Abstract
Terrestrial microalgae evolved a variety of photoprotective strategies enabling a life on land. This includes the production of sunscreen compounds, which shield cells from excess radiation. Here, we report a new genus of conjugating green algae, Serritaenia gen. nov., whose members produce extracellular mucilage with a striking pigmentation. This phenomenon is very unusual for eukaryotic algae and poses cell biological and functional questions. So far, extracellular sunscreen pigments are exclusively known from cyanobacteria, while eukaryotic algae typically contain intracellular sunscreens. We demonstrate that pigmented mucilage in Serritaenia spp. can be induced by experimental exposure to UVB in an intensity-dependent manner, and that it strongly absorbs deleterious wavebands. Microscopic details of UVR-treated cells suggest that the directional secretion of pigmented mucilage is responsible for the defined and well-oriented pigment layers observed in natural material. Even though the chemical nature of the pigment remains to be elucidated, several pieces of evidence suggest that the ‘sunscreen mucilage’ of Serritaenia represents an elaborate photoprotective adaptation, unprecedented in eukaryotic algae.
Introduction
Microalgae from diverse evolutionary lineages have a terrestrial lifestyle and colonize natural as well as anthropogenic surfaces on land (Fritsch, Citation1922; Hoffmann, Citation1989; Rindi & Guiry, Citation2004; Karsten et al., Citation2007; Ettl & Gärtner, Citation2014). Compared with their aquatic relatives, terrestrial microalgae face increased levels of solar radiation, which is considered a major stress factor (Rozema et al., Citation2002; Wynn-Williams & Edwards, Citation2002; Karsten, Citation2008; Kitzing & Karsten, Citation2015). In particular, the ultraviolet radiation (UVR) of sunlight is harmful: ultraviolet A (UVA, 315–400 nm) causes the formation of free radicals and reactive oxygen species, while ultraviolet B (UVB, 280–315 nm) can damage DNA and proteins directly (Vincent & Neale, Citation2000; Pattison & Davies, Citation2006; Hargreaves et al., Citation2007). There are a number of cellular and physiological features of terrestrial microalgae that are expected to have a photoprotective function. This includes non-photochemical quenching, self-shading (exposed cell layers protect underlying cells), and the production of sunscreen compounds, which strongly absorb harmful wavebands (Karsten, Citation2008; Gao & Garcia-Pichel, Citation2011; Karsten & Holzinger, Citation2014). Algal sunscreen compounds are chemically diverse and can be found in eukaryotic as well as prokaryotic microalgae. They comprise mycosporine-like amino acids (MAAs, e.g. in the cytoplasm of chlorophytes, streptophytes, rhodophytes, dinoflagellates and cyanobacteria; Garcia-Pichel & Castenholz, Citation1993; Karsten et al., Citation1998; Jeffrey et al., Citation1999; Řezanka et al., Citation2004; Hotter et al., Citation2018; Hartmann et al., Citation2020), secondary carotenoids (e.g. in extraplastidic lipid droplets of chlorophytes; Bidigare et al., Citation1993; Remias & Lütz, Citation2007), and the non-photosynthetic pigments scytonemin and gloeocapsin in the extracellular mucilage of cyanobacteria (Garcia-Pichel & Castenholz, Citation1991; Storme et al., Citation2015). Whereas the colourless MAAs absorb only UVR, ‘sunscreen pigments’ such as carotenoids, scytonemin and gloeocapsin show broader absorbance spectra (including visible light) that lead to their colourful appearance. Some algal sun-screen pigments still require a chemical and physiological characterization, and it is not always certain that they represent a single compound (e.g. gloeocapsin).
Here, we report unicellular ‘conjugating green algae’ (Zygnematophyceae, Streptophyta), traditionally lumped in the polyphyletic genus Mesotaenium Nägeli, that can form mass developments in terrestrial habitats and display a striking pigmentation of their extracellular mucilage. The Zygnematophyceae are the likely sister clade to land plants (Wodniok et al., Citation2011; Timme et al., Citation2012; Wickett et al., Citation2014), and their adaptations to the terrestrial environment have gained much attention in recent years (Gitzendanner et al., Citation2018; de Vries et al., Citation2018; Cheng et al., Citation2019; Philippe et al., Citation2020). Although several species live on land, this group of algae seems to lack the well-known sunscreen compounds mentioned above (Remias et al., Citation2012a; Aigner et al., Citation2013). Instead, there are accounts of reddish pigments in the vacuoles of some representatives (Nedbalová & Sklenár, Citation2008; Holzinger et al., Citation2010; Remias et al., Citation2012a, Citationb; Garduño-Solórzano et al., Citation2020) and of colourless, phenolic compounds (Pichrtová et al., Citation2013; Holzinger et al., Citation2018), both of which absorb UVR and presumably have photoprotective roles.
The colourful, mucilaginous capsules studied here differ fundamentally from these intracellular compounds and are unusual for eukaryotic microalgae in general. Instead, a similar phenomenon is known from terrestrial cyanobacteria, which accumulate extracellular sheath pigments (e.g. scytonemin) in response to UVR (Garcia-Pichel & Castenholz, Citation1991; Garcia-Pichel et al., Citation1992; Ehling-Schulz et al., Citation1997). This resemblance found across two domains of life raises the question of whether the extracellular pigmentation of the Zygnematophyceae reported here serves as a sunscreen as well. So far, pigmented mucilage in Mesotaenium species was only sporadically reported (de Bary, Citation1858; Fučíková et al., Citation2008), and we lack any understanding of its formation and biological function.
During this study we sampled unicellular Zygnematophyceae with extracellular pigmentation from several sites in Europe and North America and explored their diversity with molecular and morphological methods. Based on these data, we introduce the new genus Serritaenia gen. nov. for this widespread and distinctive group of unicellular Zygnematophyceae, taking a step towards an unambiguous taxonomy of evolutionarily interesting green algae. Using bacteria-free laboratory cultures of Serritaenia established in this study, we successfully induced pigmented mucilage with artificial UVR, determined its spectral properties, and studied its formation. We present several pieces of evidence that the pigmented capsules of Serritaenia represent an elaborate photoprotective adaptation.
Materials and methods
Sampling, isolation and maintenance of algae
Algal material was collected at several sites in Western Germany and in the Great Smoky mountains (North Carolina, USA) listed in Supplementary table S1. Blackish crusts (dry) or gelatinous masses (wet) on bryophytes and from rock surfaces were transferred to the lab, if necessary rehydrated with distilled water and stored at 4–15°C in dim light (14/10 h light/dark cycle, PAR 3–15 µmol photons m–2 s–1). To determine pH values of substrates (plant litter, bryophytes) from our main study site (Wohlsberg, Wiehl, Germany), seven samples were mixed with 100 ml distilled water each, incubated for a few hours and then measured with a SevenEasy™ pH meter (Mettler-Toledo GmbH, Germany). Algal samples were photo-documented (for details see below), then diluted with distilled water and ultrasonicated on ice (max. 10 s) with the ultrasonicator XL-2000 (Misonix Inc., New York, USA) to liberate cells from the mucilage. Resulting cell suspensions were sprayed with pressurized air onto agar plates with solidified culture medium Waris-H (with 1.5% agar; McFadden & Melkonian, Citation1986) and incubated at 16°C and dim light until bacterial colonies appeared. Bacteria-free algal cells from these plates were transferred into liquid growth medium Waris-H and grown at 16°C under a 14/10 h light/dark cycle with a photon fluence rate of 30 µmol photons m–2 s–1 (PAR), giving rise to the clonal and axenic cultures used in this study. For long-term maintenance of cultures, about 2 ml of a running culture was transferred to fresh medium every 2 months. Algal strains are available through the corresponding author and the ‘Central Collection of Algal Cultures’ (CCAC; https://www.uni-due.de/biology/ccac/).
Light microscopy and confocal laser scanning microscopy (CLSM)
Regular brightfield microscopy and photo-documentation of experimental cultures were done with the Zeiss Axiovert 200M inverted microscope equipped with the Zeiss AxioCam ICc5 camera. For high-resolution imaging, the Zeiss IM35 inverted microscope equipped with the objective lenses Plan 40×/0.65 and Planapochromat 63×/1.4 (Carl Zeiss, DE), electronic flash, and the digital single lens reflex camera Canon EOS 6D were used. Colour balance and contrast of light micrographs were adjusted with Photoshop CS4 (Adobe Inc., California, USA). Confocal laser scanning microscopy was done with a Zeiss LSM 710 and the Zen software (Carl Zeiss, DE). Algal cells from actively growing cultures were collected by centrifugation (3000 g, 5 min), mixed with 0.1% Calcofluor White (CFW) in distilled water and used for conventional preparations sealed with Vaseline to prevent evaporation. The plastid morphology was visualized using chlorophyll autofluorescence (excitation: 458 nm; emission: 651–707 nm) and the algal cell wall by cellulose-bound Calcofluor White (excitation: 405 nm; emission: 410–502 nm). Z-stacks were recorded with a step size of 0.42 µm and processed with the software ZEN lite (Carl Zeiss, DE) and the image processing package Fiji (Schindelin et al., Citation2012).
DNA sequencing, alignment and molecular phylogenetics
Algal material from 2 ml of an axenic culture was collected by centrifugation (5000 g, 5 min), resuspended in sterile water, and lysed by ultrasonication on ice (5 × 5 s) with the ultrasonicator XL-2000 (Misonix Inc., New York, USA). Insoluble debris was pelleted by centrifugation (5000 g, 1 min) and the supernatant was used as template for PCR (details below). For the morphotype GSM.5.thick, which did not grow under our culture conditions, single colonies were isolated from the natural sample, photo-documented and crushed with a sterile coverslip to liberate DNA. The coverslip was then removed, the cell debris diluted with 10 µl sterile water, supplemented with 10 µl PCR-buffer (10×), heated at 95°C for 5 min and then used as template for PCR. The chloroplast encoded gene for the RuBisCO large subunit (rbcL) was amplified by a semi-nested PCR with the primers MaGo1F, MaGo2F and MaGo3R (Gontcharov et al., Citation2004) and Invitrogen Taq DNA Polymerase (Thermo Fisher Scientific, Massachusetts, USA), using the following protocol: Initial denaturation (3 min at 94°C), then 34 cycles of denaturation (45 s at 94°C), annealing (1 min at 47°C), and extension (2 min at 72°C), then final extension (5 min at 72°C). PCR products were subjected to commercial Sanger sequencing at the McGill University and Génome Québec Innovation Centre (Montreal, Canada) with the primers MaGo2F and MaGo3R. The rbcL gene sequences were assembled from the two overlapping partial reads using the program AlignIR™ 2.0 (LI-COR Biosciences, Nebraska, USA) and manually added to a comprehensive alignment of zygnematophycean sequences (plus sequences of streptophyte outgroup taxa) with the alignment editor SeaView 4.5.4 (Galtier et al., Citation1996; Gouy et al., Citation2010). The generated rbcL gene sequences have been deposited in GenBank under the accession numbers MW159369–MW159377 (see also Supplementary table S1). After pre-analyses with varying taxon sampling, a refined dataset with 43 zygnematophycean rbcL gene sequences (including all codon positions) was subjected to phylogenetic inference with Neighbour-joining (NJ), Maximum-parsimony (MP) and Maximum-likelihood (ML) methods using MEGA software version X (Kumar et al., Citation2018). NJ (distances computed with the Maximum composite likelihood method) and MP (with the Subtree-pruning-regrafting (SPR) algorithm) were done with the ‘complete deletion option’ resulting in 1033 remaining sites. The ML analysis was done with full alignment (1211 sites) using the GTR+I+G model (discrete Gamma distribution; 5 categories). To assess support of the branches, we performed 1000 bootstrap repetitions for every analysis and added the resulting values to the NJ topology shown in the results.
