Abstract
The 5-HT3 and GABAA receptors are members of the Cys-loop family of neurotransmitter-gated ion channels that also include receptors for glycine and acetylcholine. The 5-HT3 and acetylcholine receptors (cationic ion channels) and the GABAA and glycine receptors (anionic ion channels) generally depolarize or hyperpolarize, respectively, the neuronal membrane. Within the amino-terminal extracellular region, all members of this family exhibit a similar architecture of ligand binding domains and a number of key residues are completely conserved. The molecular characterization of their ligand binding and gating characteristics has benefited from the existence of a large repertoire of individual subunits that contribute to the pentameric ion channel. Although differences do exist, advances in our knowledge of one member offers valuable insight into the family as a whole. Each member of the Cys-loop receptors (and all other multimeric ion channels) must face the same challenges: How to assemble individual subunits into an ion channel and which subunits to use? How are assembled receptors distinguished from those that are unassembled or misassembled, then exported from the endoplasmic reticulum and delivered to the cell surface? How are they targeted to, and anchored at synaptic and extrasynaptic sites? How and when are they to be removed from these sites to provide long-term regulation of neuronal activity? In this review, we summarize our current knowledge for the 5-HT3 and GABAA receptors that have provided complementary information and helped us build an overall picture of how receptor biogenesis and trafficking occurs.
Introduction
The Cys-loop family of neurotransmitter-gated ion channels consists of excitatory receptors for 5-HT, and nicotine/acetylcholine (nACh), and inhibitory receptors for glycine and GABA (GABAA). Each member of this family is thought to share a conserved molecular architecture within their amino-terminal extracellular domain Citation[1]. This structural similarity has enabled much experimental data to be interpreted by homology modelling, based on the crystal structure of acetylcholine binding protein from the snail, Lymnaea stagnalis Citation[2]. Moreover, findings from one receptor type may be extrapolated to other members of the family and tested directly. This has enabled a more rapid progress of our understanding for the whole Cys-loop family.
5-HT3 receptors
The 5-HT3 receptors are unique amongst the other 5-HT (serotonin) receptors in that they are ligand gated ion channels, with the actions of the other 5-HT receptors being mediated via metabotropic G-protein coupled signalling. The 5-HT3 receptors are localized within both the peripheral and central nervous systems where they play important roles in gut motility, emesis, anxiety and cognition Citation[3]. Within the central nervous system, 5-HT3 receptors have been localized to many brain areas including the hippocampus, cortex, substantia nigra, and the brain stem Citation[4]. Despite the wide distribution of the 5-HT3 receptors and the diverse range of clinical conditions with which they have been implicated, their therapeutic use is currently limited to the treatment of emesis and irritable bowel syndrome.
5-HT3 receptors are constructed as pentameric ion channels, with the 5-HT3A homomeric receptors and 5-HT3A/3B heteromeric receptors being well characterized Citation[3]. When expressed alone, 5-HT3B does not produce functional receptors, but is retained in the endoplasmic reticulum (ER) Citation[5]. However, when rescued by the co-expression of 5-HT3A, 5-HT3A/3B heteromers are formed, with a stochiometry of 3(3B):2(3A) and the arrangement in the pentamer of B-B-A-B-A Citation[6]. Several studies have implied the existence of native receptors that cannot be explained by the formation of 5-HT3A and 5-HT3A/3B receptors and their promiscuous assembly with nicotinic acetylcholine receptor subunits has been postulated. More recently, 3 novel 5-HT3 receptor subunits, 5-HT3C-E have been cloned. Like the 5-HT3B subunit, the 5-HT3C-E subunits do not reach the cell surface or function as homomers, but they are rescued from the ER and expressed on the cell surface by co-expression with 5-HT3A and appear to exhibit functional receptors with altered efficacy for 5-HT Citation[7]. Although it appears that the incorporation of the 5-HT3A subunit is a prerequisite for receptor formation, no investigation into the existence of other di-heteromeric (for example, 5-HT3B/3C) or tri-heteromeric (for example, 5-HT3A/3B/3C) combinations have been reported.
To date, no research has yet been reported to establish and identify the existence of amino-terminal assembly signals within any of the 5-HT3 receptor subunits. This is in contrast to the extensive studies performed on the GABAA receptors (Sieghart, this issue), where a number of assembly signals have been identified. Given the conserved architecture of the Cys-loop receptors Citation[2], combined with the common formation of the neurotransmitter binding sites occurring at subunit interfaces Citation[3], it is reasonable to assume that they will be analogous to those of the GABAA receptors. Of course, this is a risky strategy and the actual assembly signals within 5-HT3 receptors need to be determined empirically.
Arguably, more important than the identity of the assembly signals within the 5-HT3 receptors, is an understanding of how this process is regulated. For example, if a cell is capable of assembling 5-HT3A receptors, how can it handle the opportunity to incorporate 5-HT3B subunits? Temporal gene expression may contribute to whether both subunits are present. However, when both subunits are available a cell would have to either select subunits randomly or show a preference. For 5-HT3A and 5-HT3B co-expression, a preference appears to be the option taken as 5-HT3A/3B receptors appear to be formed preferentially Citation[6]. How could a preference be executed? This could be achieved by a differential affinity for assembly signals. Alternatively, signals might be exposed at different points during subunit folding, giving a temporal advantage to some signals. Finally, it is possible that spatial segregation of unassembled subunits may contribute to actual subunit availability. It is not known which (if any) of these mechanisms actually operate.
