Abstract
The presence of isogenes encoding V-ATPase subunits seems to be a characteristic for plants. Twenty-eight genes encode for the 13 different subunits in Arabidopsis thaliana, 23 genes each are known in tomato (Solanum lycopersicum) and can be identified in rice (Oryza sativa), respectively. In Arabidopsis the four subunits VHA-B, -E, -G and -a are encoded by three isogenes each. The transcript levels of these subunits were analysed by in silico evaluation of transcript pattern derived from the NASC-array database and exemplarily confirmed by semiquantitative RT-PCR. A tissue specifity was observed for the isoforms of VHA-E and VHA-G, whereas expression of VHA-a isoforms appeared independent of the tissue. Inflicting environmental stresses upon plants resulted in differentiated expression patterns of VHA-isoforms. Whereas salinity had minor effect on the expression of V-ATPase genes in A. thaliana, heat and drought stress led to alterations in transcript amount and preference of isoforms. Correlation analysis identified two clusters of isoforms, which were co-regulated on the transcript level.
| Abbreviations | ||
| CS | = | cold stress |
| DS | = | drought stress |
| HS | = | heat stress |
| OS | = | osmotic stress |
| SS | = | salt stress |
| VHA | = | vacuolar H+-ATPase subunit |
Introduction
The vacuolar H+-ATPase (V-ATPase) is a transmembrane protein complex of about 800 kDa found in all eukaryotes. The V-ATPase is localized in the endomembrane system, where it acidifies diverse cell compartments and generates a proton motive force (PMF) used to energize secondary transport (Sze et al. Citation1999, Kluge et al. Citation2003). The ability of the V-ATPase to maintain the cytosolic pH homeostasis and to acidify the endomembrane compartments is important during essential processes such as growth and cell elongation (Smart et al. Citation1998, Viereck et al. Citation1996). Furthermore, the vacuole plays a central role in plant cell response to salinity of halotolerant plants. Here the V-ATPase allows sequestration of excess sodium chloride in the vacuole by energizing secondary transport needed for sodium accumulation (Golldack & Dietz, Citation2001). In plants the V-ATPase is composed of 13 VHA-subunits distributed among the cytosolic V1-domain and the membrane bound V0-domain (Sze et al. Citation2002). The V1-domain is dominated by the hexameric head assembled from three copies each of VHA-A and -B. Subunits VHA-D and -F function as central stalk and transduce conformational changes in VHA-A established by ATP-hydrolysis into rotation of the proteolipid ring. The remaining V1-subunits (VHA-E to -H) constitute at least one or possibly up to three peripheral stalks which immobilize the catalytic head during rotation of the rotor (Domgall et al. Citation2002, Wilkens et al. Citation2004). VHA-E and -G form a tetramer containing two copies of each subunit interacting with single copies of VHA-C and -H (Féthière et al. Citation2004, Seidel et al. Citation2005).
The central element of the V0-domain is the proteolipid ring consisting of 4 or 5 copies of VHA-c, one copy of VHA-c′ that is, however, absent in plants and one copy of VHA-c″. In addition, V0 contains one copy each of VHA-a, -e and -d (Sze et al. Citation2002). VHA-a is the largest subunit of the complex (∼100 kDa) and characterized by two domains (Kluge et al. Citation2004). The N-terminal domain of VHA-a takes part in the peripheral stalk formation whereas the C-terminal, transmembrane domain is indispensable for proton transfer across the membrane (Landolt-Marticorena et al. Citation2000, Kawasaki-Nishi et al. Citation2001). In yeast, VHA-subunit isoforms were identified exclusively for VHA-a. In contrast, subunits VHA-B, VHA-E, VHA-G, VHA-a, VHA-c, VHA-c″, VHA-d and VHA-e are characterized by the presence of 2 to 5 isoforms in Arabidopsis thaliana (Sze et al. Citation2002). The whole set of plant VHA-isoforms was reported in detail exclusively for A. thaliana and Lycopersicon esculentum. 28 vha-genes are found in the genome of A. thaliana and 23 vha-genes in L. esculentum (Sze et al. Citation2002, Coker et al. Citation2003). An isoform-specific expression has been studied only for few VHA-subunits in plants. In A. thaliana the isoform VHA-c1 is expressed in a tissue-specific manner in the elongation zone of roots, particularly in the root tip, in contrast to the isoform VHA-c3 which is mainly expressed in root caps and pollen (Padmanaban et al. Citation2004). A VHA-c isoform found in Pennisetum glaucum revealed tissue-specific expression in male and female reproductive organs and additionally in shoot hairs (Tyagi et al. Citation2005). In Gossypium hirsutum VHA-c isoforms were highly expressed in tissues undergoing rapid cell expansion (Hasenfratz et al. Citation1995). Additionally, tissue-specific isoforms of VHA-E were described in A. thaliana, which are essential for embryogenesis (Dettmer et al. Citation2005), pointing to a crucial role of the V-ATPase in plant development. An organelle-specific subcellular localization was observed for the VHA-G isoforms from tobacco (Rouquié et al. 1998) and the VHA-a isoforms of yeast, tobacco, Mesembryanthemum crystallinum and A. thaliana (Matsuoka et al. Citation1997, Kawasaki-Nishi et al. Citation2001, Kluge et al. Citation2004, Dettmer et al. Citation2006). The localization of VHA-A isoforms seems to vary among organisms. For example in L. esculentum a tissue-specific localization was detected (Bageshwar et al. Citation2005), whereas an organelle-specific localization was reported for carrots (Gogarten et al. Citation1992).
