ABSTRACT
This study builds on recent treatments of the marine red algal family Gracilariaceae (Gracilariales) focusing in detail on Hydropuntia and Crassiphycus. Species in these two genera often present identification problems due to high levels of morphologial similarity among genetically distinct species, and high levels of phenotypic plasticity, leading to pseudo-cryptic speciation and homoplasies. In order to resolve long standing problems, clarify some species concepts and better understand the evolution of the group, we performed phylogenetic analyses of all plastid rbcL DNA sequences available for known Hydropuntia and Crassiphycus species, including newly sequenced specimens. Our results revealed the presence of potentially undescribed species, the existence of strong phylogeographic patterns below and above the species level and helped re-delineate morphologically similar taxa. New detailed morphological descriptions for three common yet poorly known Western Atlantic species are provided: C. secundus, C. usneoides and H. rangiferina. H. rangiferina from the Indo-Pacific is a distinct species from the true H. rangiferina and represents a putative undescribed species. We also provide a time-calibrated phylogeny for the six genera in the Gracilariales to identify past geological and climatic processes associated with their origin and diversification.
Introduction
Twelve years after the creation of Gracilaria Greville (Citation1830), the genus was split into two, Gracilaria and Hydropuntia Montagne (Citation1842). Hydropuntia was typified and described with the generitype H. urvillei Montagne from specimens collected from Toud Island (Warrior Inlet) in the Torres Strait, north-eastern Australia, to represent species with strong thallus constrictions (Silva et al., Citation1996). Over a century later, Kylin (Citation1956), regarded Hydropuntia as a heterotypic synonym of Gracilaria thus merging both genera into Gracilaria. Chang & Xia (Citation1963) divided Gracilaria again and described the genus Polycavernosa on the basis of unique spermatangial conceptacles that were deeply immersed in the medulla and confluent (generitype: P. fastigiata C.F.Chang & B.-M.Xia; now a synonym of Hydropuntia edulis (Gmelin) Gurgel & Fredericq (Citation2004)). Wynne (Citation1989) treated Polycavernosa as a synonym of Hydropuntia since H. urvillei had the same type of spermatangial conceptacles found in Polycavernosa. Abbott et al. (Citation1991) subsequently reported the presence of two types of spermatangial conceptacles on the same thallus in Gracilaria mixta I.A.Abbott, J.Zhang & B.-M.Xia, leading the authors to merge Hydropuntia back into Gracilaria. Between the first generic split of Gracilaria by Montagne (Citation1842) and the second by Chang & Xia (Citation1963) to recognize Hydropuntia or Polycavernosa, respectively, Gracilaria, as a distinct genus in the Gracilariaceae, has remained divided into two or more genera for 140 years of its ~190 years of existence.
Recently, Gracilaria was split into Agarophyton Gurgel, J.N.Norris & Fredericq, Crassiphycus Guiry, J.N.Norris, Fredericq & Gurgel, and Hydropuntia (Guiry et al., Citation2018; Gurgel et al., Citation2018), based on published phylogenies (Bellorin et al., Citation2002; Gurgel & Fredericq, Citation2004; Gurgel et al., Citation2008; Soares et al., Citation2015) and new rbcL phylogenetic analyses (Gurgel et al., Citation2018). Phylogenies based on chloroplast and mitochondrial phylogenomic data (Iha et al., Citation2018) also corroborate rbcL topologies in recognizing that these four phylogenetically defined genera correspond to distinct and well-supported clades. Further details of the taxonomic history of Gracilaria, Hydropuntia and Crassiphycus can be found in Gurgel & Fredericq (Citation2004), Gurgel et al. (Citation2018) and Lyra et al. (Citation2015).
While molecular systematic studies have tended to focus on solving taxonomic problems in Gracilaria, species in Hydropuntia and Crassiphycus have not received the same attention. As a result, morphological descriptions of several Crassiphycus and Hydropuntia species remain too succinct, outdated, inadequately detailed, or lack molecular confirmation, leading to taxonomic confusions (e.g. Nunéz-Resendiz et al., Citation2015, Citation2017). In order to decrease this knowledge gap, the present study provides the first focused examination on the molecular systematics of the genera Crassiphycus and Hydropuntia, including new descriptions of poorly characterized species, and new combinations. An assessment of their evolution using a time-calibrated phylogeny for both genera also provides, for the first time, more precise dating of the most recent common ancestor of Crassiphycus and Hydropuntia and helps us identify which past climatic, geographic and oceanographic processes could be related to their origin and diversification.
Materials and methods
Morphological analysis
Specimens were collected on nearshore reefs during low tides by snorkelling or via scuba diving. Collected specimens (Supplementary table 1) were preserved in buffered 5% formalin/seawater or pressed and air-dried on high-density pH neutral paper sheets for morphological analyses. Specimens were deposited in LAF, US and SP (herbarium abbreviations follow Thiers, Citation2020). Cross-sections for anatomical studies were hand-made according to Tsuda & Abbott (Citation1985) and Fredericq & Hommersand (Citation1989). Digital photomicrographs were edited and assembled in plates using Adobe Photoshop CS6 (Adobe Systems Inc., San Jose, California, USA) and CorelDRAW Graphic Suite (Corel Corp., Ottawa, Canada).
Molecular analysis
DNA extractions were performed using the DNeasy Plant Mini Kit (QIAGEN, Valencia, CA), or were submitted to a CTAB-Cesium Chloride DNA procedure (Freshwater & Rueness, Citation1994). PCR and sequencing primers used in this study were FrbcLstart, F7, F57, F492, F577, F753, F993, R753, R1381 and RrbcSstart listed in Freshwater & Rueness (Citation1994) and Lin et al. (Citation2001). PCR and automated DNA sequencing followed protocols described in Gurgel & Fredericq (Citation2004). All rbcL DNA sequences available in GenBank were added to 26 newly generated sequences in this study (metadata in Supplementary table 1).
Two alignments were constructed. A comprehensive alignment, designed to evaluate phylogenetic hypotheses within and between Crassiphycus and Hydropuntia taxa, comprised 147 taxa including newly sequenced and all rbcL DNA sequences of Crassiphycus and Hydropuntia published to date, plus selected Gracilaria species and two Agarophyton sequences as outgroups. A reduced alignment, designed to assess ages of the most recent common ancestor between lineages via a time-calibrated phylogenetic analysis, comprised 45 taxa and included at least a single representative sequence of each Crassiphycus and Hydropuntia species identified from results of the first dataset, selected Gracilaria and Agarophyton species, plus one Curdiea Harvey and one Melanthalia Montagne species as outgroups. JModeltest2 under the Akaike information criterion (Darriba et al., Citation2012) identified the GTR+I+G (general time reversible model with gamma distribution and invariable sites) as the best model of molecular evolution for both alignments.
Bayesian phylogenetic analysis for the comprehensive alignment was executed in MrBayes 3.2.6 (Ronquist et al., Citation2012) on the XSEDE platform available online in the CIPRES Science Gateway website (Miller et al., Citation2010). We applied two independent MCMC runs for 20 million generations, sampling one tree every 1000 generations. Stationarity was determined based on both the average standard deviation of split frequencies between both runs reaching values below 0.001 (below 0.01 is considered good) and visually by plotting the log likelihood of the sampled trees against generation time. The burn-in was set at 10%. Posterior probabilities were determined from all trees saved after burn-in removal from both MCMC runs (sumt command), totalling 18 000 trees. The selected phylogram, saved after burn-in removal, corresponded to the tree with the highest maximum likelihood value.
The time-calibrated Bayesian phylogenetic analysis was implemented in Beast 2.6 (Bouckaert et al., Citation2019). Three calibration points (node ages), obtained from results from the most recent time-calibrated red algal tree based on full chloroplastidial phylogenomic analysis by Nan et al. (Citation2017, fig. 5A), were used in our analysis. Therefore, our calibrations did not use either fossil data or gene substitution rate data but relied on the results of estimated node ages for the red algae from another study. Calibration points included the most recent common ancestor (mrca) between Agarophyton chilense (C.J.Bird, McLachlan & E.C.Oliveira) Gurgel, J.N.Norris & Fredericq and A. tenuistipitatum (C.F.Chang & B.-M.Xia) Gurgel, J.N.Norris & Fredericq (48.97 million years ago, mya), the mrca for subfamily Gracilarioideae (171.99 mya), and the mrca for tribe Gracilarieae (122.05 mya). To produce good quality node age estimates for the multi-calibration, we followed Duchêne et al. (Citation2014), by implementing calibrations close to the node.