In addition to the contribution of assembly signals, post-translational modifications appear to play a role in receptor biogenesis Citation[1], Citation[3], Citation[8]. An interesting example of this has been reported for the N-linked glycosylation of murine 5-HT3A receptors. N-glycosylation at N109 Citation[1], Citation[3] is required for 5-HT3A receptor assembly, as indicated by the failure to produce ligand binding sites when this site is mutated Citation[8]. This glycosylation site is variably present in other members of the Cys-loop family and is located within a region containing multiple GABAA receptor assembly signals responsible for inter-subunit interactions Citation[1]. Interestingly, N-glycosylation at N190, appears to be required, not for receptor assembly (as ligand binding sites are formed), but for transport to the cell surface. In contrast, N-glycosylation at N174 is not required for receptor assembly or transport to the cell surface, but does appear to inhibit receptor function Citation[8]. Thus, N-glycosylation of 5-HT3A may participate at multiple stages of receptor maturation, transport and function. However, a similar study on human 5-HT3A determined that all these N-glycosylation sites were required for receptor assembly (ligand binding) and cell surface expression Citation[9].
Extrapolation from our knowledge of assembly signals within the large extracellular amino-terminus of GABAA receptors (Sieghart, this issue), it seems reasonable to assume that 5-HT3B is unable to assemble in the absence of the 5-HT3A subunit. However, the use of subunit chimaeras between 5-HT3B and 5-HT3A revealed that the 5-HT3B subunit appears to be able to assemble, but not traffic beyond the ER due to the existence of cytoplasmic ER retrieval signals (ERS) Citation[10]. The ERS are responsible for returning proteins from the cis-Golgi to the ER and so prevent forward transport to the cell surface. One ERS was identified within the first cytoplasmic loop (between TMI-II) of 5-HT3B and shown to cause ER retention unless masked by the co-expression of 5-HT3A Citation[10]. A similar situation is observed for the γ2 subunit of GABAA receptors, in which a short splice variant (γ2S) is capable of exiting the ER and reaching the cell surface when expressed alone. In contrast, the long splice variant (γ2L), containing an 8 amino acid insert, is retained in the ER unless co-expressed with both α1 and β2 Citation[11]. Thus, in addition to a strict quality control mechanism operating on receptor folding and assembly within the lumen of the ER, an additional mechanism exists on the cytoplasmic side of the ER membrane. Together, these mechanisms combine to determine which surface expressed receptor compositions are permitted.
Although, the identification of assembly and ERS signals provides insight into the receptor combinations that are capable of generating cell surface functional ion channels, they provide no information regarding how this process is orchestrated. Although a number of generalized ER chaperone molecules, such as Immunoglobulin binding protein (BiP), calnexin and protein disulphide isomerase, have been shown to orchestrate receptor folding and glycosylation and return immature subunits to the ER, they operate indiscriminately Citation[5], Citation[12]. The recent discovery of RIC-3 (resistance to inhibitors of acetylcholinesterase 3) has opened the doors to the existence of discriminatory ER chaperone molecules. Like the general ER chaperones, RIC-3 is ER-localized (). RIC-3 is a transmembrane protein that exhibits chaperone activity on 5-HT3 and nicotinic acetylcholine receptors, but not on glutamate or GABAA receptors (at least for the subunit combinations examined). RIC-3 promotes the folding, assembly and/or exit from the ER of 5-HT3A receptors Citation[13]. Similar properties have been reported for its effects on nicotinic acetylcholine receptors Citation[14]. Although the 5-HT3B subunit is preferentially incorporated into the pentamer Citation[6] at sites that the 5-HT3A subunit could occupy, upon the co-expression of 5-HT3A and 5-HT3B with RIC-3, homomeric 5-HT3A receptor assembly is favoured Citation[15]. Therefore, RIC-3 expression appears to reverse the normal preference for 5-HT3B subunits and so manipulate 5-HT3 receptor composition.
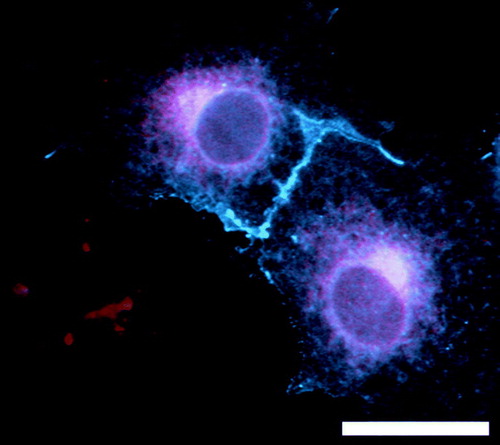
Figure 1. COS7 cells expressing RIC3(isoform d)-DsRed (red) and 5-HT3A-CFP (blue). RIC3 is restricted to the ER and associated vesicular structures that overlap with 5-HT3A receptors. In addition, 5-HT3A receptors are observed on the cell surface in microspikes and lamellopodia. Scale bar = 15 µm.
A large amount of controversy exists around RIC-3, with significant species differences being reported Citation[14]. Mammalian RIC-3 has been shown to possess a cleavable signal sequence Citation[15]. However, in RIC-3 from invertebrates, this region is predicted to form a non-cleaveable transmembrane domain. Moreover, whereas RIC-3 was shown to inhibit the functional expression of certain (α3β4 and α4β2) nACh receptors in Xenopus oocytes, in mammalian cells, a paradoxical enhancement of these receptors was observed Citation[16]. Similarly, human RIC-3 has been reported to abolish the functional expression of murine 5-HT3A receptors in oocytes Citation[17], yet enhance the functional expression of human 5-HT3A in mammalian cells Citation[13], Citation[15]. Finally, drosophila RIC-3 promotes the functional expression of nACh receptors (mammalian or drosophila receptors) more efficiently in drosophila cells, than in human cells Citation[18]. The reciprocal is also true for human RIC-3. Together, these results point to the existence of other, host cell specific, accessory cofactors.