UV-PAR exposure experiments
Algae used for UV-PAR exposure experiments (strains GSM.5.thin and OBE.1) were grown under ‘PAR only’ conditions as detailed above to a sufficient density, mixed with fresh culture medium Waris-H (ratio 1:1), dispensed in six-well multi-titre plates or 60 mm Petri dishes (both Fischer, Germany), and then exposed to the experimental conditions with varying UV-PAR intensities (14/10 h light/dark cycle) but stable temperature of 16°C (in triplicate). During these experiments treated algae were analysed with respect to extracellular pigmentation, presence/absence of mucilage, colony formation, and the number of dead cells. To test for the effects of different UV-PAR intensities on the algae, cells were exposed to the Arcadia D3+ Reptile Lamp T5 with 12% UVB (Arcadia, UK) in five irradiance settings adjusted by distance to the lamp (see Results for details), and observed and photo-documented over 14 days (7 days for replicates). The effect of specific wavebands on the production of extracellular pigment was assessed with algal material exposed to optimal UV-PAR intensities for pigment production (as determined before) but covered by optical filters that block UVB (longpass filter WG-320, Schott, Germany) or total UVR (longpass filter GG-385, Schott, Germany; 6 mm polycarbonate sheets Makrolon®, Covestro AG, Germany). Transmission spectra of used filters and consumables were recorded with the Epoch Microplate Spectrophotometer (BioTek Instruments Inc., Vermont, USA) and are shown in the results. In two further experiments, cells of strain GSM.5.thin were exposed to UVB from (1) the UVB Broadband TL fluorescent tube lamp, and (2) the UVB Narrowband TL fluorescent tube lamp, respectively (both 20W, Philips, the Netherlands). The UVB treatments of 0.8–3 W m–2 were for 4 h per day and supplemented with 30–100 µmol photons m–2 s–1 PAR (14 h per day from SunLike LEDs, 5000 K, Seoul Semiconductor, Korea). To study the effects of high ‘PAR only’ intensities on the algae, cells were exposed to a 25 W SunLike high-power LED (5000 K; Seoul Semiconductor, Korea) with photon fluence rates of 200, 300, 400, 600, 700 and 1000 µmol photons m–2 s–1 and a 14/10 h light/dark cycle and observed for 7 days. PAR intensities were measured with the MQ-500 Full-Spectrum Quantum Sensor (Apogee Instruments Inc., Utah, USA), UVA and UVB intensities with the digital UV radiometers Solarmeter® Model 4.2 and Solarmeter® Model 6.2, respectively (both Solar Light Company Inc., Pennsylvania, USA).
Microspectrophotometry
The absorbance spectra of pigmented and non-pigmented mucilage of S. testaceovaginata (strain GSM.5.thin) from the light experiments detailed above (UV-PAR and PAR only) were recorded with a CRAIC QDI 2010 UV-VIS-NIR Microspectrophotometer (CRAIC Technologies Inc., California, USA) in transmission mode at the NanoScale Fabrication and Characterization Facility at the University of Pittsburgh, USA. Algal cells were analysed in conventional wet mounts with UV-transparent quartz glass coverslips and microscope slides (both Ted Pella Inc., California, USA). Using a 36× mirror objective (numerical aperture 0.5), the absorbance of defined areas (15 × 15 µm) was sampled over 200–1600 nm in sub-nanometer intervals. We made 54 measurements of pigmented mucilage and 15 measurements of non-pigmented mucilage. Each measurement contained 25 averaged sample scans. The absorbance spectra obtained from the regions outside of the colonies (only culture medium) served as reference. Digital micrographs of the analysed specimens documenting the sampling area were taken with the DFK 41AF02 colour industrial camera (The Imaging Source Europe GmbH, Germany).
Pigment extraction attempts
Cells of S. testaceovaginata (strain GSM.5.thin) with strongly pigmented mucilage (induced under UVR as detailed above) were concentrated by centrifugation (3000 g, 8 min), washed with distilled water, collected by centrifugation (10 000 g, 5 min), frozen in liquid nitrogen, and lyophilized with the Christ Alpha 1-4 LSC freeze-dryer (Christ, Germany). Freeze-dried cells were suspended in acetone, methanol, acidic methanol (with 0.5% concentrated HCl = 0.05 N), diethyl ether, trichloromethane, n-hexane, respectively, and incubated at room temperature for at least 72 h. Cells and their mucilage were then examined for pigment loss under the Motic AE2000 inverted microscope (Motic Deutschland GmbH, Germany).
Results and Discussion
Serritaenia species with pigmented mucilage colonize various terrestrial substrates
In several forests of Western Germany (listed in Supplementary table S1) we observed macroscopic, gelatinous crusts composed of unicellular conjugating green algae, which resembled Mesotaenium braunii de Bary (and similar species) and are here described as Serritaenia spp. (taxonomic details below). The forests comprise spruce monocultures (Picea) and deciduous trees (e.g. Fagus, Quercus, Betula), and harbour a prominent bryophyte flora () due to the warm ‘oceanic’ climate (Cfb after Köppen-Geiger) and significant rainfall (www.climate-data.org). At several sites in the studied areas, the algae formed mass developments, which appeared as dry, black crusts during summer, covering decomposing tree logs, tree bark, plant litter and bryophytes (). Pleurocarpous and acrocarpous mosses, and leafy liverworts have been found heavily colonized by the algae, and, in some instances, entire plants appeared black due to the algal biofilm (). We assume that such algal mass developments have unrecognized, detrimental effects on bryophytes, similar to those of well-known fungal infections (Fenton, Citation1983; Davey & Currah, Citation2006; Tamura et al., Citation2019; Rosa et al., Citation2020).
Figs 1–9. Habitat of Serritaenia species in western Germany, macroscopic appearance and microscopic details of natural material. Fig. 1. Exposed slope in spruce monoculture (Wiehl, DE) with black crusts formed by Serritaenia sp. (dashed square). Fig. 2. Close-up of dry crusts covering dead plant litter and bryophytes. Fig. 3. Pleurocarpous and acrocarpous mosses covered by black algal crusts. Fig. 4. Hydrated algal colonies (dashed square) on the leaflets of Polytrichum formosum. Fig. 5. Serritaenia colonies with brown pigmentation (arrows) next to other gelatinous green algae (Chlorophyta) on a leaflet of Polytrichum formosum. Figs. 6, 7. Serritaenia cells with extracellular pigmentation of different colour (arrowheads) found in the same sample (Wiehl, DE). Fig. 8. Serritaenia sp. (Bad Kreuznach, DE) showing intense, unilateral pigmentation of the mucilaginous capsules (arrowhead). Fig. 9. Small-celled Serritaenia species (asterisk) co-occurring with a large-celled species (Wiehl, DE). Both species exhibit zones of brown mucilage (arrowheads). Scale bars: 10 µm.

As revealed by hydrated material, the crusts were either formed by homogeneous populations of Serritaenia species, or by more complex assemblages comprising other gelatinous algae as well (e.g. chlorophytes resembling Coccomyxa). Even if macroscopic crusts were absent, seemingly unaffected bryophyte plants were frequently found colonized by such algae, e.g. in the form of microscopic colonies on the leaflets (). These colonies represented nearly spherical, gelatinous clusters of cells, each cell bounded by a mucilaginous capsule. As indicated by the hierarchical mucilage layers surrounding subgroups of cells, the Serritaenia colonies emerged from serial cell divisions and secretion of copious mucilage. In algae from all study sites, we observed a striking pigmentation of extracellular mucilage, ranging from blackish-violet to red-brown (). The strength of pigmentation varied from a diffuse tint to intensely pigmented zones. Interestingly, the pigment distribution was not homogeneous, and we frequently observed a prominent, unilateral pigmentation of the mucilaginous capsules (). Furthermore, a single natural sample could contain algal mucilage of different colour () as well as pigmented cells of different sizes (), indicating a yet unrecognized diversity of Zygnematophyceae with extracellular pigmentation.
We also sampled the type locality of Mesotaenium testaceovaginatum, a species reported to have brick-red mucilage (Fučíková et al., Citation2008). This alga was described from the ‘wet walls’ in the Great Smoky Mountains National Park (North Carolina, USA), a vertical, exposed rock surface with acidic water, harbouring a diversity of prokaryotic and eukaryotic microalgae (; Furey et al., Citation2007; Lowe et al., Citation2007). We found red-brown biofilm (), which, surprisingly, contained two Mesotaenium-like algae with reddish mucilage (morphotypes ‘GSM.5.thin’ and ‘GSM.5.thick’; here assigned to Serritaenia). Both formed irregular, mucilaginous colonies, but differed consistently in cell diameter (15 vs. 20 µm) and the strength of extracellular pigmentation (). There might be physiological differences as well, as we were not able to cultivate GSM.5.thick (several attempts), while GSM.5.thin grew well under our culture conditions. The original description of M. testaceovaginatum was probably based on cells of both morphotypes (see Supplementary text for details) and requires an emendation (see below).
Figs 10–13. ‘Wet walls’ near Clingmans Dome in the Great Smoky Mountains National Park (North Carolina, USA) and the two Serritaenia morphotypes found in this habitat. Fig. 10. Sampling area at ‘wet walls’ (left side). Fig.11. Red-brown biofilm on rock surface containing various microalgae, including two Serritaenia morphotypes. Fig. 12. Cells of morphotype GSM.5.thin loosely arranged in copious, reddish mucilage. Fig. 13. Colony of morphotype GSM.5.thick with well-defined, intensely pigmented capsules. Note the difference in cell width to cells shown in (12). Scale bars: 20 µm.

