Abstract
Many members of the TRP superfamily oligomerize in the ER before trafficking to the plasma membrane. For membrane localization of the non-selective cation channel TRPV4 specific domains in the N-terminus are required, but the role of the C-terminus in the oligomerization and trafficking process has been not determined until now. Therefore, the localization of recombinant TRPV4 in two cell models was analyzed: HaCaT keratinocytes that express TRPV4 endogenously were compared to CHO cells that are devoid of endogenous TRPV4. When deletions were introduced in the C-terminal domain three states of TRPV4 localization were defined: a truncated TRPV4 protein of 855 amino acids was exported to the plasma membrane like the full-length channel (871 aa) and was also functional. Mutants with a length of 828 to 844 amino acids remained in the ER of CHO cells, but in HaCaT cells plasma membrane localization was partially rescued by oligomerization with endogenous TRPV4. This was confirmed by coexpression of recombinant full-length TRPV4 together with these deletion mutants, which resulted in an almost complete plasma membrane localization of both proteins and significant FRET in the plasma membrane and the ER. All deletions upstream of amino acid 828 resulted in total ER retention that could not rescued by coexpression with the full-length protein. However, these deletion mutants did not impair export of full-length TRPV4, implying that no oligomerization took place. These data indicate that the C-terminus of TRPV4 is required for oligomerization, which takes place in the ER and precedes plasma membrane trafficking.
| Abbreviations | ||
| aa | = | amino acid |
| CFP | = | cyan fluorescent protein |
| ER | = | endoplasmatic reticulum |
| FRET | = | Förster resonance energy transfer |
| GFP | = | green fluorescent protein |
| LSM | = | confocal laser scanning microscopy/microscope |
| MIP | = | maximum intensity projection |
| RFP | = | monomeric red fluorescent protein |
| ROI | = | region of interest |
| TRPV4 | = | transient receptor potential vanilloid 4 |
| YFP | = | yellow fluorescent protein |
Introduction
The transient receptor potential (TRP) superfamily encompasses now seven subfamilies. TRPV4 (transient receptor potential vanilloid 4) is one of the six members of the TRPV subfamily and acts as a non-selective cation channel (Pedersen et al. [Citation2005]). Activation of TRPV4 can be achieved by hypotonicity, moderate heat, synthetic ligands like 4α-phorbol 12,13-didecanoate (4α-PDD) and endogenous agonists like arachidonic acid (Güler et al. [Citation2002], Watanabe et al. [Citation2002a], Watanabe et al. [Citation2002b], Watanabe et al. [Citation2003]). TRPV4 exhibits a functional role in thermosensation (Lee et al. [Citation2005]), cell volume regulation (Becker et al. [Citation2005]), epithelial permeability (Reiter et al. [Citation2006]), cystic fibrosis (Arniges et al. [Citation2004]), nociception (Alessandri-Haber et al. [Citation2005]) and most probably also mechanotransduction (Gao et al. [Citation2003], Andrade et al. [Citation2005], Kohler et al. [Citation2006]). According to the different functions of TRPV4, expression of the channel has been detected in various tissues. The predicted structure of TRPV4 displays six membrane-spanning domains with a pore loop; the N-terminal domain with three ankyrin repeats as well as the C-terminal domain are located within the cytoplasm (Liedtke et al. [Citation2000], Wissenbach et al. [Citation2000]). It has been shown that TRPV1, TRPV5 and TRPV6 form tetramers (Kedei et al. [Citation2001], Jahnel et al. [Citation2001], Hoenderop et al. [Citation2003]) and experimental evidence suggests that also TRPV4 exists in the plasma membrane in an oligomeric state, preferentially as a homooligomer (Hellwig et al. [Citation2005], Arniges et al. [Citation2006]). Assembly of TRP channels is a complex process (Ambudkar [Citation2007], Schindl & Romanin [Citation2007]). In TRPV4 the deletion of the ankyrin domains in the N-terminus resulted in the loss of oligomerization and retention of the channel in the ER (Arniges et al. [Citation2006]). Replacement of the N-terminal domain with the one of TRPV1 lead to reduced assembly (Hellwig et al. [Citation2005]), indicating a significant role of the N-terminal domain for correct localization and oligomerization of TRPV4. The ankyrin domains in the N-terminus are also engaged in the assembly of TRPV5 and TRPV6 (Chang et al. [Citation2004], Erler et al. [Citation2004]). However, the role of the C-terminal domain of TRPVs, especially of TRPV4, is less clear. FRET studies with a chimeric channel where the transmembrane region and the C-terminal domain of TRPV4 had been replaced with the respective domains of TRPV1, still showed interaction with the wild type TRPV4 as measured by FRET, but about 25% less as when the C-terminal domain of TRPV4 was present in the chimeric construct (Hellwig et al. [Citation2005]). In oligomerization of TRPV1 a coiled-coil region in the C-terminus termed TRP-like domain is involved (Garcia-Sanz et al. [Citation2004], Hellwig et al. [Citation2005]) and also in TRPM8 a coiled-coil domain in the C-terminus is participating in the assembly (Erler et al. [Citation2006], Tsuruda et al. [Citation2006]).
Therefore, we determined the impact of the C-terminal domain on the assembly and the localization of TRPV4. As a cellular model system with endogenous TRPV4 expression HaCaT keratinocytes were used. In contrast, CHO cells are devoid of endogenous TRPV4 (Liedtke et al. [Citation2000], Suzuki et al. [Citation2003], Becker et al. [Citation2005]), thus offering a complementary approach for analyzing TRPV4 assembly in a reconstituted cell system. Localization studies of full-length TRPV4 and of various C-terminal deletion mutants by confocal microscopy provided a powerful tool to analyze the involvement of different regions of the C-terminus in assembly and trafficking of TRPV4. Hereby we observed three different states localization of TRPV4 deletions mutants: proteins truncated after aa 855 were exported like the full-length TRPV4. Further truncation till aa 828 still allowed ER export when the full-length TRPV4 was coexpressed. Further deletions resulted in complete ER retention of the mutants also after coexpression, indicating that the oligomerization process was disturbed. In addition, all truncated channels that were integrated into the plasma membrane were fully functional.
Material and methods
Plasmids and deletion mutants
Construction of TRPV4-GFP has been described previously (Becker et al. [Citation2005]). Shortly, the ORF of human TRPV4 was amplified from HaCaT cDNA and cloned in the BglII and BamH1 sites of the eukaryotic expression vector pEGFP N3 (Clontech, California, USA). To obtain RFP-TRPV4, TRPV4-GFP was digested with with BglII and BamH1 and the TRPV4 insert was cloned in the BamH1 site of the vector dsRed-Monomer Fluorescent Protein C1 (Clontech, California, USA) (A).
Figure 1. Analysis of the C-terminal domain of TRPV4. (A) Schematic drawing of the CFP, GFP, YFP and RFP-tagged TRPV4 constructs. (B) Various tagged sequential C-terminal deletion mutants were constructed (see Material and methods).