We estimated all site model parameters, including base frequency parameters, and used a strict molecular-clock model (with default parameters) and the Yule speciation model (Heled & Drummond, Citation2011), starting from a random tree. Preliminary analyses using the random local clock option did not produce results significantly better than the strict molecular-clock model (Bayes factor = 1.035, data not shown). Variation in the time-calibration priors followed a normal distribution with 3.0 standard deviations from the mean. All other prior variations assumed a Gamma function with default values. Twenty million MCMC generations sampling one tree every 1000 generations were implemented. MCMC trace files, stationarity and parameter estimates were analysed in Tracer 1.7.1 (Rambaut et al., Citation2018). Bayesian posterior probabilities after burn-in removal and the maximum clade credibility tree (MCC tree) were calculated in TreeAnnotator 2.6 (Rambaut & Drummond, Citation2014). Trees were formatted with FigTree 1.4.2 (Rambaut, Citation2012) and further edited in InkScape 0.92 (http://inkscape.org). Genetic distances were calculated using ‘p’ distances.
Results
Phylogenetic analysis
The 147 taxon rbcL alignment comprised 1405 base pairs (bp) of which 915 bp (65%) were constant, 490 bp varied at least once, and 391 bp were parsimony informative (including the outgroups). Bayesian analysis reached stationarity after a short number of MCMC generations and hence Bayesian posterior probability (BI) was obtained for 38 000 saved trees. Bayesian analysis produced a fully resolved tree with the exception of the relationships among intra-specific haplotypes. The Bayesian analysis identified with high phylogenetic support the monophyly of the genus Crassiphycus (BI = 1.0), Gracilaria (BI = 0.96) and Hydropuntia (BI = 1.0) (). The ML results showed the same tree topology but overall lower phylogenetic support, particularly among internal nodes, compared with the Bayesian results: Crassiphycus (Bootstrap Proportions, BP = 84%), Gracilaria (BP = 57%) and Hydropuntia (BP = 100%) (Supplementary fig. 1).
Fig. 1. Bayesian phylogenetic tree of the genera Crassiphycus, Hydropuntia and other closely related Gracilariaceae based on rbcL DNA sequences. Numbers above branches correspond to posterior probabilities and maximum likelihood bootstrap probabilities based on 1000 replications, respectively (PP/BP). Branches marked with a star have full phylogenetic support: PP = 1.0 and BP = 100%. Recognized phylogeographic groups: Indo-Malay Phylogroup (Gulf of Thailand Sea and Mallaca Strait), South China Sea Phylogroup (South China Sea and the Philippines), North-eastern (NE) Brazil Phylogroup, South-eastern (SE) Brazil Phylogroup, and the North-western (NW) Phylogroup. * = actual branch length is 50% reduced for aesthetical purposes. Scale bar = substitutions per site
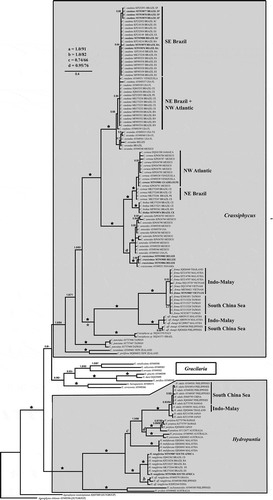
Crassiphycus
All western Atlantic Crassiphycus species formed a single clade with maximum support (BI = 1.0, BP = 100%, , Supplementary fig. 1). The western Atlantic Crassiphycus clade contained three sub-clades composed of sister species pairs: (1) C. corneus – C. birdiae, (2) C. usneoides – C. crassissimus and (3) C. caudatus – C. secundus (). Crassiphycus also included five Indo-Pacific and Australasian species that formed a paraphyletic lineage to the Atlantic clade: C. firmus, Crassiphycus aff. changii, C. proliferus, C. punctatus and C. secundatus (, Supplementary fig. 1). Crassiphycus paraphyletic lineages also included an unidentified species from the Mediterranean Sea, Crassiphycus sp., Crassiphycus aff. changii and C. firmus formed two fully supported sister clades restricted to the Indo-Pacific Ocean (, Supplementary fig. 1). Intraspecific and interspecific genetic diversity found among Crassiphycus species varied between 0.0–0.7% and 0.5–8.94%, respectively.
Hydropuntia
The genus Hydropuntia presented seven molecularly distinct species predominantly restricted to the Indian and Pacific Oceans, i.e. H. edulis, H. perplexa, H. preissiana, H. multifurcata, H. rangiferina, H. eucheumatoides and H. urvillei (, Supplementary fig. 1). The new record of H. rangiferina from southern KwaZulu-Natal, eastern South Africa, represents the same species collected in Brazil (, Supplementary fig. 1). Hydropuntia rangiferina was resolved as two distinct species based on branch length. One comprised specimens from Brazil and South Africa, and the other specimens from Ghana, India and the Philippines. Since the type locality for H. rangiferina is located in north-eastern Brazil (Silva et al., Citation1996: 175), we recognized the clade containing the Brazilian specimens as H. rangiferina sensu stricto. The only nodes in Hydropuntia that did not receive high (BI > 0.95, BP > 80%) or full support (BI = 1.0, BP = 100%) corresponded to the phylogenetic placement of H. multifurcata (BI = 0.74, BP = 66%), and the relationship between H. preissiana and H. perplexa that received full Bayesian support (BI = 1.0) but low boostrap support (BP = 76%). All intraspecific relationships received no phylogenetic support due to very low levels of genetic diversity (, Supplementary fig. 1). Intraspecific and interspecific genetic diversity found among Hydropuntia species varied between 0.0–3.1% and 2.6–10.4%, respectively.
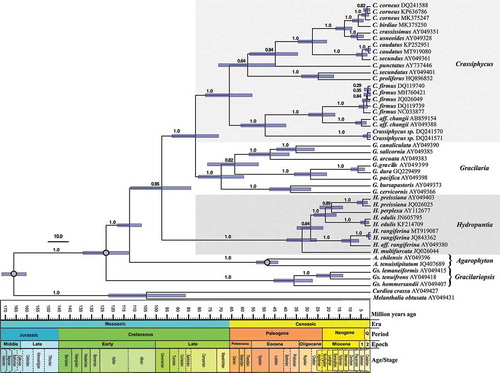
Chronogram
The reduced alignment was also 1405 bp long and presented 877 constant bp, 528 variable bp and 425 bp that were parsimony informative (including outgroups). Bayesian analysis also reached stationarity very quickly and produced a fully resolved ultrametric tree with high phylogenetic support for most nodes (). Chronogram topology presented high agreement with results from the comprehensive alignment (, , Supplementary fig. 1). Estimated mrca ages for extant species within each Gracilarieae genus were 49.54 (± 5.3), 32.85 (± 7.2), 67.69 (± 11.3) and 71.62 (± 11) mya for Agarophyton, Hydropuntia, Crassiphycus and Gracilaria, respectively. Of the genera in the tribe Gracilarioideae, Hydropuntia presented the oldest origin, (87–) 100 (–116) mya, between the early and late Cretaceous, followed by the most recent diversification observed among all Gracilariales genera included in our analysis, at the end of the Eocene, in the Paleogene (). The split between Crassiphycus and Gracilaria was estimated to have occurred (73.9–) 83.91 (–96.5) mya in the late Cretaceous, and the separation between the Pacific and the Atlantic Crassiphycus clades was estimated to have occurred (57.58–) 67.69 (–80.29) mya during the end of the Cretaceous and beginning of the Paleogene. Gracilaria presented the oldest mrca for its extant species, (61.63–) 71.62 (–83.63) mya showing a radiation that also started at the end of the Cretaceous. Mrca ages among intraspecific haplotypes were less than 2.2 mya (e.g. C. caudatus, C. corneus, C. firmus; ).
