Abstract
The coast of India is reported to have eight species of bladed Bangiales, five assigned to Porphyra and three to Phycocalidia. Species tend to be seasonal, occurring particularly in the winter and monsoon season. During a reassessment of the diversity of these algae on the Indian coast, a new species of bladed Bangiales, Phycocalidia sukshma M.G.Kavale & M.A.Kazi sp. nov., was discovered and is described from Karwar on the west coast of India. It was collected intertidally during the monsoon season, particularly from the uppermost part of the shore. The species is monostromatic, thin and delicate in texture with 16 spermatia per spermatangium and eight zygotospores per zygotosporangium. The blade margin is undulate with very rare denticulations. Analysis of plastid rbcL and mitochondrial COI-5P resolved Ph. sukshma as a distinct clade, sister to Brazilian and Indian specimens of Ph. vietnamensis. The COI-5P data also revealed that the species known as Porphyra kanyakumariensis Krishnamurthy & Baluswami formed a clade within the genus Phycocalidia and a new combination is made. Phycocalidia kanyakumariensis was collected during the monsoon from the Kanyakumari coast, Tamil Nadu and Mulloor and Leela beach (Kovalam), Kerala. Our work adds to the growing number of warm water species known in this group, indicating the need for further revision of the bladed Bangiales of India and globally.
Introduction
The bladed Bangiales is one of the largest groups of red algae, occurring in the intertidal and shallow subtidal from tropical to cold temperate regions (Brodie et al., Citation2008). Their simple morphology and limited distinguishing characters make species identification difficult and belie the extent of genetic diversity revealed by molecular taxonomic studies (Nelson et al., Citation1999; Sutherland et al., Citation2011; Sánchez et al., Citation2014, Citation2015; Yang et al., Citation2020). Until recently, the bladed (foliose) members of the order were all assigned to Porphyra C.Agardh. Now, major taxonomic revisions of the bladed Bangiales have resulted in 14 genera. Sutherland et al. (Citation2011) divided Porphyra sensu lato into nine genera: Boreophyllum, Clymene, Fuscifolium, Lysithea, Miuraea, Porphyra, Pyropia and Wildemania; Sánchez et al. (Citation2014) added Neothemis. In the latest taxonomic revision, Yang et al. (Citation2020) separated Pyropia into the following genera: Pyropia, Calidia, Neoporphyra, Neopyropia, Uedaea and Porphyrella. Subsequently, Santiañez & Wynne (Citation2020) showed that as Calidia was an illegitimate name, a homonym of the lichen genus Calidia J.Stirton which necessitated a new name, and they proposed Phycocalidia.
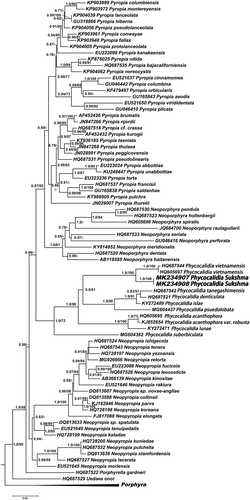
India has a long coastline with a wide range of habitats that support a rich seaweed diversity although few species are taxonomically documented (Mantri et al., Citation2020). Earlier studies in the Indian region documented eight species of Porphyra which were reported to appear seasonally, particularly in winter and monsoon season (). After Sutherland et al.’s (Citation2011), revision, we reassessed the diversity of these algae as part of a biodiversity study of the economically important seaweeds from the west coast of India. Together with previous studies, five Porphyra species (P. indica, P. okhaensis, P. chauhanii, P. malvanansis and P. kanyakumariensis) and three Phycocalidia species (Ph. suborbiculata, Ph. vietnamensis and Ph. acanthophora var. robusta) were recognized for the bladed Bangiales from India (). Among these, Ph. suborbiculata was the first authentic report of the genus in India (Krishnamurthy & Baluswami, Citation1984). Kavale et al. (Citation2015a, Citation2015b) confirmed the presence of two more Phycocalidia species from Indian waters based on molecular analysis: Ph. vietnamensis from the coast of Maharashtra, Goa and Kerala states and a new variety Ph. acanthophora var. robusta from Goa, later also found in the central west coast of India at Redi, Malvan, Vayangani, the coast of Maharashtra State and Dona Paula from the Goa State (Kavale et al., Citation2017).