Sequencing of the Arabidopsis genome enabled the development of powerful tools for expression analyses such as the whole genome affimetrix gene chip. Significant amounts of data on transcript accumulation have been deposited in publicly available data bases and offer access to an understanding of gene family transcript regulation. Employing this approach the transcript patterns of A. thaliana vha-isoforms were investigated in order to get new insight into the role of the different isoforms within the complex. Thus, this work focused on the regulational understanding of the three isoforms each of VHA-B, -E, -G and -a.
Materials and methods
Data collection
Transcript data were obtained from the NASC array database using the ‘bulk gene download’ tool (Craigon et al. Citation2004). All data available for V-ATPase subunits were downloaded and 172 independent datasets using wild type plants were selected for further analysis. The following subunits were available: At1g78900 (A), At1g76030 (B1), At1g12840 (C), At3g58730 (D), At4g11150 (E1), At3g08560 (E2), At1g64200 (E3), At4g02620 (F), At3g01390 (G1), At4g23710 (G2), At4g25950 (G3), At3g42050 (H), At2g28520 (a1), At2g21410 (a2), At4g39080 (a3), At4g34720 (c1), At1g75630 (c4), At2g16510 (c5), At3g28715 (d2), At5g55290 (e1) and At4g26710 (e2).
Data analysis
To obtain the average expression level of each subunit, the signal values were normalized by setting the highest signal value of each VHA isoform to 100% (normalized value%=(100% * signal value)/maximal signal value) and the median of all normalized values was defined as average expression level. Relative expression level was defined as the single isoform expression level related to the sum of the expression level of all three isoforms. For graphic display normalized signal values were sorted into classes of defined signal strength. Signal values within a range of 10% signal intensity were assigned to the same class. Expression levels were compared by calculation of Pearson's correlation factor. Only correlation-factors equal or greater than 0.5 were considered to be significant.
Determination of tissue specific expression
Data derived from experiments excluding mutants and stress treatments were sorted according to plant tissues: ‘leaves’ containing 49 experiments with single leaves and complete rosettes, ‘petiole’ representing three experiments, ‘seedling’ containing 14 experiments with seedlings in different developmental stages, ‘stem’ containing five experiments with inflorescences, ‘flowers’ containing six experiments with flower buds, ‘whole plants’ containing 10 experiments with whole plants and all aerial tissue, ‘pollen’ containing eight experiments with pollen in different stages of development, and ‘roots’ containing four experiments.
RT-PCR
A. thaliana plants (Columbia) were grown in hydroponics under control and stress conditions. The different treatments as indicated in the text were administered by adding 125 mM NaCl (salt stress) and 150 mM mannitol (osmotic stress), respectively, to modified Hoagland's solution (Golldack et al. Citation2002) or by placing the pots into a 4°C climate chamber for 6 h with the same conditions as the control. RNA was extracted from plant tissues and cDNA was synthesized as described before (Golldack & Dietz Citation2001). Gene specific primers were synthesized and the expression of the V-ATPase isogenes a, B, E and G was quantified via semiquantitative RT-PCR (see ).
Table I. Primers for transcript analysis by semiquantitative RT PCR.
Protein preparation, 2D gel electrophoresis and Western blot analysis
A. thaliana plants (Columbia) were grown under control conditions and separated in root and shoot (Kandlbinder et al. Citation2004). Protein samples were prepared under reducing conditions and 2D gel electrophoresis was performed as described in Heiber et al. (Citation2007), using 18 cm-strips with immobilized pH gradient 3–10 NL (GE healthcare/Amersham). Western blot analysis was performed with an antiserum raised against heterologously expressed AtVHA-E1 (At4g11150) and SuperSignal West Pico Chemiluminescent Substrate (Pierce).
Results
The work aimed at quantifying the expressional pattern of VHA subunits that are represented by three copies each in the Arabidopsis thaliana genome (VHA-a, -B, -E, -G) in order to identify functional interrelations. Amino acid sequence similarity among the isoforms is high as depicted in . However, data on transcript accumulation for the isoforms VHA-B1 and -B3 were absent from the database. Thus the three isoforms of VHA-B were omitted from most of the database analyses. A set of 172 experiments with transcript data for a wide range of conditions and tissues was selected to gain new insight into co-regulation of the different isoforms of the V-ATPase subunits E, G and a. shows the average expression levels of the three isoforms E, G and a in terms of the arithmetic mean and median, as well as the highest and lowest signal value observed for any given subunit in the analyzed experiments. For further analysis the median value which is less sensitive towards extreme outliers was used to define the average expression level as well as the tissue- and stress-dependent expression levels. Stress-dependent regulation of the VHA-isogenes was analyzed using data from At-genexpress experiments (http://web.uni-frankfurt.de/fb15/botanik/mcb/AFGN/atgenex.htm) concerning control plants and plants stressed by drought, heat, cold, salt and osmotic stress. The data were first analysed separately for shoots and roots in all conditions. However, the only difference in regulation between both tissues was observed in regulation of VHA-G under salt and cold stress (not shown). In all other cases no major tissue-specificity could be seen. Therefore only the results with shoot tissue were presented in detail.
Table II. Amino acid sequence identity and average expression level of VHA subunits. (A) Comparison of amino acid sequence identity between the isoforms. The genes are identified by their At-number. (B, C) Average expression data are given as arithmetic mean and median, maximum and minimum values as calculated from 172 experiments. (B)% indicates the percent expression level compared to the total expression of this particular subunit. (C)%* indicates the percent expression of each subunit present without isoforms as compared to the average expression of all single isoform subunits.
