ABSTRACT
Until recently, there was no agreement on species delimitation within the morphologically similar chrysophycean genera Uroglena, Uroglenopsis and Urostipulosphaera. In this study, we aimed at a modern taxonomic revision based on the combination of morphological characters (ultrastructure of cysts, cell and colony features) and a multigene phylogeny (SSU, ITS rDNA and rbcL sequences), with ecology taken into account. Of more than 650 explored localities, only approximately one in 10 hosted a viable and detectable population of these colonial chrysophytes at the time of sampling. We established and examined 189 short-term cultures along with single colony isolates, derived mostly from blooming or encysting populations. We obtained the cyst morphology for four species and two lineages of Uroglena, two species of Uroglenopsis, and four species of Urostipulosphaera. A total of 12 resolved lineages could be attributed to previously described species or new species (Uroglena imitata sp. nov., Urostipulosphaera granulata sp. nov.). Based on our molecular analyses and morphological observations, we assign all the previously described Uroglena-like taxa to newly recognized genera and propose a key to identification. Consequently, Uroglena now includes 16 species and two varieties, Uroglenopsis contains four species and Urostipulosphaera encompasses nine species. Within Uroglena and Urostipulosphaera, species are defined by the ultrastructure of their cysts. On the contrary, as Uroglenopsis has simple cysts, species are defined by cell and colony characteristics.
Introduction
The term ‘species’ represents one of the cornerstones of both old and modern biology because of the permanent need to categorize and identify organisms. Nevertheless, alternative taxonomy-independent methods of biodiversity research have grown (Sun et al., Citation2012; Apotheloz-Perret-Gentil et al., Citation2017). Since the introduction of binomial nomenclature by Linnaeus (Citation1753), the nature of species changed with evolutionary concepts. Darwin’s theory of evolution (Darwin, Citation1859) accelerated the so-called ‘species problem’ and the discussion continues. Although we consider ‘species’ as a hypothesis (Bonde, Citation1977) when using more or less transient or artificial boundaries in nature, ‘species’ acts as the fundamental framework in many fields of biological research. Different species concepts (from the morphological species concept to multidisciplinary approaches) have been introduced and are usually applied differently to particular taxonomic groups.
Hey (Citation2001) stated that ‘the species problem is the long-standing failure of biologists to agree on how we should identify species and how we should define the word species’. de Queiroz (Citation2005, Citation2007) introduced the unified species concept which clearly separates the issues of species conceptualization and species delimitation. In this view, a separately evolving metapopulation lineage is the only necessary property of a species, but the species may be delimited in a variety of ways. In protists, it has been suggested that we should skip problematic searching for a correct general species concept and rather focus on clear species delimitation, ideally using more than one line of evidence and including a robust phylogenetic framework as a standard (Boenigk et al., Citation2012).
Protist taxonomy is still dealing with a high proportion of cryptic taxa within morphospecies (Howe et al., Citation2009; Škaloud & Rindi, Citation2013) and one of the main problems and challenges is incomplete reference DNA databases due to the lack of molecular data for numerous morphologically described species (Leray & Knowlton, Citation2015). The use of both molecular and morphological techniques is essential in the correct estimation of species diversity as both approaches are complementary (Škaloud et al., Citation2020). In Chrysophyceae (Stramenopiles, SAR), a diverse protist group commonly observed in planktonic freshwater communities (Finlay & Esteban, Citation1998; Wolfe & Siver, Citation2013), current knowledge of diversity is mainly based on traditional morphology, with a few exceptions.
The diversity of silica-scaled chrysophytes, particularly Synurales and Paraphysomonadida, has been studied by a multidisciplinary approach providing a robust phylogenetic framework and good species-specific morphological characters (Jo et al., Citation2013; Scoble & Cavalier-Smith, Citation2014; Škaloud et al., Citation2014). In naked chrysophytes, however, only Kremastochrysopsis (Remias et al., Citation2020), Ochromonas-like (Andersen et al., Citation2017) and Spumella-like (Findenig et al., Citation2010; Grossmann et al., Citation2016) morphotypes have been evaluated using molecular techniques. These morphotypes represent ‘prototypes’ of a single-celled naked flagellate with a basic chrysophycean, or stramenopile, respectively, cell plan (two heterokont flagella), and as such they are scattered across the whole phylogenetic tree of Chrysophyceae.
Due to the absence of solid surface structures (e.g. silica scales) and a variable cell shape, the taxonomy of naked flagellates is very problematic. Consequently, the postulated taxonomic diversity certainly does not reflect the real species richness. Fortunately, all the chrysophytes possess one solid structure in their life cycles suitable for precise morphological delineation, the stomatocyst. These silica cysts are products of both asexual and sexual reproduction and usually exhibit great ultrastructural diversity – cyst wall decoration and shape of collar(s) surrounding the pore (Sandgren, Citation1991). However, encysting populations are rarely observed since the encystment process typically takes place over a short period at the end of blooms (Agbeti & Smol, Citation1995).
Photosynthetic colonial Dinobryon, Synura and Uroglena-like flagellates often cause the well-known spring and autumn plankton blooms in meso-oligotrophic fresh waters (Anneville et al., Citation2005; Bock et al., Citation2014). Recently, Pusztai & Škaloud (Citation2019) taxonomically revised the polyphyletic Uroglena-like morphotype, which has resulted in at least three genetically and morphologically distinct lineages within the Ochromonadales (Chrysophyceae), distinguished as Uroglena Ehrenberg, Uroglenopsis Lemmermann and Urostipulosphaera Pusztai & Škaloud. So far, 35 taxa of Uroglena (the majority), Uroglenopsis and Urostipulosphaera have been validly described (Cronberg & Laugaste, Citation2005; Pusztai & Škaloud Citation2019; Guiry & Guiry, Citation2020; Index Nominum Algarum, Citation2020). Cells of these three genera are always radially arranged as a monolayer coat at the periphery of the predominantly spherical colony. Nevertheless, the genera differ in cell shape (especially in cell posterior), flagellar length ratio, and the character of the branched radial structures.
Unfortunately, these morphological characters that clearly delimit three Uroglena-like genera seem to be useless in species delimitation. The colonies and cells of species within each of the genera are generally uniform and/or exhibit the same trends in phenotypic plasticity (Wujek & Thompson, Citation2002; Pusztai & Škaloud, Citation2019). Moreover, Uroglena, Uroglenopsis and Urostipulosphaera seem to have a similar ecology. Finally, previous work has not confirmed the presence of any scale-like structures (Wujek, Citation1976; Pusztai & Škaloud, Citation2019). Therefore, in these naked chrysophytes the ultrastructure of cysts seems to be the only applicable and relatively stable morphological character for species delineation. In general, Uroglena-like taxa have smooth or decorated spherical cysts with or without a straight/curved collar or two concentric collars.
The present study represents a follow-up to our previous paper showing that the Uroglena-like morphotype includes three separate genera (Pusztai & Škaloud, Citation2019), focusing on species diversity. It is based on the examination of short-term cultures along with single colony isolates, derived mostly, but not exclusively, from blooming and encysting populations of Uroglena, Uroglenopsis and Urostipulosphaera. The goal of this work was to conduct a modern taxonomic revision at species level, based on the combination of both morphological (ultrastructure of cysts, cell and colony features) and genetic evidence (SSU, ITS rDNA and rbcL sequences), taking ecology into account.
Material and methods
Sample processing and morphological investigations
Sampling was carried out predominantly in the northern temperate zone (throughout Europe and part of North America) in 2014–2020. Isolates of Uroglena, Uroglenopsis and Urostipulosphaera (Supplementary table S1) were obtained from various freshwater bodies mostly during the spring and autumn chrysophyte blooms. Samples were collected and processed as described previously in Pusztai & Škaloud (Citation2019) but using TES-buffered WC liquid medium (pH ~7.5; Andersen et al., Citation1997) additionally. Measured values of abiotic factors (water pH, temperature, specific conductivity) were further visualized by boxplots and ecological differences between taxa were tested by parametric and non-parametric tests (t-test, Mann–Whitney test).
Morphological microscopic investigations were made as described previously in Pusztai & Škaloud (Citation2019) but using a FE-SEM ZEISS Ultra Plus (ZEISS Oberkochen, Germany) scanning electron microscope (SEM) additionally. Moreover, 50 cells from each of the six successfully maintained cultures of five different species of Urostipulosphaera strains were photographed and their shape and size analysed with ImageJ 1.45s (Schneider et al., Citation2012) for potential use in species delineation. Species determination of encysting populations was carried out according to information on ultrastructure in the original descriptions.
Sequencing and phylogenetic analysis
DNA isolation was carried out as described in Škaloudová & Škaloud (Citation2013) but using 10 ml of InstaGene matrix (Bio-Rad Laboratories) for single-colony isolates. Three loci were amplified by PCR: nuclear SSU rDNA, entire nuclear ITS rDNA region (ITS1-5.8S-ITS2) and plastid rbcL. These molecular markers should provide sufficient genus-level taxonomic resolution as well as species-level taxonomic resolution within the Chrysophyceae (Scoble & Cavalier-Smith, Citation2014; Grossmann et al., Citation2016; Andersen et al., Citation2017; Bock et al., Citation2017; Kristiansen & Škaloud, Citation2017). In addition, ITS rDNA is one of the most frequently used chrysophyte barcodes (Pawlowski et al., Citation2012). It is preferred over COI (cox1) in order to avoid the potentially misleading clustering of some strains (Jost et al., Citation2010; Bock et al., Citation2017).