Fig. 2. Time-calibrated Bayesian phylogenetic tree of the genera Crassiphycus, Hydropuntia and other closely related Gracilariaceae based on rbcL DNA sequences. Numbers above the branches correspond to posterior probabilities (PP). Analysis employed a Yule speciation model and a strict molecular clock model. Time scale in million of years before present. Calibration nodes marked with grey circles were derived from Nan et al. (Citation2017, fig. 5A). Bars at nodes represent the 95% highest posterior density interval for each most recent common ancestor age. Scale bar in millions of years before present. Period Q = Quaternary; Epoch 1 = Pliocene; Epoch 2 = Pleistocene
Taxonomy and morphological observations
Crassiphycus secundus (Gurgel & Fredericq) Gurgel, J.N.Norris & Fredericq in Guiry et al., (Citation2018: 2)
SYNTYPE LOCALITIES: Guadeloupe, French West Indies: Le Moule, E side of Grande-Terre (16º20′00′′N, 61º21′00′′W); and Grand Bourg, SW side of Marie-Galante (15º55′48′′N, 61º16′12′′W).
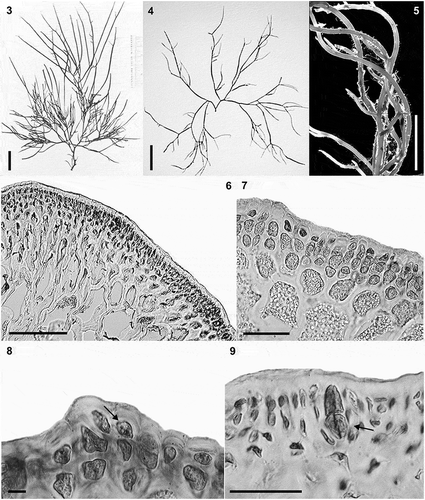
EXTENDED DESCRIPTION: Thalli erect, fleshy and cartilaginous, of loosely branched, narrow terete axes, 7–15 cm long, 1.6–2.0 mm thick (); not adhering well to paper when dry; colour variable from pale yellow, orange, pink-red to dark brown and greenish; attached by small, discoid holdfast. Main axes often not distinct from other major branches, filiform, terete, slightly tapering towards the apices. Branching sparse, subdichotomous or often irregular and secund (, ); at wide angles (70–90°), becoming entangled (). Branches long, usually all of same length; branching more or less evenly interspersed. Apices mostly obtuse, truncate, or tapering (acuminate), or sometimes caudate. Thallus anatomy compact with smooth transition in cell size between inner large medullary cells and the outer small cellular cortical region (). Cortex of 1–4 cell layers with cortical cells 6–12 µm × 6–10 µm, and subcortex of 2–4 cell layers (). Subcortex of radially elongated cells 15–21 × 3.5–13 µm (). Surface cell layer of rounded to obtuse cells, appearing dome-shaped (, arrow), more wide than tall, rarely compressed; occasionally radially elongated, 6–13 × 5.0–6.25 µm. Medulla of 8–9 cell layers (), cells rich in floridean starch. Central medullary cells polygonal, isodiametric or rounded, 99–180 × 86–136 µm, with thick walls; outer medullary cell layers of smaller, round to radially elongated cells, 54–79 × 32–52 µm (). Tetrasporangia cruciately divided (), variable in size, 27–50 µm tall × 12–16 µm in diameter.
Figs 3–9. Habit and anatomical morphology of Crassiphycus secundus. Figs 3–5. Range of habit variation and degree of branching. Fig. 3. Crassiphycus secundus type deposited in BM. Fig. 4. Voucher specimen from Capron Shoal, Fort Pierce, Florida, USA (LAF 07.98; rbcL GenBank accession number: AY049356). Fig. 5. Voucher specimen depicting a drift specimen collected at the beach behind Harbor Branch Oceanographic Institution, Indian River, Fort Pierce, Florida, USA (LAF 07.18.98.01.01; rbcL GenBank accession number: AY049361). Figs 6–9. Cross section from specimens collected in Cockroach Bay, Tampa Bay area, Florida, USA (LAF 26.10.99.01.04; rbcL GenBank accession number: AY049360). Fig. 6. Cross section showing gradual cell size transition between cortex and medulla. Fig. 7. Cross section showing detail of the transition between cortex and medulla, note extensive amount of floridean starch. Fig. 8. Cross section showing detail of cortical cells with oblique division of outer cortical cells (arrow). Fig. 9. Cross section showing mature cruciately divided tetrasporangium (arrow). Scale bars = 2 cm (Fig. 3), 4 cm (Fig. 4), 0.5 cm (Fig. 5), 100 µm (Fig. 6), 40 µm (Figs 7 & 9), 4.5 µm (Fig. 8)
REPRESENTATIVE SPECIMENS EXAMINED: See supplemental materials.
Crassiphycus usneoides (Mertens ex C.Agardh) Gurgel, J.N.Norris & Fredericq in Guiry et al. (Citation2018)
TYPE LOCALITY: Brazil. LD #29447! leg. F.K.Mertens (C. Agardh 1823: 333, as ‘Fucus usneoides Mertens mscr’).
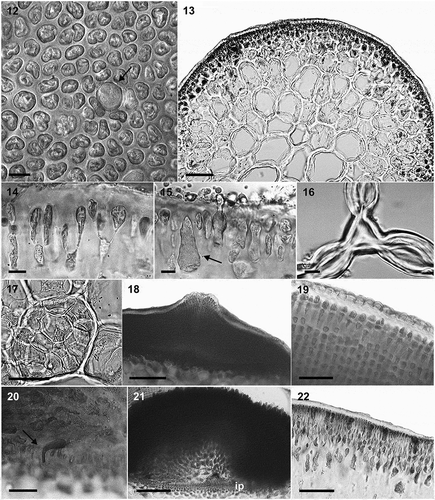
EXTENDED DESCRIPTION: Thalli 5–20 cm long, 1.1–2.2 mm in diameter, decumbent, prostrate or erect, cartilaginous, filiform, cylindrical to ellipsoid throughout. Colour from pale yellow, orange to pinkish and dark brown. Branching subdichotomous, sparse to clumped but often secund (, ). Branches sometimes bend downward, entangle or reattach to the substratum. Small terete to fusiform branchlets up to 2 cm long along axes and branches, slightly compressed at the base, and sometimes dichotomously branched at the apex. Apices obtuse, blunt. Thalli not adhering well to paper. Cortex formed by tightly packed, small, isodiametric to slightly radially elongated cells without sharp edges, interspersed with larger gland cells (). Cortex-medulla cell size transition is smooth (). In traverse section, cortex formed by two distinct cell layers. Outer layer formed by 1–2 rows of tightly packed, small, isodiametric to slightly radially elongated cells, without sharp edges, interspersed with larger gland cells (, ). Gland cells scattered or clustered, 21–40 µm × 5.5–15 µm (arrow, , ). Vegetative cortical cells 7–20 µm long × 3–8 µm in diameter; gradually enlarging to subcortex of larger cells of more variable shapes with larger intercellular spaces (). In transverse section, medulla of rounded, sometimes slightly compressed cells, 185–340 × 100–245 µm, 1.3–3.7 µm (). In longitudinal sections, medullary cells 116–260 × 27–37 µm, smaller medullary cells spherical to isodiametric, larger cells also slightly compressed. Outermost medullary cells often radially elongate, rich in floridean starch granules (). Medulla frequently with uniseriate filaments or clusters of smaller cells confined within larger medullary cells (). Medullary filaments more concentrated closer to subcortex, and more common in cystocarpic plants. Cells of medullary filaments, 37–116 × 34–87 µm, often compressed transversally. Cystocarps 0.75–1.2 mm in diameter, subglobose to dome-shaped, most not constricted at base and slightly immersed in thallus; rostrated (); scattered, isolated or in clusters, over the thallus. Pericarp with 18–22 cell layers (). Pericarpic cells in first four internal layers spherical, anticlinally elongated or star-shaped, with numerous secondary pit connections; middle cell layers become progressively more compact and rectangular-shaped; outer layer cells become more radially elongated. Outer radially elongated pericarp cells dome-shaped, 18–19 μm in height, 5–7 μm in width; forming a thick cuticle with an undulated surface appearance (). Carposporophyte broad-based, with very few lateral tubular nutritive cells (, ). Cystocarp floor of 5–6 layers of small, darkly staining, content-rich cells. Inner layer of gonimoblasts with cells of variable shape and size, mostly round; gonimoblast cells terminating in long chains of carposporangia. Fusion cell small but conspicuous, occasionally becoming elevated above cystocarp floor. Carposporangia obovate, 31–44 μm in length (). Spermatangia not found. Tetraspores 35–38 µm in length × 15–19 µm in diameter ().