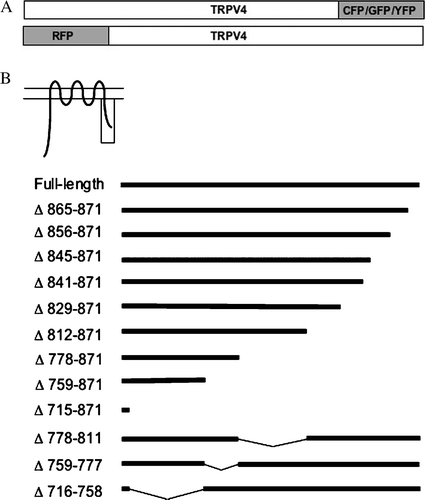
The deletion mutants Δ865-871-GFP, Δ856-871-GFP, Δ845-871-GFP, Δ841-871-GFP, Δ829-871-GFP, Δ812-871-GFP, Δ778-871-GFP, Δ759-871-GFP and Δ715-871-GFP were constructed the following way: TRPV4-GFP was digested with EcoRI (internal site) and BamH1, resulting in a new vector that was missing about 1000 bp in the 3′ region of TRPV4. The respective truncated TRPV4 3′ regions were amplified by PCR and cloned in the EcoRI and BamH1 sites of the vector. To construct Δ778-811-GFP, Δ759-777-GFP and Δ716-758-GFP the respective plasmids Δ778-871-GFP, Δ759-871-GFP and Δ715-871-GFP were used as vectors. These plasmids were digested with BamH1 and NotI and the respective inserts were amplified by PCR with Δ865-871-GFP as template and cloned in the corresponding vectors. For FRET experiments TRPV4-CFP, TRPV4-YFP, Δ841-871-YFP and Δ778-871-YFP were used. Construction of TRPV4-CFP has been reported before (Ramadass et al. [Citation2007]). To obtain the YFP-constructs, TRPV4-GFP, Δ841-871-GFP and Δ778-871-GFP were digested with BamH1 and Not1 to remove the GFP tag. This was than replaced with a CFP tag amplified by PCR with pECFP-N1 (Clontech, California, USA) as template. The identity of all constructs was verified by sequencing. To verify plasma membrane localization cells were transfected with pAcGFP1-Mem (Clontech, California, USA).
Cell culture and transfection
HaCaT cells (human keratinocyte cell line; Boukamp et al. [Citation1988]) (passages 47 to 70) and CHO-K1 cells (passages 40 to 70) were cultivated in Keratinocyte SFM medium (Invitrogen, Karlsruhe, Germany), and Ham′s F-12 (Invitrogen) medium respectively, containing 10 % FCS and 10 mM Hepes (Invitrogen) at 37°C in 5% CO2 atmosphere. On the day of transfection, cells were seeded on coverslips. 8 h after seeding cells were either single or double transfected using Effectene transfection reagent (Quiagen) according to manufacturer's instructions. 24 h after transfection medium containing the transfection reagent was replaced by normal growth medium. Cells were fixed 48 h after transfection (for kinetic studies at the indicated time points) with 4% Paraformaldehyde in Phosphate Buffered Saline (PBS; Invitrogen, mM 1.5 KH2PO4, 155.2 NaCl, 2.7 Na2HPO4, pH 7.2) for 20 min at room temperature, washed 3 times with PBS and were embedded in Mowiol.
For FRET measurements transfection via electroporation was performed. 5×107 CHO cells were electroporated with 30–50 µg of each plasmid) in an electro cell manipulator (settings: 129 Ω, 250 µF, 423 V, resulting in an impulse of 4.0–4.5 ms; BTX electro cell manipulator 600, Holliston, USA). The cells were transferred into 10 ml recovery medium (CHO growth medium with 3 mM EGTA) and incubated for 45 min in the incubator at 37°C and 5% CO2. Cells were centrifuged for 5 min and the pellet was re-suspended in 4 ml of CHO growth medium and seeded into Petri dishes onto coverslips. 48 h after electroporation cells were fixed as described above.
Microscopy
The fixed cells were imaged using 63× (plan apochromate, 1.4 NA) objective mounted on a Leica SP5 laser scanning microscope (LSM) (Leica, Bensheim, Germany). Cells transfected with GFP constructs were excited at 488 nm and the emission was recorded at 495–530 nm, while RFP-TRPV4 transfected cells were excited at 561 nm and the emission recorded at 575–620 nm. Maximum intensity projections (MIP) consisted of 20–25 single optical z-slices laid through each cell. Images were deconcolved using Autodeblur gold (version ×1.4.1, Media-Cybernetics, Silverspring, USA; five iterations with medium noise level) and visualized with Imaris (version 5.0.3, Bitplane, Saint Paul, USA) and Photoshop (version 9.0, Adobe). The images shown were representatives of at least three independent transfections. To determine the localization of TRPV4 and the deletion mutants 30 cells or more derived from at least three independent transfections were analyzed by LSM and expression of TRPV4 and the mutants was classified into plasma membrane, ER or mixed (both plasma membrane and ER localization). To confirm the plasma membrane localization of TRPV4 the ‘Image-iT™ LIVE Plasma Membrane and Nuclear Labeling Kit counterstains for GFP-expressing cells’ (Invitrogen) was used.
Acceptor bleaching FRET
Cells used for acceptor bleaching FRET experiments were transfected by electroporation as described above. Acceptor bleaching was performed using a Leica SP5 microscope with the 63× 1.4 NA plan-apochromate objective. For excitation of CFP the 458 nm laser line- and for excitation of YFP the 514 nm Argon laser line was used. CFP emission was monitored in the range of 465–505 nm, while YFP emission was monitored between 520–560 nm. The bleaching impulse at 514 nm was chosen in a way to reduce acceptor fluorescence intensity around 30–70%.
The acceptor bleaching FRET efficiency (E) was estimated by use of the formula:where IDab is the emission intensity of the donor after- and IDbb the donor emission intensity before acceptor bleaching.
[Ca2 + ]i measurements
Transiently transfected CHO cells were plated in 85 mm dishes onto glass cover slips. [Ca2 + ]i measurements in single cells were carried out 48–72 h after transfection using the fluorescence indicator fura-2-AM (Sigma-Aldrich, Taufkirchen, Germany) in combination with a monochromator-based imaging system (Attofluor Ratio Vision system) attached to an inverted microscope (Axiovert 100, Carl Zeiss, Oberkochen, Germany). Transfected CHO cells were loaded with 4 µM fura-2-AM and 0.01 % Pluronic F-127 (Sigma-Aldrich) for 30 min at 37°C in a standard solution composed of mM 138 NaCl, 6 KCl, 1 MgCl2, 2 CaCl2, 5.5 glucose, and 10 HEPES (adjusted to pH 7.4 with NaOH). Coverslips were then washed in this buffer, and mounted in a perfusion chamber on the microscope stage for 20 min before the experiment started. For [Ca2 + ]i measurements, fluorescence was excited at 340 nm and 380 nm. After 50 s osmolarity was lowered from 300 mOsm/1 to 200 mOsm/1 by adding pre-warmed distilled water to the medium. The fluorescence ratio F340/ F380 was calculated and plotted. For the delta values shown in , the maximum F340/ F380 values were determined within 150 s after hypotonic stimulus and subtracted by the average control values before lowering osmolarity for each cell. In all experiments, transfected cells were identified by their GFP fluorescence at an excitation wavelength of 480 nm.