The amplification of SSU rDNA and rbcL markers followed Pusztai & Škaloud (Citation2019), using the primers 18SF and 18SR (Katana et al., Citation2001) and our previously designed primers Chryso_SSU_F2 (5’-TGT CTC AAA GAT TAA GCC AT-3’), Chryso_SSU_R2 (5’-CTA CGG AAA CCT TGT TAC GA-3’), Chryso_rbcL_F4 (5’-TGG ACD GAY TTA TTA ACD GC-3’) and Chryso_rbcL_R7 (5’-CCW CCA CCR AAY TGT ARW A-3’). The amplification of the ITS marker was performed as described by Kynčlová et al. (Citation2010), using the newly designed primers Chryso_ITS_F (5’-ATC ATT TAG AGG AAG GTG A-3’) and Chryso_ITS_R (5’-GCT TCA CTC GCC GTT ACT-3’). The PCR products were purified and sequenced at Macrogen Inc. Sequencing of additional molecular loci was not possible due to the limited amount of DNA obtained using our single-colony isolation method. Newly determined sequences were aligned with sequences of Uroglena, Uroglenopsis and Urostipulosphaera from GenBank (Supplementary table S1) to produce three multigene alignments, one for each genus. The SSU rDNA sequences were not used in species-level analyses because they were invariant within each genus. ITS rDNA (586/604/775 bp) and rbcL (962 bp) were concatenated as alignments of 1548 bp (Uroglena), 1566 bp (Uroglenopsis) and 1737 bp (Urostipulosphaera). Single-locus alignments were used to evaluate congruence, including 18/77 unique/total sequences of Uroglena, 20/67 unique/total sequences of Uroglenopsis and 8/45 unique/total sequences of Urostipulosphaera taxa. rbcL sequences were manually aligned using MEGA 6 (Tamura et al., Citation2013), and ITS alignments were constructed using MAFFT v6, applying the Q-INS-i strategy (Katoh et al., Citation2002). Positions with deletions in a majority of sequences were removed from the alignment.
The best-fit nucleotide substitution model for each of the alignment partitions was estimated using the Bayesian Information Criterion (BIC) and Akaike Information Criterion (AIC) in jModelTest 2.1.4 (Darriba et al., Citation2012). For the ITS region, boundaries of the ITS1, 5.8S and ITS2 regions were determined by comparing them with the published 5.8S sequence of Dinobryon pediforme strain LO2_36_1 (KJ579347). These procedures selected for Uroglena the following models: HKY for ITS1 and 5.8S, HKY + I for ITS2, GTR + I for the first and second codon position of the rbcL gene, GTR + G for the third codon position of the rbcL gene; for Uroglenopsis HKY + G for ITS1 and ITS2, GTR + I for 5.8S and the rbcL second codon position, GTR for the rbcL first codon position, GTR + G for the rbcL third codon position; for Urostipulosphaera HKY + I for ITS1, GTR + I for 5.8S and the rbcL first and second codon positions, GTR + G for ITS2 and the rbcL third codon position.
Phylogenetic trees were inferred by Bayesian inference (BI) using MrBayes version 3.2.1 (Ronquist et al., Citation2012). BI analyses were run on the CIPRES Science Gateway v.3.3 web portal (Miller et al., Citation2010) with partitioned datasets using the substitution models specified above. All parameters were unlinked among partitions. Two parallel MCMC runs were carried out for 10 million generations, each with one cold and three heated chains. Trees and parameters were sampled every 100 generations. Convergence of the two cold chains was assessed during the run by calculating the average standard deviation of split frequencies (SDSF). The SDSF value was 0.0012 for Uroglena, 0.0010 for Uroglenopsis and 0.0001 for Urostipulosphaera. Finally, the burn-in value was determined using the ‘sump’ command. Bootstrap analyses were performed by maximum likelihood (ML) and weighted maximum parsimony (wMP) criteria using GARLI, version 2.01 (Zwickl, Citation2006) and PAUP*, version 4.0b10 (Swofford, Citation2002), respectively, as described in Pusztai et al. (Citation2016). Topologies of all clades in single-locus ITS and rbcL trees were congruent with the exception of UK-37 and UK-41 isolates with unsupported positions in the ITS tree.
Results
Distribution and ecology
More than 650 localities were explored, only ~10% of which hosted a detectable population of Uroglena-like colonial chrysophytes at the time of sampling. We established 189 single-colony isolates (Supplementary table S1) of Uroglena (77), Uroglenopsis (67) and Urostipulosphaera (45). The phylogenetic position of all three genera is shown on a simplified phylogram (Supplementary fig. S1) adapted from Pusztai & Škaloud (Citation2019). Many isolates originated from encysting populations (Supplementary table S1) and we successfully collected material from type localities of four taxa – Uroglena skujae Matvienko ex Pusztai & Škaloud, sp. nov. (= Uroglena europaea (Pascher) Skuja), Uroglena volvox Ehrenberg, Uroglenopsis americana (Calkins) Lemmermann and Uroglenopsis botrys (Pascher) Pascher. Further, we obtained the cyst morphology for four species and two lineages of Uroglena, two species of Uroglenopsis and four species of Urostipulosphaera. Twelve resolved lineages could be clearly attributed to previously or newly described species. Colonies usually consisted of tens to hundreds of cells, but smaller colonies with fewer cells might be produced by a large colony collapsing during observation. Smaller colonies were also formed in culture.
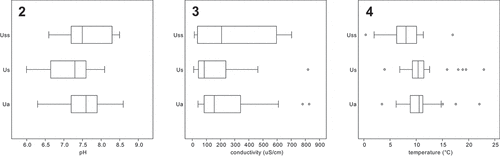
Although all three genera exhibited a similar ecology in the northern temperate zone, some differences were discovered (). Uroglena and Uroglenopsis both exhibit spring and autumn population maxima, but not Urostipulosphaera. Urostipulosphaera occurred in colder waters than Uroglena (Mann–Whitney, p = 0.021) and Uroglenopsis (Mann–Whitney, p = 0.017). Uroglenopsis occupied waters with significantly lower pH than Uroglena (t-test, p = 0.014) and Urostipulosphaera (t-test, p = 0.026) and with significantly lower conductivity than Uroglena (Mann–Whitney, p = 0.034). Values of measured environmental factors (Supplementary table S1) are shown as habitat differences at the generic level (). Unfortunately, statistical evaluation of species-level differences is not possible due to insufficient numbers of observations.
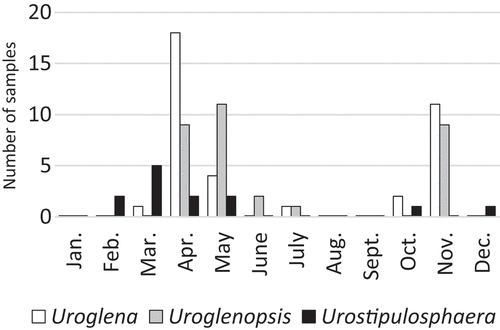
Fig. 1. Phenology of Uroglena, Uroglenopsis and Urostipulosphaera in the northern temperate zone based on all collected populations (number of samples) in the years 2014–2020. Urostipulosphaera seems to be an early spring taxon that peaked in March, while Uroglenopsis seems to be a late spring taxon that peaked in May. Uroglena peaked in April. Uroglena and Uroglenopsis exhibit significant spring and autumnal population maxima, while Urostipulosphaera does not.
Figs 2–4. Habitat differences between Uroglena (Ua), Uroglenopsis (Us) and Urostipulosphaera (Uss) in terms of measured environmental factors for all collected populations. Fig. 2. pH. Fig. 3. Conductivity. Fig. 4. Temperature. Urostipulosphaera occurred in waters with significantly lower temperature than Uroglena (Mann–Whitney, p = 0.021) and Uroglenopsis (Mann–Whitney, p = 0.017). Uroglenopsis occurred in waters with significantly lower pH than Uroglena (t-test, p = 0.014) and Urostipulosphaera (t-test, p = 0.026) and with significantly lower conductivity than Uroglena (Mann–Whitney, p = 0.034).
Uroglena
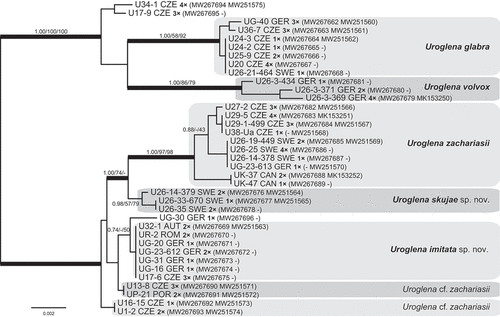
Phylogenetic analysis revealed two strongly supported major lineages within Uroglena (). The first lineage consisted of a well-resolved clade of Uroglena glabra Matvienko and U. volvox, and two isolates (U34-1 and U17-9) forming an unsupported basal clade. The second lineage included two well-resolved sister clades of Uroglena zachariasii Thompson & Wujek and Uroglena skujae Matvienko ex Pusztai & Škaloud, sp. nov., plus a group of genetically identical isolates here referred to as Uroglena imitata sp. nov., two clades both termed Uroglena cf. zachariasii, and a single isolate UG-30.