Table 1. Occurrence of Porphyra and Phycocalidia species along Indian coasts
Porphyra crispata was also reported from India but the type specimen is referable to Monostroma nitidum Wittrock (Kurogi & Yamada, Citation1984, Citation1986). Yoshida (Citation1997) synonymized the specimen of P. crispata from Ojika Island, Japan with Porphyra yamadae Yoshida. However, rbcL sequence data for P. yamadae were identical to Ph. acanthophora (E.C.Oliveira & Coll) Santiañez (Kavale et al., Citation2015a; Xie et al., Citation2015). Now, P. yamadae is considered a synonym of Ph. acanthophora (Xie et al., Citation2015). Similarly, the specimens collected previously as P. crispata from the Indian region can now be regarded as Ph. acanthophora (Xie et al., Citation2015; Dumilag & Yap, Citation2018, Citation2020).
An outcome of recent studies by Yang et al. (Citation2018, Citation2020) and Kim et al. (Citation2018) is that species biogeography can be indicative of the underlying phylogeny of bladed Bangiales. The recently described Phycocalidia is a genus of subtropical and tropical species, except for Ph. suborbiculata that extends into cold temperate regions (Milstein et al., Citation2015; Kim et al., Citation2018; Yang et al., Citation2018). During our reassessment we found a new warm water species from Karnataka and from our morphological and molecular results were able to revise the taxonomy of Porphyra kanyakumariensis from Tamil Nadu and Kerala.
Materials and methods
Collection
Specimens were collected from the upper intertidal of Karwar (14°48’40”N, 74°06’72.3”E), Karnataka State, Mulloor (8°19’ 23.8”N, 77°03’62.6”E) and Leela beach (Kovalam) (8°24’02.8”N, 76°58’35.8”E), Kerala State and Kanyakumari (8°04’43”N, 77°33’04”E), Tamil Nadu State in August 2014 and September 2018. Specimen colour and distribution were recorded in the field. The samples were washed thoroughly with seawater to remove sand particles and epiphytes and transferred to the laboratory in a coolbox. The samples were preserved at −20°C for further analysis. Herbarium specimens were deposited in the Botanical Survey of India, Kolkata, India (BSIS; abbreviations follow Thiers, Citation2020).
Morphological characterization
Specimens were initially grouped based on their colour, shape and texture. A minimum of 30 thalli of each type was used to record morphological observations. The parameters selected for morphological analysis were size, shape, texture, colour, the thickness of the thallus, presence or absence of marginal spines, number, division formulae and distribution of reproductive bodies and the thickness of mucilage. Morphological observations were carried out using a compound and phase contrast microscope (Motic, Hong Kong). Parameters selected for identification were compared with previously published reports (Supplementary table S1; Hus, Citation1902; Kurogi, Citation1972; Dhargalkar et al., Citation1981; Krishnamurthy & Baluswami, Citation1984; Chennubotla et al., Citation1990; Joshi et al., Citation1992; Anil Kumar & Panikkar, Citation1995).
Molecular characterization
Total genomic DNA was extracted with the Gene EluteTM Plant genomic DNA miniprep kit (Sigma Aldrich, St. Louis, Missouri, USA). The COI-5P (cytochrome c oxidase subunit 1) gene was amplified using primers GazF1: 5’ - TCAACAAATCATAAAGATATTGG - 3’ and GazR1: 5’ - ACTTCTGGATGTCCAAAAAAYCA - 3’, following Saunders (Citation2005). The rbcL (large subunit of ribulose 1, 5 bisphosphate carboxylase/oxygenase) gene was amplified using primers rbF: 5’ - GTAATTCCATATGCTAAAATGGG- 3’ (Freshwater & Rueness, Citation1994) and rbR: 5’ - ACATTTGCTGTTGGAGTYTC- 3’ (Kucera & Saunders, Citation2012). Amplification of PCR product was carried out for rbcL following Kucera & Saunders (Citation2012). All PCR products were purified with a Gene EluteTM Gel Extraction Kit (Sigma Aldrich) and sequenced by Xcelris Labs Ltd, India. Sequences were deposited in GenBank. For the phylogenetic analysis of COI-5P and rbcL genes, reference sequences were retrieved from GenBank (Supplementary table S2). Two separate alignments were generated for COI-5P and rbcL sequences using the ClustalW algorithm in MEGA v6 (Tamura et al., Citation2013). Each dataset was subjected to maximum likelihood (ML) phylogenetic analysis using PhyML 3.0 (Guindon et al., Citation2010). The best suited evolutionary models to describe the substitution pattern were estimated in the Smart Model Selection (SMS) program (Lefort et al., Citation2017) based on lowest Bayesian Information Criterion scores (BIC). ML analyses were carried out using the General Time Reversible substitution model with discrete Gamma distribution and invariant sites (GTR+G+I). Support for nodes was estimated by both approximations to likelihood ratio test (aLRT SH-like) and bootstrap resampling method with 1000 replications. The pairwise distance between sequences was calculated using MEGA v6.