Preference of VHA-isoforms
The relative expression levels of the three E-, G- and a-isoforms differed significantly among each subunit. The relative expression level of subunit E1 was 91.32% of the VHA-E isoforms whereas subunits E2 and E3 showed relative expression levels below 10% (B). The RT-PCR analysis confirmed the preference of VHA-E1 in shoots, while VHA-E3 was predominant in roots. VHA-E3 was expressed about three times weaker than E1, and E2 was not detectable (). On protein level VHA-E1 was the dominantly expressed isoform in both leaves and roots. The transcript results matched the polypeptide abundance in shoots as visualized by 2D gel electrophoresis and subsequent immunodetection (.A1/1.A3). Surprisingly, the relative abundance of VHA-E1 and -E3 protein in roots resembled that in shoots despite the increase in the VHA-E3 to VHA-E1-mRNA-ratio(.B1/1.B3).
Figure 1. Semi-quantification of subunit transcripts by RT-PCR and 2D-Western blot analysis. Results are shown for (A) shoots and (B) roots of plants grown under control (C) and salt stress (SS) conditions. Isoform transcripts were quantified and are depicted as relative percent ratios (A1, B1) or as median signal values (A2, B2). Representative PCR-results are also presented. The data are means of at least 4 RT PCRs from 2 independent experiments. Samples for 2D gel electrophoresis and Western blotting (A3, B3) were obtained from plants grown under control conditions. Western blot analysis was performed using antibody against AtVHA-E (1:3000), detection occurred via chemi-luminescence.
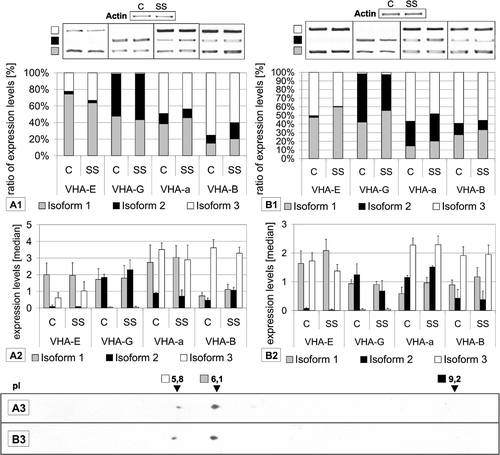
The isoforms 1 of subunit G was the dominant isoform expressed with a relative expression level of 76.44%, followed by isoform 2 (23.43%). Isoform 3 was only marginally expressed. In contrast, RT-PCR revealed the equivalence of VHA-G1 and VHA-G2, but confirmed the low expression level of VHA-G3. VHA-a1 and VHA-a2 had an approximately threefold lower relative expression level compared to VHA-a3 (B). The transcript level of VHA-a3 was most abundant among the a-subunits as demonstrated by RT-PCR. In leaves VHA-a1 showed a higher expression level than VHA-a2, in roots the ratio was inverted (). Although VHA-B isoforms were excluded from the database analysis, the transcript level was analysed by RT-PCR. The transcript pattern of the three VHA-B subunits showed the preference of isoform 3, VHA-B3 represented 75.1% of total VHA-B signal. The relative expression of VHA-B3 was lower in roots. VHA-B1 was expressed in roots at a higher level than in leaves similar to VHA-B2, which was the lowest expressed isoform (). Database analysis of the single copy subunits VHA-A, -C, -D, -F, -H was performed for comparison, the relative expression levels of VHA-C, -D, -F, -H were in the range of 83–95% of their average overall signal intensity, with exception of VHA-A, whose relative expression level was 134% (C). It is tempting to assume that the expression level of VHA-A is higher than that of all other subunits (except E1) to meet the requirements for three copies within the VHA complex.
Tissue specific expression of VHA-isoforms
Data from experiments performed under control conditions using various plant tissues were selected to detect tissue-specific regulation. Except for pollen, VHA-E1 was the dominantly expressed isoform of VHA-E in all tissues. Generally, it contributed more than 80% to total subunit E expression (B). Only in pollen it was down regulated in favour of VHA-E2. Here it was expressed at a level of 45.7% of total VHA-E expression, while VHA-E2 increased to 46.9% and VHA-E3 dropped to 7.4%. Independent of the ratio between the isoforms, the expression of VHA-E1 was highest in petiole and lowest in roots.
Figure 2. Tissue-specific expression of VHA isoforms. Expression levels were calculated as medians of signal values of the respective vha-genes using data from arrays hybridized with samples from the following tissues: leaves (l), roots (r), stems (st), petioles (pe), pollens (po), flowers (f), seedlings (se) and whole plant (wp). Diagrams on the left depict the ratio of expression levels of each isoform in percent of total expression level of all isoforms. In addition, the expression levels are presented as medians of signal values in order to indicate trends for absolute abundance.
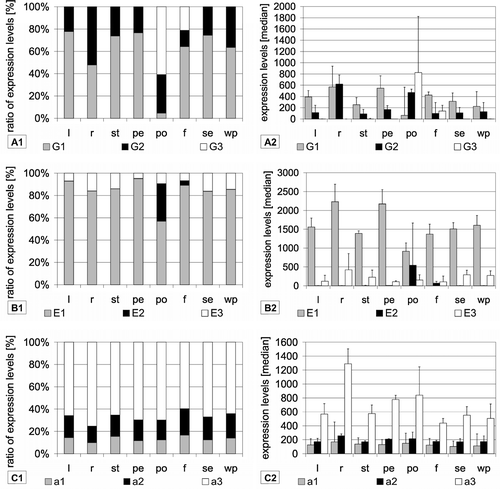
The expression pattern of VHA-G isoforms in shoots resembled that of VHA-E. VHA-G1 was the dominantly expressed isoform, followed by expression of VHA-G2 (). The relative contribution of VHA-G1 in roots and shoots was somewhat higher than measured by the RT PCR approach. The trend of decreased relative levels of VHA-G1 in roots as compared to shoots was in agreement with the RT-PCR results. Expression of VHA-G3 made up about 1% of the total VHA-G in most tissues. Only in pollen with 65.4% and flowers with 16.8% of the total VHA-G expression, VHA-G3 was induced to a significant level (A1). The expression level of VHA-G2 was highest in roots and pollen and remained nearly unchanged in all other tissues (A2).