Figs 10–11. Habit variation of Crassiphycus usneoides subsp. usneoides. Fig. 10. Holotype specimen of Crassiphycus usneoides deposited in LD. Fig. 11. Voucher specimen from Santa Rosalia bridge, Campeche Bay, Mexico (LAF 14.02.99.02.02; rbcL GenBank accession number: AY049349). Scale bars = ruler is in cm (Fig. 10), 1 cm (Fig. 11)

Figs 12–22. Anatomical features of Crassiphycus usneoides. Fig. 12. Surface view of cortex depicting variation in elliptical to irregularly shaped cortical cells and presence of large, circular glandular cortical cell flanked by smaller cortical cells. Fig. 13. Transverse section through the mid-portion of the thallus showing gradual transition between cortical and medullary cells in terms of their cell sizes. Fig. 14. Transverse section showing elongated tear-shaped cortical cells, with cells stretching at the region of their primary-pit connections, between cortical and sub-cortical cells. Details of thickened cell walls. Fig. 15. Transverse section showing detail of a glandular cortical cell and a trichocyte (arrow) bearing short hair-like extension. Fig. 16. Transverse section showing lenticular thickening of medullary cells forming interstitial cellular spaces. Fig. 17. Transverse section showing irregular roundish smaller medullary cells filling the space inside older medullary cells. Fig. 18. Transverse section through the cystocarp showing thick pericarp, pericarp ostiole, and initial phases of cystocarp development at the base of the cystocarp. Fig. 19. Transverse section through the cystocarp showing details of pericarp cell shape and organization. Detail of lenticular shape of the cuticle. Fig. 20. Transverse section through the base of the cystocarp showing nutritive tubular cells connecting gonimoblast cells to the gametophytic cells of the cystocarp floor. Fig. 21. Transverse section through the cystocarp showing detail of the carposporophyte, gonimoblasts irradiating from a small fusion cell, ip = inner pericarp composed of small gametophytic cells. Fig. 22. Transverse section through tetrasporophytic thallus showing tetrasporangia scattered across cortex, and thick cuticle. Scale bars = 4.5 µm (Figs 12, 14–16), 50 µm (Figs 17, 19–20, 2), 100 µm (Fig. 13), 200 µm (Figs 18, 21)
REPRESENTATIVE SPECIMENS EXAMINED: See supplemental materials.
Crassiphycus usneoides subsp. succosus (J.Agardh) Gurgel, Fredericq & J.N.Norris comb. nov
BASIONYM: Gracilaria usneoides var. α succosa J.Agardh, Citation1852: 595.
TYPE LOCALITY: ‘corallia, Ins. St. Crucis’, St. Croix, US Virgin Islands (Agardh Citation1852, 596).
EXTENDED DESCRIPTION: Thalli 12–25 cm tall, 2.0–4.5 mm in diameter, axes erect, cartilaginous, thick, terete throughout, axes sometimes slightly compressed (, ). Branching initially dichotomous, later becoming subdichotomous to secund above in upper 1/3 of thallus. Branches linear, uniform in thickness, with obtuse, blunt or often broken tips; not constricted at the base (, ). Colour variable, similar to that of C. usneoides subsp. usneoides. Gland cells abundant.
Figs 23–24. Habit variation of Crassiphycus usneoides subsp. succosus. Both specimens are from Santa Rosalia bridge, Campeche Bay, Mexico. Fig. 23. Voucher specimen LAF 14.02.99.02.01B. (rbcL GenBank accession number: AY049349). Fig. 24. Voucher specimen LAF 14.02.99.02.01A (rbcL GenBank accession number: AY049346). Scale bars: 1 cm (Figs 23–24)
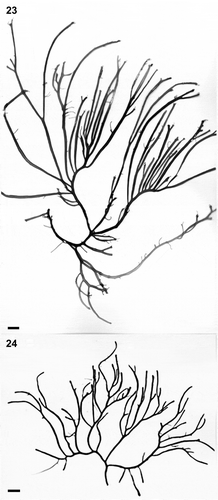
NOTE: C. usneoides subsp. succosus is larger, taller and more robust than subsp. usneoides and could represent the tetrasporophyte life stage of C. usneoides subsp. usneoides.
Hydropuntia rangiferina (Kützing) Gurgel & Fredericq
TYPE LOCALITY: Pernambuco, Brazil collected by Binder. HOLOTYPE in Leiden (L #940.284.39).
ISOTYPE in LD#29499 ().
Figs 25–30. Habit and morphology of Hydropuntia rangiferina. Figs 25–28. Habit variation. Fig. 25. Isotype specimen deposited in LD, LD 29499. Fig. 26. Voucher specimen from ES, Brazil (SP 469736; rbcL GenBank accession number: MK375244). Fig. 27. Voucher specimen from ES, Brazil (SP 469739; rbcL GenBank accession number: MK375245). Fig 28. Voucher specimen from Two-Mile Reef, Sodwana, KwaZulu Natal, South Africa (LAF 02.10.01.02.13; rbcL GenBank accession number: MT919087). Fig. 29. Cortical cells in surface view, arrows point to glandular cells. Fig. 30. Cross section showing medulla anatomy and gradual transition in cell size between medulla and cortex. Fig. 31. Cross section showing detail of cortical and subcortical cells. Scale bars = ruler on the image is in cm (Fig. 25), 1 cm (Figs 26–28), 25 µm (Fig. 29), 100 µm (Fig. 30), 25 µm (Fig. 31)
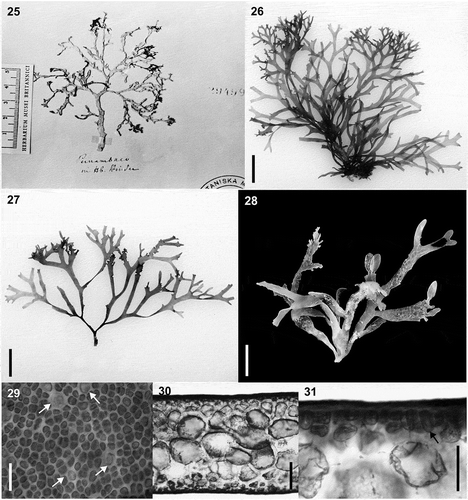
EXTENDED DESCRIPTION: Thalli flattened, erect, delicate, brown-reddish, 6–8 cm long, fixed to the substratum by a small discoid holdfast (). Axes 1–2 mm wide throughout the thallus. Specimens not adhering well to paper upon drying. Some specimens with a short stipe (), terete to compressed, up to 1.5 cm long, giving rise to axes dichotomously to trichotomously branched, in the same plane. Mature specimens fan- shaped. Apices acute or rounded. In surface view, gland cells abundant, scattered, 10–12.5 μm long, 7.5–10 μm wide (, arrows). In transverse section, 220–300 μm thick (), with 1–2 layers of pigmented cortical cells, 5–6.2 μm long, 5–7.5 μm wide, ovate to squarish; medulla composed of 2–5 layers of rounded to polygonal colourless cells, 22.5–25 μm high, 25–42.5 μm wide. Medullary cells rich in floridean starch (). Transition between cortex to medulla abrupt (, ). Fertile specimens were not observed.
REPRESENTATIVE SPECIMENS EXAMINED: See supplemental materials.
Discussion
Evidence from our results that Crassiphycus and Hydropuntia form two fully supported clades, distinct from Gracilaria and all the other genera in the Gracilariaceae (Agarophyton, Curdiea, Gracilariopsis, Melanthalia), corroborate the classification proposal by Gurgel et al. (Citation2018). Our results also provide new detailed descriptions for three common, morphologically similar yet genetically distinct species C. secundus, C. usneoides and H. rangiferina.
C. secundus and C. caudatus are morphologically similar and phylogenetically sister species. Genetic distance between these two species is low (< 2%). However, a clear phylogenetic distinction between them has been retrieved in all published Gracilariacae rbcL phylogenies (e.g. Lyra et al., Citation2015; Gurgel et al., Citation2018; Ayres-Ostrock et al., Citation2019). The original description of C. secundus in Schramm & Mazé (Citation1865) is limited to a couple of uninformative sentences. Our extended description provides morphological evidence to better recognize this species.