Fig. 5. Phylogeny of the genus Uroglena obtained by Bayesian inference of the concatenated ITS rDNA and rbcL dataset. The analysis was performed under a partitioned model, using different substitution models for each partition. Values at the nodes indicate statistical support estimated by three methods: MrBayes posterior node probability (left), maximum likelihood bootstrap (middle) and weighted maximum parsimony bootstrap (right). Only statistical supports with posterior probability higher than 0.7 are shown. Thick branches highlight nodes receiving the highest posterior probability support (1.00). Number of isolates sharing identical DNA sequences within a strain is indicated as ‘1–4×’. Scale bar represents the expected number of substitutions per site.
Uroglena cells () were always inverse-teardrop shaped, with a sharply pointed cell posterior and two unequal (ratio 1:2) anterior flagella. Cells usually contained a single girdle-shaped, bi-lobed, slightly spiral, gold-coloured plastid with an anterior stigma. Cell posterior continuing as thin, probably cytoplasmic, threads connecting individual cells by a dichotomously branching system into a more or less spherical colony; threads at colony centre sometimes thicker. Cysts were always spherical and smooth or coated with almost imperceptible very small particles, with simple or complex concentric straight collar(s). Morphological characteristics of individual species are summarized in .
Table 1. Morphological characteristics of individual Uroglena, Uroglenopsis and Urostipulosphaera species
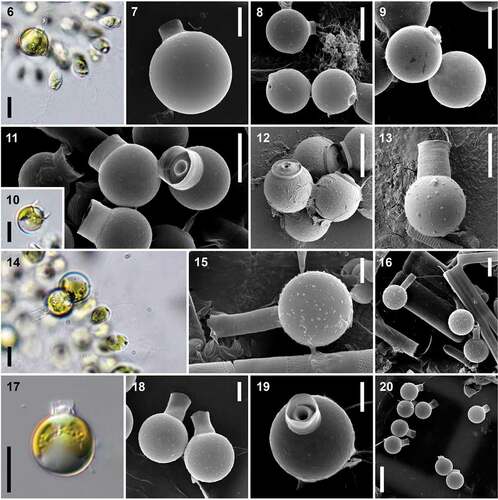
Figs 6–20. Species-specific cysts as the main morphological character for species delimitation within the genus Uroglena. Figs 6–9. U. glabra – mature cysts (Figs 6, 7) and group of immature cysts with not fully developed collar (Figs 8, 9). Figs 10–13. U. zachariasii – mature cysts (Figs 10, 11), group of immature cysts with not fully developed collars (Fig. 12) and cysts with very high secondary collar morphologically fitting U. zachariasii var. uplandica (Fig. 13). Figs 14–16. U. skujae – mature cysts (Figs 14, 15) and group of cysts with different collar lengths (Fig. 16). Figs 17–20. U. imitata – mature cysts with clearly visible primary collar (Figs 17, 19) and fully developed secondary collar (Fig. 18), group of cysts with different secondary collar lengths (Fig. 20). Scale = 20 μm (Fig. 20), 10 μm (Figs 6, 8–14, 16, 17) and 5 μm (Figs 7, 15, 18, 19). LM investigations (Figs 6, 10, 14, 17), SEM investigations (Figs 7–9, 11-13, 15, 16, 18–20).
Uroglenopsis
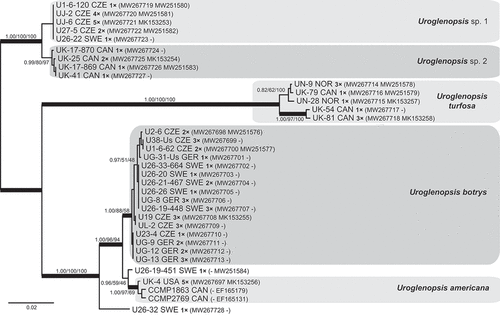
Phylogenetic analysis revealed three strongly supported major lineages within Uroglenopsis (). The first lineage consisted of two sister clades, referred to here as Uroglenopsis sp. 1 and sp. 2. We were not able to assign these two clades to any previously described species. The second lineage encompassed only isolates determined as Uroglenopsis turfosa (Skuja) Thompson & Wujek. The third lineage was composed of two robust clades of Uroglenopsis americana and Uroglenopsis botrys, and two single-sequence isolates U26-32 and U26-19-451. U. americana isolates were related to strains CCMP1863 and CCMP2769 from Canada. U. botrys was the most common species recovered in this study.
Fig. 21. Phylogeny of the genus Uroglenopsis obtained by Bayesian inference of the concatenated ITS rDNA and rbcL dataset. The analysis was performed under a partitioned model, using different substitution models for each partition. Values at the nodes indicate statistical support estimated by three methods: MrBayes posterior node probability (left), maximum likelihood bootstrap (middle) and weighted maximum parsimony bootstrap (right). Only statistical supports with posterior probability higher than 0.8 are shown. Thick branches highlight nodes receiving the highest posterior probability support (1.00). Number of isolates sharing identical DNA sequences within a strain is indicated as ‘1–5×’. Scale bar represents the expected number of substitutions per site.
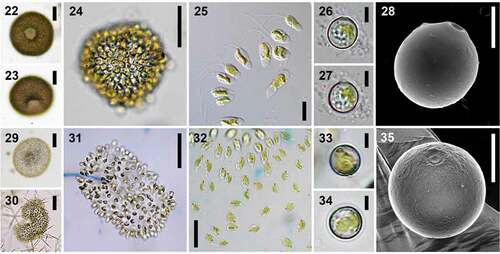
Uroglenopsis colonies and cells () were of diverse shape. Colonies were usually spherical to oval, but U. americana and U. botrys also produced elongated to irregularly multi-lobed colonies. U. turfosa had unique morphology: fresh colonies were always closely packed together, with hexagonal cells in apical view and a conspicuous hole in the spherical colony. Cells were mostly spindle-shaped, oval to slightly obovate or elongated and cylindrical, with a predominantly bluntly tapering cell posterior and two distinctly unequal (ratio ≥ 1:4) anterior flagella. Cells usually contained a single parietal, gold-coloured plastid, elongated along the cell axis, with an anterior stigma. When stained with Lugol’s iodine solution or methylene blue, cells were seen to be embedded into a compact jelly mantle with one to few thin short (1–3 µm) spine-like structures protruding posteriorly, most likely helping to fix cells within the jelly, as pointed out by Skuja (Citation1948). Cells of stationary colonies exhibited characteristic jerky movements. Cysts were almost spherical to slightly oval or oblate, smooth and without a collar. Morphological characteristics of individual species are summarized in .
Figs 22–35. Colony, cell and cyst characteristics within the genus Uroglenopsis. Figs 22–28. U. turfosa – colonies in fresh natural samples with a remarkable hole in the spherical closely packed colony (Figs 22, 23), hexagonal cells in apical view still closely packed together in young cultures (Fig. 24), colonies with cells loosely packed in old cultures (Fig. 25), mature cysts with a characteristic marginal rim surrounding the pore (Figs 26–28). Figs 29–35. U. botrys – colonies (Figs 29–31) and cells (Fig. 32) which are very diverse in shape, mature cysts exhibiting very simple ultrastructure (Figs 33–35).
Urostipulosphaera
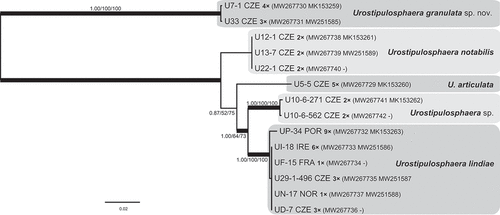
Of two strongly supported major lineages (), the first encompassed a single clade, here referred to as Urostipulosphaera granulata sp. nov. Based on the concatenated SSU rDNA and rbcL phylogeny (Pusztai & Škaloud, Citation2019), Urostipulosphaera sp. CCMP 2768 is the sister clade to U. granulata within the first lineage. The second lineage was composed of four clades, here referred to as U. notabilis (Mack) Pusztai & Škaloud, U. articulata (Korshikov) Pusztai & Škaloud comb. nov., U. lindiae (Bourrelly) Pusztai & Škaloud comb. nov. and Urostipulosphaera sp.
Fig. 36. Phylogeny of the genus Urostipulosphaera obtained by Bayesian inference of the concatenated ITS rDNA and rbcL dataset. The analysis was performed under a partitioned model, using different substitution models for each partition. Values at the nodes indicate statistical support estimated by three methods; MrBayes posterior node probability (left), maximum likelihood bootstrap (middle) and weighted maximum parsimony bootstrap (right). Thick branches highlight nodes receiving the highest posterior probability support (1.00). Number of isolates sharing identical DNA sequences within a strain is indicated as ‘1–9×’. Scale bar represents the expected number of substitutions per site.
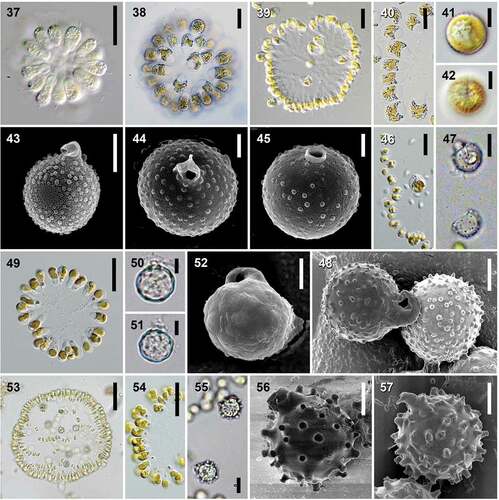
Urostipulosphaera () cells were usually obovate, with two distinctly unequal (ratio ≥ 1:4) anterior flagella. Cells usually contained a single girdle-shaped or slightly spiral, or ribbon-shaped, bi-lobed, gold-coloured plastid with an anterior stigma. Predominantly truncate or rounded cell posteriors were always connected via a dichotomously branching system of relatively thick articulated gelatinous stalks, sometimes covered with bacteria and thus made more visible. In fresh samples, colonies were usually perfectly spherical but sometimes with poorly visible stalks. Cultured colonies were sometimes oval but always with clearly visible stalks. Cysts were almost spherical to slightly oval or oblate, rough or embellished and with a curved collar. Morphological characteristics of individual species are summarized in .