The VHA-a isoforms showed no tissue-specific regulation. Their expression levels remained in a rather constant ratio in all tissues. For example in leaves VHA-a1, -a2 and -a3 shared 14.3%, 19.3% and 66.4% of total VHA-a expression, respectively (see .C1). Independent on their ratio, only VHA-a3 showed a dynamic regulation in different tissues (.C2).
Environmental stresses
Using analyses of the NASC-array transcriptional database and RT-PCR studies expression of VHA isoforms was investigated in response to the abiotic stresses salt, drought, heat, cold and osmotic stress. The most significant changes regarding the transcript levels of the three isoforms of VHA-E, -G and -a occurred under drought and heat stress. Here the transcript amounts of VHA-E2, -G3 and -a2 showed a pronounced stress-dependent increase. VHA-E1 and E3 accounted for 86.6% and 13.2%, respectively, of total VHA-E transcript under control conditions, whereas the VHA-E2 transcript was close to the detection limit (B). Under heat and drought stress VHA-E2 accumulated to 51% of total signal intensity, wheras VHA-E3 was slightly up-regulated and VHA-E1 was down-regulated (.B1). The total transcript amount of VHA-E remained roughly the same under all conditions, only the ratio of the three isoforms changed (B2). VHA-a1 and VHA-a2 were induced on the expense of VHA-a3, which dropped to 14% whereas VHA-a2 contributed 60% to total transcript amount under heat and drought (C). The expression of VHA-G isoforms was also strongly affected by heat and drought, here VHA-G3 was induced up to 29%, in parallel the transcript level of VHA-G1 decreased to 53%, while VHA-G2 was unaffected (B/3D).
Figure 3. Stress specific expression of VHA-isoforms. Expression levels were calculated as medians of signal values. Data were used from the At-genexpress experiments performed under the following conditions: control (C), salt stress (SS), osmotic stress (OS), cold stress (CS), heat stress (HS) and drought stress (DS). Results are shown separately for experiments done with shoot tissue (A–C) and root tissue (D). Diagrams on the left present the ratio of expression levels of each isoform in percent of total expression level of all isoforms. In addition, the expression levels are presented as medians of signal values in order to present trends for absolute abundance.
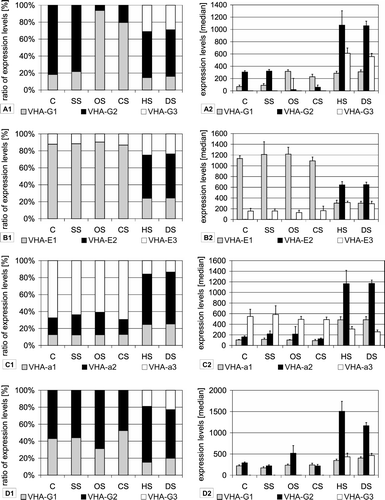
Under control conditions the ratio of VHA-G1 to VHA-G2 transcript levels was 4:1, in cold and osmotically stressed plants the ratio was inversed in shoots and VHA-G2 became the predominant isoform (B). In contrast, the ratio was unaffected in roots by the stress treatments (D).
Correlation analysis of expression pattern
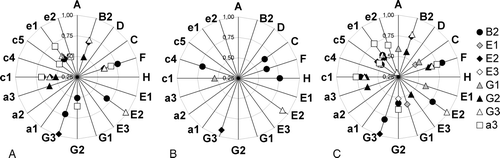
The correlation analysis was first performed on data from 172 experiments, regardless of the experimental conditions, and subsequently for control and the various stress conditions separately. Those co-regulations that were identified in the general approach could also be seen in the analysis of the control experiments. Under control conditions the correlation of subunits was sligthly stronger than in the general approach and five additional weak correlations could be observed, mostly between subunits of the peripheral stalks. The correlation analysis of the experiments done under various stress conditions showed a drastically reduced co-regulation. Only six co-regulations remained as identified by high correlation factors. Of these six, three were also found in the general analysis and that of the control experiments.
VHA-B2 was co-regulated with subunits assembling the peripheral stalk of the V-ATPase complex, but also with some V0-subunits (). The strongest correlation of VHA-B2 with subunits of the peripheral stalks was seen with VHA-E2 and -G3, which in turn seemed to be co-regulated in a tissue- and stress-dependent manner. The correlation between VHA-B2 and VHA-F and -c1, respectively, was also strong. Additionally a weak correlation of subunits VHA-G2 and -e1 could be observed (A, B). Under stress co-regulation shifted among the subunits of the peripheral stalks towards the subunits VHA-C, -F and -H, with the strongest coregulation between VHA-B2 and -H (r=0.76). Co-regulation between VHA-B2 and V0 changed here from VHA-c1 and -e1 to VHA-c4 (C). As already indicated by the tissue specifity, VHA-E2 was strongly co-regulated with VHA-G3. This co-regulation was found in all three correlation analysis approaches, and the correlation factor was always higher than 0.9. VHA-E2 correlated with the same set of subunits as VHA-B2 and VHA-G3, namely VHA-e1, -c1, -F and -B2 (A, B). On the other hand VHA-G2 expression correlated mainly with V0 subunits like VHA-a3, VHA-c1 and VHA-e1, but also with VHA-B2 and -F. Nevertheless, all of these correlations had a correlation factor in the range of 0.5–0.6, displaying weak correlations of the transcript levels of these subunits (A, B). Under stress conditions none of these co-regulations could be found at a significant level, and under control conditions alone they were only slightly higher than in the general approach (). VHA-a3 correlated mostly with subunits of the V0-domain but also to subunits VHA-F and -G2. The highest co-regulation was detected with VHA-e1 (r=0.71), followed by VHA-c1, -F, -G2, -c5 and e2 (A, B).