It remains to be established whether RIC-3 expression constitutively drives the formation of 5-HT3A receptors or if it may operate to dynamically regulate receptor composition. Moreover, how RIC-3 would handle the expression of 5-HT3C-E is currently unknown. Similar to that of RIC-3, cyclophilin A has been reported to promote the functional expression of 5-HT3A receptors. In the case of cyclophilin A this has been attributed to its peptidyl prolyl isomerase activity Citation[19]. Again, little is known regarding how this operates on 5-HT3B-E receptors.
To study receptor trafficking beyond the ER a combination of a fluorescently labelled antagonist and a fluorescent chimaera with 5-HT3A receptors were utilized Citation[20]. Using a fluorescently labelled 5-HT3A construct (5-HT3A-CFP), receptors were detectable within the ER within 3 h, the Golgi within 4 h and the surface within 4.5 h. As expected, ligand binding sites were detected within the ER, Golgi and on the cell surface. In the presence of cycloheximide, to block new protein synthesis, the majority of receptors were localized to the cell surface. The 5-HT3A receptor is trafficked to the cell surface within vesicles associated with microtubules and delivered to actin-rich surface domains such as lamellipodia and microspikes Citation[20], Citation[21] (). This localization may be relevant to the targeting of receptors to actin-rich synapses (see below). Overall, these findings are consistent with a rate-limiting step existing at the ER Citation[22] where subunits are folded and assembled prior to rapid transport to the cell surface.
The detection of native 5-HT3 receptors in neurons is difficult due their low expression levels. Nevertheless, native 5-HT3AS receptors (a short splice variant) have been identified to exist as dendritic clusters that colocalize with actin in cultured hippocampal neurons Citation[21]. When 5-HT3AS receptors were expressed recombinantly in these cells, receptors were preferentially localized to both filamentous and clustered (1–2 µm2) actin structures. The maintenance of these clusters appears to depend on the actin cytoskeleton, as the disruption of the cytoskeleton lead to a dispersal of the clusters into smaller structures (<0.5 µm2) Citation[21]. Similarly, 5-HT3 receptors have been localized to the tips of micropodia (HEK cells) and on dendritic spines Citation[23]. To date, no information is available regarding the existence of synaptic and/or actin anchoring proteins that might be responsible for 5-HT3 receptor targeting and retention at synaptic sites.
In addition to their postsynaptic localization Citation[21], Citation[23], 5-HT3A receptors have been localized to presynaptic GABAergic (inhibitory) nerve terminals in the amygdala Citation[24] and CA1 region of the hippocampus Citation[25]. Within the superficial dorsal horn, 5-HT3A receptors are localized exclusively to presynaptic glutamatergic (excitatory) synapses Citation[26]. Similarly, 5-HT3A receptors have been localized to neuronal terminals in axons and dendrites, as well as in glial cell membranes within the medial nucleus of the solitary tract Citation[27]. These findings are consistent with a role of presynaptic 5-HT3A receptors in the modulation of neurotransmitter (including GABA, 5-HT, glutamate, dopamine and acetylcholine) release, as well as in postsynaptic responses. Moreover, the localization of 5-HT3A receptors to glial processes apposing 5-HT3A receptor containing axons and dendrites suggests that the receptors may participate in glial signalling pathways in response to the same 5-HT release events.
Unusually, a large amount of intracellular 5-HT3A receptors have been observed Citation[27] suggesting that 5-HT3A receptor-containing synapses may be dynamically regulated. Although some intracellular receptors exist within the biosynthetic pathway (ER and Golgi), indicating the delivery of newly synthesized receptors, others may be derived from the endocytic removal of surface receptors from the somatodendritic (endosomes) or axonal (dense core vesicles) membranes Citation[27]. Indeed, intracellularly retained, unassembled NR1 subunits are thought to serve as substrates for the assembly of NMDA receptors Citation[28]. Moreover, intracellular AMPA receptors residing within the biosynthetic and endosomal pathways are thought to contribute to trafficking events leading to synaptic targeting and the modification of synaptic strength Citation[29].
Using novel live imaging techniques Citation[30], it was determined that a large variability in cell surface mobility of 5-HT3A receptors exists. This variability most likely reflects a heterogeneous population of receptors that are either freely diffusable or anchored to the actin cytoskeleton Citation[20], Citation[21]. Interestingly, 5-HT3A receptor mobility decreases in response to exposure to the receptor agonist, 5-HT. This raises the possibility that synaptic 5-HT3 receptor anchoring may be triggered at sites of 5-HT release. However, in support of the endocytic regulation of 5-HT3A receptors, recombinantly expressed surface receptors were shown to be dynamically removed when exposed to the continued presence of agonist, with ∼50% of surface receptors being internalized within 5 min Citation[20]. Similar receptor internalization has been observed for native receptors in myenteric neurons Citation[31]. Interestingly, actin appears to play a role in the recruitment of 5-HT3A receptors to the cell surface via the stimulation of PKC activity Citation[32]. The PKC-stimulated 5-HT3A receptor delivery to the cell surface appears to be inhibited by polymerized actin and it has been hypothesized that actin may provide an inhibitory barrier to receptor recruitment Citation[32]. Interestingly, these processes are reminiscent of GABAA receptor internalization Citation[33]. Together, these studies suggest a possible scenario for the synaptic targeting of 5-HT3 receptors, in which receptors are delivered to cell surface extrasynaptic sites and diffuse into synapses where they may be anchored to actin in response to 5-HT release. Such a scenario has been reported for the GABAA receptors (see below). To date, there is little detail regarding how 5-HT3 receptors are trafficked to the cell surface or synapses, or how they are removed. Extrapolation from the evidence obtained on other Cys-loop receptors provides some clues as to where to look. However, no trafficking or anchoring molecules have been identified that operate on 5-HT3 receptors and no receptor interacting proteins (other than RIC-3) have been reported.