Statistics and iterations
The presented images, blots and tables are representatives, derived from at least three independent transfections for each condition. Analysis of variance (ANOVA) was performed and differences were considered to be statistically significant, when p≤0.01.
Results
Localization of full-length TRPV4 and deletion mutants in different cell types
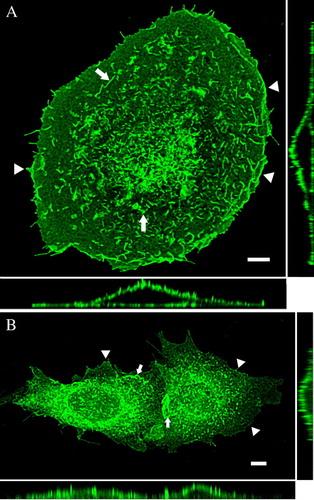
To determine the role of the C-terminus of TRPV4 in oligomerization and membrane trafficking, TRPV4 was tagged with different fluorescent tags (A). Based on the construct TRPV4-GFP sequential C-terminal domain deletions mutants were constructed (B). The intracellular localization of TRPV4-GFP and of the deletion mutants was analyzed by confocal laser scanning microscopy (LSM). For this purpose, the different constructs were transiently transfected into two different mammalian expression systems. HaCaT cells express TRPV4 endogenously, while CHO cells are devoid of endogenous TRPV4 (Liedtke et al. [Citation2000], Suzuki et al. [Citation2003], Becker et al. [Citation2005]), which makes these cell types an ideal couple to compare TRPV4 trafficking. The maximum intensity projection (MIP) images (consisting of 20 optical xy slices in different z-planes – from cells basal to apical regions) in reveal the localization of recombinant TRPV4-GFP in different plasma membrane compartments in HaCaT (A) and CHO cells (B). The orthogonal xz- and yz-sections (bottom and right) demonstrate clearly the localization of the channel in the plasma membrane; in both cell types TRPV4-GFP became accumulated in lamellipodia and filopodia (arrowhead) at the basal cell plane. In the more apical cell regions, TRPV4-GFP was densely packed in microvilli as indicated by arrows.
Figure 2. Full-length TRPV4 is localized in plasma membrane in different cell types. HaCaT keratinocytes (A) and CHO cells (B) were transiently transfected with TRPV4-GFP and fixed 48 h after transfection. The maximum intensity projections (MIP) taken with the laser scanning microscope (LSM), reveal the plasma membrane localization of TRPV4 in the different cell lines. TRPV4 is accumulated in lamellipodia and filopodia (arrowheads) as well as in microvilli (arrows). The orthogonal xz- and yz-sections (bottom and right) demonstrate clearly the localization of the channel in the plasma membrane in both cell types. In addition CHO cells display some TRPV4-GFP localization in the ER (indicated by the diffuse background fluorescence). Bar = 5 µm.
To determine the kinetics of TRPV4-GFP expression, transfected HaCaT and CHO cells were analyzed at various time points after transfection ( – online version only). Starting from 48 h after transfection, there was hardly any TRPV4-GFP remaining in the ER of HaCaT keratinocytes (A), almost all protein was transported towards the plasma membrane. CHO cells showed also TRPV4-GFP clearly in the plasma membrane but in contrast to HaCaT cells, some TRPV4-GFP protein was also retained in the ER of (B), as described before for HEK293 cells (Hellwig et al. [Citation2005]). To confirm the membrane localization of TRPV4, transfected cells were costained with the Plasma Membrane Labeling Kit (Invitrogen) ( – online version only).
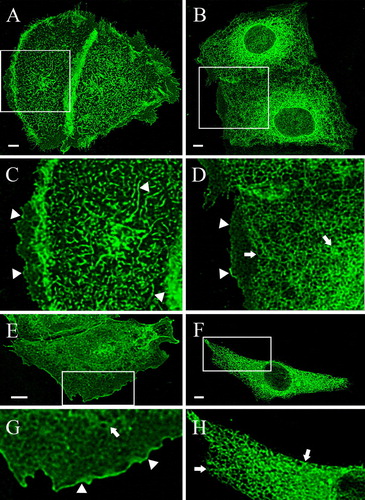
After determination of the kinetics of TRPV4-GFP expression in HaCaT and CHO cells, all deletions mutants depicted in B were transiently transfected into both cell types. The representative MIP projections in reveal the localization of Δ856-871-GFP and Δ845-871-GFP in HaCaT and CHO cells 48 h after transfection. In HaCaT (A and C) and CHO cells (E and G) the deletion up to 16 aa from the end of the C-terminal domain did not alter the localization of the channel compared to the full-length TRPV4. Δ856-871-GFP was enriched in lamellipodia and filopodia as well as in microvilli, which were especially well developed in HaCaT cells (C). In HaCaT cells the nuclei are not visible (A), while they are slightly visible in CHO cells (E), as Δ856-871-GFP remained partly in the ER (arrows), comparable to the full-length TRPV4-GFP in this cell type. The (2.5×) magnified images highlight the plasma membrane localization of Δ856-871-GFP in both cell types (arrowheads). In the next shorter deletion mutant, where eleven additional aa were deleted (Δ845-871-GFP), a significant change in the localization pattern was observed. Only a small amount of Δ845-871-GFP was localized in plasma membrane (arrowheads) of HaCaT cells (B and D), while most of the recombinant protein remained in the ER network (arrows). In transfected CHO cells however, no protein was exported towards the plasma membrane, Δ845-871-GFP remained exclusively in the ER (arrows) (F and H). These data together with the kinetic study demonstrate a very interesting difference between cells expressing endogenous TRPV4 and those devoid of it in relation to the trafficking of TRPV4.
Figure 3. Differential localization of the deletion mutants: effect of the deletions and the cell type. HaCaT keratinocytes (A–D) and CHO cells (E–H) were transfected with the indicated deletion mutants, fixed after 48 h and analyzed with the LSM. (A) In HaCaT cells Δ856-871-GFP was completely localized in the plasma membrane (A, C), while Δ845-871 remained to a considerable amount in ER (B, D). The magnified insets clarify the exclusive plasma membrane localization of Δ856-871 (arrowheads) (C) and the assorted ER (arrows) and plasma membrane distribution (arrowheads) of Δ845-871 (D). In CHO cells Δ856-871 was mainly found in plasma membrane (arrowheads) (E and the magnified inset G), while Δ845-871 was exclusively localized in the ER (arrows) (F and the magnified inset H).