Results
Molecular analysis
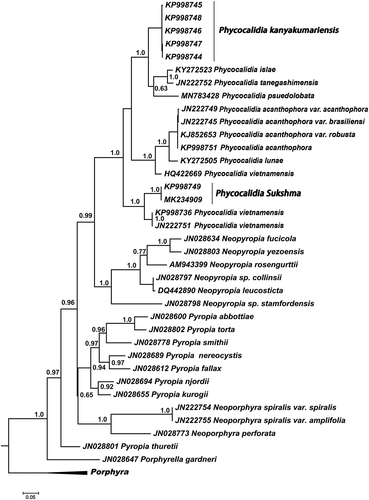
The rbcL phylogenetic data set consisted of 80 nucleotide sequences including seven outgroup taxa. In the phylogenetic tree (), Ph. vietnamensis (Brazil, Hawaii), Ph. tanegashimensis (Japan, Brazil), Ph. denticulata (Australia), Ph. islae (Philippines), Ph. pseudolobata (China), Ph. acanthophora (Brazil), Ph. acanthophora var. robusta (India), Ph. lunae (Philippines) and Ph. suborbiculata (Brazil) were resolved in the same clade as Ph. sukshma M.G.Kavale & M.A.Kazi sp. nov., which formed a well-supported monophyletic clade with Ph. vietnamensis (HQ687544 and HQ605697). The genetic divergence between these two lineages was 3%. The divergence between Ph. sukshma and other species of Phycocalidia ranged from 3% to 11%. No intraspecific variation was observed in rbcL sequences of Ph. sukshma. The COI-5P phylogenetic data set consisted of 40 nucleotide sequences including six outgroup taxa (). Ph. sukshma formed a well-supported monophyletic clade with Brazilian and Indian specimens of Ph. vietnamensis (JN222751, KP998736 and KP998743) with nucleotide divergence of 5.8–6%. The divergence between Ph. sukshma and other species of Phycocalidia ranged from 9.5–16.4%. Ph. kanyakumariensis (KP998744–KP998748) formed a strongly supported monophyletic clade with Ph. tanegashimensis (JN222752). The nucleotide divergence between these two sequences was 8.0%. Ph. sukshma and Ph. kanyakumariensis formed a well-supported monophyletic clade with a sister clade of Ph. acanthophora and Hawaiian specimen of Ph. vietnamensis (HQ422669). No intraspecific variation was observed in COI-5P sequences of either Ph. sukshma or Ph. kanyakumariensis.
Taxonomic treatments
Phycocalidia sukshma M.G.Kavale & M.A.Kazi,sp. nov
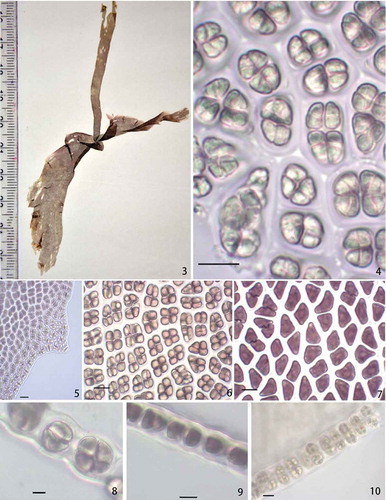
DescriptionGametangial blade () foliose, up to 10 cm long, 4 cm wide, sessile, several blades arising from a discoid holdfast, lanceolate, with cordate base, delicate texture; margin undulate rarely flat; marginal spines 1–2 cells, 3.3–9.6 µm in length (). Thalli monostromatic, 17.6–22 µm thick in vegetative parts (), 19.7–23 µm in reproductive parts (), vegetative cells triangular to polygonal with blunt corners, in surface view 9.7–16.5 µm (), in cross-section as broad as long, or sometimes broader than long, 9.3–12.3 µm long and 6.9–15.1 µm broad (), rectangular or squarish with rounded corners; each cell with a stellate plastid with central pyrenoid. Surface mucilage 3.6–4.0 µm thick on either side of the section. Colour reddish brown when live, purple brown on drying. Monoecious, spermatangia of pale yellow colour and reddish brown zygotosporangia in stripes or occasionally in irregular patches or mixed, 16 spermatia up to 3.9 µm in diameter in each spermatangium with division formula a/2, b/2, c/4, 8 zygotospores, 4.9–5.9 µm in diameter in each zygotosporangium, with a division formula a/2, b/2, c/2.