GABAA receptors
GABAA receptors are pentameric ion channels constructed from a repertoire of subunits that include α1-6, β1-3, γ1-3, δ, ε, π and θ. A detailed account of GABAA receptor assembly is presented elsewhere in this issue (Sieghart, this issue). With respect to the most common composition of GABAA receptors (α1β2γ2) in the brain, the minimal requirement for the production of functional, GABA gated, ion channels is the incorporation of α1 and β2 subunits and inclusion of the γ2 subunit is required for sensitivity to benzodiazepines Citation[12]. It has been thought that the incorporation of subunits from the remaining classes (δ, ε, π and θ) occurs as a direct replacement for the γ subunit, but this has now been questioned for the ε Citation[34], Citation[35] and θ Citation[36] subunits.
Although no proteins, such as RIC-3, have been reported to manipulate receptor composition, there is one potential candidate, that of GABARAP (GABAA receptor associated protein). GABARAP is a molecule that acts as a linker between the γ2 subunit and microtubules and promotes (but is not essential for) the synaptic clustering of GABAA receptors Citation[37]. Although GABARAP enhances the surface expression (and so synaptic localization) of GABAA receptors, it is not localized to inhibitory synapses Citation[38], ruling out a role in synaptic anchoring. Instead, GABARAP is enriched in ER and Golgi structures. GABARAP has been reported to exert a number of influences on GABAA receptors. These include decreased desensitization, a rightward shift in EC50 for GABA, increased conductance and increased synaptic targeting. All of these features are consistent with the encouragement of γ2-containing receptors. Intriguingly, when the GABAA receptor composition is constrained (by physically linking subunits into concatemers) to the production of αβγ, such that αβ receptors cannot be formed, GABARAP no longer exhibits any effects on promoting a γ2 physiological phenotype Citation[39]. Likewise, when the γ2 subunit is present in large excess (1:1:10 ratio), GABARAP appears to have no effect. Therefore, it is possible that an important function of GABARAP is to promote the incorporation of γ2 into αβγ receptors. A role for GABARAP in promoting the formation of αβγ, over αβ, GABAA receptors is reminiscent of the role RIC-3 plays in promoting the formation of 5-HT3A, at the expense of 5-HT3A/3B, receptors Citation[15].
An alternative role for GABARAP is suggested by its homology to GATE-16 (Golgi-associated transport enhancer of 16KDa), pointing to a possible role in vesicular trafficking. Interestingly, P130 competitively inhibits GABARAP binding to γ2, yet P130 is required for the efficient expression of γ2-containing receptors Citation[40]. This would be consistent with a transient requirement for the interaction of γ2 with GABARAP (during its assembly?). Subsequent trafficking steps might then require the interaction with other molecules, such as P130. GABARAP has also been shown to interact with NSF (N-ethylmalemide-sensitive factor) and GRIP-1 (glutamate receptor interacting protein 1) Citation[38], Citation[41]. Both these molecules have been shown to play a role in the trafficking and synaptic localization of AMPA receptors Citation[29], but their relevance to these processes in GABAA receptor trafficking is not established.
Once GABAA receptors have assembled in the ER, they are transported through the Golgi stacks and trans-Golgi network, prior to transport to the cell surface. This may involve Plic-1, which is associated with intracellular membranes including the ER and membranes at the edges of Golgi, but is not concentrated at sites of surface clustered GABAA receptors Citation[42]. Plic-1 interacts with the cytoplasmic domains of α and β, but not γ2 or δ subunits. Interestingly, inhibiting the Plic-1 interaction with α subunits, leads to a decrease in surface receptor levels (but no enhancement of receptor internalization). Thus, Plic-1 may operate by enhancing the recruitment of receptors to the cell surface. The observation that Plic-1 increases receptor stability is consistent with the proposed role for Plic-1 as a negative regulator of the proteasome and it has been proposed that Plic-1 traffics with the receptors, along the biosynthetic pathway, protecting them from degradation. However, an enhancement of subunit folding, receptor assembly or transport out of the ER would all lead to protection against proteasomal degradation. Indeed, these are the most likely mechanisms by which RIC-3 operates on 5-HT3 and nACh receptors. Therefore, it is possible that Plic-1 may exhibit chaperone-like activity on newly synthesized GABAA receptors that is independent of proteasomal activity. At the cell surface, it is possible that Plic-1 provides protection against proteasomal/lysosomal degradation within the endocytic pathway and so permit the recycling of internalized receptors back to the cell surface Citation[42].
Within the Golgi, GABAA receptors interact with a range of molecules involved in further trafficking steps. A palmitoyltransferase, GODZ (Golgi specific protein with the DHHC zinc finger domain) is able to interact with the γ2 subunit and trap the subunit within the Golgi unless it is assembled with α and β subunits Citation[43]. This may be relevant, given the ability of γ2S to escape the ER and traffic to the cell surface prior to assembling with α1 and β2 Citation[11]. In the presence of assembled receptors (αβγ), GODZ can be observed beneath, but not on, the cell surface Citation[43]. Thus GODZ may also be involved in the transport of GABAA receptors to the cell surface. GODZ does not form a stable interaction with the γ2 subunit, but has been shown to palmitoylate the γ2 subunit and this has been reported to be a requirement for the synaptic localization of receptors Citation[44], Citation[45]. It is important to note that surface trafficking is a prerequisite to synaptic targeting, and this has also been shown to require palmitoylation Citation[45]. Palmitoylation does not appear to be restricted to the GABAA receptor members of the Cys-loop family, both α7 nACh and 5-HT3A receptors are palmitoylated Citation[46]. In the case of these receptors, it appears that palmitoylation occurs during receptor assembly and is required for the formation of ligand binding sites and cell surface expression.