Agardh (Citation1852) described Gracilaria usneoides var. α succosa, as having a fleshy thallus and greenish colour (‘fronde virescente succulenta’), and Gracilaria usneoides var. β usneoides with a lichenoid habit (‘fronde lichenoidea’). Our examination of C. usneoides specimens agree with Agardh’s morphological observations with respect to habit morphology (but not colour). Two distinct C. usneoides morphotypes were observed in our new collections. We therefore decided to maintain Agardh’s (Citation1852) original interpretation and recognize two distinct morphotypes. Further anatomical details of C. usneoides vegetative and reproductive structures were reported by Ardito et al. (Citation2014). Agardh’s (Citation1852) original description of Crassiphycus usneoides subsp. usneoides, including the type material (), describes a habit similar to several drift specimens collected in the north-western Atlantic (e.g. in the Florida Intra-Coastal Waterways, ).
Specimens of C. usneoides subsp. succosus examined in this study were not green as originally described by Agardh (Citation1852). However, colour is not a reliable taxonomic character for the Gracilariaceae. Specimens might be sun-bleached, become darker in low-light or subtidal habitats, or lose their pigmentation as a result of light exposure, desiccation or liquid-preservation. It has also been shown that members of Gracilariaceae have distinct colour varieties with a Mendelian pattern of inheritance (Guimarães et al., Citation2003). Morphologically, C. usneoides subsp. succosus thalli tend to be taller, more erect, thicker (main axes up to 5 mm in diameter), with axes bearing more unilateral (secund) branching, and a greater number of simple (unbranched), small and thin branchlets. This subspecies shares a remarkable habit similarity with C. corneus and this similarity has been reported by other authors (Børgesen, Citation1920, as G. wrightii; Taylor, Citation1960, as G. debilis; Ardito et al., Citation2014; and Núñez-Resendiz et al., Citation2015, Citation2017).
A single anatomical character observed in tropical Western Atlantic Crassiphycus species is the unique ability of the mature medullary cells to continue to cut off smaller cells in the medulla, giving rise to the microcystidiate condition. This condition is different from another feature observed here in C. usneoides: the presence of secondary medullary cells coursing through primary medullary cells. These cell files occur either as uniseriate filaments or filling the space left by a former larger medullary cell. This seems to be a unique feature not seen in any other species of Gracilariaceae to date.
Although cited in recent checklists of marine algae for the Western Atlantic Ocean (Wynne, Citation2017), H. rangiferina remains a poorly known species in the tropical Western Atlantic Ocean, while at the same time, being one of the most commonly cited species in the tropical eastern Atlantic (as G. dentata: Fox, Citation1957; Steentoft, Citation1967; Lawson & John, Citation1982). Hydropuntia rangiferina has also been reported for the Indian Ocean from Yemen, Oman and Pakistan (Silva et al., Citation1996). Lawson & John (Citation1982) suggested that H. rangiferina may be a synonym of Gracilaria henriquesiana Hariot noting that these two could be forms of the same species based on vegetative features alone. Hydropuntia rangiferina is a flattened, subdichotomously branched species. Flat species of Gracilaria are said to be the most difficult ones to identify in the absence of reproductive structures (Yamamoto, Citation1984). For example, overlapping habit phenotypes can be found among specimes of Gracilaria silviae (Lyra et al., Citation2015), G. baiana (Lyra et al., Citation2016), G. cearensis (Soares et al., Citation2015), and H. rangiferina thalli (this study). The confirmation of these species for the Brazilian coast could only be validated by molecular data. Hydropuntia aff. rangiferina seems to correspond to another species whose taxonomy status has yet to be elucidated. Further studies on H. aff. rangiferina are needed in order to resolve its taxonomy.
With the exception of C. crassissimus, all other Crassiphycus species are characterized by cylindrical thalli and irregular branching patterns. Their habits are often extremely similar, frequently presenting overlapping external phenotypes, particularly C. caudatus, C. secundus, C. usneoides, and to a certain extent, C. corneus. This morphological overlap has led to pervading taxonomic confusion and frequent misidentifications (Ardito et al., Citation2014; Núñez-Resendiz, Citation2015, Citation2017; Vilchis et al., Citation2019). Bird et al. (Citation1986, as Gracilaria) advocated the close phylogenetic relationship among C. crassissimus and C. usneoides, did not accept C. usneoides as being taxonomically distinct from C. corneus, and suggested that C. crassissimus could be a rough water ecad of C. corneus. Recently, Vilchis et al. (Citation2019) reported on the striking morphological similarities between C. corneus and C. usneoides. In light of the many published reports, the morphological plasticity and overlap among Crassiphycus species has clearly baffled phycologists over recent decades. Bird et al. (Citation1986) also regarded G. damaecornis as a slender form of C. corneus, with more regularly dichotomous branches, and a fastigiate to corymbose habit. Regardless whether Gracilaria damaecornis is a distinct species from C. corneus, the former remains a poorly known species and future studies should clarify its status. Our results disagree with Bird et al. (Citation1986) and show that C. crassissimus and C. usneoides are distinct yet sister species (, ). Conversely, our results agree with Agardh (Citation1852) who regarded C. usneoides as a distinct species from C. corneus.
In the western Atlantic Ocean, partial phylogeographic agreement was found between two Crassiphycus species with wide geographic distributions: C. corneus and C. caudatus. Crassiphycus corneus populations seem to be genetically separated into northern and southern hemisphere populations. This phylogeographic pattern suggests that the Amazon-Orinoco Rivers, together with the different oceanic circulation patterns between the two hemispheres, represent extant barriers to gene flow. Crassiphycus caudatus, on the other hand, displays a phylogeographic disjunction located in the mid coast of Brazil. This result bears phylogeographic concordance with several other marine species such as the fishes Macrodon ancylodon and Macrodon atricauda (Santos et al., Citation2006; Carvalho-Filho et al., Citation2010), the polychaete Perinereis anderssoni (Paiva et al., Citation2019) and the marine mite Rhombognathus levigatoides (Pepato et al., Citation2019). This phylogeographic signature is attributed to a natural geographic barrier known as the Abrolhos Bank and the Vitória-Trindade Ridge. Floeter et al. (Citation2001) suggest that this topographical barrier to the Brazil Current induces fundamental changes in physical, chemical and biological features. The Abrolhos Bank and the Vitória-Trindade Ridge are also responsible for promoting genetic isolation in the marine environment during periods of Pleistocene glaciation. During glacial maxima, marine connectivity north and south of the Abrolhos Bank and the Vitória-Trindade Ridge was further decreased as shallower regions of the continental shelf in this region emerged as sea levels dropped (Pepato et al., Citation2019). The interplay between glaciation events and the Vitória-Trindade Ridge is hypothesized to have driven the evolution of several Brazilian marine benthic taxa (Pepato et al., Citation2019). Our results also corroborate the recently published phylogeographic study of C. caudatus populations along the Brazilian coast by Ayres-Ostrock et al. (Citation2019).
Belizean C. crassissimus specimens sequenced in this study correspond to more recent collections of specimens referred to as ‘H. cornea’ in Fredericq & Norris (Citation1985) and further discussed in Fredericq & Hommersand (Citation1990). Therefore, the study of male and female pre- and post-fertilization structures done by Fredericq & Norris (Citation1985) refers to C. crassissimus and not to C. corneus. Among the western Atlantic species, C. crassissimus and C. corneus are the first and second thickest species, respectively. C. crassissimus is the most morphologically variable species, with habits ranging from completely compressed, more or less prostrate, to partially erect morphs, to entirely erect cylindrical morphs (illustrated in Gurgel, Citation2017). Another similarity between C. crassissimus and C. corneus is the partial immersion of their cystocarps into the thallus. Besides these similarities, C. crassissimus is phylogenetically closer to C. usneoides than to C. corneus.