Figs 3–10. Habit and anatomy of Phycocalidia sukshma. Fig. 3. Habit. Fig. 4. Surface view of blade showing spermatangia. Fig. 5. Spinulose margin of the thallus. Fig. 6. Surface view of blade showing zygotosporangia. Fig. 7. Surface view of blade showing vegetative cells. Fig. 8. Cross-section of blade through zygotosporangia. Fig. 9. Cross-section of blade through vegetative cells. Fig. 10. Cross-section of blade through spermatangia. Scale bars = 10 µm
GENBANK NUMBERS OF HOLOTYPE: rbcL MK234907; COI-5P: KP998749; isotype: rbcL: MK234908, COI-5P: MK234909.
HOLOTYPE: CAL/ALG/050, epilithic in the upper intertidal zone, collected at Karwar, Karnataka, India (14°48ʹ40ʺN, 74°06ʹ72.3ʺE) on 14 August 2014 by M.G. Kavale and M.A. Kazi. The specimen was deposited in Central National Herbarium, Botanical Survey of India (BSIS), Kolkata, India.
ISOTYPES: CAL/ALG/50, deposited in BSIS.
ETYMOLOGY: The specific epithet is selected as the plants are delicate and small. In Karnataka, Kannada is the local language in which sukshma means delicate and small.
HABITAT AND SEASONALITY: Phycocalidia sukshma was found in the upper intertidal to splash region, growing mostly on laterite and basaltic rocks, exposed to direct wave action. It is known only from the Karwar coast of Karnataka, India. The species was found during the monsoon season from June to September.
Phycocalidia kanyakumariensis (V.Krishnamurthy & M.Baluswami) M.G.Kavale & M.A.Kazi, comb. nov
BASIONYM: Porphyra kanyakumariensis V.Krishnamurthy & M.Baluswami (Citation1984, p. 35).
TYPE LOCALITY: Kovalam, Tamil Nadu, India
DISTRIBUTION: Tamil Nadu, Kerala.
REMARKS: Phycocalidia kanyakumariensis was described by Krishnamurthy & Baluswami (Citation1984, as Porphyra kanyakumariensis) as monostromatic, attached to rock in the lower to mid-intertidal zone, growing during the monsoon season at Kovalam near Kanyakumari on the coast of Tamil Nadu, India. The material studied here showed morphological similarity with Porphyra kanyakumariensis.
Description
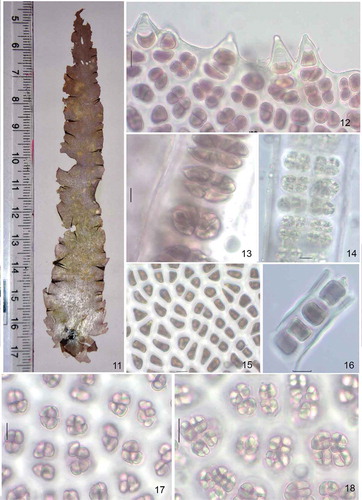
Gametangial blade up to 30 cm long, 15 cm broad (), sessile with cordate base, arising from discoid holdfast, lanceolate, oblanceolate to ovate; robust texture. Reddish brown colour when live, turning purple-brown on drying. Margin plain to undulate, ruffled surface with numerous spines, 1–5 celled, up to 28.5 µm in height (). Monostromatic, 52–58 µm thick in vegetative parts (), 55–69 µm in reproductive parts (). Vegetative cells polygonal with blunt corners, 14–21 µm in greatest dimensions (); in cross-section one to two times thicker than wider 18–23 µm long, 9.5–13 µm broad (), rectangular with rounded corners, containing a stellate plastid with central pyrenoid. Surface mucilage 4–6 µm thick on either side of the section. Spermatangia 128, colourless to pale yellow, up to 3 µm in diameter with division formula a/4,b/4,c/8; zygotosporangia 16, reddish brown colour, 4–9 µm in diameter with division formula a/2, b/2/c/4; distribution mixed or irregular patches.