Figure 4. Correlation analysis of vha-gene expression among 172 experiments. For each subunit the signal values of all experiments were correlated pairwise with each subunit. The diagrams show only correlation factors (r) higher than 0.5, which point to a significant co-regulation of both genes. The correlation factor (r) values increase from the centre to the border of the diagram. (A) The correlation among all experiments (172). (B) Under stress conditions (85) and (C) for control experiments only (87).
Discussion
The relative expression levels of the E-, G- and a-isoforms in A. thaliana differed significantly among each subunit, suggesting a specific regulation even under control conditions. A strong regulation was observed after application of abiotic stress factors, where the expression levels of VHA-isoforms changed in a very explicit way. The database analysis showed a change in expression under heat and drought for isoforms of all three subunits, in both roots and leaves. Under this conditions the pollen specific isoforms VHA-E2 and -G3 as well as VHA-a2 were up-regulated, whereas salinity did not affect the expression level of VHA-a, VHA-E and VHA-G. The RT-PCR results mostly confirmed the results of the database analysis and revealed that salt stress does not significantly influence the expression levels of the subunits. In contrast, VHA-B2 was induced twofold by salinity in shoots (A). With the exception of VHA-B2 the V-ATPase subunit transcripts did not respond to salt and osmotic stress, while the VHA-isoforms E2, E3, G2, G3, a1 and a2 responded to drought and heat stress. Since V-ATPase transcript levels do not respond to salinity in Arabidopsis (cf. also VHA-D, Kluge et al. Citation1999), up-regulation of VHA-isoforms under drought and heat stress conditions must be triggered independently using distinct signalling pathways. V-ATPase induction by NaCl occurs independent of ABA in juvenile M. crystallinum, indicating the presence of an alternative signalling pathway besides drought-induced ABA-signaling (Barkla et al. Citation1999). Since single subunit isoforms were up-regulated, but other isoforms were unaffected or down-regulated in response to stress, up-regulation of transcript levels for VHA-E, -G and -a isoforms could indicate an increased turnover of distinct subunits at the protein level. Alternatively, the assembly of specific V-ATPase complexes might occur which allow at least partial acclimation to accomplish the stress-related metabolic need.
VHA-B isogenes
The ATP-binding subunit VHA-B is highly conserved among all eukaryotes with sequence identity in the range of 86–95% on the amino acid level (). Thus, VHA-B from Zostera marina L. was able to complement yeast Vma2p mutants (Alemzadeh et al. Citation2006). Two cysteine residues are conserved in VHA-B () which might link VHA-B to redox regulation as reported for VHA-A and VHA-E (Kawamura et al. Citation2001, Tavakoli et al. Citation2001).
Figure 5. Amino acid sequence alignment of VHA-B subunits. Displayed is the alignment of VHA-B amino acid sequences from Neurospora crassa (Nc), Saccharomyces cereviseae (Sc), Homo sapiens (Hs), Oryza sativa (Os) and Arabidopsis thaliana (At). The alignment was generated with ClustalW.
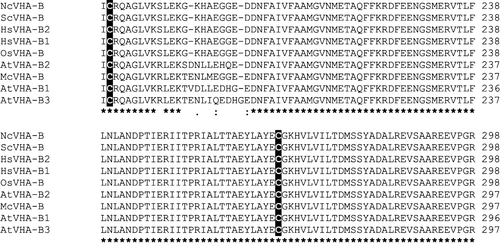
Although VHA-B was shown to be involved in regulation and targeting (Puopolo et al. Citation1992, Vasileya et al. Citation2000, Holliday et al. Citation2000, Zuo et al. Citation2006), the subunit was only hardly characterized up to now. Isoforms of VHA-B were already identified in barley roots in the early 1990s (Berkelmann et al. Citation1994) and a differential expression of VHA-B was reported for two barley cultivars in response to salinity (Wei et al. Citation2001). In A. thaliana, only VHA-B2 was affected by salinity as demonstrated by the data evaluation from cDNA arrays and the RT-PCR analysis. However, VHA-B2 was marginally expressed and the physiological relevance of its salt-dependent induction appears questionable.