Recently, Ng et al. (Citation2017) synonymized G. changii and G. firma. They based their taxonomic proposal on comparative morphology of type specimens and newly generated rbcL and cox1 DNA sequences. The holotypes of G. changii into G. firma indeed look similar (Ng, Citation2019). However, Ng et al. (Citation2017) did not address the sister clade to G. firma, herein identified as C. aff. changii. They identified this sister clade as an ‘unnamed Malaysian “Hydropuntia” species’ but this clade is characterized by plants with morphological characters typical of C. changii/C. firmus (e.g. AY049388, JQ026026). The existence of the C. aff. changii suggests that C. firmus and C. changii can represent two cryptic species where C. aff. changii can represent the true C. changii. Because no DNA sequences could be obtained from either C. firmus or G. changii holotypes, the status of the specimens sister to C. firmus remains unresolved.
Striking phylogeographic concordance can be observed within three Indo-Pacific species: Crassiphycus aff. changii, C. firmus and Hydropuntia edulis. Phylogeographic patterns within each of these species comprise two phylogroups, one identified as the Indo-Malay Phylogroup, which is so far restricted to South-Eastern Asia (Indochina), more specifically, the Gulf of Thailand and the Malacca Strait regions; and the other identified as South China Sea Phylogroup, comprised of populations located in the South China Sea and the Philippines (). Our results agree with those reported by Leliaert et al. (Citation2018) who demonstrated that dispersal, followed by isolation and allopatry, represent the major biogeographic processes promoting genetic differentiation and speciation in red algae. Strong phylogeographic signals provided by rbcL datasets have been observed in other Gracilariales species such as Gracilaria tikvahiae (Gurgel et al., Citation2004), C. caudatus (Ayres-Ostrock et al., Citation2019) and Agarophyton chilensis (Yang et al., Citation2008).
For Hydropuntia edulis, observed phylogeographic patterns recognized not only the Indo-Malay and South China Sea Phylogroups (in this particular case the latter also includes haplotypes found in Hawaii, possibly a recent introduction), but also a third phylogroup so far endemic to Japan (). Phylogeographic structure between Japan and continental Asia populations has been observed for other Gracilariaceae species such as G. textorii and G. incurvata, recent sister species restricted to continental Asia and Japan, respectively (Kim et al., Citation2006).
Our time-calibrated phylogeny agrees well with published phylogenomic chronograms by Nan et al. (Citation2017) and the time-calibrated phylogenetic analysis of the Florideophyceae based on seven genes (including rbcL) by Yang et al. (Citation2016). Our time estimate for the divergence between the tribes Gracilariopsiseae and Gracilarieae was 126 (117–139) mya compared with 140 mya in Yang et al. (Citation2016) which contained only one species of Gracilariopsis, one Agarophyton and an unidentified ‘Gracilaria sp.’. Conversely, our time estimates are different, and on average smaller, than those provided by Du et al. (Citation2016). Our mrca ages for the cladogenesis between Gracilariopsiseae and Gracilarieae, and between Agarophyton and the remaining Gracilarieae are 126 (117–140) and 115 (11–123) mya, respectively, compared with 448 (423–473) and 316 (298–334) in Du et al. (Citation2016), respectively. Unfortunately Du et al. (Citation2016) did not provide enough details on how their time-calibrated analysis was performed to help us assess potential reasons for this disagreement (e.g. clock model, speciation model, calibration values, prior distributions). We also contend that calibrations of red algal phylogenies should not be conducted using vascular plants and green algal fossil references such as those used and advocated in Du et al. (Citation2016).
The origin of the Gracilariales is considered ancient (~248–300 mya; Nan et al., Citation2017) and not the focus of this study. However, our results suggest that events in the mid and late Cretaceous played a major role towards the diversification within the tribe Gracilarieae, giving rise to the four genera currently recognzied in Gracilaria sensu lato: Agarophyton, Hydropuntia, Crassiphycus and Gracilaria. The Cretaceous marked the full development of the Tethyan Ocean, a period characterized by global warmer conditions, absence of polar ice caps, the presence of vast open oceans, higher sea levels, and a large number of warm, shallow, inland seas widespread across the planet. The interior of many continents was covered with shallow warm seas (Skelton et al., Citation2003). These geological and climatic conditons promoted the formation of a wide range of marine habitats, resulting in the diversification of marine organisms worldwide (Skelton et al., Citation2003), including genera in the Gracilarieae as observed in our results. Our results agree with the biogeographic explanation for the diversification of the Gracilariales in Hommersand (Citation1990), which identifies the Tethyan Ocean as the driver for the origin of several high taxa in the modern Florideophyceae, including the formation of a southern (Curdiea and Melanthalia) and a northern (the remaining genera) Gracilariaceae generic clade.
Hommersand (Citation1990) also identified the late Cretaceous and early Paleogene as a moment of great importance in the diversification of Gracilarieae. During this time frame, the diversification inside both Gracilaria and Crassiphycus, including the separation between the Pacific and the Atlantic Crassiphycus clades, seems to have occurred within a short period of time, over ~10 my. This was a period of major climatic changes which led to one of the major mass extinctions in the history of the planet, the K–T extinction event (Aguirre et al., Citation2000; Skelton et al., Citation2003). However, fossil records also show that some marine taxa were immune to the mass extinction event (Jablonski & Raup, Citation1995; Aguirre et al., Citation2000). We hypothesize that the split between Gracilaria and Crassiphycus and their subsequent intra-generic diversification are coupled with the potential ‘ecological release’ in marine benthic habitats following the K–T mass extinction event (~66 mya). Hydropuntia, however, shows a more recent time of intra-generic diversification, at the boundary between the Eocene and the Oligocene epochs.
Molecular-based studies help us better understand the systematics of red algal groups whose morphological variation is plagued with homoplasies, such as the Gracilariales. Our molecular phylogenies corroborate that Crassiphycus and Hydropuntia represent distinct genera in the Gracilariaceae. Our time-calibrated chronogram helped us better understand the evolutionary history of the family. This study provides a morphological characterization, guided by molecular phylogenies, for three common Gracilariaceae species in the Western Atlantic Ocean: C. secundus, C. usneoides and H. rangiferina. We anticipate that the extended species descriptions provided herein will help researchers better identify Crassiphycus and Hydropuntia species, both in the field and the laboratory. We also raise attention to other Gracilariaceae species that need further studies such as the correct taxonomic determination of Crassiphycus sp. from the Mediterranean, C. aff. changii from Malaysia and the Philippines, and H. aff. rangiferina from the Indo-Pacific. As more molecular data are generated for Crassiphycus and Hydropuntia species from different parts of the world, the more we will learn about the true diversity and evolution of this ecologically and economically important group of organisms.
Supplemental Material
Download MS Word (107.3 KB)Acknowledgements
We thank R.H. Sims and E.M. Plastino and all collectors listed in Supplementary table 1 for help with collections for help with collections; and M.C. Oliveira and G.M. Lyra for making available some unpublished C. caudatus rbcL DNA sequences. For thoughtful discussion and clarification on nomenclature issues we thank Laurence Dorr (NMNH Botany). This study corresponds to the Smithsonian Marine Station at Fort Pierce Contribution No. 1146.
Author contributions
CFD Gurgel: original concept, field and lab work, DNA sequencing, data analyses, drafting and editing manuscript; L. Soares & M.T. Fujji: field and lab work, DNA sequencing, drafting and editing manuscript; S. Fredericq & J.N. Norris: original concept, field and lab work, drafting and editing manuscript; W. Schmidt: phylogenetic analysis, editing manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2020.1814424
Supplementary table 1. Information of newly generated rbcL DNA sequences and respective collection details, including GenBank accession numbers, for Crassiphycus and Hydropuntia species used in this study.
Supplementary fig. 1. Maximum likelihood phylogram of the genera Crassiphycus (C.) and Hydropuntia (H.), including other selected species in the genus Gracilaria (G.), (Gracilariaceae, Rhodophyta) based on rbcL DNA sequences. Two Agarophyton species were used as outgroups. Numbers above branches correspond to bootstrap proportions based on 1000 replications. N = number of sequences inside the collapsed branch. Scale bar = substitutions per site.
Additional information
Funding
References
- Abbott, I.A., Zhang, J. & Xia, B. (1991). Gracilaria mixta sp. nov. and other western Pacific species of the genus (Rhodophyta, Gracilariaceae). Pacific Science, 45: 12–27.
- Agardh, J.G. (1852). Species Genera et Ordines Algarum … Vol. 2, pt. 3, fasc. 1, 701–786. Lund.