In fact, all species of the ill-defined genus Mesotaenium that match the studied algae were described on a purely morphological basis (Kützing, Citation1845; de Bary, Citation1858; Fučíková et al., Citation2008) (see Supplementary text for details). Hence, there is no information about the true diversity of these microalgae and their relationships. Specifically, the variation observed in natural populations (cell size, mucilage colour) poses the question of whether the different colours found in the extracellular mucilage of the studied algae are caused by species-specific compounds, or are a result of varying environmental conditions.
Serritaenia comprises genetically diverse microalgae with subtle morphological differences
We subjected natural material and cultivated Serritaenia strains to genetic and morphological analyses (listed in Supplementary table S1). The rbcL gene sequences generated from nine cultures and three individual colonies of the Serritaenia morphotype GSM.5.thick (picked from samples) were analysed in the framework of the Zygnematophyceae with phylogenetic methods. As known from previous studies (McCourt et al., Citation2000; Gontcharov et al., Citation2004), the molecular phylogeny based on the rbcL gene was not able to resolve the deeper branching patterns within the conjugating green algae but resolved genus-level clades. We recovered six distinct lineages with unicellular Zygnematophyceae currently assigned to the genus Mesotaenium (Supplementary fig. S1), exemplifying once more that the structurally simple, yet diverse ‘saccoderm desmids’ (traditionally Mesotaeniaceae) are still heavily under-studied (Gontcharov et al., Citation2004; Gontcharov & Melkonian, Citation2010). However, these phylogenetically diverse life forms show clear cell morphological differences (Gontcharov, Citation2008) and, recently, gained importance for evolutionary inferences (Bonnot et al., Citation2019; Cheng et al., Citation2019; Liang et al., Citation2019; Xu et al., Citation2019; Philippe et al., Citation2020), so that a taxonomic revision of these algae is desirable.
The Serritaenia strains formed a well-supported clade with considerable genetic distance from other Zygne-matophyceae (). It was most closely related to strain SAG 12.97 that was previously referred to as ‘Mesotaenium endlicherianum’ (Gontcharov et al., Citation2003, Citation2004; Gontcharov, Citation2008; Matasci et al., Citation2014; Cheng et al., Citation2019), but does not meet the original description of that species (already noticed by Gontcharov et al., Citation2003). The Serritaenia clade displayed a so far undetected genetic diversity comprising eight genotypes falling into three subclades (). The members of different subclades showed a divergence of 2–7% in the rbcL gene, which compares to interspecific distances found in many other algal and embryophyte genera (Chase et al., Citation2005; Newmaster et al., Citation2006; Hall et al., Citation2010). The three sequences derived from individual colonies of Serritaenia morphotype GSM.5.thick (natural material) were identical, but differed clearly from that of strain GSM.5.thin (in 13 nucleotides). This aligns well with the observed phenotypic and physiological differences between the two Serritaenia morphotypes, and suggests that they represent different biological entities.
Figs 14–18. Phylogeny of the genus Serritaenia and cellular details of representative strains. Fig. 14. Unrooted Neighbour-joining phylogeny of 43 zygnematophycean rbcL gene sequences (partially collapsed) displaying the genetic diversity of the genus Serritaenia. Support values are shown on the respective branches (NJ/ML/MP) when > 50%, and branches with maximum support (100/100/100) are bold. The scale bar represents 0.02 nucleotide substitution per site and branches marked with ‘//’ were reduced in length to 40%. The cell width of the strains is shown on the right side of the diagram (purple dots = mean, purple lines = size range; n = 20). The asterisk indicates the sequence of the type species. Fig. 15. Cell morphology of six Serritaenia strains illustrating the variability of the genus. Fig. 16. Large-celled Serritaenia species (strain DEL.1) in side view (left, focal series), top view (two top right micrographs) and shortly after cell division (bottom right). Fig. 17. Cells of a small-celled representative (strain OBE.sm2) in side view. Bottom micrograph displays cells shortly after division. Fig. 18. CLSM data of a large-celled Serritaenia strain (OBE.1) revealing the complexity of the chloroplast and its serrated edges (arrowhead). The middle image displays a section through the cell in two channels (orange = chlorophyll autofluorescence; cyan = Calcofluor White fluorescence), the bottom image shows a 3D-model of the chloroplast. Scale bars in micrographs: 10 µm.
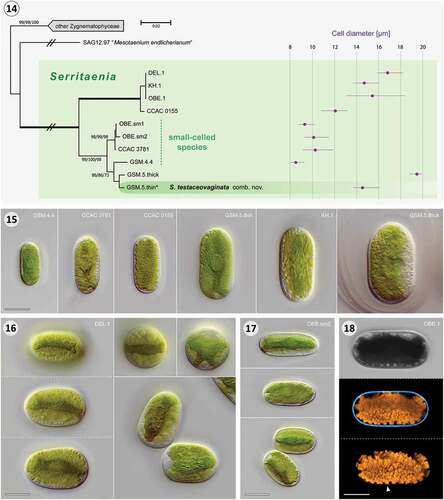
In terms of morphology, the studied Serritaenia strains displayed a set of common cellular details, which – in combination – are characteristic for these algae. During interphase, cells of all strains were cylindrical, i.e. with parallel sides, and exhibited roundish (not truncated) cell poles (). Each cell contained a single plate-like chloroplast situated in the cell’s centre, not parietal (). The chloroplast extended for the length of the entire cell and, depending on its orientation to the observer, could create two typical appearances, ‘top view’ and ‘profile view’ (compare the top two cells in ). Under laboratory conditions, in particular, the chloroplasts of all studied strains frequently exhibited one or more additional ridges, giving the chloroplast a more complex morphology (). Serritaenia chloroplasts were always characterized by serrate or crenate edges () and a single circular or elliptical pyrenoid in the chloroplast centre (). Each cell contained a rather inconspicuous nucleus with a central nucleolus, displaced towards the cell wall, while the chloroplast occupied the cell’s centre (). Furthermore, the somewhat asymmetric daughter cells resulting from cell division showed an ‘angled’ arrangement () that differed from the patterns found in many other unicellular Zygnematophyceae (including strain SAG 12.97). Taken together, the studied algae differ fundamentally from the type species of the genus Mesotaenium (M. endlicherianum Nägeli, see Supplementary text for details), and form a morphologically coherent clade, well-separated from other saccoderm desmids.
To assess the phenotypic diversity within the Serritaenia clade, we performed a comparative morphological analysis with cells of axenic cultures grown under controlled abiotic conditions (16°C, medium Waris-H, 30 µmol photons m–2 s–1 PAR). In fact, there are reports of phenotypic plasticity in some unicellular Zygnematophyceae (Brook, Citation1981; Neustupa et al., Citation2008; Černá & Neustupa, Citation2010), and we also found some variability concerning the presence of additional chloroplast ridges and the abundance of colourless globules in Serritaenia, especially in natural material. The cell width, however, was rather constant within strains, and turned out to be a clear distinguishing character for some Serritaenia genotypes recognized in our rbcL phylogeny (). This is in line with recent findings about cell width stability in genotypes of the structurally similar saccoderm desmid Cylindrocystis (Barcytė et al., Citation2020). Interestingly, the strains DEL.1 and KH.1 with identical rbcL gene sequences showed marked differences in cell width as well (), which might point to some hidden diversity, not resolved by the rbcL gene. Delimitation of biological entities in the Zygnematophyceae remains difficult and integrative species-level taxonomy is still in an early stage (Kouwets, Citation2008; Neustupa et al., Citation2011; Stastny et al., Citation2013; Schagerl & Zwirn, Citation2015). We conclude that a fine-grained taxonomy of Serritaenia species should be ideally based on more variable genetic markers, and on extended sampling in nature. Hence, we refrain from introducing new species at this point, but establish new combinations for former Mesotaenium species: Serritaenia braunii comb. nov. and Serritaenia testaceovaginata comb. nov. (see below). S. testaceovaginata is defined as the type species of the new genus and assigned to a specific genotype (strain GSM.5.thin; ). This genotype was sampled at the type locality of M. testaceovaginatum and closely matches the original description of the latter (see Supplementary text for details). As the other members of the Serritaenia clade are genetically more diverse than expected, we postpone the decision about the reference strain for S. braunii until original material of M. braunii de Bary or new samples from its type locality are analysed. Due to the excellent description of M. braunii by de Bary (Citation1858) there is no doubt that this species belongs to Serritaenia (compare Supplementary fig. S2 and our micrographs), warranting a new combination. Contrary to the information found in several monographs (e.g. Lenzenweger, Citation2003; Coesel & Meesters, Citation2007; Brook & Williamson, Citation2010; Ettl & Gärtner, Citation2014), M. braunii should not be treated as a heterotypic synonym of Mesotaenium macrococcum (first described as Palmogloea macrococca Kütz.). Unpublished observations on the holotype of P. macrococca (Germany: Oberharz, nearby Auerhahn, 1845, coll. Kütz., L.3940277 (L)) revealed that this species more resembles M. braunii var. minus de Bary in size. However, an in-depth (ideally genetic) analysis of respective type material is required before these names can be considered for certain Serritaenia strains. This also applies to Palmogloea macrococca var. nigrescens C.Cramer, while M. macrococcum var. lagerheimii Willi Krieg. and M. macrococcum var. truncatum (West & G.S.West) Willi Krieg. can be excluded due to gross morphological differences from Serritaenia (see Supplementary text for further details on relevant taxa). Based on our taxonomic assessment and the phylogenetic results, we here make a start on revising the genus-level taxonomy of Mesotaenium-like algae and introduce the genus Serritaenia gen. nov. with two new combinations.
Serritaenia A.Busch & S.Hess, gen. nov.
Description
Cells cylindrical, with rounded or slightly pointed apices and smooth cell wall. Chloroplast axial, extending for entire length of cell, plate-like or more complex due to additional ridges, typically with serrate, dentate or crenate edges, exhibiting a single, central pyrenoid. Nucleus displaced towards cell wall (never central), with distinct nucleolus. Cytoplasm colourless, with varying numbers of opaque globules, sometimes obscuring chloroplast details. Cells form colonies with confluent mucilage or well-defined, sometimes lamellated capsules. Mucilage colourless or pigmented (reddish, brown, blackish or violet). Arrangement of daughter cells shortly after cell division angled, never chain-like.
TYPE (here designated): S. testaceovaginata (Fučíková et al.) A.Busch & S.Hess, comb. nov.
ETYMOLOGY: The generic name Serritaenia is derived from Latin serra, -ae, f. [saw] and taenia, -ae f. [band], referring to the chloroplast morphology.
PhycoBank ID: http://phycobank.org/102647.
Serritaenia testaceovaginata (Fučíková et al.) A.Busch & S.Hess, comb. nov.