VHA-E isogenes
Strompen et al. (Citation2005) described pollen-specificity of VHA-E2 expression while VHA-E3 mainly accumulated in the endosperm and surrounding maternal tissues of the developing seed. VHA-E1 was shown to be the major isoform during embryogenesis required for functionality of the secretory system during somatic development but not in the haploid gametophytes (Strompen et al. Citation2005). Here, the isoforms VHA-E1 and -E3 showed relatively constant transcript ratios in the database analysis. In line with published results, VHA-E1 was the predominantly expressed isoform in leaves as semi-quantified via RT-PCR. In pea, VHA-E isoforms differ in tissue specificity. One isoform occurs ubiquitously in all tissues, whereas a second is absent from cotyledons of etiolated seedlings and can barely be detected in leaves of mature plants (Kawamura et al. Citation2000). Interestingly, circumstantial evidence suggests that the subunit composition also alters enzymatic properties, since proton pumping activity was higher in preparations of leaves than in epicotyls (Kawamura et al. Citation2000). In A. thaliana, the isoform sequence identity ranges between 81% (E1/E3), 71% (E1/E2) and 64% (E2/E3). The pollen-specific isoform E2 has the lowest degree of identity with both other isoforms, e.g., it also lacks Cys121. Since Cys-residues in VHA-E are known to be involved in catalysis (Kawamura et al. Citation2001) and redox regulation of V-ATPase (Dietz et al. Citation1998, Tavakoli et al. Citation2001), the absence of Cys121 might affect V-ATPase function. In M. crystallinum, VHA-E transcripts differently accumulated in vascular bundles, epidermis and cortex cells of leaves and roots (Golldack & Dietz Citation2001). Cell type-specific expression in the tissues cannot be distinguished in our approach. The Western blot analysis of VHA-E isoforms demonstrated that transcript abundances not directly translate into protein abundances. Apparently, translational and posttranslational regulation differs between roots and shoots.
VHA-G isogenes
The tissue specific expression of the VHA-G isogenes resembled that of the VHA-E with VHA-G3 being exclusively expressed in pollen and flowers, corresponding to VHA-E2. Thus, the observed expression level of VHA-G3 was low in most tissues. This confirmes the findings of Becker et al. (Citation2003), who found VHA-G3 to be one of 162 genes expressed only in pollen, but not in vegetative tissues. The database analysis favours VHA-G1 as the dominantly expressed isoform in leaves and roots, while G2 substitutes for G1 in roots to a major extent. In contrast, RT-PCR results revealed an even expression of VHA-G1 and -G2 in both roots and leaves.
VHA-a isogenes
Up to now, VHA-a isoforms were demonstrated to be organelle- but not tissue-specific. Similar to the presence of tonoplast- and Golgi-specific VHA-a isoforms in yeast, an organelle-specific localization was shown for the plant VHA-a isoforms as well (Kluge et al. Citation2004, Dettmer et al. Citation2006). The database analysis confirmed the absence of a major tissue-specific expression of VHA-a isoforms, albeit by RT-PCR VHA-a3 was identified as the isoform predominantly expressed in roots. In leaves transcript levels of vacuolar VHA-a3 and the trans Golgi network (TGN)-specific VHA-a1 were nearly equal. This might be caused by a differential TGN/tonoplast ratio in roots and leaves rather than a true tissue specificity.
VHA isoforms represent general feature of plant V-ATPase
The fact that VHA-E1/-E3 and VHA-G1/-G2 did not show tissue specific expression may point to a house-keeping function being subject to little regulation, or to distinct subcellular roles. For example organelle specificity was shown for VHA-G in tobacco (Rouquié et al. Citation1998). It is concluded that the presence of three isoforms each of VHA-a, -B, -G and -E in the A. thaliana genome is not related to an exclusive interaction between specific subunit isoforms in the holocomplex but implicates functional differentiation at three different levels, (i) subcellular localization, (ii) cell-type/developmental specificity and (iii) stress response. The three VHA-a isoforms as part of the V0-complex are suggested to realize both a subcellular and a stress-dependent specificity in expression and function. VHA-E and VHA-G appear to be partly redundant; E1 and E3, as well as G1 and G2 may replace each other and are suggested to have a particular function under stress, while E2 and G3 have high cell specificity as well as stress responsiveness. The results may be compared with a recent proteome analysis on rosette leaf vacuoles (Carter et al. Citation2004) where in addition to each of the single gene subunits VHA-A, -C, -D, -F and -H, the following isoforms of the multiple subunits were detected VHA-B1, -B3, -E1, -G1, -G2, -a2 and -a3. This confirms the results presented here, e.g., that VHA-E1 apparently has no specificity towards a particular VHA-G-isoform.
The possibility of V-ATPase complexes preferentially containing specific subunit isoforms in an organelle- and tissue-dependent manner was previously reported for VHA-a and -E, respectively (Kawamura et al. Citation2000, Strompen et al. Citation2005, Dettmer et al. Citation2006). VHA-isoforms were also identified in the monocot Oryza sativa, here VHA-a and VHA-E are encoded by three genes each similar to A. thaliana, but only two genes were identified coding for VHA-B and VHA-G (). This comparison allows the conclusion that there is no need for three isogenes each for VHA-a, -B, -G and -E in higher plants.
Table III. Compilation of the rice VHA-genes. Each Arabidopsis thalianaVHA-cDNA was BLAST-searched against the rice genome or taken from the annotated TIGR rice genome database (db). Coding regions with significant similarity to A. thalianaVHA-genes were included in the table. VHA genes are given with Os-numbers from the TIGR db and the NCBI db. Additionally the number of amino acids (aa), the predicted molecular weight (MW) and the isoelectric point (pI) were calculated with the protparam tool. The identity of the rice sequences to A. thaliana was determined using ClustalW.
The correlation analysis also provides evidence for tissue-dependent isoform co-regulation, but takes this concept a step further by sorting subunit isogenes in co-regulated subsets. The observation of only a very limited number of co-regulations between V-ATPase subunits under stress conditions can be explained by the number of different experimental conditions used in this analysis. These findings reveal interesting relationships even though they urge further investigation, for example by use of in vivo FRET to analyse isoform specific composition of V-ATPase complexes (Seidel et al. Citation2004, Citation2005).