- Aguirre, J., Riding, R. & Braga, J.C. (2000). Late Cretaceous incident light reduction: evidence from benthic algae. Lethaia, 33: 205–213.
- Ardito, S.M., Sentíes, A. & Dreckmann, K.M. (2014). Caracterización morfoanatómica de Hydropuntia usneoides (Gracilariaceae, Rhodophyta) para la costa venezolana. Interciencia, 39: 49–53.
- Ayres‐Ostrock, L.M., Valero, M., Mauger, S., Oliveira, M.C., Plastino, E.M., Guillemin, M.L. & Destombe, C. (2019). Dual influence of terrestrial and marine historical processes on the phylogeography of the Brazilian intertidal red alga Gracilaria caudata. Journal of Phycology, 55: 1096–1114.
- Bellorin, A.M., Oliveira, M.C. & Oliveira, E.C. (2002). Phylogeny and systematics of the marine algal family Gracilariaceae (Gracilariales, Rhodophyta) based on small subunit rDNA and ITS sequences of Atlantic and Pacific species. Journal of Phycology, 38: 551–563.
- Bird, C.J., Oliveira, E.C. & McLachlan, J. (1986). Gracilaria cornea, the correct name for the western Atlantic alga hitherto known as G. debilis (Rhodophyta, Gigartinales). Canadian Journal of Botany, 64: 2045–2051.
- Børgesen, F. (1920). The Marine Algae of the Danish West Indies, Pt. 3. Rhodophyceae (6) with addenda to the Chlorophyceae, Phaeophyceae and Rhodophyceae. Dansk Botanisk Arkiv, 4: 14–35.
- Bouckaert, R., Vaughan, T.G., Barido-Sottani, J., Duchêne, S., Fourment, M., Gavryushkina, A., Heled, J., Jones, G., Kühnert, D., De Maio, N., Matschiner, M., Mendes, F.K., Müller, N.F., Ogilvie, H.A., du Plessis, L., Popinga, A., Rambaut, A., Rasmussen, D., Siveroni, I., Suchard, M.A., Wu, C.-H., Xie, D., Zhang, C., Stadler, T. & Drummond, A.J. (2019). BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLOS Computational Biology 15: e1006650.
- Carvalho-Filho, A., Santos, S. & Sampaio, I. (2010). Macrodon atricauda (Günther, 1880) (Perciformes: Sciaenidae), a valid species from the southwestern Atlantic, with comments on its conservation. Zootaxa, 2519: 48–58.
- Chang, C.F. & Xia, B.-M. (1963). Polycavernosa, a new genus of the Gracilariaceae. Studia Marina Sinica, 3: 120–126.
- Darriba, D., Taboada, G.L., Doallo, R. & Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9: 772, https://doi.org/10.1038/nmeth.2109.
- Du, Q., Bi, G., Mao, Y. & Sui, Z. (2016). The complete chloroplast genome of Gracilariopsis lemaneiformis (Rhodophyta) gives new insight into the evolution of family Gracilariaceae. Journal of Phycology, 52: 441–450.
- Duchêne, S., Lanfear, R. & Ho, S.Y. (2014). The impact of calibration and clock-model choice on molecular estimates of divergence times. Molecular Phylogenetics and Evolution, 78: 277–289.
- Floeter, S.R., Guimarães, R.Z., Rocha, L.A., Ferreira, C.E., Rangel, C.A. & Gasparini, J.L. (2001). Geographic variation in reef‐fish assemblages along the Brazilian coast. Global Ecology and Biogeography 10: 423–431.
- Fox, M. (1957). A first list of marine algae from Nigeria. Botanical Journal of the Linnean Society, 55: 615–631.
- Fredericq, S. & Hommersand, M.H. (1989). Proposal of the Gracilariales, ord. nov. (Rhodophyta) based on an analysis of the reproductive development of Gracilaria verrucosa. Journal of Phycology, 25: 213–227.
- Fredericq, S. & Hommersand, M.H. (1990). Diagnoses and key to the genera of the Gracilariaceae (Gracilariales, Rhodophyta). Hydrobiologia, 204/205: 173–178.
- Fredericq, S. & Norris, J.N. (1985). Morphological studies on some tropical species of Gracilaria Grev. (Gracilariaceae, Rhodophyta): taxonomic concepts based on reproductive morphology. In Taxonomy of Economic Seaweeds, Vol. I. (Abbott, I.A. & Norris, J.N., editors), 137–155. California Sea Grant College Program, Univ. Calif. La Jolla.
- Freshwater, D.W. & Rueness, J. (1994). Phylogenetic relationships of some European Gelidium (Gelidiales, Rhodophyta) species based on rbcL nucleotide sequence analysis. Phycologia, 33: 187–94.
- Greville, R.K. (1830). Algae Britannicae, or descriptions of the marine and other inarticulated plants of the British Islands, belonging to the order Algae; with plates illustrative of the genera. Edinburgh: MacLachlan & Stewart; Baldwin & Cradock, [iii]+lxxxviii+218 pp., XIX pls.
- Guimarães, M., Plastino, E.M. & Destombe, C. (2003). Green mutant frequency in natural populations of Gracilaria domingensis (Gracilariales, Rhodophyta) from Brazil. European Journal of Phycology, 38: 165–169.
- Guiry, M.D., Norris, J.N., Fredericq, S. & Gurgel, C.F.D. (2018). Crassiphycus Guiry, Gurgel, J.N.Norris & Fredericq, gen. nov., a replacement name for Crassa Gurgel, J.N.Norris & Fredericq, nom. inval. (Gracilariaceae, Rhodophyta), with some additional nomenclatural notes. Notulae Algarum, 82: 1–4.
- Gurgel, C.F.D. (2017). Gracilariales. In Syllabus of Plant Families (2/2) Rhodobionta (A. Engler’s Syllabus der Pflanzenfamilien series) (Frey, W., editor). Borntraeger-Cramer Publisher, Stuttgart.
- Gurgel, C.F.D. & Fredericq, S. (2004). Systematics of the Gracilariaceae (Gracilariales, Rhodophyta): a critical assessment based on rbcL sequence analysis. Journal of Phycology, 40: 138–159.
- Gurgel, C.F.D., Fredericq, S. & Norris, J.N. (2004). Phylogeography of Gracilaria tikvahiae (Gracilariaceae, Rhodophyta): a study of genetic discontinuity in a continuously distributed species based on molecular evidence. Journal of Phycology, 40: 748–758.
- Gurgel, C.F.D., Fredericq, S., Norris, J.N. & Yoneshigue-Valentin, Y. (2008). Two new flat species of Gracilaria (Gracilariales, Rhodophyta) from Brazil: G. abyssalis sp. nov. and G. brasiliensis sp. nov. Phycologia, 47: 249–264.
- Gurgel, C.F.D., Norris, J.N., Schmidt, W.E., Le, H.N. & Fredericq, S. (2018). Systematics of the Gracilariales (Rhodophyta) including new subfamilies, tribes, subgenera and two new genera, Agarophyton gen. nov. and Crassa gen. nov. Phytotaxa, 374: 1–23.
- Heled, J. & Drummond, A.J. (2011). Calibrated tree priors for relaxed phylogenetics and divergence time estimation. Systematic Biology, 61: 138–149.
- Hommersand, M.H. (1990). Biogeography of the marine red algae of the North Atlantic Ocean. In Evolutionary Biogeography of the Marine Algae of the North Atlantic, 349–410. Springer, Berlin.
- Iha, C., Grassa, C.J., Lyra, G.M., Davis, C.C., Verbruggen, H. & Oliveira, M.C. (2018). Organellar genomics: a useful tool to study the evolutionary relationships and molecular evolution in Gracilariaceae (Rhodophyta). Journal of Phycology, 54: 775–787.
- Jablonski, D. & Raup, D.M. (1995). Selectivity of end-Cretaceous marine bivalve extinctions. Science, 268: 389–391.
- Kim, M.S., Yang, E.C. & Boo, S.M. (2006). Taxonomy and phylogeny of flattened species of Gracilaria (Gracilariaceae, Rhodophyta) from Korea based on morphology and protein-coding plastid rbcL and psbA sequences. Phycologia, 45: 520–528.
- Kylin, H. (1956). Die Gattungen der Rhodophyceen. C.W.K. Gleerups, Lund, XV+673 pp.