Figs 11–18. Habit and anatomy of Phycocalidia kanyakumariensis. Fig. 11. Habit of P. kanyakumariensis. Fig. 12. Spinulose margin of the thallus. Fig. 13. Cross-section of blade through zygotosporangia. Fig. 14. Cross-section of blade through spermatangia. Fig. 15. Surface view of blade showing vegetative cells. Fig. 16. Cross-section of blade through vegetative cells. Fig. 17. Surface view of blade showing zygotosporangia. Fig. 18. Surface view of blade showing spermatangia. Scale bars = 10 µm
Discussion
In this study, using molecular-assisted methods, we have confirmed a new species of Phycocalidia from the Indian region and added to the growing number of species in this warm water genus (Dumilag & Yap, Citation2020; Yang et al., Citation2020). Ph. sukshma is closely related to Ph. vietnamensis, a widespread species reported from Brazil, India, Thailand, China and Hawaii (Milstein et al., Citation2015), which was the name we initially assigned to our species based on the habit in the field. Ph. sukshma is much more delicate and smaller than Ph. vietnamensis and differs in thallus thickness and number of zygotospores and spermatia. Furthermore, in Ph. vietnamensis, the thalli exposed to wave action exhibit extensive marginal denticulation (Kavale et al., Citation2015b) whereas marginal spines were rare in Ph. sukshma. This study has also enabled us to add the Kanyakumari coast, Tamil Nadu, Leela beach (Kovalam) Kerala to earlier locations for the occurrence of Ph. kanyakumariensis (Krishnamurthy & Baluswami, Citation1984; Chennubotla et al., Citation1990).
In the present study, phylogenetic analyses were found to be congruent with the previous reports for the Bangiales (Milstein et al., Citation2015; Sutherland et al., Citation2011; Yang et al., Citation2020). The molecular phylogenies show results consistent with Milstein et al. (Citation2015) which group Brazilian and Indo-Pacific species and Ph. sukshma and Ph. kanyakumariensis represent new additions to this well-supported clade. According to Milstein et al. (Citation2015), the most likely reason for this geographic grouping is introduction of species to Brazilian waters from the Indo-Pacific region. There are four species each of the genus Neopyropia (N. katadae, N. leucosticta, N. koreana, N. yezoensis) and Phycocalidia (Ph. acanthophora, Ph. suborbiculata, Ph. tanegashimensis, Ph. vietnamensis) which are considered to be introductions to the Atlantic and Mediterranean coasts from the Pacific region (Dumilag & Aguinaldo, Citation2017; as Porphyra). The phylogenetic analysis in the present study also supports the hypothesis of possible introductions of Phycocalidia species to the Brazilian coast. Both rbcL and COI-5P gene analysis supported the sister relationship between Ph. sukshma and Ph. vietnamensis. The rbcL interspecific divergence estimated in Ph. sukshma and other Phycocalidia species was comparable with interspecific range reported in earlier studies (Brodie et al., Citation2007, Broom et al., Citation2010). Species discrimination based on sequence divergence in COI-5P analysis can be assessed based on previous reports by Saunders (Citation2005) and Le Gall & Saunders (Citation2010). The sequence divergence in Ph. sukshma, Ph. kanyakumariensis and other Phycocalidia species was well within the range of previous studies in the red algae. Including the present study, all the bladed Bangiales analysed using molecular data from India were placed in the genus Phycocalidia (Sutherland et al., Citation2011; Kavale et al. Citation2015a, Citation2015b; Yang et al., Citation2020).
Phycocalidia species reported from India were confirmed based on morphological, ecological and unique DNA sequences with the following exceptions. The authors failed to collect Ph. suborbiculata, P. malvanansis and P. chauhanii even after repeated surveys during 2014–2018. These species have not been collected since they were first reported which raises the question as to whether they are still present on Indian shores.
Phycocalidia suborbiculata V.Krishnamurthy & M.Baluswami (Citation1984) was described based on specimens collected in 1984 from the Gale reef which is now part of Sri Lanka. It was later reported from the Kollam coast, Kerala (Quilon coast) which was fully covered with tripods (artificially prepared cement concrete blocks used to prevent sand erosion) by Anilkumar and Panikkar. The herbarium specimens are no longer available in the academic institution where they were originally deposited (M.V.N. Panikkar, personal communication, 12 August 2014). P. chauhanii was also reported by Anilkumar & Panikkar (Citation1997) from Kollam coast but non-availability of holotype herbarium material and loss of the original collecting site prevented us from relocating the species. However, there is a possibility that P. chauhanii was Ph. acanthophora, as the majority of morphological characters were similar to Ph. acanthophora var. robusta except number of carpospores per zygotosporangia (). However, confirmation of Ph. acanthophora var. robusta as P. chauhanii is not possible due to unavailability of the holotype specimen.