Full-length TRPV4 can rescue Δ845-871-GFP, Δ841-871-GFP and Δ829-871-GFP but not shorter deletion mutants
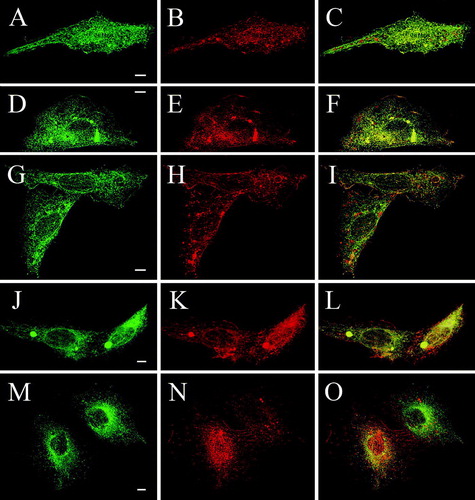
To clarify if interaction with the full-length TRPV4 or another cell type-specific difference was responsible for the differential trafficking of Δ845-871-GFP, a cross-complementation assay was devised, where GFP-tagged deletion mutants were coexpressed with full-length RFP-TRPV4. The localization of the proteins was determined as described in Material and Methods. Δ856-871-GFP and RFP-TRPV4 double transfected HaCaT cells showed an almost identical localization of both proteins almost exclusively in the plasma membrane (, A–C indicated by yellow in the merged image on the right side of the panel). After co-transfection of RFP-TRPV4 with Δ845-871, which remained in single transfected HaCaT cells mainly in the ER, the truncated channel was found to a very high amount in the plasma membrane. The merged image demonstrates the strong co-localization of Δ845-871 and RFP-TRPV4; Δ845-871-GFP was now similarly distributed as the full-length TRPV4-GFP (D–F). After coexpression of the next shorter deletion mutant Δ841-871-GFP with RFP-TRPV4, again Δ841-871-GFP was exported to the plasma membrane (G–I). The same phenomenon was observed also with the next mutant, Δ820-871-GFP (J–L), indicating indeed a strong impact of the full-length TRPV4 on localization of these three deletion mutants. In contrast, the next shorter protein (Δ812-871-GFP) remained in the ER also after coexpression with RFP-TRPV4 (M–O). The full-length TRPV4 however, was not retained in the ER by this truncated protein but trafficked to the plasma membrane. Furthermore, this experiment excluded the possibility of a GFP-RFP interaction being responsible for the observed trafficking of mutants Δ841-871-GFP, Δ845-871-GFP and Δ829-871-GFP, since Δ812-871-GFP, Δ778-811-GFP, Δ759-777-GFP and Δ716-758-GFP could not be rescued by coexpression of RFP-TRPV4 (summarized in ).
Figure 4. Cross complementation of deletion mutants with full-length TRPV4 rescues plasma membrane localization of Δ845-871, Δ841-871 and Δ829-871 in HaCaT keratinocytes. HaCaT keratinocytes were double transfected with full-length RFP-TRPV4 (red) and the GFP-tagged deletions mutants Δ856-871-GFP (A–C), Δ845-871-GFP (D–F), Δ841-871-GFP (G–I), Δ829-871-GFP (J–L) and Δ812-871-GFP (M–O) (green), fixed after 48 h and analyzed by LSM. The panels on the right display the merged images and the co-localization (yellow).
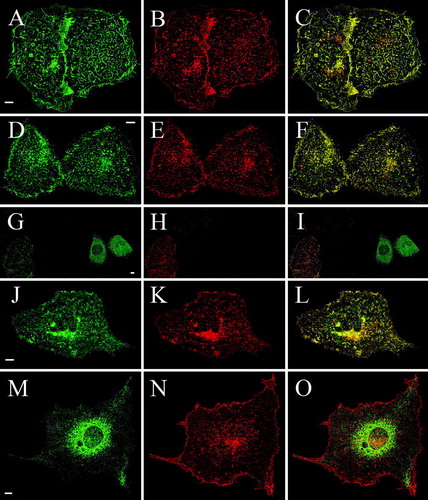
Table I. Expression patterns of TRPV4 deletion mutants after cotransfection with RFP-TRPV4 in HaCaT and CHO cells. HaCaT keratinocytes and CHO cells were double-transfected with the indicated GFP-tagged deletion mutants and RFP-TRPV4 and the localization of both recombinant proteins was analyzed by LSM. Coexpression of full-length RFP-TRPV4 with the deletion mutants Δ845-871-GFP, Δ841-871-GFP and Δ829-871-GFP (indicated in bold) showed a significant altered distribution in comparison with cells transfected with the respective mutant alone.
Table S1. Kinetics of TRPV4 expression in HaCaT keratinocytes and CHO cells. HaCaT keratinocytes and CHO cells were transfected with TRPV4-GFP and the localization of the recombinant protein was analyzed by LSM at the indicated time points. TRPV4-GFP in HaCaT cells is exported faster towards the plasma membrane than in CHO cells. Furthermore, TRPV4-GFP is HaCaT cells fully exported out of the ER in contrast to CHO cells.
The deletion mutants Δ829-871-GFP, Δ841-871-GFP, Δ845-871-GFP and Δ856-871-GFP stayed in single transfected CHO cells exclusively in the ER but after coexpression with RFP-TRPV4, they were found to a considerable amount in the plasma membrane (). The merged images (right panels) reveal the co-localization between the full-length protein and the respective truncated channel in plasma membrane structures (yellow). In comparison to HaCaT cells, the rescue was less efficient in CHO cells, some truncated protein remained in the ER. However, the mutant Δ812-871-GFP remained completely in the ER when cotransfected with RFP-TRPV4 (M–O). The plasma membrane- and ER localization of the truncated proteins are depicted in detail in the 3D shadow projection (). Coexpression of Δ778-811-GFP, Δ759-777-GFP and Δ716-758-GFP with RFP-TRPV4 in CHO did neither change the localization of the mutants nor inhibit trafficking of the full-length TRPV4 (summarized in Table 1).
Figure 5. Cross complementation of deletion mutants with full-length TRPV4 rescues plasma membrane localization of Δ845-871, Δ841-871 and Δ829-871 in CHO cells. CHO cells were double transfected with full-length RFP-TRPV4 (red) and Δ856-871-GFP (A–C), Δ845-871-GFP (D–F), Δ841-871-GFP (G–I), Δ829-871-GFP (J–L) and Δ812-871-GFP (M–O) (all green). Cells were fixed after 48 h and analyzed by LSM. On the right panels the merged images and the co-localization are displayed (yellow).