Another molecule primarily localized to the Golgi and is involved in GABAA receptor targeting is BIG2 (brefeldin A-inhibited GDP/GTP exchange factor 2) that interacts with β subunits Citation[47]. Although it is primarily localized to the trans Golgi network and at the ends of Golgi cisterae, it has also been identified within presynaptic and postsynaptic, inhibitory and excitatory, synapses, where it is associated with the synaptic membrane and intracellular vesicles. In addition, the overexpression of BIG2 has been reported to increase GABAA receptor release from the ER, a compartment that is upstream in the biosynthetic pathway. Therefore, BIG2 may be more widely distributed, dynamically moving throughout the biosynthetic and endocytic pathways and may play a general role in protein trafficking.
GABAA receptors may be selected for forward transport, or simply guided, through the biosynthetic pathway by their interaction with GRIF1 (GABAA receptor interacting factor-1, otherwise known as TRAK2). GRIF-1 is a cytoplasmically localized protein that is thought to be an adaptor, linking organelles to kinesin 1 motor proteins, to facilitate their anterograde transport Citation[48]. GRIF-1 also interacts with α and β subunits of GABAA receptors Citation[49] and may be involved in their surface and/or synaptic targeting.
At the cell surface, GABAA receptors may exist at synaptic or extrasynaptic sites where they participate in phasic (synaptically induced, by presynaptic neurotransmitter release) and tonic (intrinsic, non-synaptic) inhibition. Synaptic targeting of GABAA receptors requires the incorporation of the γ subunit Citation[50], while δ-containing receptors are found predominantly extrasynaptically Citation[51]. The GABAA receptor expression level on the neuronal surface is a major contributor to the level of neuronal inhibition. This is well illustrated in heterozygotes of γ2 knockout mice. These animals have reduced synaptic GABAA receptor levels and exhibit anxiety that may be relieved by enhancing GABAA receptor activity with benzodiazepine treatment Citation[52].
Importantly, GABAA receptors are not thought to be static portals of neuronal inhibition. Rather, they represent a dynamic pool of receptors undergoing rapid recruitment to, and removal from, the neuronal surface and synapses. Therefore, the exocytic and endocytic pathways that regulate GABAA receptor trafficking are directly relevant to the inhibitory control of neuronal activity in the brain. Further detail regarding GABAA receptor trafficking is available in recent reviews Citation[33], Citation[37], Citation[53].
To date, the only molecule implicated in the synaptic anchoring of GABAA receptors is gephyrin. Interestingly, it appears that both the γ2 subunit and gephyrin are mutually required for synaptic localization at most, but not all, synapses Citation[54]. At extrasynaptic sites GABAA receptor clusters appear to anchor to the actin cytoskeleton via an ERM (ezrin/radixin/moesin) family protein, radixin Citation[54].
Once on the cell surface, GABAA receptors exhibit significant movement as a result of lateral diffusion and constitutive exocytosis and endocytosis. By engineering a binding site for an activity-dependent, irreversible inhibitor, it has been possible to selectively inactivate receptors that were stimulated by synaptic activity Citation[55]. Using this approach to inactivate synaptic receptors, it was discovered that synaptic activity recovered rapidly, within minutes. No recovery occurred when all surface receptors were blocked. In contrast, synaptic recovery was not blocked when the exocytosis of intracellular receptors was inhibited. Therefore, these findings provide strong evidence that synaptic GABAA receptors are recruited from an extrasynaptic surface pool. This conclusion is supported by studies that determined that synaptic receptors are preferentially stabilized at synapses Citation[56]. Moreover, extrasynaptic GABAA receptors are constitutively recycled by endocytosis and exocytosis. Both the machinery for clathrin-dependent endocytosis and the sites of delivery of newly inserted receptors were found to be extrasynaptic Citation[57]. Together, these studies show that surface GABAA receptor levels are regulated at extrasynaptic sites. Synapses then draw on this mobile pool of receptors as required.
In addition to the surface extrasynaptic pool, a significant intracellular pool of receptors is also available. Indeed, there are both constitutive and regulated pathways for GABAA receptor endocytosis, and receptors may be either recycled to the cell surface or degraded Citation[58–60]. The constitutive endocytosis of GABAA receptors occurs via clathrin-coated vesicles to which GABAA receptors are recruited by a novel AP2 binding motif (KTHLRRRSSQLK) Citation[60], which is conserved in all β subunits. This process appears to require the recruitment of PRIP (phopholipase C-related catalytically inactive protein) to β subunits Citation[59]. In addition, γ2-containing GABAA receptors can undergo regulated, PKC-activated, endocytosis Citation[58]. This regulated GABAA receptor endocytosis utilizes a distinct second AP2 binding domain is based on a di-leucine motif present in β subunits Citation[61]. Interestingly, the constitutive endocytosis signal binding to AP2 is blocked by phoshorylation. Given that this motif carries a PKC phosphorylation site, constitutive endocytosis of GABAA receptors may be blocked, and regulated endocytosis potentiated, upon PKC activation. Such an event might deplete both the extrasynaptic and intracellular pools of receptors by endocytosis and degradation and so limit the normal maintenance of synaptic receptors. This process may regulate the functions of HAP-1 (Huntingtin-associated protein) and NSF in controlling GABAA receptor surface expression. HAP-1 appears to interact with β subunits and protect GABAA receptors from lysosomal degradation and so facilitate receptor recycling to the cell surface Citation[62]. NSF has been shown to interact with β subunits at, or near the cell surface and lead to a decrease in surface receptor levels Citation[63]. This is in contrast to its interaction with GABARAP that presumably (this has not been shown) promotes the delivery of GABAA receptors to the surface. The mechanism(s) by which NSF may play such opposing roles has not been established, but it may enable the recruitment of synaptic (γ2-containing) receptors via NSF-GABARAP interactions in the biosynthetic pathway, and the removal of extrasynaptic (non γ2-containing) receptors via a direct interaction with the β subunits in the endocytic pathway. These mechanisms may play important pathological roles in the reduced phasic and/or tonic inhibition observed as a result of reduced surface GABAA receptor expression during anxiety Citation[52], epilepsy Citation[64–67] and ischemia Citation[68].