≡ Mesotaenium testaceovaginatum Fučíková, J.D.Hall, J.R.Johans & R.L.Lowe. Bibliotheca Phycologica, 113: 31, Pl. VI, figs 6, 26–28. Citation2008 (basionym).
Emended Description: Cells with characters of the genus, on average about 14–15 µm wide, 18–30 µm long. Nucleus 5–7 µm, nucleolus about 2 µm. Pyrenoid circular to slightly elliptic, 4–5 × 3–4 µm.
LECTOTYPE (here designated): [icon!] Fučíková et al., Bibliotheca Phycologica, 113: pl. VI, fig. 6. Citation2008; oml trf. (reproduced in Supplementary fig. S3).
EPITYPE (here designated): Permanent slide with fixed material of strain GSM.5.thin deposited in Herbarium Berolinense (Botanic Garden and Botanical Museum Berlin), accession B 40 0001077, locality: ‘Wet walls’ on the way to Clingmans Dome, Great Smoky Mountains, North Carolina, USA; 13 September 2017, leg. A. Busch and S. Hess.
Notes: The holotype (fixed sample) was lost and likely contained more than one taxon. We select a here cited illustration, as part of the original material published along with the original description as lectotype. In addition, we designate an epitype that supports the lectotype and is associated with DNA sequence data.
REFERENCE SEQUENCE: MW159377 (rbcL gene sequence of strain GSM.5.thin).
PhycoBank ID: http://phycobank.org/102650.
Serritaenia braunii (de Bary) A.Busch & S.Hess, comb. nov.
≡ Mesotaenium braunii de Bary, Unters. Conjugaten: 74. 1858 (basionym).
TYPE: Schwarzwald (Black Forest, Germany)
PhycoBank Id: http://phycobank.org/102648.
UVB induces extracellular pigmentation in Serritaenia
All Serritaenia strains lost their extracellular pigmentation under our standard culturing conditions with PAR at about 30 µmol photons m−2 s−1. Treatment of our experimental strain GSM.5.thin (S. testaceovaginata) with the SunLike high-power LED (5000 K, 25 W, see for spectrum) at 200, 300, 400, 600, 700, and 1000 µmol photons m–2 s–1 for 7 days, still resulted in healthy, dividing cells but no extracellular pigmentation. Exposure of the algae to the Arcadia D3+ fluorescent tube lamp, which emits PAR, UVA and UVB (see for spectrum), caused clear cellular reactions depending on the five irradiance settings used (). Over 14 days under settings 1–3, cells remained in colonies, showed growth, and formed purple mucilage, which was already visible 3–5 days after starting the experiment. The extracellular pigmentation correlated with the applied irradiance and was especially pronounced under settings 2 and 3 (). Algal colonies treated with higher intensities (settings 4 and 5) disintegrated and the cells bleached, demonstrating the deleterious effect of the applied UVR (this never happened with PAR only). After 14 days of exposure, more than 40% of the cells were dead and bleached under setting 4, more than 95% under setting 5.
Figs 19–22. Effect of irradiance and light quality on the production of extracellular pigmentation in Serritaenia testaceovaginata (strain GSM.5.thin). Fig. 19. Relative spectral power distribution of the Arcadia D3+ fluorescent tube lamp (violet) and the SunLike LED (green), and relative transmittance of the polystyrene lids of the used multiwell plates (dashed blue line). Fig. 20. Brightfield images of Serritaenia cells after 14 days of exposure to the Arcadia D3+ lamp under five different irradiance settings (1–5). Algal colonies under settings 2 and 3 exhibit marked extracellular pigmentation. Fig. 21. Relative transmittance of the applied longpass filters (lines), and relative spectral power distribution of the used lamp (light grey). Fig. 22. Brightfield images of Serritaenia cells after 7 days of exposure to the Arcadia D3+ lamp (setting 3 in (20)), but covered by different longpass filters. A control sample without longpass filter is shown as well (‘No filter’). Scale bars: 100 µm.
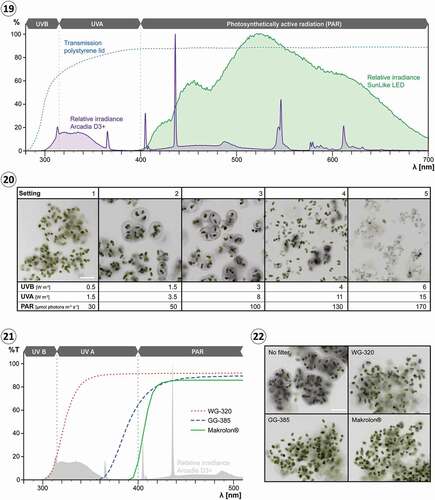
To identify the waveband of the Arcadia D3+ fluorescent tube lamp which induces pigment formation, we used longpass filters that block UVB and total UVR, respectively (see for transmission spectra). Colonies of S. testaceovaginata (strain GSM.5.thin) exposed to conditions optimal for pigment production (setting 3) but covered by filters blocking total UVR (SCHOTT GG-385, Makrolon® polycarbonate) did not show any sign of extracellular pigmentation after 7 days, while the control (without filter) contained colonies with strongly pigmented mucilage (). Algal colonies covered by a filter that reduces the UVB irradiance to 0–20% (SCHOTT WG-320) resulted only in very few slightly pigmented colonies; the vast majority of colonies still displayed colourless mucilage (). We then treated the same algal strain with the Philips UVB ‘Broadband’ and ‘Narrowband’ fluorescent tube lamps at different intensities (see Rizzini et al., Citation2011 for spectra), and confirmed the pigment-inducing effect of UVB in our experiments. In the case of both light sources, pigmentation was clearly visible in a range of 1.5–3 W m−2 UVB after 2 weeks of treatment.
We have to acknowledge that light experiments in the laboratory are highly artificial, since the spectral power distribution of the used lamps clearly differs from sunlight. Therefore, it cannot be excluded that other wavebands at higher intensities, or light with other spectral ratios can trigger similar reactions in the algae. However, UVB is already known to induce the production of screening compounds, especially MAAs, in diverse microalgae (Ehling-Schulz et al., Citation1997; Portwich & Garcia-Pichel, Citation2000; Sinha et al., Citation2001, Citation2003a, Citationb; Gröniger & Häder, Citation2002; Klisch & Häder, Citation2002), and it is probably of special relevance for Serritaenia as well. There are indications of UVB-photoreceptors in cyanobacteria and eukaryotic algae, but the molecular basis of UV perception in these life forms remains largely unknown (Portwich & Garcia-Pichel, Citation2000; Kräbs et al., Citation2002; Singh et al., Citation2010). In land plants and chlorophyte green algae, UVB is perceived by the UVR8 photoreceptor and induces photoprotective mechanisms (Rizzini et al., Citation2011; Allorent et al., Citation2016; Clayton et al., Citation2018). Homologues of UVR8 were also identified in transcriptome data of several Zygnematophyceae including Serritaenia sp. (strain CCAC 0155, formerly referred to as ‘Mesotaenium braunii’), but not yet functionally characterized (Han et al., Citation2019). It thus remains an open question whether the induction of Serritaenia’s extracellular pigment is based on such a photoreceptor or on other effects of UVB (e.g. cell damage). In any case, Serritaenia with its pronounced reaction to short-wavelength UVR might be a valuable laboratory model for future experimentation.
Pigmented mucilage absorbs deleterious radiation and can change colour
So far, we did not succeed in extracting the pigment from the mucilage using a number of solvents and acidic hydrolysis (see Methods for details), which is not unusual considering the diversity of difficult-to-dissolve, wall-bound pigments in plants, especially bryophytes (Mårtensson & Nilsson, Citation1974; Hooijmaijers & Gould, Citation2007). Hence, we used microspectrophotometry to determine the absorbance spectra of pigmented mucilage in vivo, enabling a direct assessment of the shielding effect. Transmission data of pigmented mucilage from S. testaceovaginata (strain GSM.5.thin) revealed absorbance over the entire UV-PAR spectrum (n = 54) compared with non-pigmented mucilage (n = 15; ). As illustrated by three representative measurements (sample 1–3), the absorbance spectra showed a consistent pattern with two maxima at ~300 nm and ~580 nm (; see Supplementary figs S2–S4 for all spectra recorded). The absolute maximum was always at 290–320 nm, corresponding to UVB and far UVA, the most harmful wavebands of terrestrial sunlight. In this spectral range the pigmented mucilage from experimental cultures blocked up to 60% of incoming radiation, representing UVB-screening factors comparable to those estimated for other algal sunscreens, e.g. scytonemin and MAAs of cyanobacteria (Garcia-Pichel & Castenholz, Citation1993). Differences in overall absorbance between the individual sampling spots clearly correlated with the degree of pigmentation visible in the corresponding light micrographs (). At this point, we cannot exclude the possibility that Serritaenia secretes more than one compound into the mucilage and that the observed spectra are a sum thereof. Some cyanobacteria of the genus Nostoc, for example, accumulate two complementary sunscreens (scytonemin and oligosaccharide-linked MAAs) in their extracellular glycan sheath, thereby extending the spectral range of photoprotection (Böhm et al., Citation1995; Ferroni et al., Citation2010). The observed correlation of UVR absorbance and visible pigmentation (also apparent from the spectral curves), however, lets us assume that the intensely coloured capsules of Serritaenia cells found in nature provide an effective broadband-screening including the UV waveband.
Figs 23–25. UV-VIS absorbance of pigmented and non-pigmented mucilage of Serritaenia testaceovaginata (strain GSM.5.thin), microscopic appearance of the sampled spots, and pH-dependent colour changes of pigmented mucilage. Fig. 23. Absorbance spectra of pigmented mucilage (violet lines; samples 1–3) and non-pigmented mucilage (green lines, 15 samples) determined by microspectrophotometry. Fig. 24. Brightfield micrographs displaying the spots (dashed squares, 15×15 µm) analysed for samples 1–3 shown in (23) as well as a representative sampling spot in non-pigmented mucilage. Fig. 25. Colour of pigmented mucilage of S. testaceovaginata (strain GSM.5.thin) at different pH values. Scale bar: 20 µm.