Figs 37–57. Species-specific cysts and colony characteristics within the genus Urostipulosphaera. Figs 37–45. U. granulata – strain U7-1 in a fresh natural sample with reduced plastids (Fig. 37), the same strain after one week of culturing (Fig. 38), strain U33 with clearly visible articulated stalks (Fig. 39), changes in cell shape and posterior under stress conditions during microscopy (Fig. 40), mature cysts with fully developed collar and granules (Figs 41–44) and immature cyst (Fig. 45). Figs 46-48. U. notabilis – formation of cysts within a colony in culture (Fig. 46), mature cysts (Figs 47, 48). Figs 49–52. U. articulata – cultured colony (Fig. 49), cysts possessing a typical acute rim surrounding the pore (probably immature) or only slightly incrusted collar (Figs 50, 51), a mature cyst (Fig. 52). Figs 53-57. U. lindiae – formation of cysts within a colony in natural sample (Fig. 53), colony in culture (Fig. 54), mature cysts with various paw-like hooked processes (Figs 55–57). Scale = 50 μm (Fig. 53), 20 μm (Figs 37, 39, 46, 49, 54), 10 μm (Figs 38, 40, 47, 55), 5 μm (Figs 41–43, 48, 50–52, 56, 57) and 2.5 μm (44, 45). LM investigations (Figs 37–42, 46, 47, 49–51, 53–55), SEM investigations (Figs 43–45, 48, 52, 56, 57).
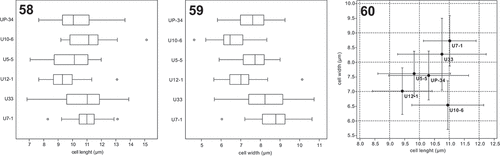
Isolates of Urostipulosphaera had a significantly higher survival rate than the two above-mentioned genera. Therefore, we were able to morphologically characterize in detail all species-level clades. Accordingly, we evaluated the usability of cell morphological features in species delineation. Our analyses show that U. notabilis (U12-1), U. articulata (U5-5) and U. lindiae (UP-34) had similar ranges of cell length and width (). Urostipulosphaera sp. (U10-6) possessed very elongated cells. U. granulata exhibited larger cells and smaller cysts than other Urostipulosphaera species. Isolates of U. granulata (U7-1 and U33) differed in their cell length/width range though they are virtually genetically identical. Moreover, the U7-1 isolate cells in the natural sample were generally longer (12–16 × 6–8.5 µm) than in the culture (7–14 × 6–10.5 µm).
Figs 58–60. Comparison of cell length and cell width between cultured Urostipulosphaera species. U. notabilis (U12-1), U. articulata (U5-5) and U. lindiae (UP-34) shared very similar range of cell length and width. Urostipulosphaera sp. (U10-6) possessed cells with clearly skewed length/width ratio in favour of length. U. granulata (U7-1 and U33) possessed generally larger cells than all the other species belonging to the second Urostipulosphaera lineage. On the other hand, two isolates of U. granulata (U7-1 and U33) differed in their cell length/width range though they are virtually genetically identical. Average values and standard deviations are given (Fig. 60).
Taxonomic revisions and diagnoses
Uroglena imitata Pusztai & Škaloud sp. nov. ()
DESCRIPTION: Colonies 120–180 µm in diameter with cells 10–12.5 µm long and 5.5–7.5(–9) µm wide. Cysts spherical, 13.3–14.8 µm in diameter with 1 µm wide pore and complex collars. Cysts usually smooth (LM) or imperfectly smooth (SEM), regularly coated with very small particles. Primary collar is 1.5–2 µm high, 2.4–2.8 µm wide. Secondary collar is (1.9–)4.8–7.9 µm high, 4.6–6.3(–8.1) µm wide. Cyst diameter/secondary collar width ratio is 2.1–2.6.
HOLOTYPE (here designated): Portion of a single gathering of cysts (strain UR-2) on SEM stub deposited at the Culture Collection of Algae of Charles University, Prague (CAUP). illustrates the holotype.
TYPE LOCALITY: Lacul Noua, Romania (45.61429’N, 25.63962’E).
ETYMOLOGY: The specific epithet ‘imitata’ reflects that cysts of U. imitata closely resemble those of U. zachariasii, the most common cyst morphotype among Uroglena, but had a significantly narrower and higher secondary collar.
REPRESENTATIVE DNA SEQUENCES: GenBank accessions no. MW267669, MW251563.
DISTRIBUTION: Currently known from Austria, Czech Republic, Portugal and Romania.
Uroglena rotundata (Skvortzov) Pusztai & Škaloud comb. nov.
BASIONYM: Uroglenopsis rotundata Skvortzov Philip. J. Sc. 86: 183, pl. 6: (Citation1958).
TYPE LOCALITY: Swamp near Harbin, China.
Notes: We did not have the opportunity to observe living material of this species. However, the original drawings of the colony with characteristic flagella length ratio unequivocally assign the species to the genus Uroglena.
Uroglena skujae Matvienko ex Pusztai & Škaloud sp. nov. ()
DESCRIPTION: Colonies 100–150 µm in diameter with cells 8.5–11.5 µm long and 7–8.5 µm wide. Cysts spherical, (11–)12.5–14.5 µm in diameter with a pronounced very long collar 8.5–14.5 µm high and 3.5–4.5 µm wide. Cysts usually smooth (LM) or imperfectly smooth (SEM), regularly coated with very small particles.
HOLOTYPE (here designated): original drawings of U. europaea by Skuja, Symb. Bot. Upsal. 9(3): p. 272, pl. 30: figs 10–12 (Citation1948).
SYNONYM: Uroglena europaea (Pascher) Skuja Citation1948: 267.
TYPE LOCALITY: Ubby-Langsjön, Sweden.
ETYMOLOGY: The specific epithet ‘skujae’ was originally proposed by Matvienko (Citation1965) for the species, named in honour of Latvian phycologist Heinrich Leonhards Skuja (1892–1972), who first described this morphology in Sweden.
REPRESENTATIVE DNA SEQUENCES: GenBank accessions no. MW267676, MW251564.
DISTRIBUTION: Currently known from Sweden and Ukraine.
Uroglenopsis troitzkajae (Korshikov) Pusztai & Škaloud comb. nov.
BASIONYM: Uroglena troitzkajae Korshikov in Korshikov & Matwienko, Uchen. Zap. Kharkovsk. Derzh, Univ., Trudy Inst. Bot. 4: 13 (Citation1941).
SYNONYM: Uroglenopsis americana (Calkins) Lemmermann sensu Troitzkaja Citation1924: 266.
TYPE LOCALITY Environs of Saint Petersburg, Russia.
Notes: We did not have the opportunity to observe living material of this species. However, the original description and drawings of the colonies and cells unequivocally assign the species to the genus Uroglenopsis.
Urostipulosphaera articulata (Korshikov) Pusztai & Škaloud comb. nov. ()
BASIONYM: Uroglena articulata Korshikov in Korshikov & Matwienko, Uchen. Zap. Kharkovsk. Derzh, Univ., Trudy Inst. Bot. 4: 5–9, (Citation1941).
SYNONYM: Uroglenopsis articulata (Korshikov) (Wujek & thompson, Citation2002: 302).
TYPE LOCALITY: Boggy lake near the village Kovda, Karelia, Russia.
REFERENCE STRAIN LOCALITY: Strain U5-5 was isolated from Kříž pond in PP Na Plachtě, Czech Republic (50.1827819’N, 15.8702700’E).
REPRESENTATIVE DNA SEQUENCES: GenBank accessions no. MW267729, MK153260.
Urostipulosphaera conimamma (Nygaard) Pusztai & Škaloud comb. nov.
BASIONYM: Uroglena conimamma Nygaard, K. Danske Vid. Selsk. Biol. Skr. 21(1): 10, fig. 7 (Citation1977).
SYNONYM: Uroglenopsis conimamma (Nygaard) Wujek & Thompson (Citation2002): 303; U. americana (Calkins) Lemmermann sensu Nygaard Citation1945: 26.
TYPE LOCALITY: Lille Gribsø, Denmark.
Notes: We did not have the opportunity to observe living material of this species. However, the original description and drawings of the colonies and cysts unequivocally assign the species to the genus Urostipulosphaera.
Urostipulosphaera europaea (Pascher) Pusztai & Škaloud comb. nov.
BASIONYM: Uroglenopsis europaea Pascher, Osterr. Bot. Z. 60: 4, pl. I: figs 15–17 (Citation1910).
SYNONYM: non Uroglena europaea (Pascher) Skuja Citation1948: 267.
TYPE LOCALITY: ‘Olsch’ bei Mugrau (pond or stream near villages Olšina or Olšov), Šumava mountains, Czech Republic.