Concluding remarks
It is clear that the pooling of knowledge from both 5-HT3 and GABAA receptors, as well as that of nACh (Millar, this issue) and glycine (Becker, this issue) receptors, strengthens our understanding of Cys-loop receptor trafficking as a whole. Clearly, more research is needed to identify the array of accessory proteins (that undoubtedly exist) involved in the regulation of 5-HT3 receptor biogenesis, trafficking and synaptic anchoring. Moreover, given the localization of several of the identified proteins to multiple points on the biosynthetic and endocytic pathways, it will be important to investigate their dynamic mobility using time-lapse microscopy. If this could be performed with respect to the receptors that they traffic, as well as the movement of the other accessory molecules, a dynamic picture of the entire process may emerge. This is an exciting prospect, especially if it could then be investigated during pathological states. This may identify critical points at which the process is perturbed in conditions such as ischemia, epilepsy, anxiety, schizophrenia and the neurodegenerative diseases. A full understanding of how these receptor trafficking events are orchestrated and regulated may provide novel therapeutic opportunities.
References
- Connolly CN, Wafford KA. The Cys-loop superfamily of ligand-gated ion channels: the impact of receptor structure on function. Biochem Soc Trans 2004; 32: 529–534
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 2001; 411: 269–276
- Thompson AJ, Lummis SC. 5-HT3 receptors. Curr pharm design 2006; 12: 3615–3630
- Brady CA, Dover TJ, Massoura AN, Princivalle AP, Hope AG, Barnes NM. Identification of 5-HT3A and 5-HT3B receptor subunits in human hippocampus. Neuropharm 2007; 52: 1284–1290
- Boyd GW, Low P, Dunlop JI, Robertson LA, Vardy A, Lambert JJ, Peters JA, Connolly CN. Assembly and cell surface expression of homomeric and heteromeric 5-HT3 receptors: the role of oligomerization and chaperone proteins. Mol Cell Neuro 2002; 21: 38–50
- Barrera NP, Herbert P, Henderson RM, Martin IL, Edwardson JM. Atomic force microscopy reveals the stoichiometry and subunit arrangement of 5-HT3 receptors. Proc Natl Acad Sci (USA) 2005; 102: 12595–12600
- Niesler B, Walstab J, Combrink S, Moller D, Kapeller J, Rietdorf J, Bonisch H, Gothert M, Rappold G, Bruss M. Characterization of the novel human serotonin receptor subunits 5-HT3C,5-HT3D, and 5-HT3E. Mol Pharm 2007; 72: 8–17
- Quirk PL, Rao S, Roth BL, Siegel RE. Three putative N-glycosylation sites within the murine 5-HT3A receptor sequence affect plasma membrane targeting, ligand binding, and calcium influx in heterologous mammalian cells. J Neuro Res 2004; 77: 498–506
- Monk SA, Williams JM, Hope AG, Barnes NM. Identification and importance of N-glycosylation of the human 5-hydroxytryptamine3A receptor subunit. Biochem Pharmacol 2004; 68: 1787–1796
- Boyd GW, Doward AI, Kirkness EF, Millar NS, Connolly CN. Cell surface expression of 5-hydroxytryptamine type 3 receptors is controlled by an endoplasmic reticulum retention signal. J Biol Chem 2003; 278: 27681–27687
- Connolly CN, Uren JM, Thomas P, Gorrie GH, Gibson A, Smart TG, Moss SJ. Subcellular localization and endocytosis of homomeric gamma2 subunit splice variants of gamma-aminobutyric acid type A receptors. Mol Cell Neuro 1999; 13: 259–271
- Connolly CN, Krishek BJ, McDonald BJ, Smart TG, Moss SJ. Assembly and cell surface expression of heteromeric and homomeric gamma-aminobutyric acid type A receptors. J Biol Chem 1996; 271: 89–96
- Cheng A, McDonald NA, Connolly CN. Cell surface expression of 5-hydroxytryptamine type 3 receptors is promoted by RIC-3. J Biol Chem 2005; 280: 22502–22507
- Millar NS. 2007. RIC-3: A nicotinic acetylcholine receptor chaperone. Brit J Pharmacol Feb 4, [epub ahead of print].
- Cheng A, Bollan KA, Greenwood SM, Irving AJ, Connolly CN. Differential subcellular localization of RIC-3 isoforms and their role in determining 5-HT3 receptor composition. J Biol Chem 2007; 282: 26158–26166
- Lansdell SJ, Gee VJ, Harkness PC, Doward AI, Baker ER, Gibb AJ, Millar NS. RIC-3 enhances functional expression of multiple nicotinic acetylcholine receptor subtypes in mammalian cells. Mol Pharm 2005; 68: 1431–1438
- Halevi S, Yassin L, Eshel M, Sala F, Sala S, Criado M, Treinin M. Conservation within the RIC-3 gene family. Effectors of mammalian nicotinic acetylcholine receptor expression. J Biol Chem 2003; 278: 34411–34417
- Lansdell S, Collins T, Yabe A, Gee VJ, Gibb AJ, Millar NS. 2008. Host-cell specific effects of the nicotinic receptor-associated protein RIC-3 revealed by a comparison of human and drosophila RIC-3 homologues. J Neurochem Jan 18, [epub ahead of print].