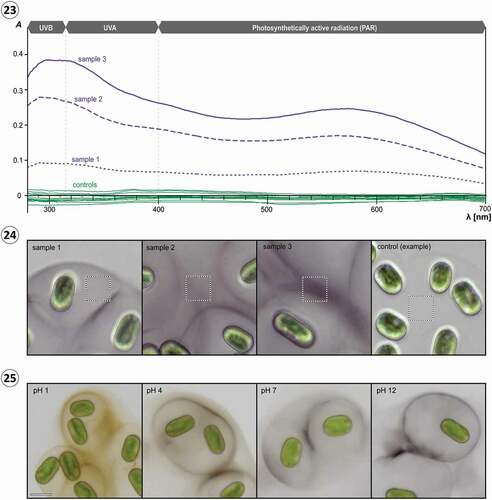
Interestingly, the pigmented mucilage of S. testaceovaginata (strain GSM.5.thin) formed under experimental conditions always showed a violet-blue colour, while this species was found with reddish mucilage in nature (compare ). Experimental changes of the pH value of the medium resulted in reversible colour changes of the pigmented mucilage, from violet-blue under alkaline and neutral conditions to black and reddish-brown under acidic conditions (). These colour changes can explain the reddish-brown tones frequently observed in mucilage from natural material. Both the ‘wet walls’ in the Great Smoky Mountains as well as the substrates (bryophytes, plant litter) at our main study site (Wohlsberg, Wiehl, Germany) showed acidic reactions, pH 4.4 (Furey et al., Citation2007) and pH 4.3–4.8 (n = 7), respectively. We assume that different Serritaenia species produce the same compound, which can show different colours depending on the chemical conditions of the substrate. Consequently, mucilage colour seems to be a trait of poor taxonomic value (which applies to gloeocapsin-containing cyanobacteria as well) and should not be used for species descriptions as done for Mesotaenium testaceovaginatum (Fučíková et al., Citation2008).
How do the pigmented capsules form?
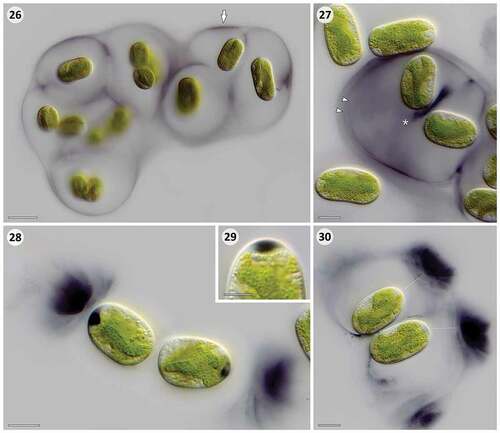
With very few exceptions such as the MAAs deposited in the silica frustules of planktonic diatoms (Ingalls et al., Citation2010), most (putative) sunscreen compounds from eukaryotic microalgae have an intracellular localization. They can accumulate in the cytoplasm (e.g. MAAs; Garcia-Pichel & Castenholz, Citation1993), in vacuoles (e.g. purpurogallin-derivatives; Remias et al., Citation2012a), or in lipid droplets (e.g. carotenoids; Bidigare et al., Citation1993). Since some of these substances might be involved in physiological processes as well (carotenoids, MAAs), their primary role as sunscreen is not always established. The Serritaenia pigment, instead, is often found in distant layers of extracellular mucilage, which is an ideal localization for effective shielding of the entire cell, including the cell periphery and plasma membrane. Furthermore, Serritaenia capsules from nature showed a striking unilateral pigmentation (), suggesting a directed (‘economic’) deposition of a sunscreen. Based on our microscopic data from experimentally treated cells, we can infer some aspects of the formation of pigmented capsules by Serritaenia. Two strains from distinct subclades, GSM.5.thin and OBE.1, produced pigmented mucilage upon exposure to moderate UV-PAR intensities (setting 3, ), but showed different patterns of mucilage secretion. In strain GSM.5.thin the outermost regions of the mucilaginous capsules exhibited an intense pigmentation, often forming a thin, discrete pigment layer (). The pigmentation in these layers was not always evenly distributed and occasionally concentrated in zones of increased density (, arrow), reminescent of the unilateral pigmentation observed in natural material. Strain OBE.1 frequently produced several nested pigmented layers within the capsules (, arrowheads) as well as pigmentation at the site of cell division and between recently divided cells (, asterisk). Furthermore, cells of this strain exhibited dark, lens-like inclusions at the cell poles, situated between the plasma membrane and the cell wall (). These inclusions were often associated with confined zones of intensely pigmented mucilage located well outside the cells (). In some instances, it became clear that these pigmented zones are in fact part of otherwise colourless or faintly pigmented, extracellular capsules (). We assume that the lens-like inclusions correspond to secreted mucilage, which normally travels through the cellulosic mesh work of the wall, but – under the experimental conditions – accumulated underneath the wall (maybe due to a sudden overreaction of the cell). It is well known from other Zygnematophyceae (e.g. Closterium, Micrasterias, Netrium, Penium) that gel-like exopolymers such as pectic substances are secreted through the existing cell wall after fusion of secretory vesicles with the plasma membrane (Oertel et al., Citation2004; Eder & Lütz-Meindl, Citation2010; Domozych et al., Citation2014). We conclude that the colourful capsules of Serritaenia are likely formed by the secretion of pigmented exopolymers, and not by the release of pigments into existing capsules. This idea is supported by the radial pigment gradients and hierarchical pigment layers often found in natural material. These layers can be easily explained by the initial deposition of pigmented mucilage followed by the secretion of (less-pigmented) mucilage that pushes the pigmented layer apart from the cell. Furthermore, the pigmented inclusions found at the cell poles in experimental material demonstrate that the secretion of pigmented mucilage can be local. We therefore assume that Serritaenia cells in the natural habitat (i.e. when the colonies are stuck to a substrate in a fixed position) are able to form well-oriented ‘sunshades’ by the directed secretion of pigmented mucilage.
Figs 26–30. Microscopic details of extracellular pigmentation in Serritaenia species induced under setting 3 for 14 days. Fig. 26. Colony of S. testaceovaginata (strain GSM.5.thin) with pigmentation in outermost layer of the mucilaginous capsules (arrow). Fig. 27. Cells of Serritaenia sp. (strain OBE.1) exhibiting several pigment layers (arrowheads) and a pigment accumulation between recently divided cells (asterisk). Fig. 28. Dark, polar inclusions in the cells of Serritaenia sp. (strain OBE.1) associated with zones of intensely pigmented mucilage. Fig. 29. Detail of lens-like pigment inclusion at the cell pole of Serritaenia sp. (strain OBE.1). Fig. 30. Two cells of Serritaenia sp. (strain OBE.1) surrounded by mucilaginous capsules with intensely pigmented zones. The assumed trajectory of secretion is indicated by dashed lines. Scale bars: Fig. 26, 20 µm; Figs 27, 28, 30, 10 µm; Fig. 29, 5 µm.
Several pieces of evidence support a role as a sunscreen
Sunscreen compounds of microbes and plants are expected to meet a number of criteria which are relevant to their function (Cockell & Knowland, Citation1999; Gao & Garcia-Pichel, Citation2011). This includes (1) a sensible (cellular) localization for effective shielding of sensitive structures, (2) the effective absorption of deleterious radiation, especially UVR and (3) the synthesis of the compound in response to elevated levels of deleterious radiation, or during life history stages which typically experience such conditions. Our microscopic and experimental data demonstrate that these criteria are met by the pigmented capsules of Serritaenia species. Ideally, there is also experimental evidence for resistance to harmful doses of respective wavebands gained by the accumulation of the compound. Indeed, we occasionally observed strongly pigmented colonies surviving the adverse conditions in our experiments (, setting 4), but could not undoubtedly prove the role of the pigmentation. In poorly known non-model organisms such as Serritaenia clear evidence for a cause-effect relationship is difficult to obtain, since the cells can potentially react in several unknown ways at the same time; e.g. on a physiological level or with repair mechanisms (Garcia-Pichel et al., Citation1993; Cockell & Knowland, Citation1999). However, some additional evidence for effective UV-screening by the pigmented Serritaenia capsules comes from the observed correlation of the strength of pigmentation and the applied UVB irradiance, pointing to self-regulatory pigment accumulation: Higher intensities of UVR require higher concentrations of the extracellular sunscreen compound to attenuate below the response threshold of the cell. An important requirement for this self-regulative effect is that the action spectrum of sunscreen synthesis aligns with the absorbance spectrum of the sunscreen to some extent, as is known from some MAAs and scytonemin (Ehling-Schulz et al., Citation1997; Cockell & Knowland, Citation1999). Indeed, the pigment-inducing waveband in Serritaenia matches the main absorbance peak of pigmented mucilage very well (both in the UVB/far UVA range). Self-regulatory pigment production could also explain the hierarchical pigment layers frequently found in larger Serritaenia colonies from natural populations. Repeated cell division and capsule formation during colony growth likely results in stretching of the outer mucilage, thereby reducing the thickness (= optical path length) and absorbance of the outer pigment layer. Due to the diminished screening effect (and increased UV-exposure), the cells are triggered to form additional, interior pigment layers to restore full photoprotection. Taken together, the pigmented capsules of Serritaenia strongly absorb deleterious short-wavelength radiation and can be induced by the same stressor. The extracellular pigment is deposited in a sunshade-like pattern, ideal to shield entire cells from excess radiation, but unlikely to play additional physiological roles (as for example known from intracellular screening compounds such as carotenoids). All this points to a primary function in photoprotection.
In addition, the secretion of light-absorbing mucilage as a strategy aligns very well with the ecology of the studied algae. Serritaenia species are found predominantly in terrestrial habitats, where they are exposed to a large range of environmental conditions, including freezing temperatures, drought, heat and increased solar radiation. The high concentrations of the extracellular pigment observed in field material suggest that the extracellular mucilage of Serritaenia not only extends the active phases of this alga by its water-holding capacity, but also represents an effective ‘broadband’ sunscreen. The pigmented mucilage shields the entire cells in the active (hydrated) and inactive (desiccated) state. This might be of particular relevance during summer, when the Serritaenia cells survive in desiccated crusts and lack the ability to react on the cellular level (e.g. with non-photochemical quenching or repair mechanisms).
Serritaenia’s sunscreen mucilage in an evolutionary context
Among eukaryotic microalgae, members of the new genus Serritaenia stand out by their ability to form heavily pigmented extracellular mucilage. This phenomenon differs drastically from the photoprotective strategies already known from other Zygnematophyceae, e.g. the reddish, water-soluble pigments found in the vacuoles of representatives from alpine and glacier environments (Remias et al., Citation2012a, Citationb; Aigner et al., Citation2013; Herburger et al., Citation2016; Garduño-Solórzano et al., Citation2020). It seems that members of different zygnematophycean lineages that colonize high-light habitats evolved different solutions for the same problem, demonstrating once more that these algae are exciting candidates for studying terrestrialization processes in a comparative way.
A stunning analogy to Serritaenia’s sunscreen capsules, however, can be found in the world of prokaryotes. Terrestrial cyanobacteria of the Chroococcales form extracellular capsules with reddish layers containing gloeocapsin, a pigment which (similar to scytonemin) is believed to act as an extracellular UV-screen (Storme et al., Citation2015). The pigmented, extracellular capsules of Serritaenia and Chroococcales display a remarkable resemblance regarding the pattern of pigment deposition, and we assume that similar evolutionary pressures resulted in the evolution of very similar photoprotective adaptations. These adaptations, however, must be based on a very different cell biological background, and represent a prime example for convergent evolution across two domains of life.