Oligomerization of full-length TRPV4 and Δ841-871
The rescue of the deletion mutants by the full-length TRPV4 occurred most probably via oligomerization of the truncated proteins with the full-length channel. To confirm this hypothesis, FRET measurements were performed (). CHO cells were double transfected with TRPV4-CFP and TRPV-YFP as positive control, Δ812-871-YFP as negative control and Δ841-871-YFP respectively (D). Acceptor bleaching of different ROIs in the plasma membrane of cells transfected with TRPV4-CFP and Δ812-871-YFP did not lead to increased donor emission intensity, as expected since Δ812-871-YFP remained completely in the ER. The calculated FRET efficiency was 0.4 ± 0.3 % (n=12). In order to determine the maximum possible FRET efficiency of the TRPV4 oligomers by this method, CHO cells were double transfected with TRPV4-CFP and TRPV4-YFP. Acceptor bleaching of different ROIs in the plasma membrane revealed a FRET efficiency of 8.1 ± 1.2 % (n=10). In cells double transfected with TRPV4-CFP (A) and the deletion mutant Δ841-871-YFP (B), both the YFP-tagged deletion mutant and the full-length protein were localized in the plasma membrane, but the truncated protein was again also found in the ER. The co-localization of TRPV4-CFP and Δ841-871-YFP is demonstrated in the merged image (C). Acceptor bleaching of ROIs in the plasma membrane resulted in a FRET efficiency of 6.8 ± 0.4% (n=15). This FRET efficiency was significantly elevated compared to TRPV4-CFP and Δ812-871-YFP double transfected cells, thus indicating not only co-localization of TRPV4-CFP and Δ841-871-YFP but in fact oligomerization. In addition, no Δ841-871-YFP was found in the plasma membrane of single transfected CHO cells, as it was the case with Δ841-871-GFP.
Figure 6. FRET of double transfected CHO cells. CHO cells were double transfected with TRPV4-CFP (A) and TRPV4-YFP (B) and fixed 48 h post transfection. The merged image (C) demonstrates the co-localization of the transfected constructs in the plasma membrane (PM) and the ER. (D) Acceptor bleaching in TRPV4-CFP and TRPV4-YFP as well as in TRPV4-CFP and Δ 841-871-YFP transfected cells exhibited a significantly higher FRET efficiency compared to TRPV4-CFP and Δ 812-871-YFP transfected cells in the PM and the ER. Bar = 10 µm.
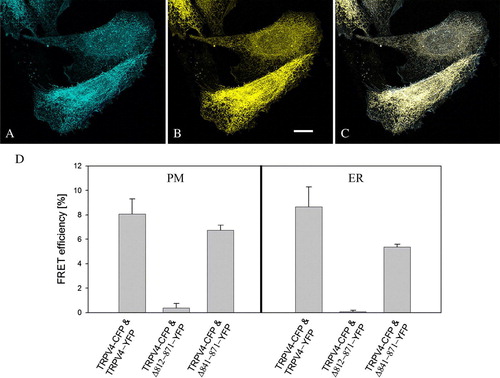
FRET efficiencies measured in the ER of cells transfected with TRPV4-CFP and either TRPV4-YFP (8.7 ± 1.6%) (n=6) or Δ 841-871-YFP (5.4 ± 0.2 %) (n=15) were comparable to FRET efficiencies obtained in the plasma membrane of the respective cells and were significantly increased in comparison to the FRET efficiencies in the ER of TRPV4-CFP and Δ 812-871-YFP (n=12) transfected cells (0.1 ± 0.1 %), indicating that oligomerization of the truncated protein with the full-length TRPV4 takes already place in the ER.
TRPV4 is functional when it is inserted in plasma membrane
Our results indicate the requirement of most of the C-terminal domain for membrane localization of TRPV4. The subsequent question was whether the whole C-terminus of TRPV4 is required for functionality of the channel. Therefore, CHO cells were transfected with Δ856-871-GFP, which is incorporated into the plasma membrane and compared to full-length TRPV4-GFP as a positive control and to Δ841-871-GFP as negative control. Hypotonicity is a potent activator of TRPV4 resulting in an influx of [Ca2 + ]i in the cytoplasm (Liedtke et al. [Citation2000], Becker et al. [Citation2005]). This reaction was consequently used to evaluate the functionality of the channel. Single transfected cells were stained with the Ca2 + sensitive dye Fura-2 and osmolarity of the medium was lowered from 300 mOsm/l to 200 mOsm/l. A demonstrates the maximum 340/380 nm values up to 150 sec after hypotonic stimulus. When Δ841-871-GFP transfected cells were exposed to hypotonicity, almost no increase of the [Ca2 + ]i occurred. The [Ca2 + ]i level remained almost constant (Δ = 0,06 ± 0,09). In contrast, CHO cells with membrane-localized Δ856-871-GFP reacted to hypotonic stimulus with a significant [Ca2 + ]i elevation of Δ = 2.5 ± 0.29. In most cases, the increase of [Ca2 + ]i occurred rapidly after lowering the osmolarity of the medium as shown by the representative trace in B. Osmotically challenged CHO cells transfected with full-length TRPV4-GFP increased their [Ca2 + ]i level by Δ = 3.52 ± 0.53. Although these Ca2 + concentrations were slightly higher than the ones that were obtained with Δ845-871-GFP transfected cells, this difference was not significant. The kinetics of the [Ca2 + ]i increase in TRPV4-GFP transfected cells were similar to those of Δ856-871-GFP transfected cells, indicating that the last 16 aa are not essential for functionality of TRPV4 after hypotonic activation and that the receptor is fully functional when it is inserted in the plasma membrane.
Figure 7. Deletion mutants are fully functional, when expressed in the plasma membrane. CHO cells were transiently transfected with different GFP deletion constructs, and after 48 h loaded with Fura-2 and exposed to hypotonicity by lowering the osmolarity from 300 to 200 mOsm/l. (A) In Δ841-871-GFP transfected cells, the [Ca2 + ]i stayed constant after hypotonic shock (n=27). In contrast [Ca2 + ]i was significantly increased in full-length TRPV4-GFP (n=14) as well as in Δ856-871-GFP transfected cells (n=21). (B) Representative traces of the three differently transfected cells were plotted. While the [Ca2 + ]i level remains constant in Δ841-871-GFP transfected cells (dotted line), Δ856-871-GFP (dashed line) and full-length TRPV4 (straight line) transfected cells reacted with a similar [Ca2 + ]i increase to the hypotonic stimulus (indicated by the line). Data demonstrated are mean values of±SEM.
![Figure 7. Deletion mutants are fully functional, when expressed in the plasma membrane. CHO cells were transiently transfected with different GFP deletion constructs, and after 48 h loaded with Fura-2 and exposed to hypotonicity by lowering the osmolarity from 300 to 200 mOsm/l. (A) In Δ841-871-GFP transfected cells, the [Ca2 + ]i stayed constant after hypotonic shock (n=27). In contrast [Ca2 + ]i was significantly increased in full-length TRPV4-GFP (n=14) as well as in Δ856-871-GFP transfected cells (n=21). (B) Representative traces of the three differently transfected cells were plotted. While the [Ca2 + ]i level remains constant in Δ841-871-GFP transfected cells (dotted line), Δ856-871-GFP (dashed line) and full-length TRPV4 (straight line) transfected cells reacted with a similar [Ca2 + ]i increase to the hypotonic stimulus (indicated by the line). Data demonstrated are mean values of±SEM.](/cms/asset/c87d70c5-7203-4b1a-ada0-61f2fb36a5c9/imbc_a_263369_f0007_b.gif)
Figure S1. Co-localization of TRPV4-GFP and a plasma membrane marker. HaCaT cells were transfected with TRPV4-GFP (green) and 48 h after transfection plasma membrane was counterstained using plasma membrane marker (see Materials and methods) (red). Nuclei were stained with Hoechst (blue). The four TRPV4-GFP transfected cells display strong co-localization with plasma membrane marker (yellow), demonstrating clearly TRPV4 localization in plasma membrane. Bar = 5 µm.