Acknowledgements
This work was performed within the framework of the SFB 613, TP A5.
References
- Alemzadeh A, Fujie M, Usami S, Yoshizaki T, Oyama K, Kawabata T, Yamada T. ZMVHA-B1, the gene for subunit B of vacuolar H+-ATPase from the eelgrass Zostera marina L. is able to replace vma2 in a yeast null mutant. J Biosci Bioengin 2006; 102: 390–395
- Bageshwar UK, Taneja-Bageshwar S, Moharram HM, Binzel ML. Two isoforms of the A subunit of the vacuolar H+-ATPase in Lycopersicon esculentum: highly similar proteins but divergent patterns of tissue localization. Planta 2005; 220: 632–643
- Barkla BJ, Vera-Estrella R, Maldonado-Gama M, Pantoja O. Abscisic acid induction of vacuolar H+-ATPase activity in Mesembryanthemum crystallinum is developmentally regulated. Plant Physiol 1999; 120: 811–820
- Becker JD, Boavida LC, Carneiro J, Haury M, Feijó JA. Transcriptional profiling of Arabidopsis tissues reveals the unique characteristics of the pollen transcriptome. Plant Physiol 2003; 133: 713–725
- Berkelmann T, Houtchens KA, DuPont FM. Two cDNA clones encoding isoforms of the B subunit of the vacuolar ATPase from barley roots. Plant Physiol 1994; 104: 287–288
- Carter C, Pan S, Zouhar J, Avila EL, Girke T, Raikhel NV. The vegetative vacuole proteome reveals predicted and unexpected proteins. Plant Cell 2004; 16: 3285–3303
- Coker JS, Jones D, Davies E. Identification, conservation and relative expression of V-ATPase cDNAs in tomato plants. Plant Mol Biol Reporter 2003; 21: 145–158
- Craigon DJ, James N, Okyere J, Higgins J, Jotham J, May S. NASCArrays: a repository for microarray data generated by NASC's transcriptomic service. Nucleic Acids Res 2004; 32: 575–577
- Dettmer J, Hong-Hermesdorf A, Stierhof YD, Schumacher K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 2006; 18: 715–730
- Dettmer J, Schubert D, Calvo-Weimar O, Stierhof YD, Schmidt R, Schumacher K. Essential role of the V-ATPase in male gametophyte development. Plant J 2005; 41: 117–124
- Dietz KJ, Heber U, Mimura T. Modulation of the vacuolar H+-ATPase by adenylates as basis for the transient CO2-dependent acidification of the leaf vacuole upon illumination. Biochim Biophys Acta 1998; 1373: 87–92
- Domgall I, Venzke D, Lüttge U, Ratajczak R, Böttcher B. Three dimensional map of a plant V-ATPase based on electron microscopy. J Biol Chem 2002; 277: 13115–13121
- Féthière J, Venzke D, Diepholz M, Seybert A, Geerlof A, Gentzel M, Wilm M, Böttcher B. Building the stator of the yeast vacuolar ATPase. J Biol Chem 2004; 279: 40670–40676
- Gogarten JP, Fichmann J, Braun Y, Morgan L, Styles P, Taiz SL, Delapp K, Taiz L. The use of antisense mRNA to inhibit the tonoplast H+ ATPase in carrot. Plant Cell 1992; 4: 851–864
- Golldack D, Dietz KJ. Salt-induced expression of the vacuolar H+-ATPase in the common ice plant is developmentally controlled and tissue specific. Plant Physiol 2001; 125: 1643–1654
- Golldack D, Popova OV, Dietz KJ. Mutation of the matrix metalloproteinase At2-MMP inhibits growth and causes late flowering and early senescence in Arabidopsis. J Biol Chem 2002; 277: 5541–5547
- Hasenfratz MP, Tsou CL, Wilkins TA. Expression of two related vacuolar H+-ATPase 16-kilodalton proteolipid genes is differentially regulated in a tissue specific manner. Plant Physiol 1995; 108: 1395–1404
- Heiber I, Ströher E, Raatz B, Busse I, Kahmann U, Bevan MW, Dietz KJ, Baier M. The Rimb (Redox Imbalanced)-mutants of Arabidopsis differentiate signalling pathways for Redox-regulation of chloroplast antioxidant enzymes. Plant Physiol 2007; 143: 1774–1788
- Holliday LS, Lu M, Lee BS, Nelson RD, Solivan S, Zhang L, Gluck SL. The aminoterminal domain of the B subunit of vacuolar H+-ATPase contains a filamentous actin binding site. J Biol Chem 2000; 275: 32331–32337
- Kandlbinder A, Finkemeier I, Wormuth D, Hanitzsch M, Dietz KJ. The antioxidant status of photosynthesizing leaves under nutrient deficiency: redox regulation, gene expression and antioxidant activity in Arabidopsis thaliana. Physiologia Plant 2004; 120: 63–73
- Kawamura Y, Arakawa K, Maeshima M, Yoshida S. Tissue specificity of E subunit isoforms of plant vacuolar H+-ATPase and existence of isotype enzymes. J Biol Chem 2000; 275: 6515–6522
- Kawamura Y, Arakawa K, Maeshima M, Yoshida S. ATP analogue binding to the A subunit induces conformational changes in the E subunit that involves a disulfide bond formation in plant V-ATPase. Eur J Biochem 2001; 268: 2801–2809
- Kawasaki-Nishi S, Bowers K, Nishi T, Forgac M, Stevens TH. The amino-terminal domain of the vacuolar proton-translocating ATPase a subunit controls targeting and in vivo dissociation, and the carboxyl-terminal domain affects coupling of proton transport and ATP-hydrolysis. J Biol Chem 2001; 276: 47411–47420