Table 2. Comparative morphological analysis of Phycocalidia species
The holotype of P. malvanansis was deposited in the Botanical survey of India, and molecular characterization is essential to confirm its existence as a distinct species. Repeat surveys are essential in the winter season to re-locate P. okhaensis and P. indica. The reassessment of the Bangiales from the Indian region in this study and our previous work reports (Kavale et al., Citation2015a, Citation2015b) confirm the presence of four species in the genus Phycocalidia: Ph. acanthophora, Ph. kanyakumariensis, Ph. sukshma and Ph. vietnamensis during monsoon season. There is a need for additional taxonomic studies on the bladed Bangiales of our coastline and from other tropical and subtropical regions, given the ongoing discovery of the species of Phycocalidia (Dumilag & Yap, Citation2020; Yang et al., Citation2020) and that large areas of the Indian coastline are yet to be explored.
Supplementary table S1. Specimen collection information, voucher numbers and references of species used for morphological comparison.
Supplementary table S2. Specimen collection information, GenBank accession numbers of sequences used in phylogenetic analysis and references.
Author contributions
M.G. Kavale: original concept, field sampling, morphological observations, manuscript drafting and editing; M.A. Kazi, molecular and phylogenetic analyses, manuscript drafting and editing; J. Brodie: manuscript revision and editing.
TEJP-2019-0101-File007.docx
Download MS Word (28.3 KB)TEJP-2019-0101-File006.docx
Download MS Word (15.9 KB)Acknowledgements
Part of this work was carried out in 2011–15 at ICS College, Khed, University of Mumbai, Maharashtra; the rest was completed in CSIR-CSMCRI, Bhavnagar, where the corresponding author is currently working. The authors are grateful to the Director, CSMCRI, Bhavnagar for encouraging us to continue the work. We acknowledge DC, Division of Biotechnology and Phycology for their valuable comments and suggestions. The authors are grateful to reviewers and editor for their helpful suggestions and comments on the manuscript. This contribution has CSIR-CSMCRI PRIS Registration No. 109.
Disclosure statement
No potential conflict of interest was reported by the authors
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2020.1829714
Additional information
Funding
References
- Anilkumar, C. & Panikkar, M.V.N. (1995). The species of Porphyra C.A. Agardh from Kerala. Seaweed Research Utilization, 17: 151–160.
- Anilkumar, C. & Panikkar, M.V.N. (1997). Indian species of Porphyra (Rhodophyceae, Bangiales). Feddes Repertorium, 108: 419–423.
- Anilkumar, C. & Rao, P.S.N. (2005). A new species of Porphyra (Rhodophyta, Bangiales) from the Malvan coast of Maharashtra (India). Feddes Repertorium, 116: 222–225.
- Brodie, J., Bartsch, I., Neefus, C., Orfanidis, S., Bray, T. & Mathieson, A.C. (2007). New insights into the cryptic diversity of the North Atlantic–Mediterranean ‘Porphyra leucosticta’ complex: P. olivii sp. nov. and P. rosengurttii (Bangiales, Rhodophyta). European Journal of Phycology, 42: 3–28.
- Brodie, J., Mols Mortensen, A.M., Ramirez, M.E., Russell, S. & Rinkel, B. (2008). Making the links: towards a global taxonomy for the red algal genus Porphyra (Bangiales, Rhodophyta). Journal of Applied Phycology, 20: 939–949.
- Broom, J.E.S., Nelson, W.A., Farr, T.J., Phillips, L.E. & Clayton, M. (2010). Relationships of the Porphyra (Bangiales, Rhodophyta) flora of the Falkland Islands: a molecular survey using rbcL and nSSU sequence data. Australian Systematic Botany, 23: 27–37.
- Chennubotla, V.S.K., Mathew, S. & Joseph, I. (1990). A note on the occurrence of Porphyra kanyakumariensis (Bangiales: Rhodophyta) along the Kerala coast. Seaweed Research Utilization, 13: 1–4.