Notes: We did not have the opportunity to observe living material of this species. However, the original description and drawings of the colonies and cells unequivocally assign the species to the genus Urostipulosphaera.
Urostipulosphaera eustylis (Skuja) Pusztai & Škaloud comb. nov.
BASIONYM: Uroglena eustylis Skuja, Symb. Bot. Upsal. 9(3): p. 272, pl. 30: figs 16–18 (Citation1948).
SYNONYM: Uroglenopsis eustylis (Skuja) (Wujek & Thompson, Citation2002: 302).
TYPE LOCALITY: Ämsjön, Uppland, Sweden.
Notes: We did not have the opportunity to observe living material of this species. However, the original description and drawings of the colonies unequivocally assign the species to the genus Urostipulosphaera.
Urostipulosphaera granulata Pusztai & Škaloud, sp. nov. ()
Description: Colonies 40–80(–100) µm in diameter with cells (7–)10–16 µm long and 6–9.5(–10.5) µm wide. Cysts almost spherical to slightly oblate or slightly oval, 9.5–12 µm wide and 7–11(–12.5) µm in length. Cysts usually equally embellished with numerous regular granules (0.3–)0.4–0.6(–0.7) µm in diameter and clearly visible in both LM and SEM. Pore (0.4–0.9 µm in diameter) is surrounded by 1.7–2.3 µm wide, curved, collapsed, tubular collar.
HOLOTYPE (here designated): Portion of a single gathering of cysts (strain U7-1) on SEM stub deposited at the Culture Collection of Algae of Charles University, Prague (CAUP). illustrates the holotype.
REFERENCE STRAIN: The culture of strain U7-1 has been deposited as CAUP B 801 in the Culture Collection of Algae of Charles University in Prague, Czech Republic.
TYPE LOCALITY: Small pool in the Botanical Garden of Charles University, Prague, Czech Republic (50.0710836’N, 14.4206419’E), ~50 m from our office.
ETYMOLOGY: The specific epithet ‘granulata’ refers to cysts of U. granulata being decorated by numerous small granules.
REPRESENTATIVE DNA SEQUENCES: GenBank accessions no. MW267730, MK153259.
DISTRIBUTION: Currently only known from two localities in Prague, Czech Republic.
Urostipulosphaera lindiae (Bourrelly) Pusztai & Škaloud comb. nov. ()
BASIONYM: Uroglena lindiae Bourrelly, Rev. Alg., Mém. Hors-sér. 1: 155, pl. 1: figs 35–38 (Citation1957).
SYNONYM: Uroglenopsis lindiae Bourrelly in (Wujek & Thompson, Citation2002: 302).
TYPE LOCALITY: Forêt de Sénart, Paris, France.
REFERENCE STRAIN LOCALITY: Strain U29-1-496 was isolated from Vydýmač pond, Czech Republic (48.9617636’N, 14.9525025’E).
REPRESENTATIVE DNA SEQUENCES: GenBank accessions no. MW267732, MK153263.
Urostipulosphaera proxima (Korshikov & Matvienko) Pusztai & Škaloud comb. nov.
BASIONYM: Uroglena proxima Korshikov & Matvienko, Uchen. Zap. Kharkovsk. Derzh, Univ., Trudy Inst. Bot. 4: 9–14, figs 5–9 (Citation1941).
TYPE LOCALITY: Near Kharkiv, Ukraine.
Notes: We did not have the opportunity to observe living material of this species. However, the original description and drawings of the colonies unequivocally assign the species to the genus Urostipulosphaera.
Urostipulosphaera soniaca (Conrad) Pusztai & Škaloud comb. nov.
BASIONYM: Uroglena soniaca Conrad, Bull. Mus. R. Hist. Nat. Belg. 14(42): 1, figs A–E, H, pl. I, II (Citation1938).
SYNONYM: Uroglenopsis soniaca (Conrad) (Wujek & Thompson, Citation2002: 301.
TYPE LOCALITY: Forêt de Soignes, Belgium.
Notes: We did not have the opportunity to observe living material of this species. However, the original description and drawings of the colonies unequivocally assign the species to the genus Urostipulosphaera.
Discussion
Morphological features and species delimitation
Originally, Uroglena-like taxa were predominantly defined by the morphology of colonies, cells and plastids. However, this often proved to be insufficient to distinguish species. Comparing five Urostipulosphaera species cultivated under the same conditions, their dimensions overlap quite a lot (). In addition, these features are plastic and variable during ontogenesis, due to environmental conditions or due to stress from heating and drying of the sample during LM observations (Wujek & Thompson, Citation2002; Pusztai & Škaloud, Citation2019). Differences in cell shape and size between algae grown in cultures, and in field conditions, are also known from the first experiments with culturing (Andersen, Citation2005). Cultured cells (e.g. U. granulata sp. nov.) are generally smaller and more globular when compared with fresh natural samples or dimensions given by other authors (summarized in Starmach, Citation1985). Even Uroglenopsis and Urostipulosphaera can produce thin, short unbranched threads when stressed () and the use of fixation or dyes can cause artefacts (Conrad, Citation1938). Therefore, the precise examination of cysts using SEM is not only a great advantage, but a necessity.
The use of cysts in taxonomy is not without complications. Cysts are not known for all the described species. For such species, it is possible, though challenging, to re-collect encysting material from the type locality. The intriguing question is whether the cyst ultrastructure of Uroglena-like colonial flagellates really has species-specific characters (Skuja, Citation1948; Wujek & Thompson, Citation2002; Cronberg & Laugaste, Citation2005). We are aware that in some chrysophyte genera (e.g. some Synura species), the cysts are not species-specific due to their simplicity, resembling immature not fully developed cysts (Duff et al., Citation1995). Holen (Citation2014) showed that in monoclonal chrysophyte cultures, the cyst diameter may be relatively stable in some populations but show a huge range in others. Moreover, sexual and asexual cysts share, in general, the same morphology, differing only in their diameter (Sandgren, Citation1983). On the other hand, the length of both the spines and the collars is markedly variable (Bourrelly, Citation1957; Nygaard, Citation1977) and influenced by temperature during the encystment process (Sandgren, Citation1983).
According to our observations and taxonomic revision, Urostipulosphaera and Uroglena cysts seem to be truly species-specific when observed by SEM. Uroglenopsis species generally have morphologically highly similar cysts, differing only in their diameter. Neverthless, it seems that cysts of many Uroglenopsis species may be determined by cell and colony characteristics.
Ecological differences among lineages
An overall goal of this study was to obtain a sufficient number of single colony isolates (or short-term cultures) from encysting populations. The proportion of encysting populations in sampled populations differed between the genera. The highest proportion was observed in Urostipulosphaera and Uroglena where cysts were successfully acquired for nearly all the revealed lineages. On the contrary, in Uroglenopsis we only obtained cysts for U. turfosa (strain UK-81) encysting in culture, and for U. botrys (UL-2). U. botrys was collected directly from the encysting population in summer after many regular inspections at the site since its spring population bloomed in April.
One possible explanation of this difference in encystment lies in our newly discovered ecological preference of Uroglenopsis in the northern temperate zone. Uroglenopsis seems to be a predominantly late spring to summer taxon, peaking in May and occurring from April to July. Since our sampling effort was typically focused on spring and autumn chrysophyte maxima, potential summer under-sampling could have affected our dataset. The long-term examination of encysting Uroglenopsis populations by Skuja (Citation1948, Citation1956) in Sweden supports this explanation. He found U. americana sensu Skuja and U. turfosa from spring onwards, but they peaked and encysted later, from summer to autumn. He further found that U. irregularis, which had already peaked during the spring, produced cysts again from summer to autumn.
According to our observations of ecological () and habitat (Supplementary table S1) preferences in the northern temperate zone, it is evident that Urostipulosphaera inhabits man-made and strongly influenced habitats, such as ponds, where it peaks in early spring waters with significantly lower temperature when compared with Uroglena and Uroglenopsis. The vast majority of Urostipulosphaera isolates came from ponds in the Czech Republic, distinctive by their high productivity, trophic state, and phytoplankton biomass (IUCN, Citation1997). Conversely, Uroglenopsis inhabits pristine habitats, such as drinking water reservoirs or lakes, usually situated in mountainous regions, and often among coniferous forests with low pH and trophic states, as well as having a delayed start to the season compared with lowland ponds. This is in accordance with our presumption of a late summer encystment process in Uroglenopsis. Finally, Uroglena exhibits intermediate ecological preferences. On the other hand, all three genera can be found in one location sharing the same planktonic habitat, but they differ in their phenology and seasonal dynamics.
Taxonomy
Of previous taxonomies for Uroglena-like flagellates, the closest to the present one was the system of Korshikov & Matvienko (Citation1941) and Matvienko (Citation1965), where Urostipulosphaera species were placed into Uroglena s.l. The common element was the presence of the system of dichotomously branched radial structures connecting cells in the colony. Two or three sections (not formally established), differing in thread/stalk thickness, were distinguished within Uroglena s.l. In contrast, Urostipulosphaera species were placed into Uroglenopsis s.l. by Wujek & Thompson (Citation2002), pointing to thin threads as a synapomorphy for Uroglena, and reflecting the sometimes poorly visible stalks of (still undescribed) Urostipulosphaera.