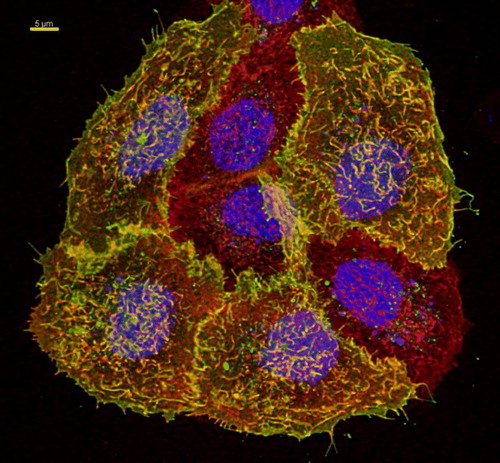
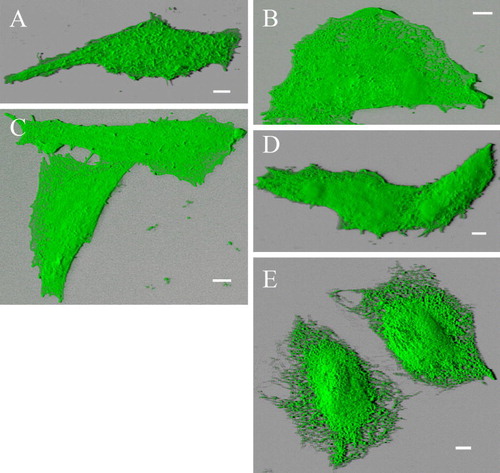
Figure S2. Blended 3D projection of the GFP channel reveals plasma membrane- and ER-localization of different deletion mutants. Alternative blended shadow projection reveals evidently plasma membrane localization of truncation mutants Δ856-871-GFP (A), Δ845-871-GFP (B), Δ841-871-GFP (C) and Δ829-871-GFP (D) double transfected with RFP-TRPV4 in CHO cells. In contrast Δ829-871-GFP (E) is exclusively localized in ER. Δ856-871-GFP transfected cell displayed more Microvilli structures than the other cells, resulting in rougher plasma membrane surface. Bar = 5 µm.
Discussion
We investigated the role of the C-terminus of TRPV4 for assembly and trafficking to the plasma membrane in two different cell systems. Recent data imply that TRPV4 exists in the plasma membrane as an oligomer (Hellwig et al. [Citation2005], Arniges et al. [Citation2006]) and for TRPV1, TRPV5 and TRPV6 it has been already shown that these channels occur in a tetrameric state (Kedei et al. [Citation2001], Jahnel et al. [Citation2001], Hoenderop et al. [Citation2003]). Several GFP-tagged mutants with deletions in the C-terminal domain of TRPV4 were constructed, transiently transfected and their localization analyzed by LSM. Surprisingly, the length of the C-terminus essential for insertion of TRPV4 in the plasma membrane was depending on the cell type that was used for the expression studies. In CHO cells only Δ865-871 and Δ856-871 trafficked towards the plasma membrane, where they were also functional. Shorter mutants, starting from Δ845-871, remained exclusively in the ER of CHO cells and consequently evoked no reaction to hypotonic stress. In HaCaT cells endogenous full-length TRPV4 was able to ‘pull out’ Δ845-871 to some extend and hereby able to override the ER retention phenotype of the mutants. The levels of endogenous TRPV4 could not be determined due to the lack of a TRPV4 antibody, but most probably the different expression levels of endogenous TRPV4 and recombinant Δ845-871 were causing the only partial rescue of the mutant. This hypothesis was confirmed when full-length recombinant TRPV4 was coexpressed with the mutants Δ845-871, Δ841-871 and Δ829-871; in these cases the majority of both proteins was found in the plasma membrane in HaCaT cells as well as in CHO cells. Thus, the complete ER retention of Δ845-871, Δ841-871 and Δ829-871 was not caused by the CHO cell system per se, but resulted from the lack of endogenous TRPV4. It seems unlikely that the RFP- or GFP-tag by itself was influencing the trafficking in double-transfected cells, since Δ812-871, Δ778-811, Δ759-777 and Δ716-758 never were exported to the plasma membrane after coexpression with full-length TRPV4.
While Δ812-871 remained in the ER, most of the full-length RFP-TRPV4 was found in the plasma membrane after coexpression. This differs e.g. from the observation that coexpression of the wild-type dopamine transporter or a recombinant full-length, CFP-tagged dopamine transporter with an ER-retained mutant resulted in the retention of the full-length protein in the ER. In this case a heterooligomerization of the mutant and the full-length protein was responsible for the ER retention (Sorkina et al. [Citation2003]). The same phenomenon was reported for two members of the Frizzled (Fz) family of Wnt receptors, Fz1 and Fz4, where also heterooligomerization of the wild-type receptor with an ER-retained variant inhibited the trafficking of the wild-type to the plasma membrane (Kaykas et al. [Citation2004]). A convincing explanation why RFP-TRPV4 is not retained by Δ812-871 would be that the truncated protein can not oligomerize with the full-length TRPV4 anymore, most probably due to the deletion of a domain essential for interaction (see below). It has been shown for membrane proteins of different classes that they are retained in the ER when the oligomerization process failed (Letourneur et al. [Citation1995], Scott et al. [Citation2001], Sato et al. [Citation2004], Lobito et al. [Citation2006]). Also analysis of different N-terminal splice variants of TRPV4 indicates that oligomerization of TRPV4 takes place in the ER and that the lack of oligomerization is inhibiting trafficking and causing ER retention (Arniges et al. [Citation2006]). To confirm that indeed oligomerization is responsible for the trafficking of the truncated channels, FRET measurements were performed that allow predictions about oligomerization of proteins, since very close proximity (<100 Ångstrom) is needed to induce FRET. The absolute FRET efficiencies can vary since this is dependent on used methodology, chromophores and in this case composition of the oligomers (ratio of CFP to YFP) as well as the different expression levels (Förster [Citation1948], Selvin [Citation2000]). The acceptor bleaching experiments revealed significant FRET efficiencies in the ER of TRPV4-CFP and TRPV4-YFP as well as in TRPV4-CFP and Δ841-871-YFP double transfected cells, while no FRET occurred in the ER in TRPV4-CFP and Δ778-871-YFP transfected cells. Also in the plasma membrane, double transfection with either TRPV4-CFP and TRPV4-YFP or TRPV4-CFP and Δ841-871-YFP resulted in significant FRET efficiencies, strongly implying that oligomerization of TRPV4 with mutants Δ845-871, Δ841-871 and Δ829-871 occurs in the ER and allows trafficking. The FRET efficiency of TRPV4-CFP and Δ841-871-YFP