposure to cyanobacterial lyophilizate reveals stronger effects than exposure to pure microcystins – a MRI study. Radiology and Oncology, 36: 165–167.
- Svirčev, Z., Drobac, D., Tokodi, N., Mijović, B., Codd, G.A. & Meriluoto, J. (2017). Toxicology of microcystins with reference to cases of human intoxications and epidemiological investigations of exposures to cyanobacteria and cyanotoxins. Archives of Toxicology, 91: 621–650.
- Welker, M. & von Döhren, H. (2006). Cyanobacterial peptides – nature’s own combinatorial biosynthesis. FEMS Microbiology Reviews, 30: 530–563.
- Wilk-Woźniak, E. (1998). Late autumn mass development of Woronichinia naegeliana (Cyanophyceae) in a dam reservoir in Southern Poland. Biologia, Bratislava, 53: 1–5.
- Wilk-Woźniak, E.& Mazurkiewicz-Boroń, G. (2003). The autumn dominance of cyanoprokaryotes in a deep meso-eutrophic submontane reservoir. Biologia, Bartislava, 58: 17–24.
- Willame, R., Jurczak, T., Iffly, J.-F., Kull, T., Meriluoto, J.& Hoffmann, L. (2005). Distribution of hepatotoxic cyanobacterial blooms in Belgium and Luxembourg. Hydrobiologia, 551: 99–117.