- Helekar SA, Patrick J. Peptidyl prolyl cis-trans isomerase activity of cyclophilin A in functional homo-oligomeric receptor expression. Proc Natl Acad Sci (USA) 1997; 94: 5432–5437
- Ilegems E, Pick HM, Deluz C, Kellenberger S, Vogel H. Noninvasive imaging of 5-HT3 receptor trafficking in live cells: from biosynthesis to endocytosis. J Biol Chem 2004; 279: 53346–53352
- Emerit MB, Doucet E, Darmon M, Hamon M. Native and cloned 5-HT(3A)(S) receptors are anchored to F-actin in clonal cells and neurons. Mol Cell Neuro 2002; 20: 110–124
- Green WN, Millar NS. Ion-channel assembly. Trends Neorosci. 1995; 18: 280–287
- Grailhe R, de Carvalho LP, Paas Y, Le Poupon C, Soudant M, Bregestovski P, Changeux JP, Corringer PJ. Distinct subcellular targeting of fluorescent nicotinic alpha 3 beta 4 and serotoninergic 5-HT3A receptors in hippocampal neurons. Eur J Neurosci 2004; 19: 855–862
- Koyama S, Matsumoto N, Kubo C, Akaike N. Presynaptic 5-HT3 receptor-mediated modulation of synaptic GABA release in the mechanically dissociated rat amygdala neurons. J Physiol 2000; 529: 373–383
- Katsurabayashi S, Kubota H, Tokutomi N, Akaike N. A distinct distribution of functional presynaptic 5-HT receptor subtypes on GABAergic nerve terminals projecting to single hippocampal CA1 pyramidal neurons. Neuropharm 2003; 44: 1022–1030
- Conte D, Legg ED, McCourt AC, Silajdzic E, Nagy GG, Maxwell DJ. Transmitter content, origins and connections of axons in the spinal cord that possess the serotonin (5-hydroxytryptamine) 3 receptor. Neuroscience 2005; 134: 165–173
- Huang J, Spier AD, Pickel VM. 5-HT3A receptor subunits in the rat medial nucleus of the solitary tract: subcellular distribution and relation to the serotonin transporter. Brain Res 2004; 1028: 156–169
- Atlason PT, Garside ML, Meddows E, Whiting P, McIlhinney RA. N-Methyl-D-aspartate (NMDA) receptor subunit NR1 forms the substrate for oligomeric assembly of the NMDA receptor. J Biol Chem 2007; 282: 25299–25307
- Greger IH, Esteban JA. AMPA receptor biogenesis and trafficking. Curr Opin Neurobiol 2007; 17: 289–297
- Guignet EG, Segura JM, Hovius R, Vogel H. Repetitive reversible labeling of proteins at polyhistidine sequences for single-molecule imaging in live cells. Chemphyschem 2007; 8: 1221–1227
- Freeman SL, Glatzle J, Robin CS, Valdellon M, Sternini C, Sharp JW, Raybould HE. Ligand-induced 5-HT3 receptor internalization in enteric neurons in rat ileum. Gastroenterol 2006; 131: 97–107
- Sun H, Hu XQ, Moradel EM, Weight FF, Zhang L. Modulation of 5-HT3 receptor-mediated response and trafficking by activation of protein kinase C. J Biol Chem 2003; 278: 34150–34157
- Leidenheimer NJ. Regulation of excitation by GABA(A) receptor internalization. Res Prob Cell Diff 2008; 44: 1–28
- Bollan KA, Baur R, Hales TG, Sigel E, Connolly CN. 2008. The promiscuous role of the epsilon subunit in GABAA receptor biogenesis. Mol Cell Neuro Dec 15, [epub ahead of print].
- Jones BL, Henderson LP. Trafficking and potential assembly patterns of epsilon-containing GABAA receptors. J Neurochem 2007; 103: 1258–1271
- Bonnert TP, McKernan RM, Farrar S, le Bourdelles B, Heavens RP, Smith DW, Hewson L, Rigby MR, Sirinathsinghji DJ, Brown N, Wafford KA, Whiting PJ. theta, a novel gamma-aminobutyric acid type A receptor subunit. Proc Natl Acad Sci (USA) 1999; 96: 9891–9896
- Chen ZW, Olsen RW. GABAA receptor associated proteins: a key factor regulating GABAA receptor function. J Neurochem 2007; 100: 279–294
- Kittler JT, Rostaing P, Schiavo G, Fritschy JM, Olsen R, Triller A, Moss SJ. The subcellular distribution of GABARAP and its ability to interact with NSF suggest a role for this protein in the intracellular transport of GABA(A) receptors. Mol Cell Neuro 2001; 18: 13–25
- Boileau AJ, Pearce RA, Czajkowski C. Tandem subunits effectively constrain GABAA receptor stoichiometry and recapitulate receptor kinetics but are insensitive to GABAA receptor-associated protein. J Neurosci 2005; 25: 11219–11230
- Kanematsu T, Jang IS, Yamaguchi T, Nagahama H, Yoshimura K, Hidaka K, Matsuda M, Takeuchi H, Misumi Y, Nakayama K, Yamamoto T, Akaike N, Hirata M, Nakayama K. Role of the PLC-related, catalytically inactive protein p130 in GABA(A) receptor function. EMBO J 2002; 21: 1004–1011
- Li RW, Serwanski DR, Miralles CP, Li X, Charych E, Riquelme R, Huganir RL., de Blas AL. GRIP1 in GABAergic synapses. J Comp Neurol 2005; 488: 11–27
- Bedford FK, Kittler JT, Muller E, Thomas P, Uren JM, Merlo D, Wisden W, Triller A, Smart TG, Moss SJ. GABA(A) receptor cell surface number and subunit stability are regulated by the ubiquitin-like protein Plic-1. Nat Neuro 2001; 4: 908–916
- Keller CA, Yuan X, Panzanelli P, Martin ML, Alldred M, Sassoe-Pognetto M, Luscher B. The gamma2 subunit of GABA(A) receptors is a substrate for palmitoylation by GODZ. J Neurosci 2004; 24: 5881–5891
- Fang C, Deng L, Keller CA, Fukata M, Fukata Y, Chen G, Luscher B. GODZ-mediated palmitoylation of GABA(A) receptors is required for normal assembly and function of GABAergic inhibitory synapses. J Neurosci 2006; 26: 12758–12768