- Dhargalkar, V.K., Agadi, V.V. &Untawale, A.G. (1981). Occurrence of Porphyra vietnamensis (Bangiales, Rhodophyta) along the Goa coast. Mahasagar – Bulletin of the National Institute of Oceanography, 14: 75–77.
- Dumilag, R.V. & Aguinaldo, Z.A. (2017). Genetic differentiation and distribution of Pyropia acanthophora (Bangiales, Rhodophyta) in the Philippines. European Journal of Phycology, 52: 104–115.
- Dumilag, R.V. & Yap, S.L. (2020). Pyropia lunae sp. nov. and Pyropia islae sp. nov. (Bangiales, Rhodophyta) from the Philippines. Botanica Marina, 61: 467–480.
- Freshwater, D.W. & Rueness. J. (1994). Phylogenetic relationship of some European Gelidium (Gelidiales, Rhodophyta) species based on rbcL nucleotide sequence analysis. Phycologia, 33: 187–194.
- Guindon, S., Dufayard, J.F., Lefort, V., Anisimova, M., Hordijk, W. & Gascuel, O. (2010). New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology, 59: 307–321.
- Hus, H.T.A. (1902). An account of the species of Porphyra found on the Pacific coast of North America. Proceedings of the California Academy of Sciences. Third series. Botany, 2: 173–239.
- Joshi, H.V., Oza, R.M. & Tewari, A. (1992). Life cycle and growth of Porphyra okhaensis sp. nov. in culture. Indian Journal of Geo-Marine Sciences, 21: 262–267.
- Kavale, M.G., Kazi, M.A. & Sreenadhan, N. (2015a). Pyropia acanthophora var. robusta var. nov. (Bangiales, Rhodophyta) from Goa, India. Indian Journal of Geo-Marine Sciences, 44: 1–8.
- Kavale, M.G., Kazi, M.A., Sreenadhan, N. & Murugan P. (2017). Nutritional profiling of Pyropia acanthophora var. robusta (Bangiales, Rhodophyta) from Indian waters. Journal of Applied Phycology, 29: 2013–2020.
- Kavale, M.G., Kazi, M.A., Sreenadhan, N. & Singh V.V. (2015b). Morphological, ecological and molecular characterization of Pyropia vietnamensis (Bangiales, Rhodophyta) from Konkan Region, India. Phytotaxa, 224: 45–58.
- Kim, S.-M., Choi, H.-G., Hwang, M.-S. & Kim, H.-S. (2018). Biogeographic pattern of four endemic Pyropia from the east cost of Korea, including a new species Pyropia retorta (Bangiaceae, Rhodophyta). Algae, 33: 55–68.
- Krishnamurthy, V. & Baluswami, M. (1984). The species of Porphyra from the Indian region. Seaweed Research Utilization, 7: 31–38.
- Kucera, H. & Saunders, G.W. (2012). A survey of Bangiales (Rhodophyta) based on multiple molecular markers reveals cryptic diversity. Journal of Phycology, 48: 869–882.
- Kurogi, M. (1972). Systematics of Porphyra in Japan. In Contributions to the Systematics of Benthic Marine Algae of the North Pacific (Abbott, I.A. & Kurogi, M. editors), 167–91. Japanese Society of Phycology, Kobe.
- Kurogi, M. & Yamada, I. (1986). New knowledge obtained by the observation on the original specimens of F.R. Kjellman: Japan skaarterafslagtet Porphyra (in Japanese). Japanese Journal of Phycology, 34: 62.
- Le Gall, L. & Saunders, G.W. (2010). DNA barcoding is a powerful tool to uncover algal diversity: a case study of the Phyllophoraceae (Gigartinales, Rhodophyta) in the Canadian flora. Journal of Phycology, 46: 374–389.
- Lefort, V., Longueville, J. & Gascuel, O. (2017). SMS: smart model selection in PhyML. Molecular Biology and Evolution, 34: 2422–2424.
- Mantri, V.A., Kavale, M.G. & Kazi, M.A. (2020). Seaweed biodiversity of India: reviewing current knowledge to identify gaps, challenges and opportunities. Diversity, 12, 13: doi: 10.3390/d12010013.
- Milstein, D., Medeiros, A.S., Oliveira, E.C. & Oliveira, M.C. (2015). Native or introduced? A re-evaluation of Pyropia species (Bangiales, Rhodophyta) from Brazil based on molecular analyses. European Journal of Phycology, 50: 37–45.