- Kluge C, Golldack D, Dietz KJ. Subunit D of the vacuolar H+-ATPase of Arabidopsis thaliana. Biochim Biophys Acta 1999; 1419: 105–110
- Kluge C, Lamkemeyer P, Tavakoli N, Golldack D, Kandlbinder A, Dietz KJ. cDNA cloning of 12 subunits of the V-type ATPase from Mesembryanthemum crystallinum and their expression under stress. Mol Membr Biol 2003; 20: 171–183
- Kluge C, Seidel T, Bolte S, Sharma SS, Hanitzsch M, Satiat-Jeunemaitre B, Ross J, Sauer M, Golldack D, Dietz KJ. Subcellular distribution of the V-ATPase complex in plant cells, and in vivo localisation of the 100 kDa subunit VHA-a within the complex. BMC Cell Biol 2004; 5: 1–18
- Kluge C, Lahr L, Hanitzsch L, Bolte S, Golldack D, Dietz KJ. New insight into the structure and regulation of the plant vacuolar V-ATPase. J Bioenerg Biomemb 2003; 35: 377–388
- Landolt-Marticorena C, Williams KM, Correa J, Chen W, Manolson MF. Evidence that the NH2 terminus of Vph1p, an integral subunit of the vector V0 sector of the yeast V-ATPase, interacts directly with the Vma1p and Vma13p subunits of the V1 sector. J Biol Chem 2000; 275: 15449–15457
- Matsuoka K, Higuchi T, Maeshima M, Nakamura K. A vacuolar-type H+-ATPase in a nonvacuolar organelle is required for the sorting of soluble vacuolar protein precursors in tobacco cells. Plant Cell 1997; 9: 533–546
- Padmanaban S, Lin X, Perera I, Kawamura Y, Sze H. Differential expression of vacuolar H+-ATPase subunit c genes in tissues active in membrane trafficking and their roles in plant growth as revealed by RNAi. Plant Physiol 2004; 134: 1514–1526
- Puopolo K, Kumamoto C, Adachi I, Magner R, Forgac M. Differential expression of the “B” subunit of the vacuolar H(+)-ATPase in bovine tissues. J Biol Chem 1992; 267: 3696–3706
- Rouquié D, Toumarie-Roux C, Szponarski W, Rossignol M, Doumas P. Cloning of the V-ATPase subunit G in plant: functional expression and sub-cellular localization. FEBS Lett 1998; 437: 287–292
- Seidel T, Golldack D, Dietz KJ. Mapping of C-termini of V-ATPase subunits by in vivo-FRET measurements. FEBS Lett 2005; 579: 4374–4382
- Seidel T, Kluge C, Hanitzsch M, Ross J, Sauer M, Dietz KJ, Golldack D. Colocalization and FRET-analysis of subunits c and a of the vacuolar H+-ATPase in living plant cells. J Biotechnol 2004; 112: 165–175
- Smart LB, Vojdani F, Maeshima M, Wilkins TA. Genes involved in osmoregulation during turgor-driven cell expansion of developing cotton fibers are differentially regulated. Plant Physiol 1998; 116: 1539–1549
- Strompen G, Dettmer J, Stierhof YD, Schumacher K, Jürgens G, Mayer U. Arabidopsis vacuolar H+-ATPase subunit E isoform 1 is required for Golgi organization and vacuolar function in embryogenesis. Plant J 2005; 41: 125–132
- Sze H, Schumacher K, Müller ML, Padmanaban S, Taiz L. A simple nomenclature for a complex proton pump: VHA genes encode the vacuolar H+-ATPase. Trends Plant Sci 2002; 7: 157–161
- Sze H, Li X, Palmgren MG. Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. Plant Cell 1999; 11: 677–690
- Tavakoli N, Kluge C, Golldack D, Mimura T, Dietz KJ. Reversible redox control of plant vacuolar H+-ATPase activity is related to disulfide bridge formation in subunit E as well as subunit A. Plant J 2001; 28: 51–59
- Tyagi W, Rajagopal D, Singla-Pareek SL, Reddy MK, Sopory SK. Cloning and regulation of a stress-regulated Pennisetum glaucum vacuolar ATPase c gene and characterization of its promotor that is expressed in shoot hairs and floral organs. Plant Cell Physiol 2005; 46: 1411–1422
- Vasileya E, Liu Q, MacLeod KJ, Baleja JD, Forgac M. Cysteine scanning mutagenesis of the noncatalytic nucleotide binding site of the yeast V-ATPase. J Biol Chem 2000; 275: 255–260
- Viereck R, Kirsch M, Löw R, Rausch T. Down-regulation of plant V-type H+-ATPase genes after light induced inhibition of growth. FEBS Lett 1996; 384: 285–288
- Wei W, Bilsborrow P, Hooley P, Fincham D, Forster B. Variation between two near isogenic barley (Hordeum vulgare) cultivars in expression of the B subunit of the vacuolar ATPase in response to salinity. Hereditas 2001; 135: 227–231
- Wilkens S, Inoue T, Forgac M. Three-dimensional structure of the vacuolar ATPase from electron microscopy. J Biol Chem 2004; 279: 41942–41949
- Zuo J, Jiang J, Chen SH, Vergara S, Gong Y, Xue J, Huang H, Kaku M, Holliday LS. Actin binding activity of subunit B of vacuolar H+-ATPase is involved in its targeting to ruffled membranes of osteoclasts. J Bone Miner Res 2006; 21: 714–721