The long-standing discrepancy of the concepts ‘all are Uroglena’ vs. ‘Uroglenopsis exist’, has been resolved by the latest taxonomic revision and by the introduction of Urostipulosphaera (Pusztai & Škaloud, Citation2019). According to our molecular analyses and morphological observations, we were able to assign all the previously described taxa to recognized genera (Supplementary table S2). Uroglena now includes 16 species and two varieties, Uroglenopsis contains four species, and Urostipulosphaera encompasses nine species. Some previously described species were placed in synonymy. Below, we provide a taxonomic overview for all three genera. In addition, a key to the determination of genera and species, mainly based on differences in cyst morphology, is provided in Supplementary table S3.
Uroglena Ehrenberg, 1834
The type species U. volvox was re-collected from its type locality in Berlin (Germany), and determined according to its original description by Ehrenberg (for more details see Pusztai & Škaloud, Citation2019).
Although Skuja (Citation1948, Citation1956) recognized only the genus Uroglena, based on his detailed drawings, it is possible to assign his taxa to newly circumscribed genera. Accordingly, U. europaea, with a newly associated species-specific smooth cyst with a very long collar is, with no doubt, a new Uroglena species. Unfortunately, these Uroglena cyst and colony types were incorrectly assigned by Skuja (Citation1948) to a previously described species Uroglenopsis europaea, and characteristics of both taxa were mixed in the new ‘hybrid’ combination Uroglena europaea. This problem was pointed out by Matvienko (Citation1965) and the new species was, invalidly (missing Latin diagnosis), described according to Skuja’s previous observations as Uroglena skujae. Therefore, Uroglena europaea should be placed in synonymy with the newly proposed Uroglena skujae Matvienko ex Pusztai & Škaloud, sp. nov. U. skujae was re-collected from its type locality (Ubby-Langsjön, Sweden) more than 60 years after cysts with such morphology were first described by Skuja (Citation1948).
In the second species with a newly assigned cyst, sensu Skuja, U. botrys (Pascher) Conrad, the species-specific cyst is identical to that of the previously described species U. glabra. The same cyst type was, however, later incorrectly assigned by previous authors to a species of Uroglenopsis, U. botrys, and characteristics of both genera were mixed in the ‘hybrid’ new combination Uroglena botrys as a species with a characteristic smooth cyst with a low collar. Conrad (Citation1938) knew that Schiller (Citation1926) added a different cyst type to U. botrys, but he ignored this cyst as immature. Therefore, Uroglena botrys and Uroglena with such cysts, sensu Skuja, should be placed in synonymy with Uroglena glabra. Skuja’s cyst type was originally described from Sweden, and we found such cysts in Swedish locations. Our SEM findings of the cyst ultrastructure further indicate that in U. glabra, collar production starts as a very low thick-walled rounded marginal rim around the pore (immature cysts) and is followed by the production of a low collar with an acute rim, or with a false complex collar. This may explain deviations in the collar characteristics (mainly length) given by different authors.
Based on comparison of our material with the dimensions and figures of material originally examined by Zacharias (Citation1895) and later by Wujek & Thompson (Citation2002), we were able to undoubtedly assign one well-supported clade to U. zachariasii (= U. volvox sensu Zacharias). U. zachariasii represents a genetically diverse clade encompassing three lineages which may belong to different populations (as considered here) or different species. All three lineages are geographically distinct with the first lineage (UK-37, UK-41) from North America, not Europe. In the second lineage, mainly from Sweden, cysts of U. zachariasii var. uplandica were recovered in one natural sample (U26-14); further evaluation is needed.
Based on genetic data and a specific cyst diameter: secondary collar width ratio, we propose a new species with a cyst morphology similar to U. zachariasii, U. imitata sp. nov. All populations of U. zachariasii showed a ratio of 1.3–1.8, corresponding to the literature. Conversely, populations of U. imitata showed a ratio of 2.1–2.6. According to older publications, only the description by Geissbühler (Citation1933) fits this newly recognized species. The remaining two clades with cysts similar to U. zachariasii or U. imitata had isolates originating from poorly encysting populations so we were unable to precisely evaluate their characteristics in SEM, and will need further examination.
Based on the original descriptions of material with species-specific cysts, U. collaris Thompson & Wujek, U. dendracantha Cronberg, U. estonica Cronberg & Laugaste, U. kukkii Cronberg & Laugaste, U. marina Büttner, U. nygaardii Bourrelly, U. pikamae Cronberg & Laugaste, and U. spinosa Cronberg & Laugaste represent well-delimited species with precise descriptions distinguishing them from any other Uroglena species. The taxonomic status of U. conradii Schiller and U. conradii var. gallica Bourrelly will need further evaluation. Their cysts were described (the first only verbally) as globular and smooth in LM without any collars, only slightly thickened around the pore in the second species. Thus, they resemble any Uroglena immature cyst. Similarly, U. volvox var. verrucosa (Mack) Thompson & Wujek (= U. botrys var. verrucosa Mack), with variable cysts (LM only) resembling U. pikamae and U. glabra, will need further evaluation of its taxonomic status. In U. radiata Calkins, which was originally described from the USA from material lacking cysts, finding encysting populations will be of great value to provide a more detailed description. However, the original description and drawings of the colonies unequivocally assign the species to the genus Uroglena, and its later displacement into Uroglenopsis by Lemmermann (Citation1899) is in conflict with the current taxonomic revision. According to the original description, U. radiata Calkins exhibited thin threads unlike Uroglenopsis americana (Calkins) Lemmermann in which no such structures were observed.
For U. rotundata comb. nov., originally described from China from material lacking cysts, a more detailed description is required. Despite the original description and drawings being vague, this species can be unequivocally assigned to the genus Uroglena according to its characteristic flagella length ratio.
Uroglenopsis Lemmermann, Citation1899
The type species U. americana was re-collected from the type locality and determined according to its original description given by Calkins (Citation1892); for more details see Pusztai & Škaloud (Citation2019). U. americana is closely related to U. botrys according to molecular genetic data as well as the specific morphology of the multilobed colonies, which they share. Considering our isolates of U. americana from the type locality, as well as older sequenced isolates (from Canada), it seems that U. americana is not common in Europe. This is in accordance with observations on Uroglenopsis by Schiller (Citation1926) who stated that U. americana very rarely occurred in Europe. Therefore, many previous European observations of U. americana very likely belonged to the widespread U. botrys and related species (see below). Matvienko (Citation1965) recognized that cells of U. americana sensu Skuja differed significantly in shape and dimensions from true U. americana, so she erected a new species, Uroglenopsis skujae Matvienko.
Pascher (Citation1910, Citation1913) distinguished Uroglenopsis, lacking the system of dichotomously branched radial structures that is clearly visible in Uroglena, and he was clearly observing Uroglenopsis botrys. Our isolated U. botrys re-collected from the type locality Máchovo jezero, Czech Republic, and from other localities, was in accordance with the original description. Unfortunately, Pascher did not observe cysts and our material from the type locality also lacked any cysts. Fortunately, we collected U. botrys from many other localities and one population (UL-2) was producing cysts. These cysts correspond to cysts additionally assigned to U. botrys by Schiller (Citation1926), or to cysts found later by Skuja (Citation1948, Citation1956) in Scandinavian U. skujae and U. irregularis.
Interestingly, colonies and cells of different U. botrys populations were very diverse in shape and therefore it was even possible to assign different U. botrys populations to different previously described species – U. apiculata, U. irregularis and U. skujae (Supplementary table S4). In the light of such natural variability of colony, cell shape and dimensions within a single species, it is more likely that U. apiculata, U. irregularis and U. skujae are ecomorphs. This hypothesis is further supported by Wujek & Thompson (Citation2002). Moreover, U. botrys was the most commonly observed species within Uroglenopsis representing nearly every second Uroglenopsis sequence obtained within this survey. Therefore, we propose that U. apiculata, U. irregularis and U. skujae should be placed in synonymy with U. botrys.
U. turfosa (= Eusphaerella turfosa Skuja) colonies were unequivocally determined according to their species-specific morphology. The cyst of U. turfosa was originally only verbally described as almost spherical to slightly roundly obovate, 13–15 µm in diameter, and with a very low, 3.5–3.8 µm wide, collar (marginal rim). Cysts of U. turfosa found by us (in culture) were almost spherical to slightly oval and smooth, 9.5–10 µm wide and 10–10.5 µm in length. The concave pore was surrounded by a 2 µm wide, rounded and slightly conical marginal rim < 1 µm. In other chrysophytes the cyst diameter may be generally invariant among the populations (Sandgren, Citation1983). Two main lineages were resolved within U. turfosa, considered here as different populations of the single species.
According to cell and plastid characteristics, U. troitzkajae comb. nov. certainly belongs to Uroglenopsis. This species was originally described from Russia from material lacking cysts. However, U. troitzkajae has unique invaginations of the gel matrix among cells. According to Conrad (Citation1938), the ‘fibrous’ structures observed by some authors were an artefact of the use of unsuitable dyes, which could lead to wrinkles in the shrunken gelatinous mass of the colony. Whether this is true or not for U. troitzkajae, there is other evidence for colony invaginations (gel matrix with the cells) in U. turfosa.
Finally, we cannot assign Uroglenopsis sp. 1 and Uroglenopsis sp. 2 to any previously described species, so we do not treat these lineages taxonomically.
Urostipulosphaera Pusztai & Škaloud, Citation2019
Based on either morphological and molecular data, or the original descriptions and drawings, we can unequivocally assign five previously described species (U. articulata, U. lindiae, U. notabilis, U. conimamma and U. eustylis) to the genus Urostipulosphaera (see Taxonomic revisions and diagnoses).