- Lawson, G.W. & John, D.M. (1982). The marine algae and coastal environment of tropical West Africa. Nova Hedwigia, 70: 1–455.
- Leliaert, F., Payo, D.A., Gurgel, C.F.D., Schils, T., Draisma, S.G., Saunders, G.W., (…) & Le Gall, L. (2018). Patterns and drivers of species diversity in the Indo‐Pacific red seaweed Portieria. Journal of Biogeography, 45: 2299–2313.
- Lin, S.M., Fredericq, S. & Hommersand, M.H. (2001). Systematics of the Delesseriaceae (Ceramiales, Rhodophyta) based on LSU rDNA and rbcL sequences, including the Phycodroideae, subfam. nov. Journal of Phycology, 37: 881–899.
- Lyra, G.M., Costa, E.S., De Jesus, P.B., De Matos, J.C.G., Caires, T.A., Oliveira, M.C., Oliveira, E.C., Xi, Z., Nunes, J.M.C & Davis, C.C. (2015). Phylogeny of Gracilariaceae (Rhodophyta): evidence from plastid and mitochondrial nucleotide sequences. Journal of Phycology, 51: 356–366.
- Lyra, G.M., Gurgel, C.F.D., Costa, E.S., Jesus, P.B., Oliveira, M.C., Oliveira, E.C., Davis, C.C. & Nunes, J.M.C. (2016). Delimitating cryptic species in the Gracilaria domingensis complex (Gracilariaceae, Rhodophyta) using morphological and molecular data. Journal of Phycology, 52: 997–1017.
- Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In Proceedings of the Gateway Computing Environments Workshop (GCE), 1–8. 14 Nov. 2010, New Orleans, LA.
- Montagne, C. (1842). Prodromus generum specierumque phycearum novarum …, [1]–16. Gide, Paris.
- Nan, F., Feng, J., Lv, J., Liu, Q., Fang, K., Gong, C. & Xie, S. (2017). Origin and evolutionary history of freshwater Rhodophyta: further insights based on phylogenomic evidence. Scientific Reports, 7: 1–12.
- Ng, P.-K. (2019). Molecular data complement morphological observations in the systematics of Gracilariaceae. In Taxonomy of Southeastern Asian Seaweeds (Phang, S.-M., Song, S.-L. & Lim, P.-E., editors), Institute of Ocean & Earth Sciences, University of Malaya, Monograph Series, 17: 93–113.
- Ng, P.-K., Lin, S.-M., Lim, P.-E., Hurtado, A.Q., Phang, S.-M., Yow, Y.-Y. & Sun, Z. (2017). Genetic and morphological analyses of Gracilaria firma and G. changii (Gracilariaceae, Rhodophyta), the commercially important agarophytes in western Pacific. PLoS ONE, 12: e0182176.
- Núñez-Resendiz, M.L., Dreckmann, K.M., Sentíes, A., Díaz-Larrea, J. & Zuccarello, G.C. (2015). Genetically recognizable but not morphologically: the cryptic nature of Hydropuntia cornea and H. usneoides (Gracilariales, Rhodophyta) in the Yucatan Peninsula. Phycologia, 54: 407–416.
- Núñez-Resendiz, M.L., Zuccarello, G.C., Dreckmann, K.M., & Sentíes, A. (2017). Phylogeography of Hydropuntia cornea/Hydropuntia usneoides complex (Gracilariales, Rhodophyta) in the Yucatan Peninsula. Phycologia, 56: 14–20.
- Paiva, P.C., Mutaquilha, B.F., Coutinho, M.C.L. & Santos, C.S. (2019). Comparative phylogeography of two coastal species of Perinereis Kinberg, 1865 (Annelida, Polychaeta) in the South Atlantic. Marine Biodiversity, 49: 1537–1551.
- Pepato, A.R., Vidigal, T.H. & Klimov, P.B. (2019). Evaluating the boundaries of marine biogeographic regions of the Southwestern Atlantic using halacarid mites (Halacaridae), meiobenthic organisms with a low dispersal potential. Ecology and Evolution, 9: 13359–13374.
- Plastino, E.M. & Oliveira, E.C. (1997). Gracilaria caudata J. Agardh (Gracilariales, Rhodophyta) restoring an old name for a common western Atlantic alga. Phycologia, 36: 225–232.
- Rambaut, A. (2012). FigTree v1.4.2: Tree figure drawing tool. http://tree.bio.ed.ac.uk/software/figtree/; searched on 21 June 2019.
- Rambaut, A. & Drummond, A.J. (2014). TreeAnnotator v2. 1.2. Edinburgh: University of Edinburgh, Institute of Evolutionary Biology.
- Rambaut, A., Drummond, A.J., Xie, D., Baele, G. & Suchard, M.A. (2018). Posterior summarisation in Bayesian phylogenetics using Tracer 1.7. Systematic Biology, 67: 901–904.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Höhna, S., Larget, B., Liu, L., Surchard, M.A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Santos, S., Hrbek, T., Farias, I. P., Schneider, H. & Sampaio, I. (2006). Population genetic structuring of the king weakfish, Macrodon ancylodon (Sciaenidae), in Atlantic coastal waters of South America: deep genetic divergence without morphological change. Molecular Ecology, 15: 4361–4373.
- Schramm, A. & Mazé, H. (1865). Essai de classification des algues de la Guadeloupe. [Ed.1a]. [iii]+52 pp. Basse-Terré, Guadeloupe: Imprimerie du Gouvernement.
- Silva, P.C., Basson, P.W. & Moe, R.L. (1996). Catalogue of the Marine Algae of the Indian Ocean. University of California Publications in Botany, 79: 1–1259.
- Skelton, P.W., Spicer, R.A., Kelley, S.P. & Gilmour, I. (2003). The Cretaceous World. Cambridge University Press, Cambridge.
- Soares, L.P., Gurgel, C.F.D. & Fujii, M.T. (2015). Taxonomic reassessment of Gracilaria cearensis (Rhodophyta, Gracilariales), a poorly defined yet common flattened species based on morphological and molecular analysis including topotype collections. Phytotaxa, 201: 241–255.
- Steentoft, M. (1967). A revision of the marine algae of São Tomé and Príncipe (Gulf of Guinea). Journal of the Linnean Society of London, Botany, 60: 99–146.
- Taylor, W.R. (1960). Marine Algae of the Eastern Tropical and Subtropical Coasts of the Americas. University of Michigan Press, Ann Arbor.
- Thiers, B. (2020). Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/ih/[continuously updated].
- Tsuda, R.T. & Abbott, I.A. (1985). Collecting, handling, preservation, and logistics. In Handbook of Phycological Methods, Vol. IV, Ecological Field Methods: Macroalgae (Littler, M.M. & Littler, D.S., editors), 67–86. Cambridge University Press, Cambridge.
- Vilchis, M.I., Neustupa, J., Dreckmann, K., Quintanar, A. & Sentíes, A. (2019). Discrimination of the species of the Crassiphycus corneus/C. usneoides complex (Gracilariaceae, Rhodophyta) through geometric morphometric analysis. Nova Hedwigia, 109: 291–301.
- Wynne, M.J. (1989). The re-instatement of Hydropuntia Montagne (Gracilariaceae, Rhodophyta). Taxon, 38: 476–479.
- Wynne, M.J. (2017). A checklist of the benthic marine algae of the tropical and subtropical western Atlantic: fourth revision. Nova Hedwigia Beihefte, 145: 1–202.
- Yamamoto, H. (1984). An evaluation of some vegetative features and some interesting problems in Japanese populations of Gracilaria. Hydrobiologia, 116/117: 51–54.
- Yang, E.C., Kim, M.S., Geraldino, P.J.L., Sahoo, D., Shin, J.A. & Boo, S.M. (2008). Mitochondrial cox1 and plastid rbcL genes of Gracilaria vermiculophylla (Gracilariaceae, Rhodophyta). Journal of Applied Phycology, 20: 161–168.
- Yang, E.C., Boo, S.M., Bhattacharya, D., Saunders, G.W., Knoll, A.H., Fredericq, S., Graf, L., & Yoon, H.S. (2016). Divergence time estimates and the evolution of major lineages in the florideophyte red algae. Scientific Reports, 6: 1–11.