The closest potential homologies to Serritaenia’s sunscreen mucilage might be found among the colourful cell walls of plants (e.g. various bryophytes) and their algal relatives. Topologically, cell walls correspond to the same cellular compartment as secreted mucilage, namely the extracellular space (apoplast). The cell walls of Zygnematophyceae are typically colourless, but some representatives of the genera Spirogyra, Zygnema and Zygnemopsis form zygospores with blue, brown or reddish spore walls (Stancheva et al., Citation2012, Citation2013; Pichrtová et al., Citation2018; Takano et al., Citation2019). Although there are no physicochemical data about these zygospore pigments, a photoprotective role in these propagules of aquatic Zygnematophyceae is not unlikely. In future, modern molecular and analytical techniques (e.g. transcriptomics and metabolomics) applied to non-model organisms like Serritaenia and relatives might provide deeper insights into the physiology and evolution of photoprotective strategies found in the ‘green lineage’ of life.
Supplementary table S1. Studied Serritaenia strains and associated data (sampling sites and accession numbers of the Central Collection of Algal Cultures (CCAC) and rbcL gene sequences).
Supplementary fig. S1. Unrooted Neighbour-joining phylogeny of 43 zygnematophycean rbcL gene sequences displaying the polyphyly of Mesotaenium (red) and the position of the new genus Serritaenia (green). Support values are shown on the respective branches (NJ/ML). Branches with maximum support (100/100) are in bold. The scale bar represents 0.02 nucleotide substitution per site.
Supplementary figs S2 and S3. Illustrations published with the original descriptions of Mesotaenium braunii (S2: A, 1-8), M. braunii var. minus (S2: A, 9-11) and M. testaceovaginatum (S3). The illustration of M. testaceovaginatum (S3) is designated as lectotype for this species.
Supplementary figs S4–S6. Microspectrophotometric measurements taken from the mucilage of Serritaenia testaceovaginata (strain GSM.5.thin) over a spectral range of 200–1600 nm. S4 and S5 display absorbance spectra of mucilage with varying degree of pigmentation from two independent wet mounts with 21 and 33 measurements, respectively. S6 displays 15 absorbance measurements of non-pigmented mucilage for comparison.
Supplementary text. Rationale for the new genus Serritaenia and taxonomy of its members.
Author contributions
SH conceived the study. AB and SH designed and performed the experiments, analysed and interpreted the data, and wrote the manuscript.
Supplemental Material
Download PDF (1.4 MB)Acknowledgements
We thank Barbara and Michael Melkonian (formerly Culture Collection of Algae at the University of Cologne, CCAC) for providing algal strains for research, Daniel N. Lamont (Nanoscale Fabrication & Characterization Facility, University of Pittsburgh) for taking microspectrophotometric measurements, Frances M. Baines (UV Guide UK) for providing lamp emission spectra, Karolina Fučíková (Assumption College, Worcester) and Jeffrey R. Johansen (John Carroll University, University Heights) for information about M. testaceovaginatum, and Wolf-Henning Kusber (Freie Universität Berlin) for advice on botanical nomenclature. Marnel W. M. Scherrenberg (Naturalis Biodiversity Center, Leiden) sampled and provided the original material of Palmogloea macrococca, which is highly appreciated. Alastair G. B. Simpson (Dalhousie University, Halifax) kindly provided laboratory resources.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary information
The following supplementary material is accessible via the Supplementary Content tab on the article’s online page at https://doi.org/10.1080/09670262.2021.1898677
Additional information
Funding
References
- Aigner, S., Remias, D., Karsten, U. & Holzinger, A. (2013). Unusual phenolic compounds contribute to ecophysiological performance in the purple-colored green alga Zygogonium ericetorum (Zygnematophyceae, Streptophyta) from a high-alpine habitat. Journal of Phycology, 49: 648–660.
- Allorent, G., Lefebvre-Legendre, L., Chappuis, R., Kuntz, M., Truong, T.B., Niyogi, K.K., Ulm, R. & Goldschmidt-Clermont, M. (2016). UV-B photoreceptor-mediated protection of the photosynthetic machinery in Chlamydomonas reinhardtii. Proceedings of the National Academy of Sciences USA, 113: 14864–14869.
- Barcytė, D., Pilátová, J., Mojzeš, P. & Nedbalová, L. (2020). The arctic Cylindrocystis (Zygnematophyceae, Streptophyta) green algae are genetically and morphologically diverse and exhibit effective accumulation of polyphosphate. Journal of Phycology, 56: 217–232.
- de Bary, A. (1858). Untersuchungen über die Familie der Conjugaten (Zygnemeen und Desmidieen): Ein Beitrag zur physiologischen und beschreibenden Botanik. Förstnersche Buchhandlung, Leipzig.
- Bidigare, R.R., Ondrusek, M.E., Kennicutt, M.C., Iturriaga, R., Harvey, H.R., Hoham, R.W. & Macko, S.A. (1993). Evidence for a photoprotective function for secondary carotenoids of snow algae. Journal of Phycology, 29: 427–434.
- Böhm, G.A., Pfleiderer, W., Böger, P. & Scherer, S. (1995). Structure of a novel oligosaccharide-mycosporine-amino acid ultraviolet A/B sunscreen pigment from the terrestrial cyanobacterium Nostoc commune. Journal of Biological Chemistry, 270: 8536–8539.
- Bonnot, C., Hetherington, A.J., Champion, C., Breuninger, H., Kelly, S. & Dolan, L. (2019). Neofunctionalisation of basic helix−loop−helix proteins occurred when embryophytes colonised the land. New Phytologist, 223: 993–1008.
- Brook, A.J. (1981). The Biology of Desmids. University of California Press, Berkeley, CA.
- Brook, A.J. & Williamson, D.B. (2010). A Monograph on some British Desmids. The Ray Society, London.
- Černá, K. & Neustupa, J. (2010). The pH-related morphological variations of two acidophilic species of Desmidiales (Viridiplantae) isolated from a lowland peat bog, Czech Republic. Aquatic Ecology, 44: 409–419.
- Chase, M.W., Salamin, N., Wilkinson, M., Dunwell, J.M., Kesanakurthi, R.P., Haidar, N. & Savolainen, V. (2005). Land plants and DNA barcodes: short-term and long-term goals. Philosophical Transactions of the Royal Society B: Biological Sciences, 360: 1889–1895.
- Cheng, S., Xian, W., Fu, Y., Marin, B., Keller, J., Wu, T., Sun, W., Li, X., Xu, Y., Zhang, Y., Wittek, S., Reder, T., Günther, G., Gontcharov, A., Wang, S., Li, L., Liu, X., Wang, J., Yang, H., Xu, X., Delaux, P.-M., Melkonian, B., Wong, G.K.-S. & Melkonian, M. (2019). Genomes of subaerial Zygnematophyceae provide insights into land plant evolution. Cell, 179: 1057–1067.
- Clayton, W.A., Albert, N.W., Thrimawithana, A.H., McGhie, T.K., Deroles, S.C., Schwinn, K.E., Warren, B.A., McLachlan, A.R.G., Bowman, J.L., Jordan, B.R. & Davies, K.M. (2018). UVR8-mediated induction of flavonoid biosynthesis for UVB tolerance is conserved between the liverwort Marchantia polymorpha and flowering plants. The Plant Journal, 96: 503–517.
- Cockell, C.S. & Knowland, J. (1999). Ultraviolet radiation screening compounds. Biological Reviews, 74: 311–345.
- Coesel, P.F.M. & Meesters, K.J. (2007). Desmids of the Lowlands: Mesotaeniaceae and Desmidiaceae of the European Lowlands. KNNV Publishing, Zeist.
- Davey, M.L. & Currah, R.S. (2006). Interactions between mosses (Bryophyta) and fungi. Canadian Journal of Botany, 84: 1509–1519.
- Domozych, D.S., Sørensen, I., Popper, Z.A., Ochs, J., Andreas, A., Fangel, J.U., Pielach, A., Sacks, C., Brechka, H., Ruisi-Besares, P., Willats, W.G.T. & Rose, J.K.C. (2014). Pectin metabolism and assembly in the cell wall of the charophyte green alga Penium margaritaceum. Plant Physiology, 165: 105–118.
- Eder, M. & Lütz-Meindl, U. (2010). Analyses and localization of pectin-like carbohydrates in cell wall and mucilage of the green alga Netrium digitus. Protoplasma, 243: 25–38.
- Ehling-Schulz, M., Bilger, W. & Scherer, S. (1997). UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. Journal of Bacteriology, 179: 1940–1945.
- Ettl, H. & Gärtner, G. (2014). Syllabus der Boden-, Luft- und Flechtenalgen. 2nd ed. Springer, Berlin.
- Fenton, J.H.C. (1983). Concentric fungal rings in Antarctic moss communities. Transactions of the British Mycological Society, 80: 415–420.
- Ferroni, L., Klisch, M., Pancaldi, S. & Häder, D.-P. (2010). Complementary UV-absorption of mycosporine-like amino acids and scytonemin is responsible for the UV-insensitivity of photosynthesis in Nostoc flagelliforme. Marine Drugs, 8: 106–121.
- Fritsch, F.E. (1922). The terrestrial alga. Journal of Ecology, 10: 220–236.
- Fučíková, K., Hall, J.D., Johansen, J.R. & Lowe, R. (2008). Desmid flora of the Great Smoky Mountains National Park, USA. Bibliotheca Phycologica, 113: 1–59.
- Furey, P.C., Lowe, R.L. & Johansen, J.R. (2007). Wet wall algal community response to in-field nutrient manipulation in the Great Smoky Mountains National Park, USA. Algological Studies, 125: 17–43.
- Galtier, N., Gouy, M. & Gautier, C. (1996). SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Bioinformatics, 12: 543–548.
- Gao, Q. & Garcia-Pichel, F. (2011). Microbial ultraviolet sunscreens. Nature Reviews Microbiology, 9: 791–802.
- Garcia-Pichel, F. & Castenholz, R.W. (1991). Characterization and biological implications of scytonemin, a cyanobacterial sheath pigment. Journal of Phycology, 27: 395–409.
- Garcia-Pichel, F. & Castenholz, R.W. (1993). Occurrence of UV-absorbing, mycosporine-like compounds among cyanobacterial isolates and an estimate of their screening capacity. Applied and Environmental Microbiology, 59: 163–169.
- Garcia-Pichel, F., Sherry, N.D. & Castenholz, R.W. (1992). Evidence for an ultraviolet sunscreen role of the extracellular pigment scytonemin in the terrestrial cyanobacterium Chlorogloeopsis sp. Photochemistry and Photobiology, 56: 17–23.