- Rathenberg J, Kittler JT, Moss SJ. Palmitoylation regulates the clustering and cell surface stability of GABAA receptors. Mol Cell Neuro 2004; 26: 251–257
- Drisdel RC, Manzana E, Green WN. The role of palmitoylation in functional expression of nicotinic alpha7 receptors. J Neurosci 2004; 24: 10502–10510
- Charych EI, Yu W, Miralles CP, Serwanski DR, Li X, Rubio M, De Blas AL. The brefeldin A-inhibited GDP/GTP exchange factor 2, a protein involved in vesicular trafficking, interacts with the beta subunits of the GABA receptors. J Neurochem 2004; 90: 173–189
- Brickley K, Smith MJ, Beck M, Stephenson FA. GRIF-1 and OIP106, members of a novel gene family of coiled-coil domain proteins: association in vivo and in vitro with kinesin. J Biol Chem 2005; 280: 14723–14732
- Beck M, Brickley K, Wilkinson HL, Sharma S, Smith M, Chazot PL, Pollard S, Stephenson FA. Identification, molecular cloning, and characterization of a novel GABAA receptor-associated protein, GRIF-1. J Biol Chem 2002; 277: 30079–30090
- Baer K, Essrich C, Benson JA, Benke D, Bluethmann H, Fritschy JM, Luscher B. Postsynaptic clustering of gamma-aminobutyric acid type A receptors by the gamma3 subunit in vivo. Proc Natl Acad Sci (USA) 1999; 96: 12860–12865
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci 1998; 18: 1693–1703
- Anagnostaras SG, Craske MG, Fanselow MS. Anxiety: at the intersection of genes and experience. Nat Neuro 1999; 2: 780–782
- Michels G, Moss SJ. GABAA receptors: properties and trafficking. Crit Rev Biochem Mol Biol 2007; 42: 3–14
- Kneussel M, Loebrich S. Trafficking and synaptic anchoring of ionotropic inhibitory neurotransmitter receptors. Biology of the cell/under the auspices of the E Cell Biol Org 2007; 99: 297–309
- Thomas P, Mortensen M, Hosie AM, Smart TG. Dynamic mobility of functional GABAA receptors at inhibitory synapses. Nat Neuro 2005; 8: 889–897
- Jacob TC, Bogdanov YD, Magnus C, Saliba RS, Kittler JT, Haydon PG, Moss SJ. Gephyrin regulates the cell surface dynamics of synaptic GABAA receptors. J Neurosci 2005; 25: 10469–10478
- Bogdanov Y, Michels G, Armstrong-Gold C, Haydon PG, Lindstrom J, Pangalos M, Moss SJ. Synaptic GABAA receptors are directly recruited from their extrasynaptic counterparts. EMBO J 2006; 25: 4381–4389
- Connolly C N, Kittler JT, Thomas P, Uren JM, Brandon NJ, Smart TG, Moss SJ. Cell surface stability of gamma-aminobutyric acid type A receptors. Dependence on protein kinase C activity and subunit composition. J Biol Chem 1999; 274: 36565–36572
- Kanematsu T, Fujii M, Mizokami A, Kittler JT, Nabekura J, Moss SJ, Hirata M. Phospholipase C-related inactive protein is implicated in the constitutive internalization of GABAA receptors mediated by clathrin and AP2 adaptor complex. J Neurochem 2007; 101: 898–905
- Kittler JT, Chen G, Honing S, Bogdanov Y, McAinsh K, Arancibia-Carcamo IL, Jovanovic JN, Pangalos MN, Haucke V, Yan Z, Moss SJ. Phospho-dependent binding of the clathrin AP2 adaptor complex to GABAA receptors regulates the efficacy of inhibitory synaptic transmission. Proc Natl Acad Sci (USA) 2005; 102: 14871–14876
- Herring D, Huang R, Singh M, Dillon GH, Leidenheimer NJ. PKC modulation of GABAA receptor endocytosis and function is inhibited by mutation of a dileucine motif within the receptor beta 2 subunit. Neuropharm 2005; 48: 181–194
- Kittler JT, Thomas P, Tretter V, Bogdanov YD, Haucke V, Smart TG, Moss SJ. Huntingtin-associated protein 1 regulates inhibitory synaptic transmission by modulating gamma-aminobutyric acid type A receptor membrane trafficking. Proc Natl Acad Sci (USA) 2004; 101: 12736–12741
- Goto H, Terunuma M, Kanematsu T, Misumi Y, Moss SJ, Hirata M. Direct interaction of N-ethylmaleimide-sensitive factor with GABA(A) receptor beta subunits. Mol Cell Neuro 2005; 30: 197–206
- Blair RE, Sombati S, Lawrence DC, McCay BD, DeLorenzo RJ. Epileptogenesis causes acute and chronic increases in GABAA receptor endocytosis that contributes to the induction and maintenance of seizures in the hippocampal culture model of acquired epilepsy. J Pharm Exp Ther 2004; 310: 871–880
- Goodkin HP, Sun C, Yeh JL, Mangan PS, Kapur J. GABA(A) receptor internalization during seizures. Epilepsia (Suppl.) 5 2007; 48: 109–113
- Kang JQ, Shen W, Macdonald RL. Why does fever trigger febrile seizures? GABAA receptor gamma2 subunit mutations associated with idiopathic generalized epilepsies have temperature-dependent trafficking deficiencies. J Neurosci 2006; 26: 2590–2597
- Naylor DE, Liu H, Wasterlain CG. Trafficking of GABA(A) receptors, loss of inhibition, and a mechanism for pharmacoresistance in status epilepticus. J Neurosci 2005; 25: 7724–7733
- Mielke JG, Wang YT. Insulin exerts neuroprotection by counteracting the decrease in cell-surface GABA receptors following oxygen-glucose deprivation in cultured cortical neurons. J Neurochem 2005; 92: 103–113