U. proxima comb. nov. was originally described from Ukraine from material with species-specific cysts differing from any other Urostipulosphaera species. U. proxima has all the characteristics of Urostipulosphaera except for the articulated stalks. Korshikov & Matvienko (Citation1941) stained colonies with several dyes but did not find any septa. In cultured material, we have found articulated stalks in all Urostipulosphaera lineages genetically characterized in our study. However, in fresh material from natural samples, whole stalks were sometimes nearly invisible and therefore, septa were not detected. From this perspective, U. proxima certainly belongs to Urostipulosphaera, but it may form a separate lineage possessing unarticulated stalks.
U. soniaca comb. nov. was originally described from Belgium from material with species-specific cysts with a hook-like projection, differing from any other Urostipulosphaera species. U. soniaca was based on material containing both Uroglena and Urostipulosphaera taxa mixed in the sample and was, unfortunately, confusingly interpreted by Conrad (Citation1938) as young and old colonies.
In U. europaea comb. nov., which was originally described from Czech Republic from material lacking cysts, the original description and drawings unequivocally assign the species into the genus Urostipulosphaera according to plastid and cell characteristics, together with the flagella length ratio (but with not visible stalks as it sometimes can happen with fresh material). Pascher (Citation1910) listed one plastid in smaller cells, and two plastids in larger cells. These larger cells were probably already deformed due to microscopy (heating stress, etc.), and their plastid was typically split into two smaller ones.
U. granulata sp. nov. was newly erected from the Czech Republic based on material with species-specific cysts clearly differing, morphologically, from all previously described Urostipulosphaera species, and it is therefore described as a new species.
Finally, we cannot assign Urostipulosphaera sp. to any previously described species, but due to lack of knowledge of its cyst morphology, we cannot be sure if it is a new species or an already described species. In order to finally decide this issue, further examination of an encysting Urostipulosphaera sp. population is needed.
Supplementary table S1. Strains of the genera Uroglena, Uroglenopsis and Urostipulosphaera used in this study. Additional information is provided for each strain: number of identical isolates (N-isol.), sampling site (Locality) along with geographic coordinates (GPS), date, physico-chemical water parameters (pH, conductivity, temperature) and the GenBank accession numbers for their ITS, rbcL and SSU gene sequences. Those strains sharing identical DNA sequences are marked with lower case letters.
Supplementary table S2. Taxonomic overview for all three genera (Uroglena, Uroglenopsis and Urostipulosphaera). According to our molecular analyses and morphological observations, we were able to assign all the previously described taxa to recognized genera.
Supplementary table S3. Key to the determination of genera and species within Uroglena-like chrysophytes.
Supplementary table S4. Cell characteristics of different U. botrys populations.
Supplementary fig. S1. Phylogeny of the Chrysophyceae showing position of Uroglena, Uroglenopsis and Urostipulosphaera within the Ochromonadales clade (adopted from Pusztai & Škaloud, Citation2019).
Author contributions
M. Pusztai: drafting and editing manuscript, sampling, morphological investigations (LM, SEM), culturing, acquiring molecular data, phylogenetic analysis; P. Škaloud: original concept, editing manuscript, sampling, phylogenetic analysis.
Supplemental Material
Download PDF (268.4 KB)Supplemental Material
Download MS Excel (10 KB)Supplemental Material
Download MS Excel (12.4 KB)Supplemental Material
Download MS Excel (13.6 KB)Supplemental Material
Download MS Excel (21.2 KB)Acknowledgements
The authors would like to thank D. Čertnerová, L. Flašková, R. Geriš, I. Jadrná, M. Klimešová, P.T. Mutinová, K. Procházková, P. Pumann, Z. Pusztaiová, M. Škaloudová, T. Šoljaková, J. Šťastný and K. Trumhová for their help with the sampling campaign and/or laboratory procedures, to M. Hyliš, P. Kejzlar and A. Schröfel for their help with electron microscopy and to V. Mrkvička and librarians from Radboud University for their help with finding and scanning old taxonomic papers. The authors also would like to thank the reviewers and editors for helpful comments on the manuscript, especially to Professor Christine Maggs, Editor-in-Chief, for her significant work for improvement of our manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary Information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2021.1892196
Additional information
Funding
References
- Agbeti, M.D. & Smol, J.P. (1995). Chrysophyte population and encystment patterns in two Canadian lakes. Journal of Phycology, 31: 70–78.
- Andersen, R.A. (2005). Algal Culturing Techniques. Elsevier Academic Press, San Diego.
- Andersen, R.A., Morton, S.L. & Sexton, J.P. (1997). Provasoli‐Guillard National Center for culture of marine phytoplankton 1997 list of strains. Journal of Phycology, 33: 1–75.
- Andersen, R.A., Graf, L., Malakhov, Y. & Yoon, H.S. (2017). Rediscovery of the Ochromonas type species Ochromonas triangulata (Chrysophyceae) from its type locality (Lake Veysove, Donetsk region, Ukraine). Phycologia, 56: 591–604.
- Anneville, O., Gammeter, S. & Straile, D. (2005). Phosphorus decrease and climate variability: mediators of synchrony in phytoplankton changes among European peri‐alpine lakes. Freshwater Biology, 50: 1731–1746.
- Apotheloz-Perret-Gentil, L., Cordonier, A., Straub, F., Iseli, J., Esling, P. & Pawlowski, J. (2017). Taxonomy-free molecular diatom index for high-throughput eDNA biomonitoring. Molecular Ecology Resources, 17: 1231–1242.
- Bock, C., Medinger, R., Jost, S., Psenner, R. & Boenigk, J. (2014). Seasonal variation of planktonic chrysophytes with special focus on Dinobryon. Fottea, 14: 179–190.
- Bock, C., Chatzinotas, A. & Boenigk, J. (2017). Genetic diversity in chrysophytes: comparison of different gene markers. Fottea, 17: 209–221.
- Boenigk, J., Ereshefsky, M., Hoef-Emden, K., Mallet, J. & Bass, D. (2012). Concepts in protistology: species definitions and boundaries. European Journal of Protistology, 48: 96–102.
- Bonde, N. (1977). Cladistic classification as applied to vertebrates. In Major Patterns in Vertebrate Evolution (Hecht, M.K., Goody, P.C. & Hecht, B.M., editors), 741–804. Plenum Press, New York.
- Bourelly, P. (1957). Recherches sur les Chrysophycées. Revue Algologique: Mémoire Hors-Série, 1: 1–412.
- Calkins, G.N. (1892). On Uroglena, a genus of colony building infusoria observed in certain water supplies of Massachusetts. Annual Reports of the State Board of Health of Massachusetts, 23: 647–657.
- Conrad, W. (1938). Notes protistologiques. V. Observations sur Uroglena soniaca n. sp. et remarques sur le genre Uroglena Ehr. (incl. Uroglenopsis Lemm.). Bulletin du Musée Royal d’Histoire Naturelle de Belgique, 14: 1–27.
- Cronberg, G. & Laugaste, R. (2005). New species of Uroglena and Ochromonas (Chromulinales, Chrysophyceae) from Estonia. Nova Hedwigia Supplement, 128: 43–63.
- Darriba, D., Taboada, G.L., Doallo, R. & Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nature Methods, 9: 772.
- Darwin, C. (1859). On the Origin of Species by Means of Natural Selection, or the Preservation of Favoured Races in the Struggle for Life. John Murray, London.
- de Queiroz, K. (2005). A unified concept of species and its consequences for the future of taxonomy. Proceedings of the California Academy of Sciences, 56: 196–215.
- de Queiroz, K. (2007). Species concepts and species delimitation. Systematic Biology 56: 879–886.
- Duff, K., Zeeb, B. & Smol, J. (1995). Atlas of chrysophycean cysts. Developments in Hydrobiology, 99: 1–189.
- Findenig, B.M., Chatzinotas, A. & Boenigk, J. (2010). Taxonomic and ecological characterization of stomatocysts of Spumella‐like flagellates (Chrysophyceae). Journal of Phycology, 46: 868–881.
- Finlay, B.J. & Esteban, G.F. (1998). Freshwater protozoa: biodiversity and ecological function. Biodiversity & Conservation, 7: 1163–1186.
- Geissbühler, J. (1933). Grundlagen zu einer Algenflora einiger oberthurgauischer Moore. Mittheilungen der Thuroauischen Natururforschenden Gesellschaft, 29: 3–65.
- Grossmann, L., Bock, C., Schweikert, M. & Boenigk, J. (2016). Small but manifold – hidden diversity in “Spumella‐like flagellates”. Journal of Eukaryotic Microbiology, 63: 419–439.
- Guiry, M.D. & Guiry, G.M. (2020). AlgaeBase. World-wide electronic publication, National University of Ireland, Galway. Available at: http://www.algaebase.org.
- Hey, J. (2001). The mind of the species problem. Trends in Ecology & Evolution, 16: 326–329.
- Holen, D.A. (2014). Chrysophyte stomatocyst production in laboratory culture and descriptions of seven cyst morphotypes. Phycologia, 53: 426–432.
- Howe, A.T., Bass, D., Vickerman, K., Chao, E.E. & Cavalier-Smith, T. (2009). Phylogeny, taxonomy, and astounding genetic diversity of Glissomonadida ord. nov., the dominant gliding zooflagellates in soil (Protozoa: Cercozoa). Protist, 160: 159–189.