- Garcia-Pichel, F., Wingard, C.E. & Castenholz, R.W. (1993). Evidence regarding the UV sunscreen role of a mycosporine-like compound in the cyanobacterium Gloeocapsa sp. Applied and Environmental Microbiology, 59: 170–176.
- Garduño-Solórzano, G., Martínez-García, M., Scotta Hentschke, G., Lopes, G., Castelo Branco, R., Vasconcelos, V.M.O., Campos, J.E., López-Cano, R. & Quintanar-Zúñiga, R.E. (2020). The phylogenetic placement of Temnogametum (Zygnemataceae) and description of Temnogametum iztacalense sp. nov., from a tropical high mountain lake in Mexico. European Journal of Phycology: doi: https://doi.org/10.1080/09670262.2020.1789226.
- Gitzendanner, M.A., Soltis, P.S., Wong, G.K.-S., Ruhfel, B.R. & Soltis, D.E. (2018). Plastid phylogenomic analysis of green plants: a billion years of evolutionary history. American Journal of Botany, 105: 291–301.
- Gontcharov, A.A. (2008). Phylogeny and classification of Zygnematophyceae (Streptophyta): current state of affairs. Fottea, 8: 87–104.
- Gontcharov, A.A. & Melkonian, M. (2010). Molecular phylogeny and revision of the genus Netrium (Zygnematophyceae, Streptophyta): Nucleotaenium gen. nov. Journal of Phycology, 46: 346–362.
- Gontcharov, A.A., Marin, B. & Melkonian, M. (2003). Molecular phylogeny of conjugating green algae (Zygnemophyceae, Streptophyta) inferred from SSU rDNA sequence comparisons. Journal of Molecular Evolution, 56: 89–104.
- Gontcharov, A.A., Marin, B. & Melkonian, M. (2004). Are combined analyses better than single gene phylogenies? A case study using SSU rDNA and rbcL sequence comparisons in the Zygnematophyceae (Streptophyta). Molecular Biology and Evolution, 21: 612–624.
- Gouy, M., Guindon, S. & Gascuel, O. (2010). SeaView version 4: a multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution, 27: 221–224.
- Gröniger, A. & Häder, D.-P. (2002). Induction of the synthesis of an UV-absorbing substance in the green alga Prasiola stipitata. Journal of Photochemistry and Photobiology B: Biology, 66: 54–59.
- Hall, J.D., Fučíková, K., Lewis, L.A. & Karol, K.G. (2010). An assessment of proposed DNA barcodes in freshwater green algae. Cryptogamie Algologie, 31: 529–555.
- Han, X., Chang, X., Zhang, Z., Chen, H., He, H., Zhong, B. & Deng, X.W. (2019). Origin and evolution of core components responsible for monitoring light environment changes during plant terrestrialization. Molecular Plant, 12: 847–862.
- Hargreaves, A., Taiwo, F.A., Duggan, O., Kirk, S.H. & Ahmad, S.I. (2007). Near-ultraviolet photolysis of β-phenylpyruvic acid generates free radicals and results in DNA damage. Journal of Photochemistry and Photobiology B: Biology, 89: 110–116.
- Hartmann, A., Glaser, K., Holzinger, A., Ganzera, M. & Karsten, U. (2020). Klebsormidin A and B, two new UV-sunscreen compounds in green microalgal Interfilum and Klebsormidium species (Streptophyta) from terrestrial habitats. Frontiers in Microbiology, 11: 499.
- Herburger, K., Remias, D. & Holzinger, A. (2016). The green alga Zygogonium ericetorum (Zygnematophyceae, Charophyta) shows high iron and aluminium tolerance: protection mechanisms and photosynthetic performance. FEMS Microbiology Ecology, 92: doi: https://doi.org/10.1093/femsec/fiw103.
- Hoffmann, L. (1989). Algae of terrestrial habitats. The Botanical Review, 55: 77–105.
- Holzinger, A., Albert, A., Aigner, S., Uhl, J., Schmitt-Kopplin, P., Trumhová, K. & Pichrtová, M. (2018). Arctic, Antarctic, and temperate green algae Zygnema spp. under UV-B stress: vegetative cells perform better than pre-akinetes. Protoplasma, 255: 1239–1252.
- Holzinger, A., Tschaikner, A. & Remias, D. (2010). Cytoarchitecture of the desiccation-tolerant green alga Zygogonium ericetorum. Protoplasma, 243: 15–24.
- Hooijmaijers, C.A.M. & Gould, K.S. (2007). Photoprotective pigments in red and green gametophytes of two New Zealand liverworts. New Zealand Journal of Botany, 45: 451–461.
- Hotter, V., Glaser, K., Hartmann, A., Ganzera, M. & Karsten, U. (2018). Polyols and UV-sunscreens in the Prasiola-clade (Trebouxiophyceae, Chlorophyta) as metabolites for stress response and chemotaxonomy. Journal of Phycology, 54: 264–274.
- Ingalls, A.E., Whitehead, K. & Bridoux, M.C. (2010). Tinted windows: the presence of the UV absorbing compounds called mycosporine-like amino acids embedded in the frustules of marine diatoms. Geochimica et Cosmochimica Acta, 74: 104–115.
- Jeffrey, S.W., MacTavish, H.S., Dunlap, W.C., Vesk, M. & Groenewoud, K. (1999). Occurrence of UVA- and UVB-absorbing compounds in 152 species (206 strains) of marine microalgae. Marine Ecology Progress Series, 189: 35–51.
- Karsten, U. (2008). Defense strategies of algae and cyanobacteria against solar ultraviolet radiation. In Algal Chemical Ecology (Amsler, C.D., editor), 273–296. Springer, Berlin.
- Karsten, U. & Holzinger, A. (2014). Green algae in alpine biological soil crust communities: acclimation strategies against ultraviolet radiation and dehydration. Biodiversity and Conservation, 23: 1845–1858.
- Karsten, U., Franklin, L.A., Lüning, K. & Wiencke, C. (1998). Natural ultraviolet radiation and photosynthetically active radiation induce formation of mycosporine-like amino acids in the marine macroalga Chondrus crispus (Rhodophyta). Planta, 205: 257–262.
- Karsten, U., Schumann, R. & Mostaert, A. (2007). Aeroterrestrial algae growing on man-made surfaces. In Algae and Cyanobacteria in Extreme Environments ( Seckbach, J., editor), 583–597. Springer, Dordrecht.
- Kitzing, C. & Karsten, U. (2015). Effects of UV radiation on optimum quantum yield and sunscreen contents in members of the genera Interfilum, Klebsormidium, Hormidiella and Entransia (Klebsormidiophyceae, Streptophyta). European Journal of Phycology, 50: 279–287.
- Klisch, M. & Häder, D.-P. (2002). Wavelength dependence of mycosporine-like amino acid synthesis in Gyrodinium dorsum. Journal of Photochemistry and Photobiology B: Biology, 66: 60–66.
- Kouwets, F. (2008). The species concept in desmids: the problem of variability, infraspecific taxa and the monothetic species definition. Biologia, 63: 881–887.
- Kräbs, G., Bischof, K., Hanelt, D., Karsten, U. & Wiencke, C. (2002). Wavelength-dependent induction of UV-absorbing mycosporine-like amino acids in the red alga Chondrus crispus under natural solar radiation. Journal of Experimental Marine Biology and Ecology, 268: 69–82.
- Kumar, S., Stecher, G., Li, M., Knyaz, C. & Tamura, K. (2018). MEGA X: molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35: 1547–1549.
- Kützing, F.T. (1845). Phycologia germanica, d. i. Deutschlands Algen in bündigen Beschreibungen. Nebst einer Anleitung zum Untersuchen und Bestimmen dieser Gewächse für Anfänger. pp. [i]–x, [1] –340 [‘240’]. Nordhausen: zu finden bei Wilh. Köhne.
- Lenzenweger, R. (2003). Desmidiaceenflora von Österreich, Teil 4. In Bibliotheca Phycologica (Cramer, J., editor), 1–87. Schweizerbart Science Publishers, Stuttgart.
- Liang, H., Wei, T., Xu, Y., Li, L., Kumar Sahu, S., Wang, H., Li, H., Fu, X., Zhang, G., Melkonian, M., Liu, X., Wang, S. & Liu, H. (2019). Phylogenomics provides new insights into gains and losses of selenoproteins among Archaeplastida. International Journal of Molecular Sciences, 20: 3020.
- Lowe, R.L., Furey, P.C., Ress, J.A. & Johansen, J.R. (2007). Diatom biodiversity and distribution on wetwalls in Great Smoky Mountains National Park. Southeastern Naturalist, 6: 135–152.
- Mårtensson, O. & Nilsson, E. (1974). On the morphological colour of bryophytes. Lindbergia, 2: 145–159.
- Matasci, N., Hung, L.-H., Yan, Z., Carpenter, E.J., Wickett, N.J., Mirarab, S., Nguyen, N., Warnow, T., Ayyampalayam, S., Barker, M., Burleigh, J.G., Gitzendanner, M.A., Wafula E., Der, J.P., dePamphilis, C.W., Roure, B., Philippe, H., Ruhfel, B.R., Miles, N.W., Graham, S.W., Mathews, S., Surek, B., Melkonian, M., Soltis, D.E., Soltis, P.S., Rothfels, C., Pokorny, L., Shaw, J.A., DeGironimo, L., Stevenson, D.W., Villarreal, J.C., Chen, T., Kutchan, T.M., Rolf, M., Baucom, R.S., Deyholos, M.K., Samudrala, R., Tian, Z., Wu, X., Sun, X., Zhang, Y., Wang, J., Leebens-Mack, J. & Wong, G.K.-S. (2014). Data access for the 1,000 Plants (1KP) project. Gigascience, 3: 2047-217X-3-17.
- McCourt, R.M., Karol, K.G., Bell, J., Helm-Bychowski, K.M., Grajewska, A., Wojciechowski, M.F. & Hoshaw, R.W. (2000). Phylogeny of the conjugating green algae (Zygnemophyceae) based on rbcL sequences. Journal of Phycology, 36: 747–758.
- McFadden, G.I. & Melkonian, M. (1986). Use of Hepes buffer for microalgal culture media and fixation for electron microscopy. Phycologia, 25: 551–557.
- Nedbalová, L. & Sklenár, P. (2008). New records of snow algae from the Andes of Ecuador. Arnaldoa, 15: 17–20.
- Neustupa, J., Stastny, J. & Hodac, L. (2008). Temperature-related phenotypic plasticity in the green microalga Micrasterias rotata. Aquatic Microbial Ecology, 51: 77–86.