- Nelson, W.A., Brodie, J. & Guiry, M.D. (1999). Terminology used to describe reproduction and life history stages in the genus Porphyra (Bangiales, Rhodophyta). Journal of Applied Phycology, 11: 407–410.
- Sánchez, N., Vergés, A., Peteiro, C., Sutherland, J.E. & Brodie, J. (2014). Diversity of bladed Bangiales (Rhodophyta) in western Mediterranean: recognition of the genus Themis and descriptions of T. ballesterosii sp. nov., T. iberica and Pyropia parva sp. nov. Journal of Phycology, 50: 908–929.
- Sánchez, N., Vergés, A., Peteiro, C., Sutherland, J.E. & Brodie, J. (2015). Corrigendum to Diversity of bladed bangiales (Rhodophyta) in Western Mediterranean: Recognition of the genus Themis and descriptions of T. ballesterosii sp. nov., T. iberica sp. nov. and Pyropia parva sp. nov. Journal of Phycology, 51 (2): 401.
- Santiañez, W.J.E. & Wynne, M.J. (2020). Proposal of Phycocalidia Santiañez & M.J.Wynne nom. nov. to replace Calidia L.-E.Yang & J.Brodie nom. illeg. (Bangiales, Rhodophyta). Notulae algarum, 140. ISSN 2009–8987.
- Saunders, G.W. (2005). Applying DNA barcoding to red macro-algae: a preliminary appraisal holds promise for future applications. Philosophical Transactions of the Royal Society B, 360: 1879–1888.
- Shinmura, I. (1974). Porphyra tanegashimensis, a new species of Rhodophyceae from Tanegashima Island in Southern Japan. Bulletin of the Japanese Society of Scientific Fisheries, 40: 735–748.
- Sutherland, J.E., Lindstrom, S.C., Nelson, W.A., Brodie, J., Lynch, M.D.J., Hwang, M.S., Choi, H-G., Miyata, M., Kikuchi, N., Oliveira, M.C., Farr, T., Neefus, C., Mols-Mortensen, A., Milstein, D. & Müller, K.M. (2011). A new look at an ancient order: generic revision of the Bangiales (Rhodophyta). Journal of Phycology, 47: 1131–1151.
- Svedelius, N. (1906). Ueber die Algenvegetation eines ceylonsichen korallenriffes mit besonderer Rücksicht auf ihre Periodizitat Botaniska Studier till F.R. Kjellman, Uppsala, pp. 184–220.
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013). MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Molecular Biology and Evolution, 30: 2725–2729.
- Tanaka, T. & Ho, P.H. (1962). Notes on some marine algae from Viet-Nam-I. Memoirs of Faculty of Fisheries Kagoshima University, 11(1): 34–36.
- Thiers, B. (2020). Index Herbariorum: A Global Directory of Public Herbaria and Associated Staff. New York Botanical Garden’s Virtual Herbarium, Bronx, NY (continuously updated, http://sweetgum.nybg.org/ih/).
- Umamaheswara Rao, U.M. & Sreeramulu, T. (1963). Vertical zonation and seasonal variation in the growth of Porphyra on Visakhapatnam coast. Current Science, 32: 173–174.
- Xie, Z.Y., Lin, S.M., Liu, L.C., Ang, P.O. Jr. & Shyu, J.F. (2015). Genetic diversity and taxonomy of foliose Bangiales (Rhodophyta) from Taiwan based on rbcL and COI-5P sequences. Botanica Marina, 58: 189–202.
- Yang, L.-E., Deng, Y.-Y., Xu, G.-P., Russell, S., Lu, Q.-Q. & Brodie, J. (2020). Redefining Pyropia (Bangiales, Rhodophyta): four new genera, resurrection of Porphyrella and description of Calidia pseudolobata sp. nov. from China. Journal of Phycology. doi:10.1111/jpy.12992‐19‐192.
- Yang, L.-E., Zhou, W., Hu, C.-M., Deng, Y.-Y., Xu, G.-P., Zhang, T., Russel, S., Zhu, J.-Y., Lu, Q.-Q. & Brodie, J. (2018). A molecular phylogeny of the bladed Bangiales (Rhodophyta) in China provide insights in to biodiversity and biogeography of the genus Pyropia. Molecular Phylogenetics and evolution, 120: 94–102.
- Yoshida, T. (1997). Japanese marine algae: new combinations, new names and new species. Phycological Research, 45: 163–167.