- Index Nominum Algarum (2020). Index Nominum Algarum, University Herbarium, University of California, Berkeley. Compiled by Paul Silva. Available at http://ucjeps.berkeley.edu/CPD.
- IUCN (1997). Fishing for a living – the ecology and economics of fishponds in central Europe. Environmental Research Series, 11: 1–184.
- Jo, B.Y., Shin, W., Kim, H.S., Siver, P.A. & Andersen, R.A. (2013). Phylogeny of the genus Mallomonas (Synurophyceae) and descriptions of five new species on the basis of morphological evidence. Phycologia, 52: 266–278.
- Jost, S., Medinger, R. & Boenigk, J. (2010). Cultivation-independent species identification of Dinobryon species (Chrysophyceae) by means of multiplex single-cell PCR. Journal of Phycology, 46: 901–906.
- Katana, A., Kwiatowski, J., Spalik, K., Zakryś, B., Szalacha, E. & Szymańska, H. (2001). Phylogenetic position of Koliella (Chlorophyta) as inferred from nuclear and chloroplast small subunit rDNA. Journal of Phycology, 37: 443–451.
- Katoh, K., Misawa, K., Kuma, K.I. & Miyata, T. (2002). MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Research, 30: 3059–3066.
- Korshikov A.A. & Matvienko A.M. (1941). Pro dva novi vidi Uroglena Ehr. Trudy Institutu Botaniki, Kharkov 4: 5–17.
- Kristiansen, J. & Škaloud, P. (2017). Chrysophyta. In Handbook of the Protists (Archibald J., Simpson A., Slamovits C., editors), 331–366. Springer, Cham.
- Kynčlová, A., Škaloud, P. & Škaloudová, M. (2010). Unveiling hidden diversity in the Synura petersenii species complex (Synurophyceae, Heterokontophyta). Nova Hedwigia, Beiheft 136: 283–298.
- Lemmermann, E. (1899). Das Phytoplankton sächsischer Teiche. Forschungsberichte aus der Biologischen Station zu Plön, 7: 96–135.
- Leray, M. & Knowlton, N. (2015). DNA barcoding and metabarcoding of standardized samples reveal patterns of marine benthic diversity. Proceedings of the National Academy of Sciences USA, 112: 2076–2081.
- Linnaeus, C. (1753). Species Plantarum. Laurentius Salvius, Stockholm.
- Matvienko, O.M. (1965). Vyznachnyk prisnovodnykh vodorostei Ukrainskoi RSR. III (1) Zolotysti vodorosti – Chrysophyta. Naukova Dumka, Kyjv.
- Miller, M.A., Pfeiffer, W. & Schwartz, T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE), 1–8.
- Nygaard, G. (1977). New or interesting plankton algae: with a contribution on their ecology. Det Kongelige Danske Videnskabernes Selskab. Biologiske Skrifter, 21: 1–107.
- Pascher, A. (1910). Chrysomonaden aus dem Hirschberger Grossteiche: Untersuchungen über die Flora des Hirschberger Grossteiches. I. Teil. Monographien und Abhandlungen zur Internationale Revue der gesamten Hydrobiologie und Hydrographie, 1: 1–66.
- Pascher, A. (1913). Flagellatae II. Chrysomonadinae. In Die Süsswasserflora Deutschlands, Österreichs und der Schweiz. Heft 2 (Pascher, A. & Lemmermann, E., editors), 1–95. Verlag von Gustav Fischer, Jena.
- Pawlowski, J., Audic, S., Adl, S., Bass, D., Belbahri, L., Berney, C., et al. (2012). CBOL protist working group: barcoding eukaryotic richness beyond the animal, plant, and fungal kingdoms. PLoS Biology, 10: e1001419.
- Pusztai, M. & Škaloud, P. (2019). Elucidating the evolution and diversity of Uroglena-like colonial flagellates (Chrysophyceae), polyphyletic origin of the morphotype. European Journal of Phycology, 54: 404–416.
- Pusztai, M., Čertnerová, D., Škaloudová, M. & Škaloud, P. (2016). Elucidating the phylogeny and taxonomic position of the genus Chrysodidymus Prowse (Chrysophyceae, Synurales). Cryptogamie, Algologie, 37: 297–307.
- Remias, D., Procházková, L., Nedbalová, L., Andersen, R.A. & Valentin, K. (2020). Two new Kremastochrysopsis species, K. austriaca sp. nov. and K. americana sp. nov. (Chrysophyceae). Journal of Phycology, 56: 135–145.
- Ronquist, F., Teslenko, M., van der Mark, P., Ayres, D.L., Darling, A., Hohna, S., Larget, B., Liu, L., Suchard, M.A. & Huelsenbeck, J.P. (2012). MrBayes 3.2: efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61: 539–542.
- Sandgren, C.D. (1983). Morphological variability in populations of chrysophycean resting cysts. I. Genetic (interclonal) and encystment temperature effects on morphology. Journal of Phycology, 19: 64–70.
- Sandgren, C.D. (1991). Chrysophyte reproduction and resting cysts: a paleolimnologist’s primer. Journal of Paleolimnology, 5: 1–9.
- Schiller, J. (1926). Der thermische Einfluss und die Wirkung des Eises auf die planktischen Herbstvegetationen in den Altwässern der Donau bei Wien nach regelmäßiger Beobachtung von Oktober 1918 bis Ende 1925. Archiv für Hydrobiologie, 56: 1–62.
- Schneider, C.A., Rasband, W.S. & Eliceiri, K.W. (2012). NIH Image to ImageJ: 25 years of image analysis. Nature Methods, 9: 671–675.
- Scoble, J.M. & Cavalier-Smith, T. (2014). Scale evolution in Paraphysomonadida (Chrysophyceae): sequence phylogeny and revised taxonomy of Paraphysomonas, new genus Clathromonas, and 25 new species. European Journal of Protistology, 50: 551–592.
- Škaloud, P. & Rindi, F. (2013). Ecological differentiation of cryptic species within an asexual protist morphospecies: a case study of filamentous green alga Klebsormidium (Streptophyta). Journal of Eukaryotic Microbiology, 60: 350–362.
- Škaloud, P., Škaloudová, M., Procházková, A. & Němcová, Y. (2014). Morphological delineation and distribution patterns of four newly described species within the Synura petersenii species complex (Chrysophyceae, Stramenopiles). European Journal of Phycology, 49: 213–229.
- Škaloud, P., Škaloudová, M., Jadrná, I., Bestová, H., Pusztai, M., Kapustin, D. & Siver, P.A. (2020). Comparing morphological and molecular estimates of species diversity in the freshwater genus Synura (Stramenopiles): a model for understanding diversity of eukaryotic microorganisms. Journal of Phycology 56: 574–591.
- Škaloudová, M. & Škaloud, P. (2013). A new species of Chrysosphaerella (Chrysophyceae: Chromulinales), Chrysosphaerella rotundata sp. nov., from Finland. Phytotaxa, 130: 34–42.
- Skuja, H. (1948). Taxonomie des Phytoplanktons einiger Seen in Uppland, Schweden. Symbolae Botanicae Upsalienses, 9: 1–399.
- Skuja, H. (1956). Taxonomische und biologische Studien über das Phytoplankton schwedischer Binnengewässer. Nova Acta Regiae Societatis Scientiarum Upsaliensis, Series IV, 16: 1–404.
- Skvortzov B.W. (1958). New and rare flagellatae from Manchuria, Eastern Asia. The Philippine Journal of Science, 86: 139–202.
- Starmach, K. (1985). Chrysophyceae and Haptophyceae. In Süßwasserflora von Mitteleuropa, (Ettl, H., Gerloff, J., Heynig, H. & Mollenhauer, D., editors), Band 1, 1–322. Gustav Fischer Verlag, Stuttgart.
- Sun, Y., Cai, Y., Huse, S.M., Knight, R., Farmerie, W.G., Wang, X. & Mai, V. (2012). A large-scale benchmark study of existing algorithms for taxonomy-independent microbial community analysis. Briefings in bioinformatics, 13: 107–121.
- Swofford, D.L. (2002). PAUP*. Phylogenetic Analysis Using Parsimony (*and Other Methods) Version 4. 0 b10. Sinauer Associates, Sunderland, MA.
- Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. (2013). MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular Biology and Evolution, 30: 2725–2729.
- Troitzkaja, O.V. (1924). Zur Morphologie und Entwicklungsgeschichte von Uroglenopsis americana (Calkins) Lemmerm. Archiv für Protistenkunde, 49: 260–277.
- Wolfe, A.P. & Siver, P.A. (2013). A hypothesis linking chrysophyte microfossils to lake carbon dynamics on ecological and evolutionary time scales. Global and Planetary Change, 111: 189–198.
- Wujek, D.E. (1976). Ultrastructure of flagellated chrysophytes II. Uroglena and Uroglenopsis. Cytologia, 41: 665–670.
- Wujek, D.E. & Thompson, R.H. (2002). The genera Uroglena, Uroglenopsis, and Eusphaerella (Chrysophyceae). Phycologia, 41: 293–305.
- Zacharias, O. (1895). Über den Bau der Monaden und Familienstöcke von Uroglena volvox. Forschungsberichte aus der Biologischen Station zu Plön, 3: 353–356.
- Zwickl, D.J. (2006). Genetic algorithm approaches for the phylogenetic analysis of large biological sequence datasets under the maximum likelihood criterion. PhD dissertation, University of Texas at Austin, Austin.