appeared lower than in TRPV4-CFP and TRPV4-YFP transfected cells, although this difference was not significant. But it is possible that homooligomerization of full-length TRPV4 is a little more effective than heterooligomerization with the truncated protein. An important question is which part of the C-terminal domain is important for oligomerization and/or trafficking. Δ845-871, Δ841-871 and Δ829-871 were exported after coexpression with the full-length construct, implying that misfolding of the truncated channels is probably not responsible for ER retention. A possible explanation could be that the two RXR motifs encompassing positions 816-821 (RLRRDR) are exposed in Δ845-871, Δ841-871 and Δ829-871 and thus promote ER retention in single transfected cells. Heterooligomerization with full-length TRPV4 could mask the ER retention motif, resulting in trafficking of the heterooligomers to the plasma membrane. This has been shown for the GABAB receptor and the ATP-sensitive K+ channel, where heterooligomerization of different subunits is masking the ER retention motif, thus allowing ER export (Zerangue et al. [Citation1999], Margeta-Mitrovic et al. [Citation2001]). If this scenario were true, the region downstream of aa 828 would be not necessary for oligomerization. For TRPV1 a domain at the start of the C-terminus, the so-called ‘TRP-like domain’, has been shown to participate in tetramerization (Garcia-Sanz et al. 2004). Sequence alignment of the C-termini of TRPV1 and TRPV4 showed indeed a high homology in the ‘TRP-like domain’ (position 716-758, 63% aa identical, data not shown), and deletion of the ‘TRP-like domain’ in TRPV4 (Δ716-758) did result in retention of mutant Δ716-758 in the ER, comparable to TRPV1 (). However, the ‘TRP-like domain’ is intact in the mutants Δ812-871, Δ778-871, Δ759-871, Δ778-811 and Δ759-777, that were all unable to traffic to the plasma membrane, even after coexpression with full-length TRPV4. Also the characteristic coiled-coil motif in the C-terminal domain of TRPV1 was missing in TRPV4 (predicted with Coils (Lupas et al. [Citation1991]), data not shown). Therefore, we conclude that in contrast to TRPV1 the C-terminal domain till position 828 is necessary for TRPV4 oligomerization and trafficking to the membrane. This is also in agreement with data from Hellwig et al. ([Citation2005]), who indicated a role of the C-terminus for oligomerization. However, our data differ from results of Suzuki et al. ([Citation2003]), where a C-terminal deletion mutant of TRPV4 (Δ809-871) whose localization had not been demonstrated was still able to interact with the microtubule-associated protein 7 (MAP7). In both our cell systems a comparable deletion mutant (Δ812-871) remained in the ER.
Taken together our data demonstrates that deletions in the C-terminus had different effects on TRPV4 trafficking. Deletions up to position 856 did not alter localization or functionality of the channel. Further deletions till 828 resulted in partial retention of the channel in the ER. This phenotype could be overridden by oligomerization with full-length TRPV4. Further deletions however, were always retained in the ER. Therefore, we conclude that in addition to the N-terminal domain with the three ankyrin domains (Hellwig et al. [Citation2005], Arniges et al. [Citation2006]) also the C-terminal domain until position 828 participates in oligomerization and trafficking of TRPV4.
tMBC 263369 Jendrach Supplementary material for online version only.
The kinetics of TRPV4-GFP expression in transfected HaCaT and CHO cells were analyzed at various time points after transfection as described in Materials and methods (Table SI). Although in general the localization of TRPV4-GFP in the plasma membrane of HaCaT and CHO cells was comparable, two differences became apparent. First, while TRPV4-GFP was detectable in the ER 6 h after transfection in both cell types, trafficking to the plasma membrane occurred earlier in HaCaT keratinocytes than in CHO cells. Whereas in HaCaT cells a small amount of TRPV4 was already detectable in the plasma membrane 12 h after transfection, it took 24 h until a comparable amount of TRPV4 was detected in the plasma membrane in CHO cells. Secondly, in CHO cells a part of the TRPV4-GFP protein often remained in the ER and in cytosolic vesicles as described before for HEK293 cells (Hellwig et al. [Citation2005]). After 48 h of transfection, there was hardly any TRPV4-GFP remaining in the ER of HaCaT keratinocytes, almost all protein had trafficked to the plasma membrane. In contrast, a part of TRPV4-GFP was often retained in the ER of CHO cells; even 72 h after transfection (compare the orthogonal xz- and yz-sections of HaCaT cells (Figure 2A) and CHO cells (Figure 2B)). In contrast, a small plasma membrane marker protein (pAcGFP1-Mem) was already 24 h after transfection almost exclusively integrated into the plasma membrane (data not shown).
Acknowledgements
We are very indebted to Prof. Dr Jürgen Bereiter-Hahn (Johann Wolfgang Goethe University, Frankfurt/Main, Germany) for stimulating discussions and critical reading of the manuscript. We gratefully acknowledge Prof. Dr Herbert Zimmermann (Johann Wolfgang Goethe University, Germany) for the use of his electroporation equipment. This work was supported by the SFB 628 ‘Functional Membrane Proteomics’ (P9) and the Center for Membrane Proteomics (CPM) Frankfurt/Main Germany.