- Neustupa, J., Stastny, J., Nemjová, K., Mazalová, P., Goodyer, E., Poulíčková, A. & Škaloud, P. (2011). A novel, combined approach to assessing species delimitation and biogeography within the well-known desmid species Micrasterias fimbriata and M. rotata (Desmidiales, Steptophyta). Hydrobiologia, 667: 223–239.
- Newmaster, S.G., Fazekas, A.J. & Ragupathy, S. (2006). DNA barcoding in land plants: evaluation of rbcL in a multigene tiered approach. Botany, 84: 335–341.
- Oertel, A., Aichinger, N., Hochreiter, R., Thalhamer, J. & Lütz-Meindl, U. (2004). Analysis of mucilage secretion and excretion in Micrasterias (Chlorophyta) by means of immunoelectron microscopy and digital time lapse video microscopy. Journal of Phycology, 40: 711–720.
- Pattison, D.I. & Davies, M.J. (2006). Actions of ultraviolet light on cellular structures. In Cancer: Cell Structures, Carcinogens and Genomic Instability (Bignold, L.P., editor), 131–157. Birkhäuser, Basel.
- Philippe, G., Sørensen, I., Jiao, C., Sun, X., Fei, Z., Domozych, D.S. & Rose, J.K. (2020). Cutin and suberin: assembly and origins of specialized lipidic cell wall scaffolds. Current Opinion in Plant Biology, 55: 11–20.
- Pichrtová, M., Remias, D., Lewis, L.A. & Holzinger, A. (2013). Changes in phenolic compounds and cellular ultrastructure of Arctic and Antarctic strains of Zygnema (Zygnematophyceae, Streptophyta) after exposure to experimentally enhanced UV to PAR ratio. Microbial Ecology, 65: 68–83.
- Pichrtová, M., Holzinger, A., Kulichová, J., Ryšánek, D., Šoljaková, T., Trumhová, K. & Nemcova, Y. (2018). Molecular and morphological diversity of Zygnema and Zygnemopsis (Zygnematophyceae, Streptophyta) from Svalbard (high Arctic). European Journal of Phycology, 53: 492–508.
- Portwich, A. & Garcia-Pichel, F. (2000). A novel prokaryotic UVB photoreceptor in the cyanobacterium Chlorogloeopsis PCC 6912. Photochemistry and Photobiology, 71: 493–498.
- Remias, D. & Lütz, C. (2007). Characterisation of esterified secondary carotenoids and of their isomers in green algae: a HPLC approach. Algological Studies, 124: 85–94.
- Remias, D., Holzinger, A., Aigner, S. & Lütz, C. (2012a). Ecophysiology and ultrastructure of Ancylonema nordenskiöldii (Zygnematales, Streptophyta), causing brown ice on glaciers in Svalbard (high Arctic). Polar Biology, 35: 899–908.
- Remias, D., Schwaiger, S., Aigner, S., Leya, T., Stuppner, H. & Lütz, C. (2012b). Characterization of an UV- and VIS-absorbing, purpurogallin-derived secondary pigment new to algae and highly abundant in Mesotaenium berggrenii (Zygnematophyceae, Chlorophyta), an extremophyte living on glaciers. FEMS Microbiology Ecology, 79: 638–648.
- Řezanka, T., Temina, M., Tolstikov, A.G. & Dembitsky, V.M. (2004). Natural microbial UV radiation filters – Mycosporine-like amino acids. Folia Microbiologica, 49: 339–352.
- Rindi, F. & Guiry, M.D. (2004). Composition and spatial variability of terrestrial algal assemblages occurring at the bases of urban walls in Europe. Phycologia, 43: 225–235.
- Rizzini, L., Favory, J.-J., Cloix, C., Faggionato, D., O’Hara, A., Kaiserli, E., Baumeister, R., Schäfer, E., Nagy, F., Jenkins, G.I. & Ulm, R. (2011). Perception of UV-B by the Arabidopsis UVR8 protein. Science, 332: 103–106.
- Rosa, L.H., de Sousa, J.R.P., de Menezes, G.C.A., da Costa Coelho, L., Carvalho-Silva, M., Convey, P. & Câmara, P.E.A.S. (2020). Opportunistic fungi found in fairy rings are present on different moss species in the Antarctic Peninsula. Polar Biology, 43: 587–596.
- Rozema, J., Björn, L.O., Bornman, J.F., Gaberščik, A., Häder, D.-P., Trošt, T., Germ, M., Klisch, M., Gröniger, A., Sinha, R.P., Lebert, M., He, Y.-Y., Buffoni-Hall, R., de Bakker, N.V.J., van de Staaij J. & Meijkamp, B.B. (2002). The role of UV-B radiation in aquatic and terrestrial ecosystems – an experimental and functional analysis of the evolution of UV-absorbing compounds. Journal of Photochemistry and Photobiology B: Biology, 66: 2–12.
- Schagerl, M. & Zwirn, M. (2015). A brief introduction to the morphological species concept of Spirogyra and emanating problems. Algological Studies, 148: 67–86.
- Schindelin, J., Arganda-Carreras, I., Frise, E., Kaynig, V., Longair, M., Pietzsch, T., Preibisch, S., Rueden, C., Saalfeld, S., Schmid, B., Tinevez, J.-Y., White, D.J., Hartenstein, V., Eliceiri, K., Tomancak, P. & Cardona, A. (2012). Fiji: an open-source platform for biological-image analysis. Nature Methods, 9: 676–682.
- Singh, S.P., Häder, D.-P. & Sinha, R.P. (2010). Cyanobacteria and ultraviolet radiation (UVR) stress: mitigation strategies. Ageing Research Reviews, 9: 79–90.
- Sinha, R.P., Klisch, M., Helbling, E.W. & Häder, D.-P. (2001). Induction of mycosporine-like amino acids (MAAs) in cyanobacteria by solar ultraviolet-B radiation. Journal of Photochemistry and Photobiology B: Biology, 60: 129–135.
- Sinha, R.P., Ambasht, N.K., Sinha, J.P. & Häder, D.-P. (2003a). Wavelength-dependent induction of a mycosporine-like amino acid in a rice-field cyanobacterium, Nostoc commune: role of inhibitors and salt stress. Photochemical & Photobiological Sciences, 2: 171–176.
- Sinha, R.P., Ambasht, N.K., Sinha, J.P., Klisch, M. & Häder, D.-P. (2003b). UV-B-induced synthesis of mycosporine-like amino acids in three strains of Nodularia (cyanobacteria). Journal of Photochemistry and Photobiology B: Biology, 71: 51–58.
- Stancheva, R., Sheath, R.G. & Hall, J.D. (2012). Systematics of the genus Zygnema (Zygnematophyceae, Charophyta) from Californian watersheds. Journal of Phycology, 48: 409–422.
- Stancheva, R., Hall, J.D., McCourt, R.M. & Sheath, R.G. (2013). Identity and phylogenetic placement of Spirogyra species (Zygnematophyceae, Charophyta) from California streams and elsewhere. Journal of Phycology, 49: 588–607.
- Stastny, J., Skaloud, P., Langenbach, D., Nemjova, K. & Neustupa, J. (2013). Polyphasic evaluation of Xanthidium antilopaeum and Xanthidium cristatum (Zygnematophyceae, Streptophyta) species complex. Journal of Phycology, 49: 401–416.
- Storme, J.-Y., Golubic, S., Wilmotte, A., Kleinteich, J., Velázquez, D. & Javaux, E.J. (2015). Raman characterization of the UV-protective pigment gloeocapsin and its role in the survival of cyanobacteria. Astrobiology, 15: 843–857.
- Takano, T., Higuchi, S., Ikegaya, H., Matsuzaki, R., Kawachi, M., Takahashi, F. & Nozaki, H. (2019). Identification of 13 Spirogyra species (Zygnemataceae) by traits of sexual reproduction induced under laboratory culture conditions. Scientific Reports, 9: 1–11.
- Tamura, M., Tanabe, M., Valkonen, J.P.T. & Akita, M. (2019). Sunagoke moss (Racomitrium japonicum) used for greening roofs is severely damaged by Sclerotium delphinii and protected by a putative Bacillus amyloliquefaciens isolate. Frontiers in Microbiology, 10, doi: https://doi.org/10.3389/fmicb.2019.00372.
- Timme, R.E., Bachvaroff, T.R. & Delwiche, C.F. (2012). Broad phylogenomic sampling and the sister lineage of land plants. PLoS ONE, 7: e29696. doi: https://doi.org/10.1371/journal.pone.0029696.
- Vincent, W.F. & Neale, P.J. (2000). Mechanisms of UV damage to aquatic organisms. In The Effects of UV Radiation in the Marine Environment (de Mora, S.J., Demers, S. & Vernet, M., editors), 149–176. Cambridge University Press, Cambridge.
- de Vries, J., Curtis, B.A., Gould, S.B. & Archibald, J.M. (2018). Embryophyte stress signaling evolved in the algal progenitors of land plants. Proceedings of the National Academy of Sciences USA, 115: E3471–E3480.
- Wickett, N.J., Mirarab, S., Nguyen, N., Warnow, T., Carpenter, E., Matasci, N., Ayyampalayam, S., Barker, M.S., Burleigh, J.G., Gitzendanner, M.A., Ruhfel, B.R., Wafula, E., Der, J.P., Graham, S.W., Mathews, S., Melkonian, M., Soltis, D.E., Soltis, P.S., Miles, N.W., Rothfels, C.J., Pokorny, L., Shaw, A.J., DeGironimo, L., Stevenson, D.W., Surek, B., Villarreal, J.C., Roure, B., Philippe, H., dePamphilis, C.W., Chen, T., Deyholos, M.K., Baucom, R.S., Kutchan, T.M., Augustin, M.M., Wang, J., Zhang, Y., Tian, Z., Yan, Z., Wu, X., Sun, X., Wong, G.K.-S. & Leebens-Mack, J. (2014). Phylotranscriptomic analysis of the origin and early diversification of land plants. Proceedings of the National Academy of Sciences USA, 111: E4859–E4868.
- Wodniok, S., Brinkmann, H., Glöckner, G., Heidel, A.J., Philippe, H., Melkonian, M. & Becker, B. (2011). Origin of land plants: do conjugating green algae hold the key? BMC Evolutionary Biology, 11: 1–10.
- Wynn-Williams, D.D. & Edwards, H.G. (2002). Environmental UV radiation: biological strategies for protection and avoidance. In Astrobiology (Horneck, G. & Baumstark-Khan, C., editors), 245–260. Springer, Heidelberg.
- Xu, Y., Wang, S., Li, L., Sahu, S.K., Petersen, M., Liu, X., Melkonian, M., Zhang, G. & Liu, H. (2019). Molecular evidence for origin, diversification and ancient gene duplication of plant subtilases (SBTs). Scientific Reports, 9: 1–10.