References
- Alessandri-Haber N, Joseph E, Dina OA, Liedtke W, Levine JD. TRPV4 mediates pain-related behavior induced by mild hypertonic stimuli in the presence of inflammatory mediator. Pain 2005; 118: 70–79
- Ambudkar IS. Trafficking of TRP channels: determinants of channel function. Handb Exp Pharmacol 2007; 179: 541–557
- Andrade YN, Fernandes J, Vazquez E, Fernandez-Fernandez JM, Arniges M, Sanchez TM, Villalon M, Valverde MA. TRPV4 channel is involved in the coupling of fluid viscosity changes to epithelial ciliary activity. J Cell Biol 2005; 168: 869–874
- Arniges M, Fernandez-Fernandez JM, Albrecht N, Schaefer M, Valverde MA. Human TRPV4 channel splice variants revealed a key role of ankyrin domains in multimerization and trafficking. J Biol Chem 2006; 281: 1580–1586
- Arniges M, Vazquez E, Fernandez-Fernandez JM, Valverde MA. Swelling-activated Ca2 + entry via TRPV4 channel is defective in cystic fibrosis airway epithelia. J Biol Chem 2004; 279: 54062–54068
- Becker D, Blase C, Bereiter-Hahn J, Jendrach M. TRPV4 exhibits a functional role in cell-volume regulation. J Cell Science 2005; 118: 2435–2440
- Boukamp P, Petrussevska RT, Breitkreutz D, Hornung J, Markham A, Fusenig NE. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol 1988; 106: 761–771
- Chang Q, Gyftogianni E, van de Graaf SF, Hoefs S, Weidema FA, Bindels RJ, Hoenderop JG. Molecular determinants in TRPV5 channel assembly. J Biol Chem 2004; 279: 54304–54311
- Erler I, Al-Ansary DM, Wissenbach U, Wagner TF, Flockerzi V, Niemeyer BA. Trafficking and assembly of the cold-sensitive TRPM8 channel. J Biol Chem 2006; 281: 38396–38404
- Erler I, Hirnet D, Wissenbach U, Flockerzi V, Niemeyer BA. Ca2 + -selective transient receptor potential V channel architecture and function require a specific ankyrin repeat. J Biol Chem 2004; 279: 34456–34463
- Förster T. Zwischenmolekulare energiewanderung und fluoreszenz. Ann Phys 1948; 6: 55–75
- Garcia-Sanz N, Fernandez-Carvajal A, Morenilla-Palao C, Planells-Cases R, Fajardo-Sanchez E, Fernandez-Ballester G, Ferrer-Montiel A. Identification of a tetramerization domain in the C terminus of the vanilloid receptor. J Neurosci 2004; 24: 5307–5314
- Gao X, Wu L, O′Neil RG. Temperature-modulated diversity of TRPV4 channel gating: activation by physical stresses and phorbol ester derivatives through protein kinase C-dependent and -independent pathways. J Biol Chem 2003; 278: 27129–27137
- Güler AD, Lee H, Iida T, Shimizu I, Tominaga M, Caterina M. Heat-evoked activation of the ion channel, TRPV4. J Neurosci 2002; 22: 6408–6414
- Hellwig N, Albrecht N, Harteneck C, Schultz G, Schaefer M. Homo- and heteromeric assembly of TRPV channel subunits. J Cell Sci 2005; 118: 917–928
- Hoenderop JG, Voets T, Hoefs S, Weidema F, Prenen J, Nilius B, Bindels RJ. Homo- and heterotetrameric architecture of the epithelial Ca2 + channels TRPV5 and TRPV6. EMBO J 2003; 22: 776–785
- Jahnel R, Dreger M, Gillen C, Bender O, Kurreck J, Hucho F. Biochemical characterization of the vanilloid receptor 1 expressed in a dorsal root ganglia derived cell line. Eur J Biochem 2001; 268: 5489–5496
- Kaykas A, Yang-Snyder J, Heroux M, Shah KV, Bouvier M, Moon RT. Mutant Frizzled 4 associated with vitreoretinopathy traps wild-type Frizzled in the endoplasmic reticulum by oligomerization. Nat Cell Biol 2004; 6: 52–58
- Kedei N, Szabo T, Lile JD, Treanor JJ, Olah Z, Iadarola MJ, Blumberg PM. Analysis of the native quaternary structure of vanilloid receptor 1. J Biol Chem 2001; 276: 28613–28619
- Kohler R, Heyken WT, Heinau P, Schubert R, Si H, Kacik M, Busch C, Grgic I, Maier T, Hoyer J. Evidence for a functional role of endothelial transient receptor potential V4 in shear stress-induced vasodilatation. Arterioscler Thromb Vasc Biol 2006; 26: 1495–1502
- Lee H, Iida T, Mizuno A, Suzuki M, Caterina MJ. Altered thermal selection behavior in mice lacking transient receptor potential vanilloid 4. J Neurosci 2005; 25: 1304–1310
- Letourneur F, Hennecke S, Demolliere C, Cosson P. Steric masking of a dilysine endoplasmic reticulum retention motif during assembly of the human high affinity receptor for immunoglobulin E. J Cell Biol 1995; 129: 971–978
- Liedtke W, Choe Y, Marti-Renom MA, Bell AM, Denis CS, Sali A, Hudspeth AJ, Friedman JM, Heller S. Vanilloid receptor-related osmotically activated channel (VR-OAC), a candidate vertebrate osmoreceptor. Cell 2000; 103: 525–535
- Lobito AA, Kimberley FC, Muppidi JR, Komarow H, Jackson AJ, Hull KM, Kastner DL, Screaton GR, Siegel RM. Abnormal disulfide-linked oligomerization results in ER retention and altered signaling by TNFR1 mutants in TNFR1-associated periodic fever syndrome (TRAPS). Blood 2006; 108: 1320–1327
- Lupas A, Van Dyke M, Stock J. Predicting coled coils from protein sequences. Science 1991; 252: 1162–1164
- Margeta-Mitrovic M, Jan YN, Jan LY. Ligand-induced signal transduction within heterodimeric GABAB receptor. Proc Natl Acad Sci USA 2001; 98: 14643–14648
- Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium 2005; 38: 233–252
- Ramadass R, Becker B, Jendrach M, Bereiter-Hahn J. Spectrally and spatially resolved fluorescence lifetime imaging in living cells: TRPV4-microfilament interactions. Arch Biochem Biophys 2007; 463: 27–36
- Reiter B, Kraft R, Gunzel D, Zeissig S, Schulzke JD, Fromm M, Harteneck C. TRPV4-mediated regulation of epithelial permeability. FASEB J 2006; 20: 1802–1812
- Sato M, Sato K, Nakano A. Endoplasmic reticulum quality control of unassembled iron transporter depends on Rer1p-mediated retrieval from the golgi. Mol Biol Cell 2004; 15: 1417–1424
- Schindl R, Romanin C. Assembly domains in TRP channels. Biochem Soc Trans 2007; 35: 84–85
- Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci 2001; 21: 3063–3072
- Selvin PR. The renaissance of fluorescence resonance energy transfer. Nat Struct Biol 2000; 7: 730–734
- Sorkina T, Doolen S, Galperin E, Zahniser NR, Sorkin A. Oligomerization of dopamine transporters visualized in living cells by fluorescence resonance energy transfer microscopy. J Biol Chem 2003; 278: 28274–28283
- Suzuki M, Hirao A, Mizuno A. Microfilament-associated protein 7 increases the membrane expression of transient receptor potential vanilloid 4 (TRPV4). J Biol Chem 2003; 278: 51448–51453
- Tsuruda PR, Julius D, Minor DL, Jr. Coiled coils direct assembly of a cold-activated TRP channel. Neuron 2006; 51: 201–212
- Watanabe H, Davis JB, Smart D, Jerman JC, Smith GD, Hayes P, Vriens J, Cairns W, Wissenbach U, Prenen J, Flockerzi V, Droogmans G, Benham CD, Nilius B. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J Biol Chem 2002b; 277: 13569–13577
- Watanabe H, Vriens J, Suh SH, Benham CD, Droogmans G, Nilius B. Heat-evoked activation of TRPV4 channels in a HEK293 cell expression system and in native mouse aorta endothelial cells. J Biol Chem 2002a; 277: 47044–47051
- Watanabe H, Vriens J, Prenen J, Droogmans G, Voets T, Nilius B. Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 2003; 424: 434–438
- Wissenbach U, Bodding M, Freichel M, Flockerzi V. Trp12, a novel Trp related protein from kidney. FEBS Lett 2000; 485: 127–134
- Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron 1999; 22: 537–548